Abstract
Introduction
The effectiveness of gastric lavage in organophosphorus (OP) poisoning has not been established. We assessed the ability of gastric lavage to remove OP insecticides as a preliminary stage in assessing effectiveness.
Patients and methods
Organophosphorus poisoning patients presenting within 6 hours were included, irrespective of prior gastric lavage. A nasogastric tube was placed and gastric contents aspirated, followed by at least three cycles of gastric lavage with 200 mL of water. Samples from the initial aspirate and the first three lavage cycles were sent for identification and quantification of the OP compounds. Patients were monitored for complications of gastric lavage.
Results
Around 42 patients underwent gastric lavage. Eight (19.0%) patients were excluded from the study because of a lack of analytical standards for ingested compounds. Insecticides were detectable in the lavage samples of 24 of 34 (70.6%) patients. Lipophilic OP compounds were detected in 23 of 24 patients, while no hydrophilic OP compounds could be detected in six patients with reported ingestion of hydrophilic compounds. For chlorpyrifos poisoning (n = 10), only 0.65 mg (SD 1.2) of the estimated ingested amount (n = 5) of 8,600 mg (SD 3,200) was recovered by gastric lavage. The mean proportion of the compound removed by initial gastric aspirate was 79.4% and subsequent three cycles removed 11.5, 6.6, and 2.7%.
Conclusion
Lipophilic OP insecticides could be quantified in the stomach contents of OP poisoning patients with the first aspiration or lavage being most effective. The amount removed was very low; hence, routine use of gastric lavage for OP poisoning patients arriving within 6 hours is unlikely to be beneficial.
How to cite this article
Mathansingh AJ, Jose A, Fleming JJ, Abhilash KPP, Chandiraseharan VK, Lenin A, et al. Quantification of Organophosphorus Insecticide Removed by Gastric Lavage in Acutely Poisoned Patients: An Observational Study. Indian J Crit Care Med 2023;27(6):397–402.
Keywords: Efficacy, Gastric lavage, Insecticide poisoning, Organophosphorus
Introduction
The role of gastric nasogastric (NG) lavage is still being debated due to a lack of good-quality studies. This study aims to explore the potential benefit of gastric lavage in patients with acute organophosphorus (OP) insecticide poisoning, by measuring the amount of insecticide removed from the stomach by gastric lavage.
Organophosphorus insecticide poisoning is an important cause of deliberate self-harm in southern India and across Asia.1–4 The mainstay of treatment of OP poisoning is mechanical ventilation, anticholinergics, and reactivators of acetylcholinesterase.5 Gastric lavage is widely used in the management of OP poisoning in developing countries.6
The role of orogastric lavage is still being debated due to a lack of good-quality studies.7 Various complications have been reported with gastric lavage, aspiration pneumonia, gastric perforation, arrhythmias, and cardiac arrest.8–10 The American Association of Clinical Toxicology and the European Association of Poisons Centres and Clinical Toxicologists have recommended that gastric lavage should not be used routinely in the management of poisoned patients.10 It is still a commonly performed procedure in South Asia and a standard of care in India with legal requirement.11
This study aims to explore the potential benefit of gastric lavage in patients with acute OP insecticide poisoning by measuring the amount of insecticide removed from the stomach by gastric lavage.
Patients and Methods
Study Setting
Prospective observational study was carried out between April 2015 and June 2016 in a 2,800-bed tertiary care referral center in south India and approved by the Institutional Review Board (no. 9141; December 11, 2014).
Participants
Patients above 18 years of age who presented to the emergency department within 6 hours with a history of OP consumption, identified by bringing an empty pesticide container or a written transfer document were included. Patients presenting with a history of unknown pesticide poisoning with a typical toxidrome of cholinergic crises and low butyrylcholinesterase (BuCHE) were also included.
