Abstract
Introduction
Gram-negative sepsis remains one of the most difficult to treat infections in intensive care units (ICUs). Carbapenems are often considered to be robust and reliable options for treating infections due to Gram-negative bacteria. The dominance of carbapenem-resistant enterobacteriaceae (CRE) has emerged as one of the greatest challenges faced by the medical community today. Carbapenem-resistant enterobacteriaceae may be resistant to all beta lactam antimicrobials including carbapenems and often, are even resistant to other classes of drugs. There are limited studies comparing polymyxin-based therapies with ceftazidime-avibactam (CAZ-AVI)-based therapies for treating infections caused by CRE.
Methods
A retrospective study comparing outcomes between patients with bacteremia caused by CRE treated with polymyxin-based combination therapy and CAZ-AVI-based therapy (with or without aztreonam).
Results
Of total 104 patients, 78 (75%) were in the CAZ-AVI group. There was no significant difference in the underlying comorbidities between the two groups. The incidence of nephrotoxicity was significantly higher in the polymyxin group (p = 0.017). Ceftazidime-avibactam-based therapy was 66% less likely to be associated with day 14 mortality (p = 0.048) and 67% less likely to be associated with day 28 mortality (p = 0.039) as compared with polymyxin-based therapy.
Conclusion
Ceftazidime-avibactam-based therapy may be a superior option to polymyxin-based therapy for infections caused by CRE. This can have significant practical applications, in terms of optimizing therapy for the individual patient as well as sparing polymyxins and reducing the use of polymyxins in our hospitals.
How to cite this article
Prayag PS, Patwardhan SA, Panchakshari S, Sambasivam R, Dhupad S, Soman RN, et al. Ceftazidime-avibactam with or without Aztreonam vs Polymyxin-based Combination Therapy for Carbapenem-resistant Enterobacteriaceae: A Retrospective Analysis. Indian J Crit Care Med 2023;27(6):444–450.
Keywords: Carbapenems, Carbapenem-resistant enterobacteriaceae, Ceftazidime-avibactam, Gram-negative sepsis, Polymyxin
Highlights
Ceftazidime-avibactam-based therapy is less associated with day 14 mortality than polymyxin.
Ceftazidime-avibactam-based therapy is less associated with day 28 mortality than polymyxin.
Ceftazidime-avibactam-based therapy is a superior option to polymyxin-based therapy for CRE.
Introduction
Gram-negative sepsis remains one of the most difficult to treat infections in intensive care units (ICUs). Infections due to enterobacteriaceae pose a grave challenge. Worsening susceptibility patterns, lack of uniform availability of effective antibiotics, cost constraints, and lack of diagnostic infrastructure compounds the challenge of treating Gram-negative infections, especially in the Indian scenario. Carbapenems are often considered to be robust and reliable options for treating infections due to Gram-negative bacteria. However, the global emergence of carbapenem-resistant organisms has posed a serious threat.1 A study published in 2022 demonstrated that there were an estimated 4.95 million (3.62–6.57) deaths associated with bacterial antimicrobial resistance (AMR) in 2019, including 1.27 million (95% confidence interval (CI) 0.911–1.71) deaths attributable to bacterial AMR.2 In Indian ICUs, Gram-negative organisms are often carbapenem resistant. The dominance of carbapenem-resistant enterobacteriaceae (CRE) has emerged as one of the greatest challenges faced by the medical community today. Carbapenem-resistant enterobacteriaceae may be resistant to all beta lactam antimicrobials including carbapenems and often, are even resistant to other classes of drugs. This leaves very few options for combating this menace. Further, reliable detection of resistance mechanisms is challenging.3 In many centers across India, there is limited expertise and diagnostic infrastructure available to detect the mechanisms of resistance and treat these infections in a timely and appropriate manner. A meta-analysis published in 2018 showed that in comparison with carbapenem susceptible CRE, CRE demonstrated a significantly higher association with the overall mortality risk.4 Also in this study, outcomes when monotherapy was used were worse than when combination therapy was used to combat infections caused by CRE.4 Polymyxins are often considered as the last resort in the setting of infections caused by carbapenem-resistant organisms. However, there are several limitations of polymyxin-based therapy. Pharmacokinetic and pharmacodynamic constraints, toxicity concerns, lack of uniform availability of accurate minimum inhibitory concentrations (MICs) and poor penetration into certain body sites are all factors that merit attention when using polymyxin-based therapies.5
