Abstract
Background
Lack of eyecare protective measures especially in unconscious and sedated critically ill patients, make them prone to ocular surface diseases (OSDs), e.g., exposure keratopathy. This study is aimed to frame an algorithm-based approach to eyecare via eyecare bundle to bring down the burden of OSDs in critically ill patients especially in resource-limited settings.
Materials and methods
After clearance from institutional ethical committee, a quasi-experimental single center study was conducted over a period of 6 months. Incidence of exposure keratopathy was calculated before and after induction of eyecare bundle and was compared. Statistical analysis was done using SPSS software v20. p-value of less than 0.05 was considered significant.
Results
A total of 218 patients were enrolled in the study after obtaining informed written consent and after fulfilling inclusion criteria. Patients were divided into control and experimental groups, with baseline characteristics similar in both the groups, respectively, in terms of gender, age (40 years), APACHE II score, and specialty distribution except predominantly medical patients in experimental group. In control group (n = 99), total 69 patients (41 medical and 28 surgical) developed exposure keratopathy, while in experimental group (n = 109) only 15 patients (6 medical and 9 surgical) developed exposure keratopathy, hence a significant reduction was observed. Further follow-up of patients in the experimental group was also done on Days 5 and 7, respectively.
Conclusion
The proposed protocolized algorithm-based eyecare bundle significantly reduced the incidence of exposure keratopathy in sedated, mechanically ventilated, and vulnerable critically ill patients.
How to cite this article
Sama S, Abrol R, Dhasmana R, Sharma N, Khandhuri S, Chauhan R, et al. Effect of Implementation of an Eyecare Bundle on Incidence of Exposure Keratopathy in Intensive Care Unit of Tertiary Care Center in North India. Indian J Crit Care Med 2023;27(6):426–432.
Keywords: Exposure keratopathy, Eyecare bundle, Ocular surface diseases
Introduction
Several studies have been conducted to evaluate the incidence of ocular surface diseases (OSDs) in critically ill patients with loss of normal eye protective reflexes, with special focus on exposure keratopathy (EK).1–5 As per the literature, despite studying various risk factors and possible interventions for OSDs, there is a general disregard for appropriate and timely eyecare in an intensive care unit (ICU) setting.6 This could be attributed to a critical care setting placing more value on life-saving interventions over seemingly minor eyecare issues. However, this leads to high incidence of preventable cases of EK which could lead to microbial keratopathy and eventually blindness. We feel this is partially due to a lack of algorithmic approach to eyecare in an ICU. Certain centers have an eyecare protocol in place; however, they are generally unregimented and based more on common practice rather than scientific evidence. Further still, implementation of these protocols is neither followed up nor emphasized upon due to the greater importance given to other areas of treatment. This practice leads to the occurrence of preventable ailments.
The defense mechanisms of the eye are three-fold anatomical, physiological, and mechanical. These include eyelid closure, an intact corneal epithelium, and the constant blinking action of the eyelid necessary for adequate distribution of the tear film over the ocular surface.7 Absence of the tear film and corneal reflex in unconscious and sedated patients make them prone to OSDs due to incomplete eye closure. Depending on the grade of improper closure as well as the duration, the patient may manifest with varying degrees of EK. The breakdown of natural defences, steroid therapy, and immunodeficiency puts the patients at additional risk for opportunistic infection.8 Issues related to mechanical ventilation such as application of positive end-expiratory pressure or prone ventilation can cause leaking capillaries and fluid retention leading to peripheral and conjunctival edema thus causing increased exposure risk. An exposed cornea always carries risks of drying, infection, and scarring, which may lead to permanent visual loss.
Several studies have been conducted evaluating the efficacy of eyecare protective measures such as taping, artificial tears, and lubricating gel.9–12 General consensus states that application of lubricating gel as well as eye drops seems to be far more effective than mere patching; however, any intervention seems to be significantly better than eye cleaning alone. Regular monitoring of the corneal surface in predisposed patients ensures early detection and management. Conflicting opinions exist when it comes to combination approaches where some studies suggest polyethylene covers over taping alongside lubricating gels while others suggest the gel alone. However, no standard guideline exists to suggest one over the other but all guidelines state the any intervention is better than no intervention.