Data and Sample Collection
Patients were managed according to standard protocols followed in our hospital. Data were collected by the principal investigator (PI) and entered in clinical research form, including compound characteristics (class, quantity, whether pyrethroid combination product), time to presentation, and prior treatment and outcomes. Patients are classified according to the Namba Poisoning Severity Score.12
After initial stabilization of the patient, a NG tube was inserted and stomach contents aspirated as per standard protocol. Ten milliliters of the “initial gastric aspirate” was collected in a bottle labeled “A,” and the total volume of aspirate was recorded. Gastric lavage was then performed by the PI by repeatedly instilling 200 mL of tap water through the NG and then re-aspirating until samples were cleared and there was no smell of OP. From the first three cycles of lavage after the aspiration, 10 mL samples were collected in bottles labeled B, C, and D, with the total volume of each gastric lavage labeled. The initial gastric aspirate and three lavage samples were sent to the laboratory.
Patients were carefully monitored for procedure-related complications like laryngospasm, esophageal perforation, arrhythmias, aspiration pneumonia, or cardiac arrest. They were monitored for intermediate syndrome (weakness of muscles of respiration, proximal limb, motor cranial nerves, and neck flexors occurring within 24–96 hours).
Laboratory Analysis
Analytical standards were available, allowing high-performance liquid chromatography (HPLC) analysis, for the following lipophilic OP compounds: acephate, chlorpyrifos, malathion, methyl parathion, phorate, profenofos, quinalphos, and triazophos and the following hydrophilic compounds: monocrotophos and dimethoate. The assays had been established for earlier research studies by the co-investigators.13,14 Between-day assay variation was measured using internal quality controls (IQCs) for monocrotophos and quinalphos and these data were assumed to be representative of assay performance for all hydrophilic and hydrophobic compounds.
HPLC of Hydrophilic OP Compounds
Around 200 µL of plasma was taken in a 1.7-mL vial and 400 µL of acetonitrile was added; the mixture was vortexed for 30 seconds. The vial was then centrifuged at 13,000 RPM. Approximately, 300 µL of supernatant was carefully separated into a glass vial and evaporated to dryness at 37 °C by blowing down with an inert gas (nitrogen). The dried residue was reconstituted with 200 µL mobile phase.
HPLC analysis for hydrophilic OP compounds was performed on a Perkin Elmer Series 200 HPLC system with a 25 cm x 4.6 mm, 5 µm Supelco, Discovery HS C18 column (Supelco, Sigma Aldrich). The mobile phase used for hydrophilic compounds was 30% acetonitrile at a flow rate of 1 mL/minute. The run time was set for 30 minutes and 100 µL of extracted sample was injected onto the column. The detector used was a Perkin Elmer diode array detector at 200 nm.
The monocrotophos IQC had a mean value and SD of 40.14 ± 1.17 µg/mL (n = 12) with a between-day assay percent coefficient of variation of 2.91%.
HPLC of Lipophilic OP Compounds
Short solid-phase C18 extraction mini columns from Phenomenex (USA) were used for the separation and clean-up of samples. One milliliter of methanol was added to the C18 column for conditioning. A vacuum manifold was then used for draining the column. Each column was equilibrated using 1 mL water, and then drained using negative pressure. One milliliter of plasma was acidified with 20 μL of perchloric acid, centrifuged at 13,000 RPM, and then added to the column. It was allowed to stand and then drained slowly using vacuum. One milliliter of 5% methanol added to the column and washed and drained. The OP compounds were then eluted using 1 mL 100% methanol. This eluent was collected and evaporated to dryness under nitrogen and the residue was reconstituted in 200 μL mobile phase and 100 μL of this was injected to the HPLC system.
HPLC analysis for lipophilic OP compounds was performed on the same HPLC system with a C18 column (Supelco, Sigma Aldrich). The mobile phase used for lipophilic compounds was 80% methanol at a flow rate of 1 mL/min. The run time was set for 30 minutes and 100 mL of the extracted sample was injected onto the column. The detector used is a diode array detector set at 240 nm.
The Quinalphos IQC had a mean value and SD of 4.96 ± 0.22 µg/mL (n = 8) with a between-day assay percent coefficient of variation of 4.07%.