In the Indian scenario, CRE often harbor the New Delhi metallo-beta-lactamase (NDM) and OXA-48 enzymes.6,7 Ceftazidime-avibactam (CAZ-AVI) is a novel beta lactam-beta lactamase inhibitor that has shown promise in combating infections caused by CRE. However, the NDM enzyme is not inhibited by avibactam. Aztreonam (ATM), on the other hand, remains stable and is not effectively hydrolyzed by the NDM enzyme. Hence, in the Indian scenario, especially when dealing with CRE-producing multiple beta-lactamase enzymes, combining CAZ-AVI and ATM can be an effective way of treating infections caused by CRE. An in vitro study published by Pragasam et al. found that the combination of CAZ-AVI and ATM can be effective for CRE isolates harboring the NDM and OXA-48 enzymes.8
The Infectious Diseases Society of America has recommended CAZ-AVI as the first-line therapy for carbapenem-resistant Enterobacterales producing the klebsiella pneumoniae carbapenemase (KPC) or OXA-48 beta lactamases with proven in vitro susceptibility to CAZ-AVI.9 The Indian Council of Medical Research has recommended that CAZ-AVI be used as first-line therapy for Enterobacterales-producing OXA-48 like beta lactamases.10 The Indian consensus on the management of CRE infection in critically ill patients study proposed 46 key consensus statements on the management of infections caused by CRE.11 This study highlighted the fact that there is a dearth of high-quality local data on CRE.11 A retrospective study published by Nagvekar et al.12 from India found that CAZ-AVI-based therapy is a viable option in the Indian settings. The predominant genetic mechanism in the isolates in this study was a combination of NDM and OXA-48.12
There are very few studies comparing polymyxin-based therapies with CAZ-AVI-based therapies for treating infections caused by CRE. A study comparing outcomes in patients treated with either CAZ-AVI or colistin for CRE infections who were selected from the Consortium on Resistance Against Carbapenems in Klebsiella and other Enterobacteriaceae study found that the mortality was higher in the colistin group.13 A retrospective study from China published in 2021 which included patients with carbapenem-resistant Klebsiella infections showed that patients in the CAZ-AVI group had significantly lower rates of 28-day mortality, higher microbiological eradication, and 28-day clinical success.14
However, there is an urgent need to generate more local data regarding the comparative efficacy of therapies in the setting of resistant Gram-negative infections as the enzyme patterns and challenges may vary from one region to the other. There is no study from India comparing the efficacy of polymyxin-based therapy and CAZ-AVI-based therapy for treating infections caused by CRE. We conducted a retrospective study comparing outcomes between patients with bacteremia caused by CRE who were treated with polymyxin-based combination therapy and CAZ-AVI-based therapy (with or without ATM).
Methods
Patient Selection
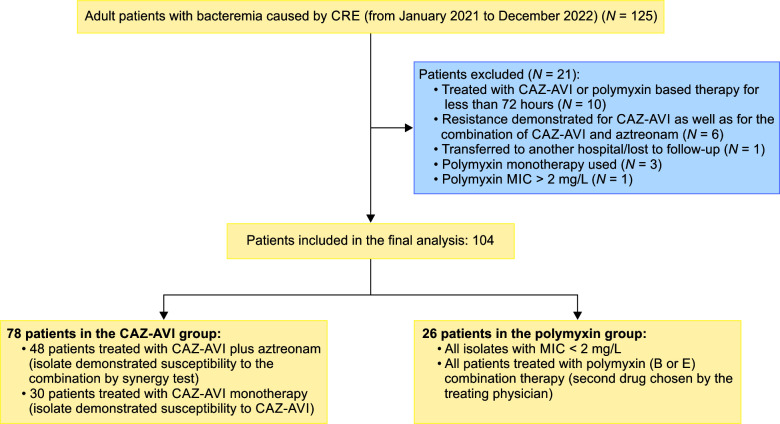
All adult patients with CRE bacteremia (from January 2021 to December 2022) who were treated for at least 72 hours with either CAZ-AVI-based therapy (with or without ATM) or polymyxin-based therapy (in combination with another drug other than CAZ-AVI) were included in the analysis. The combination drug was chosen by the treating physician. Figure 1 shows the selection process as well as the exclusion criteria used in the study. Patients were excluded if there was resistance demonstrated for CAZ-AVI as well as the combination of CAZ-AVI and ATM (on the synergy test). Also, patients were excluded if polymyxin monotherapy was used, or if the polymyxin MIC was >2 μg/mL. An institutional ethics committee approval was obtained prior to commencing this study.