This oversight unfortunately results in OSD in the ICU in 23–60% of patients.13 However, if a defined process for assessment and intervention is followed, most of these problems can be avoided.14 This study aimed to provide an algorithmic approach to eyecare via an eyecare bundle with appropriate implementation and accountability. It is specifically tailored for an Indian setting that has limited resources and manpower, thereby making an effort to develop a national eyecare policy for India. We developed a checklist-based strategy and an easy-to-follow eyecare bundle tailored for a tertiary care center in north India.
Materials and Methods
The Institute ethics committee approved this prospective, observational study. The study was registered with the Clinical Trials Registry of India (CTRI Registration No: CTRI/2022/10/046492). All intubated and sedated patients admitted in the institute's ICU for a period of greater than 48 hours or those with a neurological diagnosis that limits the blink reflex were included in the study. Conscious patients with intact blink reflex, those with known pre-existing ocular conditions like orbital trauma, ectropion, entropion, and rheumatological disorders making the patient prone to dry eye and those whose caregivers refuses to give consent were excluded from the study. Written, informed consent was obtained from the caregivers of all 218 participants included in the study. The entire study was conducted over a period of six months.
On commencement of the study, the patients were first divided into two groups—those enrolled before the implementation of the eyecare bundle (control group; n = 99) and those enrolled after the implementation of eyecare bundle (experimental group; n = 109). Incidence of exposure keratopathy was calculated on day 3 in each group respectively. Patients in the experimental group were further followed up on days 5 and 7 also look for further progression of disease and also effectiveness of eye bundle care (Flowchart 1). All patients fulfilling the inclusion criteria were subjected to corneal surface examination with a fluorescein stain strip placed on the lower lid of the eye and manually closing the upper eyelid to ensure spread of dye (Fig. 1). The corneal surface was then evaluated using a blue filter of an ophthalmoscope and findings noted. Eyecare bundle was implemented and the frequency of eyecare was decided on grading of eye closure. Grading of eye closure (lagophthalmos) was graded as:
Flowchart 1.
CONSORT 2010 flow diagram
Fig. 1.

Exposure keratopathy as exhibited using fluorescein dye under blue filter of an ophthalmoscope
Grade I, no sclera visible: Eyecare bundle was implemented three times a day.
Grade II, conjunctiva seen: Eyecare bundle was implemented three times a day with eye tapping.
Grade III, cornea seen: Eyecare bundle was implemented four times a day with eye tapping.
The particulars of eyecare bundle and the steps followed during implementation are mentioned in Flowchart 2.
Flowchart 2.
Steps of implementation of eyecare bundle
The Statistical Package for Social Sciences software was used to enter the data (version 20). The Kolmogorov and Smirnov test was used to determine the normality of the data. All categorical variables are described in terms of frequencies and proportions, while the continuous variables are characterized as median ± standard deviation. Both the Chi-square test and the Fisher exact test were used to ascertain the relationship between two discrete variables. p-value lesser than 0.05 were deemed significant. Additionally, Microsoft Excel was also used to create graphs and charts.
Results
After compiling all the data and statistical analysis, following results were seen in each group, respectively.
Control Group
When comparing the baseline characteristics in the control group that constituted 99 patients fulfilling the inclusion criteria, total number of patients who developed EK on day 3 were 69, here the median age of patients with and without exposure keratopathy were 56.0 ± 16.9 years (n = 69) and 57.0 ± 17.4 years (n = 30) (Table 1). The APACHE scores were 16.0 ± 5.4 and 16.5 ± 5.4 in patients who developed exposure keratopathy and those who did not in the control group.
Table 1.