Statistical Analysis
A sample size calculation was not possible as there were no similar previous studies. A pilot study on 40 patients was targeted. The approximate estimation of OP ingested was calculated as the concentration of the compound × the total volume consumed (as reported by the patient). The total OP removed was calculated = (initial gastric aspirate OP concentration × volume] + (1st gastric lavage OP concentration × volume] + (2nd gastric lavage OP concentration × volume] + (3rd gastric lavage OP concentration × volume].
Data were analyzed with SPSS software using frequency distributions, Box–Cox plots, and histograms. The estimate of the OP compound was presented as a point estimate with a 95% confidence interval. A paired t-test was done to test whether there was a difference between the concentration of OP before and after lavage. The association between certain determinant variables and the amount of OP in the stomach was done using Chi-square test/ Fisher's exact test.
Results
Demography
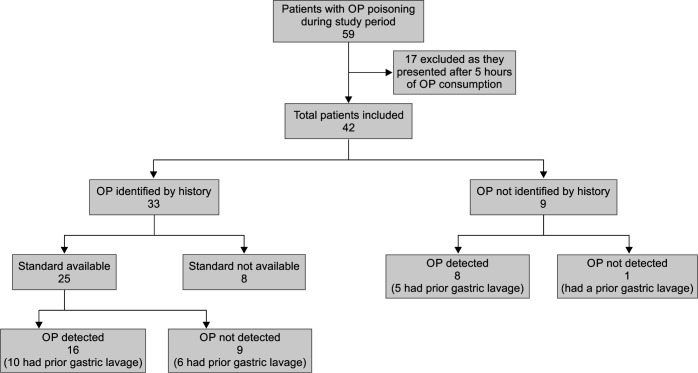
The study included 42 patients with acute OP poisoning (Fig. 1). Patient characteristics are summarized in Table 1.
Fig. 1.
STROBE figure
Table 1.
Baseline characteristics of patients with organophosphorus poisoning
| Profile | Details |
|---|---|
| Total participants | 42 |
| Median age (IQR), years | 26.5 (23.0–33.8) |
| Male:female ratio (%) | 22 (52.4%):20 (47.6%) |
| Compound identified (%) by Pesticide container Written transfer document Oral report |
33 (78.6%) 26 7 9 |
| OP compounds identified by History Triazophos Chlorpyrifos Phorate Profenofos Dichlorvos Dimethoate Monocrotophos Others* |
(n = 33) 6 5 4 4 3 3 3 5 |
| Diethyl OP compounds (%) Dimethyl OP compounds (%) S-alkyl OP compounds (%) |
16/33 (45.5%) 13/33 (39.4%) 4/33 (12.1%) |
| Pyrethroid combination with OP Profenofos + cypermethrin Triazofos + deltamethrin Chlorpyrifos + cypermethrin Chlorpyrifos + deltamethrin Ethion + cypermethrin |
9/33 (27.3%) 3 3 1 1 1 |
| Median S. BuChE (IQR), μL; (EC 3.1.1.8) (normal activity – 3000–8000 μL) Activity <3000 (%) |
355.5 (IQR 143–3352) 31/42 (73.8%) |
| Severity of poisoning (Namba Scale) Latent Mild Moderate Severe |
6/42 (14.3%) 14/42 (33.3%) 4/42 (9.5%) 18/42 (42.8%) |
| Prior medical aid Prior decontamination procedures: yes Forced emesis Gastric lavage Activated charcoal Atropinization Pralidoxime |
31/42 (73.8%) 31/42 (73.8%) 6 (14.3%) 27 (64.3%) 1 (2.4%) 22/42 (52.4%) 5/42 (11.9%) |
| Median time (IQR) from consumption to presentation at any medical center, hours Median time (IQR) from consumption to presentation to our hospital, hours |
1 (0.5–1.5) 2.8 (2–4) |
| Clinical features at presentation Vomiting Salivation Altered sensorium Breathlessness Sweating Defecation Seizures urination Lacrimation |
35 (83.3%) 26 (61.9%) 21 (50.0%) 14 (33.3%) 10 (23.8%) 8 (19.0%) 7 (16.7%) 6 (14.3%) 5 (11.9%) |
*One each of malathion, methyl parathion, phenthoate, quinalphos, and ethion. BuChE, butyrylcholinesterase; IQR, interquartile range
The OP compounds were identified from the history of 33 of 42 (78.6%) patients. Twelve compounds were reported; many (17 of 33, 51.5%) had consumed WHO class I OP compounds. Nine had ingested OP insecticides co-formulated with pyrethroid (cypermethrin n = 5 or deltamethrin n = 4). The two most commonly ingested OP compounds were triazophos (n = 3 alone, n = 3 in combination with pyrethroid) and chlorpyrifos (n = 3 alone, n = 2 in combination with pyrethroid; Table 1).