Fig. 1.
Process of patient selection
Microbiological Methods
Blood Culture and Bacterial Identification
Blood culture was performed using an automated BD BACTEC Fx system. Characteristic colonies were subjected to genus and species level bacterial identification by using the Matrix Assisted Laser Desorption and Ionization - Time of Flight Mass Spectrometry technology on the MALDI Biotyper Sirius (Bruker Daltonics Bremen, Germany).
Ceftazidime-avibactam Susceptibility Testing
Susceptibility to CAZ-AVI was determined by the commercial broth microdilution system, BD Phoenix™ M50 using the BD CPO NMIC 500 antimicrobial susceptibility testing panels. Susceptibility interpretation for CAZ-AVI as sensitive or resistant was done using MIC breakpoints set by Clinical and Laboratory standards Institute (CLSI, Wayne, PA, USA).
Ceftazidime-avibactam and Aztreonam Synergy Testing
In vitro efficacy of CAZ-AVI and ATM combination was performed using the double disc sandwich diffusion method. Sandwich disc of CAZ-AVI/ATM was prepared by placing ATM disc moistened with sterile normal saline directly over CAZ-AVI disc, dried for 30 minutes before use. Standardized suspension (0.5 McFarland Standard) of pure culture of the test isolate was prepared in cation-adjusted Mueller Hinton broth and inoculated on cation-adjusted Mueller Hinton agar as a lawn culture. Discs of CAZ, ATM, CAZ-AVI, and CAZ-AVI/ATM were placed onto the MH agar plates 20-millimeters (mm) apart and incubated overnight at 37°C, ambient air. Diameter of zone of inhibition was measured in mm and susceptibility interpretation as sensitive or resistant was done as per the CLSI guidelines.
Molecular Enzyme Profiling
Molecular characterization of beta lactamases was done by Sepsis Flow Chip Assay (Master Diagnostica, Granada, Spain). Sepsis flow is a Conformité Européene In-Vitro Diagnostic Devices Directive (CE-IVD) marked test based on the principle of multiplex polymerase chain reaction (PCR) followed by reverse dot blot hybridization on a membrane chip for simultaneous identification of 36 bacteria and 20 antibiotic-resistance markers.
Colistin Broth Disc Elution Test
Colistin susceptibility was performed using the Colistin Broth Disc Elution test. Colistin Broth Disc Elution is recommended by the CLSI as an easy-to-perform test that gives comparable accuracy with broth microdilution.
Statistical Analysis
The data on categorical variables were shown as n (percent of cases) and the data on continuous variables was shown as median (minimum–maximum). Chi-square test or Fisher's exact probability tests was used for comparing the categorical variables. The Mann–Whitney U test was used to evaluate the distribution of medians of continuous variables. Multivariate logistic regression analysis was used to ascertain the independent determinants of mortality. The fundamental normality assumption was tested prior to exposing the study variables to the Mann–Whitney U test. P-values lower than 0.05 were assumed to be of statistical significance. The Statistical Package for Social Sciences (SPSS version 24.0, IBM Corporation, Armonk, United States) was utilized for analyzing the data.
Definitions Used in the Study
Clinical cure was defined as cessation of all antimicrobials and survival for at least 72 hours without the need to restart antibiotics. Microbiological cure was defined as repeat blood cultures being negative (sent after at least 72 hours of definitive therapy).