Comparison of study participants with/without exposure keratopathy with respect to gender, age distribution, APACHE-II scores, and specialty distribution in the control group (n = 99)
| Control group (n = 99) | Exposure keratopathy present (n = 69) | Exposure keratopathy absent (n = 30) | p-value |
|---|---|---|---|
| Gender Male Female |
44 (74.6) [63.8] 25 (62.5) [36.2] |
15 (25.4) [50.0] 15 (37.5) [50.0] |
0.199 |
| Age (years) <40 >40 |
56.0 ± 16.9 (median ± SD) 17 (65.4) [24.6] 52 (71.2) [75.4] |
57.0 ± 17.4 (median ± SD) 9 (34.6) [30.0] 21 (28.8) [70.0] |
0.577 |
| APACHE score <17 (low mortality risk) >17 (high mortality risk) |
16.0 ± 5.4 (median ± SD) 36 (69.2) [52.2] 33 (70.2) [47.8] |
16.5 ± 5.4 (median ± SD) 16 (30.8) [53.3] 14 (29.8) [46.7] |
0.915 |
| Specialty Medical Surgical |
41 (64.1) [59.4] 28 (80.0) [40.6] |
23 (35.9) [76.7] 7 (20.0) [23.3] |
0.099 |
Values inside the parentheses indicate row percentages. Values inside the square brackets indicate column percentages
Of the 69 patients in the control group (n = 69) who were identified to have exposure keratopathy, 44 (63.8%) were male and 25 (36.2%) were female, a greater preponderance of patients 52 (75.4%) was observed to be over the age of 40 years. Nearly equal numbers of patients with low (36, 52.2%) and high (33, 47.8%) APACHE scores were observed in this group (Table 1).
In the 30 patients who did not have exposure keratopathy, male and female proportions were found to be equal. Almost equal numbers of patients with low 16 (53.3%) and high 14 (46.7%) APACHE scores were observed (Table 1). When we compared age, sex, and APACHE-II scores in patients with and without EK in the control group, they were not statistically significant (Table 1).
On further divisions into medical and surgical patients in the control group, 59.4% of the patients in the medical group developed exposure keratopathy as compared with 40.6% of patients in the surgical group, but this difference was statistical non-significant (Table 1).
Experimental Group
When comparing the baseline characteristics in the experimental group that constituted 109 patients fulfilling the inclusion criteria. On initial assessment 11% of patients had exposure keratopathy on day 3. Incidence of exposure keratopathy calculated was 12.7% on day 5 and 11.2% on day 7, respectively. Percentage of total patients lost to follow-up on days 5 and 7 were 6.4% and 10.1%, respectively (Fig. 2). The cumulative incidence of exposure keratopathy was 13.8% in the experimental group (Fig. 3).
Fig. 2.

Percentage of patients in the experimental group with and without exposure keratopathy and the percentage of patients that could not be assessed on days 3, 5 and 7
Fig. 3.

Incidence of exposure keratopathy before and after implementation of eyecare bundle
The median age of patients with and without exposure keratopathy was 47.0 ± 18.0 years (n = 15) and 54.0 ± 19.2 years (n = 94). The median APACHE scores were 22.0 +/–5.8 and 22.0 +/–5.1 in patients who developed exposure keratopathy and those who did not in the test group (Table 2).
Table 2.
Comparison of study participants with/without exposure keratopathy with respect to gender, age distribution, APACHE-II scores, and specialty distribution in the experimental group (n = 109)
| Variables | Experimental group (n = 109) | p-value | |
|---|---|---|---|
| Exposure keratopathy present (n = 15) | Exposure keratopathy absent (n = 94) | ||
| Gender Male Female |
12 (17.1) [80.0] 3 (7.7) [20.0] |
58 (82.9) [61.7] 36 (92.3) [38.3] |
0.170 |
| Age (in years) <40 >40 |
47.0 ± 18.0 (median ± SD) 6 (19.4) [40.0] 9 (11.5) [60.0] |
54.0 ± 19.2 (median ± SD) 25 (80.6) [26.6] 69 (88.5) [73.4] |
0.356 |
| APACHE-II score <17 (Low mortality risk) >17 (high mortality risk) |
22.0 ± 5.8 (median ± SD) 2 (16.7) [13.3] 13 (13.4) [86.7] |
22.0 ± 5.1 (median ± SD) 10 (83.3) [10.6] 84 (86.6) [89.4] |
0.670 |
| Specialty Medical Surgical |
6 (7.2) [40.0] 9 (34.6) [60.0] |
77 (92.8) [81.9] 17 (65.4) [18.1] |
0.001 |
Values inside the parentheses indicate row percentages. Values inside the square brackets indicate column percentages
Of the 15 patients in the experimental group (n = 109) who were identified to have exposure keratopathy, 12 (80 %) were male and 3 (20%) were female, a greater preponderance of patients (60%) was observed to be over the age of 40 years.