Most patients (31 of 42, 73.8%) had received prior medical aid before reaching our center (Table 1). In those who had received prior medical aid, the median time to presentation to our hospital was 3 hours [Interquartile range (IQR 2–4)] as compared to 2 hours (IQR 1, 3) in those who came straight to our hospital. Around 27 of 42 (64.3%) had prior gastric lavage and the median (IQR) time to admissions for those with and without previous lavage was 3 hours (IQR 2–4.5) and 2.5 hours [IQR 1.5–4], respectively.
Clinical Outcome
Twenty (47.6%) patients were admitted to the intensive care unit (ICU) for mechanical ventilation. Seven (16.7%) developed intermediate syndrome. Three developed ventilator-associated pneumonia, while two died (4.8%).
Thirty patients (71.4%) had received gastric decontamination before presenting to our hospital and 27 (64.3%) had received gastric lavage. There was no significant difference in outcomes of ICU admission (p = 0.461), mechanical ventilation (p = 0.461), intermediate syndrome (p = 0.225), or death (p = 0.361) in those who had prior gastric lavage compared to those who did not have prior gastric lavage.
Detection of OP in Gastric Lavage Samples
Organophosphorus compounds ingested were known from the patient's history for 33 of 42 patients. Eight of these 33 patients were excluded because of a lack of standards for laboratory analysis for these compounds (phorate 4, dichlorvos 3, and ethion 1). Twenty-five patients with a history of OP ingestion for which we had analytical standards, the OP compound was detected in the gastric lavage of 16 patients (64.0%). Of the nine patients who did not provide a history of the particular OP insecticide ingested, eight had a detectable OP compound in the gastric lavage. Thus, there were overall 24 patients with detectable OP in the gastric lavage (Fig. 1). Lipophilic OP compounds were detected in the gastric samples of 23 of 24 patients, while no hydrophilic OP compounds could be detected in any of the six patients with reported hydrophilic compound ingestion. In 10 patients who did not have detectable OP in gastric lavage, nine had ingested known OP compounds (six hydrophilic compounds and three lipophilic compounds; Table 2).
Table 2.
Various OP compounds based on their detection in stomach contents
| Compound | OP detected (24) | OP not detected (10) |
|---|---|---|
| Lipophilic | ||
| Chlorpyrifos | 10 (41.7%) | 0 |
| Triazophos | 5 (20.8%) | 1 (10%) |
| Profenofos | 3 (12.5%) | 0 |
| Methyl parathion | 3 (12.5%) | 0 |
| Malathion | 1 (4.2%) | 1 (10%) |
| Quinalphos | 1 (4.2%) | 1 (10%) |
| Hydrophilic | ||
| Monocrotophos | 0 | 3 (30%) |
| Dimethoate | 0 | 3 (30%) |
| Unknown | 1 (4.2%)* | 1 (10%) |
*Patient gave a history of ingestion of an unknown OP compound; however, OP was detected in the gastric lavage sample but no peaks were identified under any known compound
Initial gastric aspirate sample was collected from 27 of 42 patients (no aspirate was obtained from the remaining patients), of whom 17 had prior gastric lavage and 10 had no prior lavage.
The mean amount of OP removed for each insecticide varied from about 0.6 mg for chlorpyrifos to 9.3 mg for triazophos (Table 3). The amount of OP insecticide consumed based on history was estimated to be 8,600 ± 3,200 mg (n = 5) for chlorpyrifos, 4,000 and 3,900 mg (n = 2) for triazophos, 1,500 and 2,000 mg (n = 2) for profenofos, and 2,000 mg (n = 1) for methyl parathion.