Results
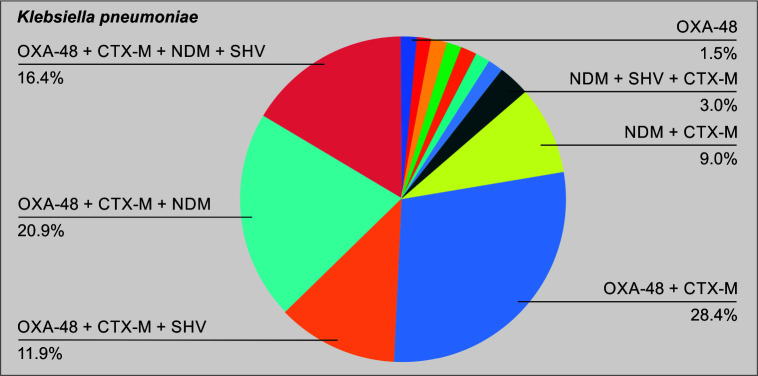
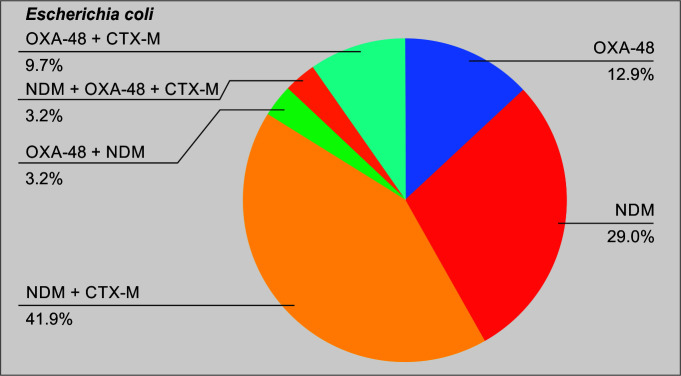
A total of 104 patients were included in the final analysis (Table 1). Figure 1 puts into perspective the relative numbers in each group. Of the total cases, 78 (75%) belonged to the CAZ-AVI group. Table 2 shows the demographics of the cases included in the study. The median age of the cases in groups A and B was 46.5 and 52.5 years, respectively. There was no significant difference in the underlying comorbidities between the two groups. Table 3 demonstrates the microbiology data including the enzymes of the CRE isolate included in each of the two groups. As shown in the table, 62.5% of the total isolates produced the NDM. Figures 2 and 3 show the relative distribution of the beta lactamase enzymes produced by the Klebsiella pneumoniae (Fig. 2) and Escherichia coli (Fig. 3) isolated from the patients included in our study. As shown in Figure 3, most of the Klebsiella isolates in our settings are multiple enzyme producers, and OXA-48 and NDM are the most predominant carbapenemases. As shown in Figure 4, NDM is the most predominant enzyme in our settings. Table 4 shows the agents used for treating the episodes of CRE bacteremia. All patients in the polymyxin group were treated with polymyxin (B/E) and another antimicrobial, as chosen by the treating team. As shown in the table, 61.5% patients in the CAZ-AVI group received ATM, as the isolate was a NDM enzyme producer. Table 5 shows the incidence of nephrotoxicity and the outcomes in the two study groups. The incidence of nephrotoxicity was higher in the polymyxin group and was statistically significant. Table 6 shows the multivariate regression analysis to obtain the factors independently associated with mortality. Ceftazidime-avibactam-based therapy was 66% less likely to be associated [change in odds % (0.34–1) x 100 = −66%] with day 14 mortality as compared with polymyxin E/B-based therapy, after adjusting for Pitt Bacteremia score and Charlson comorbidity index (CCI) score. The 95% CI for the odds ratio comparing CAZ-AVI vs polymyxin E/B for day 14 mortality was not very wide (0.118–0.989) showing the consistent empirical evidence. Table 7 shows the multivariate regression analysis to obtain the factors independently associated with day 28 mortality. Ceftazidime-avibactam-based therapy was 67% less likely to be associated with day 28 mortality [change in odds % (0.33–1) x 100 = −67%] compared with polymyxin E/B-based combination therapy after adjusting for Pitt Bacteremia score and CCI score. The 95% CI for the odds ratio comparing CAZ-AVI vs polymyxin E/B for day 28 mortality was not very wide (0.116–0.946) showing the consistent empirical evidence.
Table 1.
Total number of patients in each of the two groups included in the study
| Group code | Group description | No. of cases | Percentage of cases |
|---|---|---|---|
| Group A | CAZ-AVI based therapy | 78 | 75.0 |
| Group B | Polymyxin B/E-based combination therapy | 26 (23: polymyxin B, 3: polymyxin E) | 25.0 |
| Total | 104 | 100.0 |
Table 2.