In 94 patients who did not have exposure keratopathy, the number of male patients was higher at 58 (61.7%) as compared with female at 36 (38.3%). Majority of patients with or without EK had high APACHE scores (86.7% and 89.4%) respectively. Age, sex, and APACHE-II scores were comparable in patients with and without EK (Table 2).
Among the medical and surgical groups here, number of patients with exposure keratopathy in the surgical group (60%) were statistically significantly higher as compared with the medical group (40%) with a p-value of less than 0.05 (Table 2).
Overall, the incidence of exposure keratopathy prior to the implementation of the eyecare bundle (control group) was 69.7%; however, after implementation of the same (experimental group) it was measured to be 13.8% thus exhibiting a marked reduction in the incidence by following the bundled care approach (Fig. 3).
When comparing the presence and absence of other eye changes, percentage of infective changes in the eyes was 3.3%, percentage of corneal changes was 4.6%, and percentage of abnormal lid position was 77.15% in the control group as compared with 0.5%, 1%, and 22.9%, respectively, in each category in the experimental group. Hand hygiene was adequate in both groups as staff was already trained in hand hygiene measures (Fig. 4).
Fig. 4.
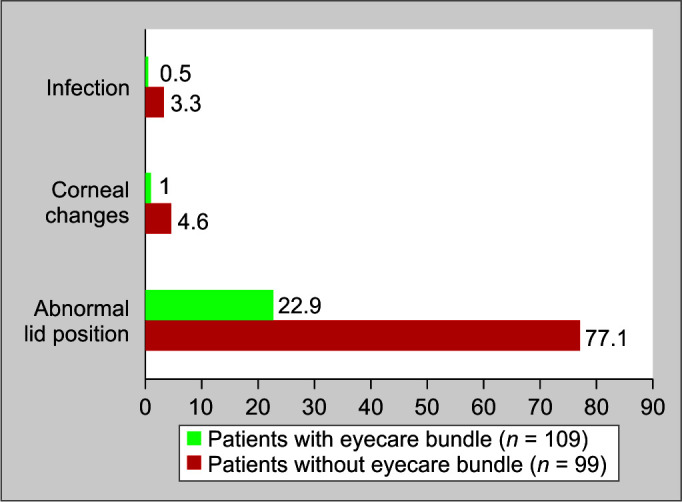
Other changes noticed in eyes of patients with and without eyecare bundle
Discussion
Exposure keratopathy entails dryness of cornea due to improper eye closure resulting in evaporation of the tear film and subsequent findings of erosions and ulcerations. In the initial stages, EK is generally benign and may be easily treated; however, it is commonly missed in critically ill patients due to more focus on life-saving measures. Such cases when left to their own, may progress to more severe cases of EK which may be potentially devastating for the patient and can lead to vision loss.6
Critically ill patients are more vulnerable to severe forms of EK due to decreased tear formation, incomplete eye closure, suppressed blink reflex, and altered vascular permeability.15 Further still, as the patient's other medical complaints take precedence over the eye-related illnesses, EK is often undiagnosed or unevaluated. However, something as simple as regular evaluation could counter this issue and lead to a significant decline in morbidity of the patients. Owing to varying definitions and study methodologies in the literature, the agreed upon value of incidence of EK in an ICU setting varies and ranges from 23% to 60%.16 However, two facts remain consistent—the value is invariably high and the most effective strategy to counter this is “Preventive”. Protocolized eyecare algorithms or education and awareness programs are believed to be effective in reducing the incidence of EK but the limited training of healthcare professionals in eyecare, makes it cumbersome and impractical exercise. Thus, making specific protocols which are easy to implement and practice by all healthcare professionals an effective tool. Several studies have identified the efficacy of such protocolized care.17,18
Kousha et al. introduced an eyecare protocol using eye lubricant (Lacri-lube) application like our study; however, they chose not to include taping citing patient distress. One investigator evaluated all included patients via portable slit lamp examination to remove inter-observer variability. So is in our study, we appointed a single investigator for similar reasons; however, we implemented bedside diagnosis utilizing the blue filter of an ophthalmoscope and fluorescein strips for ease of use and lack of availability of a portable slit lamp in ICU. As per Kousha et al., with a regimented protocol and other support, the incidence of EK was reduced from 56% to 2.6%. Further still, they also noted that APACHE-II scores had no statistical significance in patients developing EK. Our study mirrored the same findings with reduction of incidence of EK from 69.7% to 13.8%.16 For streamlining the assessment of patients, the presence of shock, multiorgan failure, use of vasopressors and its effect on EK was assessed using APACHE-II scoring system. In our study also the median APACHE-II scores had no statistical significance between the two groups. The incidence of exposure keratopathy was comparable in patients with low and high APACHE-II scores.