Table 3.
Mean amount of OP compounds removed by gastric lavage for individual compounds in those who had a prior gastric lavage vs those who did not have a prior gastric lavage
| OP compound | Mean ± SD amount removed (mg) (N) | Mean ± SD amount removed in those who had a prior lavage (mg) (N) | Mean amount removed in those who did not have a prior lavage (mg) (N) | ||
|---|---|---|---|---|---|
| <=2 h | >2 h | <=2 h | >2 h | ||
| Chlorpyrifos | 0.65 ± 1.23 (10) |
1.03 ± 1.51 (6) |
0.08 ± 0.06 (3) |
– | 0.10 (1)a |
| Triazophos | 9.28 ± 12.35 (5) |
– | 0.84 (1)a |
20.38, 0.14 (2)b |
24.99, 0.03 (2)b |
| Profenofos | 0.12 ± 0.11 (3) |
0.04 (1) |
– | 0.08, 0.24 (2)b |
– |
| Methyl parathion | 6.92 ± 11.80 (3) |
0.13 (1) |
20.54, 0.07 (2)b |
||
| Malathion | 0.07 (1)a |
– | 0.07 (1)a |
– | – |
| Quinalphos | 0.33 (1)a |
0.33 (1)a |
– | – | – |
aActual value for one patient;
bActual value for 2 patients
Amount of OP Compound Removed in Relation to Time to Presentation and Prior Gastric Lavage
Overall, there were 27 of 41 who had received a prior gastric lavage, out of which 14 of 27 had an identifiable compound on analysis of the gastric lavage sample (chlorpyrifos — 9, triazophos — 1, profenofos — 1, methyl parathion — 1, malathion — 1, and quinalphos — 1).
The mean amount of chlorpyrifos removed in those with and without prior lavage was 0.72 mg (n = 9) and 0.10 mg (n = 1), respectively. The mean amount of chlorpyrifos removed in those presenting for <2 hours and >2 hours was 1.03 mg (n = 6) and 0.09 mg (n = 4), respectively (see Table 3 and Fig. 2). The amount of triazophos removed in patients with and without prior gastric lavage was 0.84 mg (n = 1) and 11.38 mg (n = 4), respectively. The amount of triazophos removed in patients presenting for <2 hours and >2 hours was 20.38 and 0.14 mg (n = 2) compared to 24.99, 0.03, and 0.84 mg (n = 3) (Table 3).
Fig. 2.
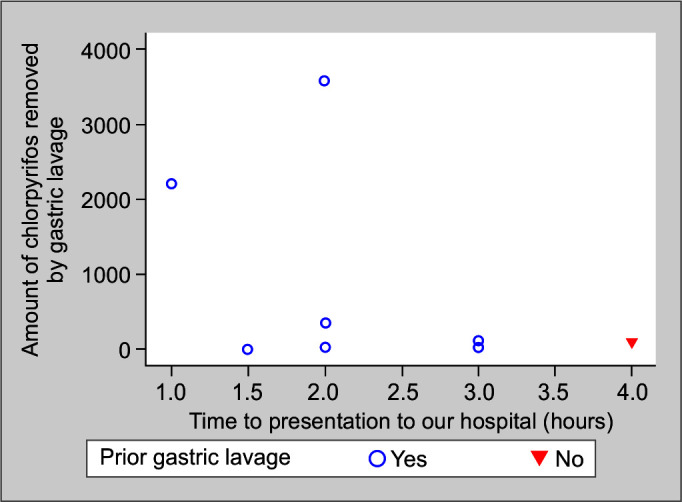
Amount of chlorpyrifos removed vs time to presentation to our hospital in those who had and did not have a prior gastric lavage
Proportion of OP Compound Removed with Gastric Aspirate and Cycles of Gastric Lavage
The mean proportion of total compound removed by the initial gastric aspirate was 79.4% compared to the subsequent three cycles, which removed 11.5, 6.6, and 2.7%. In those from whom an initial aspirate could not be taken, 57.7% of the OP compound was removed by the first cycle of gastric lavage and the subsequent cycles removed 29.2 and 13.07%.