Demographic characteristics and underlying comorbid illnesses in the two groups
| Study group | ||||||
|---|---|---|---|---|---|---|
| Parameters | Group A (n = 78) | Group B (n = 26) | All (n = 104) | p-value | ||
| Age (years) | Median (range)§ | 46.5 (9–80) | 52.5 (2–80) | 47.5 (2–80) | 0.870NS | |
| Male sex | n(%) | 50 (64.1) | 15 (57.7) | 65 (62.5) | 0.559NS | |
| Clinical profile/risk factors | Solid organ transplant Bone marrow transplant Hematological malignancy Oncological malignancy Chronic liver disease Chronic kidney disease ICU requirement Central line Prior antibiotic therapy* Immunosuppressive therapy Diabetes mellitus |
n(%) | 1 (1.3) 11 (14.1) 39 (50.0) 11 (14.1) 3 (3.8) 50 (64.1) 55 (70.5) 46 (59.0) 48 (61.5) 14 (17.9) |
1 (3.8) 1 (3.8) 9 (34.6) 2 (7.7) 3 (11.5) 0 16 (61.5) 13 (50.0) 18 (69.2) 15 (57.7) 6 (23.1) |
2 (1.9) 12 (11.5) 48 (46.2) 13 (12.5) 6 (5.8)2 (1.9) 66 (63.5) 68 (65.4) 64 (61.5) 63 (60.6) 20 (19.2) |
0.439NS 0.286NS 0.173NS 0.510NS 0.163NS 0.999NS 0.814NS 0.057NS 0.352NS 0.728NS 0.566NS |
§P value by Mann–Whitney U test; the rest of p values by Chi-square test or Fisher's exact probability test. p-value < 0.05 is considered to be statistically significant. NS, statistically non-significant;
*Received antibiotics in the three months preceding the episode of CRE bacteremia
Table 3.
Microbiology data of the CRE isolates, including the enzyme analysis
| Study group | ||||||
|---|---|---|---|---|---|---|
| Group A (n = 78) | Group B (n = 26) | All (n = 104) | p-value | |||
| Phenotypic ID |
Klebsiella Escherichia coli Other |
51 (65.4%) 21 (26.9%) 6 (7.7%) |
16 (61.5%) 10 (38.5%) 0 |
67 (64.4%) 31 (29.8%) 6 (5.7) |
0.722NS 0.265NS |
|
| Sepsis flow-resistance markers | OXA-48 NMD SHV CTX-M VIM IMP KPC |
n (%) | 55 (70.5) 48 (61.5) 19 (24.4) 62 (79.5) 0 0 4 (5.1) |
14 (53.8) 17 (65.4) 9 (34.6) 18 (69.2) 0 0 0 |
69 (66.3) 65 (62.5) 28 (26.9) 80 (76.9) 0 0 4 (3.8) |
0.119NS 0.726NS 0.307NS 0.282NS 0.999NS 0.999NS 0.570NS |
KPC,Klebsiella pneumoniae carbapenemase
Fig. 2.
Relative number of patients in the CAZ-AVI group and the polymyxin group
Fig. 3.
Pattern of enzymes produced by the Klebsiella pneumoniae isolates
Fig. 4.
Pattern of enzymes produced by the Escherichia coli isolates
Table 4.
Antibiotics used in the ceftazidime-avibactam and polymyxin (B/E) groups
| Study group | ||||||
|---|---|---|---|---|---|---|
| Group A (n = 78) | Group B (n = 26) | All (n = 104) | p-value | |||
| Additional agent used | Aztreonam Meropenem Fosfomycin Tigecycline Meropenem + tigecycline Minocycline |
n (%) | 48 (61.5) 0 (0) 0 (0) 0 (0) 0 (0) 0 |
0 9 (34.6) 7 (26.9) 2 (7.7) 3 (11.5) 5 (19.2) |
48 (46.1) 9 (8.6) 7 (6.7) 2 (1.9) 3 (2.9) 5 (4.8) |
|
| Additional treatment duration (days) | Median (range)§ | 8 (4–14) | 8 (5–15) | 8 (4–15) | 0.908NS |
§p-value by Mann–Whitney U test; the rest of the p-values by Chi-square test or Fisher's exact probability test. p-value < 0.05 is considered to be statistically significant. NS, statistically non-significant
Table 5.