Kuruvilla et al. identified various contributing factors such as abnormal eyelid position, mechanical ventilation, duration of mechanical ventilation, sedation, and primary illness. They did not implement any protocol; however, they acknowledged that regular eye evaluations by ophthalmologist as well as the increased compliance toward eyecare by nursing staff could be very useful in reducing preventable cases of EK and the same could be done by all physicians if dedicated ophthalmology services were not available.6
In our study, we found that in the experimental group surgical patients were more prone to develop exposure keratopathy even after implementation of eyecare bundle, this result was also statistically significant, but this result might be due to low number of patients who developed exposure keratopathy in the experimental group or because of other factors such as presence of previous eye conditions such as already existing edema, thus it may require further studies that correlate the incidence of exposure keratopathy in these patients. Apart from this, no statistical significance was noted in patients developing exposure keratopathy before or after implementation of our eyecare bundle. Thus, implying that the patient was more likely to develop EK due to lack of proper care and evaluation than pre-existing or current illnesses or demographic features. This makes the issue more iatrogenic than previously thought, thus further highlighting the importance of protocolized eyecare.
An interesting point noted in all the above-mentioned studies as well as ours was the presence of Hawthorne effect which is a phenomenon where participants in a study alter their behavior when they know they are being observed. Nursing staff in our ICU adhered to the eyecare protocol which was well supplemented by regular examination by the critical care physician or any appointed healthcare worker thus nullifying such effect on any reduction of incidence noted in our study or any of those mentioned above.
It is important to note that the study investigators who were involved in the implementation of the eyecare bundle, collection of data regarding EK as well as the investigators analyzing the collected data were blinded from the other two events thus increasing the sensitivity of our findings.
Conclusion
In conclusion, protocolized eyecare management with regular examinations is associated with a lower incidence of EK and no cases of severe EK. A high incidence of EK in critically ill patients who are ventilated and sedated exhibits a vulnerable group for whom meticulous eyecare could be a boon in preventing iatrogenic causes of blindness. Our eyecare bundle is a first of its kind example of protocolized eyecare for an Indian or resource-deficient setting and has exhibited excellent results in our study. The same may be helpful in all critical care units in similar settings.
CTRI registered trial (Registration No.) - CTRI/2022/10/046492
Limitations
A limitation of our study could be the unavailability of a portable slit lamp which may have helped detect more cases of mild EK in critically ill patients. The study being quasi-experimental, hampers the internal validity and as study participants were not randomized, thus systematic biases may have influenced the study results. Propensity score matching could have been done. Another limitation is that patients were not differentiated according to immune status like presence of diabetes etc., this opens up further possibilities of larger studies assessing the effect of these conditions on EK.
Supplementary Materials
All the supplementary materials are available on the website of www.ijccm.org.