Complications of Gastric Lavage
Careful monitoring showed no post-procedural complications, such as laryngospasm, esophageal perforation, arrhythmias, aspiration pneumonia, or cardiac arrest.
Discussion
While gastric lavage is widely used in the management of OP insecticide poisoning, there is a lack of evidence regarding its potential benefit. The study was designed to document the amount of pesticide removed during gastric lavage and its potential therapeutic benefit.
Around 64% had gastric lavage before coming to the hospital showing that gastric lavage is widely used at the first point of care. Approximately, 70.5% of patients who underwent gastric lavage had detectable OP compounds in their stomachs. All the OP compounds identified in the gastric lavage samples were fat-soluble compounds. This was a counter-intuitive observation, as normally lipid-soluble compounds should be able to penetrate membranes and should be readily absorbed.15 Water-soluble OP compounds, monocrotophos and dimethoate, could not be identified in the gastric samples. Yet we know that they have the highest serum concentrations14 and have high concentrations in urine.13
We found in this study that the amount of OP insecticide that could be removed by aspiration and three lavages was miniscule (1,000- to 10,000-fold less than the reported dose ingested). Most OP removals occurred with the initial gastric aspirate or first cycle of gastric lavage with a declining trend in subsequent cycles. Similar findings were demonstrated individually for the four fat-soluble OP compounds: chlorpyrifos, triazophos, profenofos, and methyl parathion.
Half of the patients with detected OP compounds presented within 2 hours of ingestion. There was no clear difference in the amount of chlorpyrifos and triazophos removed in patients presenting within 2 hours and between 2 and 6 hours. Nor was there apparent difference in the amount of OP compound removed between patients who did and did not have prior lavage. These findings are limited by the small number of patients with individual compound poisoning and the majority of patients having had prior lavage.
Based on LD 50 in the rat for chlorpyrifos of 95 to 270 mg/kg16 and triazophos of 57 mg/kg,17 we estimate that the amount of chlorpyrifos (0.65 mg) and triazophos (9.2 mg) removed by gastric lavage in this study was 0.0001 and 0.26% of the lethal dose, respectively. This suggests that gastric lavage is unlikely to offer any clinical benefit and this lack of benefit should be balanced against the risk of harm. Evidence is now required to show that lavage removes a substantial proportion of the ingested dose if performed within 1 hour.
Limitations
The study was done as a pilot study with small numbers to check its feasibility. Most patients presented to our hospital after 1 hour of OP consumption and had prior decontamination procedures. HPLC testing was done to detect OP compounds instead of mass spectrometry, which may have reduced the sensitivity of the assays. Of the 10 patients in whom OP could not be detected, nine had reported ingestion of known OP compounds. Misclassification of these nine OP compounds was unlikely as they were identified based on pesticide containers (six of nine) and written transfer documents (three of nine). Pesticide assays on blood samples could not be done. Standards for lab analysis were not available for all OP compounds. The eight excluded patients’ gastric lavage samples were also analyzed for other OP compounds and did not show any peaks.
The dose ingested was calculated based on the history and, therefore, could be inaccurate. However, the concentration of the OP insecticide formulations is high and the log orders are greater than the quantity removed in our study. Therefore, a modest inaccuracy in the estimated dose is unlikely to change the conclusions of the study.
Conclusion
We were able to detect lipophilic OP compounds in the gastric lavage samples of patients presenting at a median time of 2.8 hours. The majority of the OP compound was removed by the first gastric aspirate or first cycle of lavage; however, the amount of insecticide removed was very low, irrespective of prior gastric lavage or time to presentation. This suggests that gastric lavage is unlikely to offer any clinical benefit to patients arriving at the hospital within 6 hours and that this lack of benefit should be balanced against the risk of harm. Evidence is now required to show that lavage removes a substantial proportion of the ingested dose if performed within 1 hour.