Incidence of nephrotoxicity and outcomes in the two study groups
| Study group | ||||||
|---|---|---|---|---|---|---|
| Group A (n = 78) | Group B (n = 26) | All (n = 104) | p-value | |||
| Side effects | Nephrotoxicity | n (%) | 5 (6.4) | 6 (23.1) | 11 (10.6) | 0.017* |
| Cure | Clinical Microbiological |
n (%) | 57 (73.1) 39/43 (90.7) |
12 (52.2) 6/8 (75.0) |
69 (68.3) 45 (88.2) |
0.058NS 0.234NS |
| Pitt Bacteremia score | 0–4 > 4 Median (range)§ |
n (%) | 72 (92.3) 6 (7.7) 2.0 (0–7) |
25 (96.2) 1 (3.8) 2.0 (0–8) |
97 (93.3) 7 (6.7) 2.0 (0–8) |
0.677NS 0.498NS 0.895NS |
| CCI score | 0 1–2 3–4 ≥5 Median (range)§ |
n (%) | 13 (16.7) 48 (61.5) 10 (12.8) 7 (9.7) 2.0 (0–6) |
6 (23.1) 16 (61.5) 4 (15.4) 0 2.0 (0–4) |
19 (18.3) 64 (61.5)1 14 (13.5) 7 (6.7) 2.0 (0–6) |
0.413NS 1.0NS 0.740NS 0.165NS |
| Mortality | Day 14 Day 28 |
n (%) | 17 (21.8) 22 (28.2) |
10 (38.5) 12 (46.2) |
27 (26.0) 34 (32.7) |
0.093NS 0.091NS |
§p-value by Mann–Whitney U test; the rest of the p values by Chi-square test or Fisher's exact probability test. p-value < 0.05 is considered to be statistically significant.
*p-value < 0.05, NS, statistically nonsignificant; CCI, charlson comorbidity index
Table 6.
Multivariate logistic regression analysis to obtain the independent determinants of incidence of mortality (day 14)
| Risk factors (variables entered in the model) | Odds ratio | 95% CI for odds ratio | p-value | |
|---|---|---|---|---|
| Pitt Bacteremia score | 0–4 >4 |
1.00 1.561 |
– 1.162–2.096 |
– 0.003** |
| CCI score | 0 >0 |
1.00 1.347 |
– 0.980–1.853 |
– 0.066NS |
| Study group | Group A (CAZ-AVI)§ Group B (polymyxin E/B)§ |
0.342 1.00 |
0.118–0.989 – |
0.048* – |
§Odds ratio = 1: reference category. Dependent variable: Mortality at day 14.
*p-value < 0.05,
**p-value < 0.01, NS, statistically nonsignificant; CCI, charlson comorbidity index
Table 7.
Multivariate logistic regression analysis to obtain the independent determinants of incidence of mortality (day 28)
| Risk factors (variables entered in the model) | Odds ratio | 95% CI for odds ratio | p-value | |
|---|---|---|---|---|
| Pitt Bacteremia score | 0–4 >4 |
1.00 1.755 |
– 1.292–2.385 |
– 0.001*** |
| CCI score | 0 >0 |
1.00 1.329 |
– 0.975–1.810 |
– 0.072NS |
| Study group | Group A (CAZ-AVI)§ Group B (polymyxin E/B)§ |
0.331 1.00 |
0.116–0.946 – |
0.039* – |
§Odds ratio = 1: reference category. Dependent variable: Mortality at day 28.
*p-value < 0.05,
***p-value < 0.001, NS, statistically nonsignificant; CCI, Charlson comorbidity index
Discussion
Infections caused by CRE remain a serious challenge in the Indian setting. There is a need to generate high-quality data regarding the local resistance mechanisms and resistance patterns. This is the first study from India that compares the outcomes of CAZ-AVI (with or without ATM) and polymyxin B-based combination therapy. This is important, given the fact that local resistance mechanisms are different.