Orcid
Rahul Chauhan https://orcid.org/0000-0003-0038-5855
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Hernandez EV, Mannis MJ. Superficial keratopathy in intensive care unit patients. Am J Ophthalmol. 1997;124(2):212–216. doi: 10.1016/s0002-9394(14)70786-x. [DOI] [PubMed] [Google Scholar]
- 2.Mercieca F, Suresh P, Morton A, Tullo A. Ocular surface disease in intensive care unit patients. Eye. 1999;13(2):231–236. doi: 10.1038/eye.1999.57. [DOI] [PubMed] [Google Scholar]
- 3.Bates J, Dwyer R, O’Toole L, Kevin L. Corneal protection in critically ill patients: A randomized controlled trial of three methods. Clin Intens Care. 2004;15(1):23–26. doi: 10.1080/09563070410001661759. [DOI] [Google Scholar]
- 4.Dua HS. Bacterial keratitis in the critically ill and comatose patient. Lancet. 1998;351(9100):387–388. doi: 10.1016/S0140-6736(05)78351-3. [DOI] [PubMed] [Google Scholar]
- 5.Marshall AP, Elliott R, Rolls K, Schacht S, Boyle M. Eyecare in the critically ill: Clinical practice guideline. Austr Crit Care. 2008;21(2):97–109. doi: 10.1016/j.aucc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kuruvilla S, Peter J, David S, Premkumar PS, Ramakrishna K, Thomas L, et al. Incidence and risk factor evaluation of exposure keratopathy in critically ill patients: A cohort study. J Crit Care. 2015;30(2):400–404. doi: 10.1016/j.jcrc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Dartt DA, Willcox MDP. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013;117:1–3. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirwan JF, Potamitis T, El-Kasaby H, Hope-Ross MW, Sutton GA. Clinical review. Lesson of the week: Microbial keratitis in intensive care. BMJ. 1997;314(7078):433–434. doi: 10.1136/bmj.314.7078.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koroloff N, Boots R, Lipman J, Thomas P, Rickard C, Coyer F. A randomised controlled study of the efficacy of hypromellose and Lacri-Lube combination versus polyethylene/Cling wrap to prevent corneal epithelial breakdown in the semiconscious intensive car. Intensive Care Med. 2004;30(6):1122–1126. doi: 10.1007/s00134-004-2203-y. [DOI] [PubMed] [Google Scholar]
- 10.Kalhori RP, Ehsani S, Daneshgar F, Ashtarian H, Rezaei M. Different nursing care methods for prevention of keratopathy among intensive care unit patients. Glob J Health Sci. 2015;8(7):212–217. doi: 10.5539/gjhs.v8n7p212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezra DG, Chan MP, Solebo LM, Malik AP, Crane E, Coombes A, et al. Randomised trial comparing ocular lubricants and polyacrylamide hydrogel dressings in the prevention of exposure keratopathy in the critically ill. Intensive Care Med. 2009;35(3):455–461. doi: 10.1007/s00134-008-1284-4. [DOI] [PubMed] [Google Scholar]
- 12.So HM, Lee CC, Leung AK, Lim JM, Chan CS, Yan WW. Comparing the effectiveness of polyethylene covers (Gladwrap) with lanolin (Duratears) eye ointment to prevent corneal abrasions in critically ill patients: a randomized controlled study. Int J Nurs Stud. 2008;45(11):1565–1571. doi: 10.1016/j.ijnurstu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Johnson K, Rolls K. Chatswood; NSW: Agency for Clinical Innovation: 2014. Eyecare for critically Ill dults, pp. 1–43. version 2, pp. [Google Scholar]
- 14.Dawson D. Development of a new eyecare guideline for critically ill patients. Intensive Crit Care Nurs. 2005;21(2):119–122. doi: 10.1016/j.iccn.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Jammal H, Khader Y, Shihadeh W, Ababneh L, Aljizawi G, AlQasem A. Exposure keratopathy in sedated and ventilated patients. J Crit Care. 2012;27(6):537–541. doi: 10.1016/j.jcrc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Kousha O, Kousha Z, Paddle J. Exposure keratopathy: Incidence, risk factors and impact of protocolised care on exposure keratopathy in critically ill adults. J Crit Care. 2018;44:413–418. doi: 10.1016/j.jcrc.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Azfar MF, Khan MF, Alzeer AH. Protocolized eyecare prevents corneal complications in ventilated patients in a medical intensive care unit. Saudi J Anaesthesia. 2013;7(1):33–36. doi: 10.4103/1658-354X.109805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirel S, Cumurcu T, Fırat P, Aydogan MS, Doğanay S. Effective management of exposure keratopathy developed in intensive care units: The impact of an evidence based eyecare education programme. Intensive Crit Care Nurs. 2014;30(1):38–44. doi: 10.1016/j.iccn.2013.08.001. [DOI] [PubMed] [Google Scholar]