Our enzyme epidemiology is different compared with countries like the United States, where the KPC enzyme is endemic and NDM as well as OXA-48 enzymes are reported as sporadic cases.15 A study published in 2015 from 7 metropolitan areas in the United States showed that the overall incidence of CRE was 2.93 cases per 100,000 population; and KPC was the most predominant enzyme in this study.16 In our study, 62.5% of the total isolates harbored the NDM enzyme. Also 66.3% of the isolates harbored the OXA-48 enzyme. In contrast, only 3.8% of the total CRE isolates in our study were found to have the KPC enzyme, thus exemplifying the differences in enzyme epidemiology in India and the western world. Also, the SHV and CTX-M enzymes were produced by 26.9% and 76.9% of the isolates. This shows that several isolates in our study were multiple enzyme producers and this complicates the management of such patients in the Indian setting.
The study included only those patients who were treated with combination therapy in the polymyxin group. Though the data are not conclusive, observational studies with a focus on the treatment of bloodstream infections caused by CRE point to a survival advantage of combination therapy as against monotherapy.17 A multinational retrospective observational study (INCREMENT) showed that when a score that assessed the mortality risk (the INCREMENT-CPE score) was incorporated into the analysis, the association of combination therapy with mortality was lower than those on monotherapy in the high-mortality-score stratum. Considering the overall evidence for combination therapy for CRE, we decided to include only those patients who were treated with combination therapy. Meropenem was the most frequently used agent in combination with polymyxin, followed by fosfomycin and minocycline. Also, there was no significant difference between the underlying clinical profiles or comorbid illnesses between the two groups.
As shown in Table 5, the use of polymyxin was associated with a higher incidence of nephrotoxicity. This reiterates the safety concerns with the usage of polymyxins. Polymyxins have a narrow therapeutic window, and with drug levels not being readily available it becomes very difficult to balance efficacy and toxicity. In our study, the usage of CAZ-AVI-based therapy was associated with better outcomes compared with polymyxin-based combination therapy. On multivariate analysis, CAZ-AVI-based therapy was independently associated with a lower 14- and 28-day mortality. The microbiological and clinical cure rates were also higher in the CAZ-AVI group, though they did not achieve statistical significance. These findings show that even in a setting dominated by NDM and OXA-48-producing isolates, CAZ-AVI-based therapy may be favored over polymyxin-based therapy. The pharmacodynamic concerns with polymyxins, lack of availability of drug levels, challenges with susceptibility testing, and suboptimal efficacy need to be seriously considered before using polymyxin-based therapy for CRE. However, careful selection of patients is important, and susceptibility to the combination of CAZ-AVI and ATM should be demonstrated by in vitro synergy testing.
Overall, our findings demonstrate that in terms of efficacy and safety, CAZ-AVI (with or without ATM) may be a better choice for infections caused by CRE in our settings, provided patients have been carefully selected after demonstration of in vitro susceptibility to either CAZ-AVI or to the combination of CAZ-AVI and ATM. This can also be an effective polymyxin-sparing strategy and reduce the usage of polymyxins in our settings. The approach should be determined in consultation with infectious diseases specialists and microbiologists.
Conclusion
This is the first study from India that compares polymyxin-based therapy with CAZ-AVI-based therapy in a setting predominated by the NDM enzyme, along with OXA-48. Our study shows that CAZ-AVI-based therapy may be a superior option to polymyxin-based therapy for infections caused by CRE. This can have significant practical applications, both in terms of optimizing therapy for the individual patient as well as sparing polymyxins and reducing the use of polymyxins in our hospitals. Further larger studies as well as randomized trials are needed to confirm these findings and optimize the therapy of this common clinical challenge in our settings.
Limitations
This was a retrospective observational study. Also, the sample size is relatively smaller. Furthermore, the choice of combination therapy was left to the treating physician.
Orcid
Parikshit S Prayag https://orcid.org/0000-0003-2102-7627
Sampada A Patwardhan https://orcid.org/0000-0003-0998-5742
Shweta Panchakshari https://orcid.org/0000-0002-5708-3287
Ramya Sambasivam https://orcid.org/0000-0003-0673-5684
Surabhi Dhupad https://orcid.org/0000-0003-4251-6411
Rajeev N Soman https://orcid.org/0000-0003-1578-9932
Amrita P Prayag https://orcid.org/0000-0002-2498-9576
Footnotes
Source of support: Nil
Conflict of interest: Dr Amrita P Prayag is associated as the Associate Editor of this journal and this manuscript was subjected to this journal's standard review procedures, with this peer review handled independently of this editorial board member and her research group.
References
- 1.Patel G, Bonomo RA. “Stormy waters ahead”: Global emergence of carbapenemases. Front Microbiol. 2013;4(48) doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis [published correction appears in Lancet. 2022 Oct 1;400(10358):1102]. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutgring JD, Limbago BM. The problem of carbapenemase-producing-carbapenem-resistant-enterobacteriaceae Detection. J Clin Microbiol. 2016;54(3):529–534. doi: 10.1128/JCM.02771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: Results of a systematic literature review and meta-analysis. Open Forum Infect Dis. 2018;5(7):ofy150. doi: 10.1093/ofid/ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soman R, Bakthavatchalam YD, Nadarajan A, Dwarakanathan HT, Venkatasubramanian R, Veeraraghavan B. Is it time to move away from polymyxins? Evidence and alternatives. Eur J Clin Microbiol Infect Dis. 2021;40(3):461–475. doi: 10.1007/s10096-020-04053-w. [DOI] [PubMed] [Google Scholar]
- 6.Veeraraghavan B, Shankar C, Karunasree S, Kumari S, Ravi R, Ralph R. Carbapenem resistant Klebsiella pneumoniae isolated from bloodstream infection: Indian experience. Pathog Glob Health. 2017;111(5):240–246. doi: 10.1080/20477724.2017.1340128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazi M, Khot R, Shetty A, Rodrigues C. Rapid detection of the commonly encountered carbapenemases (New Delhi metallo-β-lactamase, OXA-48/181) directly from various clinical samples using multiplex real-time polymerase chain reaction assay. Indian J Med Microbiol. 2018;36(3):369–375. doi: 10.4103/ijmm.IJMM_18_324. [DOI] [PubMed] [Google Scholar]
- 8.Pragasam AK, Veeraraghavan B, Shankar BA, Bakthavatchalam YD, Mathuram A, George B, et al. Will ceftazidime/avibactam plus aztreonam be effective for NDM and OXA-48-Like producing organisms: Lessons learnt from in vitro study. Indian J Med Microbiol. 2019;37(1):34–41. doi: 10.4103/ijmm.IJMM_19_189. [DOI] [PubMed] [Google Scholar]
- 9.Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTRP. aeruginosa) (2022). 2022 doi: 10.1093/cid/ciac268. Accessed June 2022. https://www.idsociety.org/globalassets/idsa/practice-guidelines/amr-guidance/1.0/idsa-amr-guidance-v1.1.pdf. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annual Report: Antimicrobial Resistance Research and Surveillance Network. 2022 Accessed June 2022. https://main.icmr.nic.in/sites/default/files/guidelines/AMRSN_annual_report_2020.pdf. [Google Scholar]
- 11.Soman R, Veeraraghavan B, Hegde A, Jiandani P, Mehta Y, Nagavekar V, et al. Indian consensus on the management of CRE infection in critically ill patients (ICONIC) - India. Expert Rev Anti Infect Ther. 2019;17(8):647–660. doi: 10.1080/14787210.2019.1647103. [DOI] [PubMed] [Google Scholar]
- 12.Nagvekar V, Shah A, Unadkat VP, Chavan A, Kohil R, Hodgar S, et al. Clinical outcome of patients on ceftazidime-avibactam and combination therapy in carbapenem-resistant enterobacteriaceae. Indian J Crit Care Med. 2021;25(7):780–784. doi: 10.5005/jp-journals-10071-23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J, Li H, Zhang M, Shi G, Liu M, Wang Y, et al. Efficacy of ceftazidime-avibactam versus polymyxin b and risk factors affecting clinical outcomes in patients with carbapenem-resistant. Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12:780940. doi: 10.3389/fphar.2021.780940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant enterobacteriaceae: The impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, et al. Epidemiology of carbapenem-resistant enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314(14):1479–1487. doi: 10.1001/jama.2015.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez F, El Chakhtoura NG, Yasmin M, Bonomo RA. Polymyxins: To combine or not to combine? Antibiotics (Basel) 2019;8(2):38. doi: 10.3390/antibiotics8020038. [DOI] [PMC free article] [PubMed] [Google Scholar]