Author Contributions
AZ and MAJ conceived the study idea, designed the study and conducted the data collection process. AJ and JJF provided clinical biochemistry laboratory expertise. KPPA, VKC, AL, SGH, RI, and JVP supervised the conduct of the trial and data collection. TM provided statistics and modeling expertise. JVP and ME assisted with the literature review and quality assurance. MAJ drafted the manuscript and all authors contributed to the article substantially to its revision. AZ takes responsibility for the paper as a whole.
Orcid
M Asisha Janeela https://orcid.org/0000-0002-7184-0627
Arun Jose Nell https://orcid.org/0000-0002-7445-0042
Kundavaram Paul Prabhakar Abhilash https://orcid.org/0000-0002-2382-4411
Vignesh Kumar Chandiraseharan https://orcid.org/0000-0001-9117-1996
Audrin Lenin https://orcid.org/0000-0001-9230-2791
Samuel George Hansdak https://orcid.org/0000-0002-3389-1220
Ramya Iyyadurai https://orcid.org/0000-0001-8453-6205
Thenmozhi Mani https://orcid.org/0000-0002-3932-5625
John Victor Peter https://orcid.org/0000-0002-3423-1830
Michael Eddleston https://orcid.org/0000-0002-6857-3441
Anand Zachariah https://orcid.org/0000-0002-9039-364X
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Bertolote JM, Fleischmann A, Butchart A, Besbelli N. Suicide, suicide attempts and pesticides: A major hidden public health problem. Bull World Health Organ. 2006;84(4):260. doi: 10.2471/blt.06.030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: A continuing tragedy in developing countries. Int J Epidemiol. 2003;32(6):902–909. doi: 10.1093/ije/dyg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007;7(1):357. doi: 10.1186/1471-2458-7-357. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao CS, Venkateswarlu V, Surender T, Eddleston M, Buckley NA. Pesticide poisoning in south India – Opportunities for prevention and improved medical management. Trop Med Int Health. 2005;10(6):581–588. doi: 10.1111/j.1365-3156.2005.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A, Azher S, et al. Early management after self-poisoning with an organophosphorus or carbamate pesticide – A treatment protocol for junior doctors. Crit Care. 2004;8(6):R391–397. doi: 10.1186/cc2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meulemans A, Van den Berghe G, Winnen B, Delooz H. Gastrointestinal decontamination for acute poisoning. Acta Clin Belg Suppl. 1990;13:13–19. doi: 10.1080/17843286.1990.11718123. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Tse ML, Gawarammana I, Buckley N, Eddleston M. Systematic review of controlled clinical trials of gastric lavage in acute organophosphorus pesticide poisoning. Clin Toxicol. 2009;47(3):179–192. doi: 10.1080/15563650701846262. [DOI] [PubMed] [Google Scholar]
- 8.Wang C-Y, Wu C-L, Tsan Y-T, Hsu J-Y, Hung D-Z, Wang C-H. Early onset pneumonia in patients with cholinesterase inhibitor poisoning. Respirology. 2010;15(6):961–968. doi: 10.1111/j.1440-1843.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 9.Eddleston M, Haggalla S, Reginald K, Sudarshan K, Senthilkumaran M, Karalliedde L, et al. The hazards of gastric lavage for intentional self-poisoning in a resource poor location. Clin Toxicol (Phila) 2007;45(2):136–143. doi: 10.1080/15563650601006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson BE, Hoppu K, Troutman WG, Bedry R, Erdman A, Höjer J, et al. Position paper update: Gastric lavage for gastrointestinal decontamination. Clin Toxicol (Phila) 2013;51(3):140–146. doi: 10.3109/15563650.2013.770154. [DOI] [PubMed] [Google Scholar]
- 11.Naderi S. The use of gastric lavage in India for poisoned patients. J Clin Toxicol. 2012;2(1):118. Available from: https://www.omicsonline.org/the-use-of-gastric-lavage-in-india-for-poisoned-patients-2161-0495.1000118.php?aid=4192. [Google Scholar]
- 12.Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides: Acute and chronic manifestations. Am J Med. 1971;50(4):475–492. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- 13.Jose A, Selvakumar R, Peter JV, Karthik G, Fleming DH, Fleming JJ. Estimation of monocrotophos renal elimination half-life in humans. Clin Toxicol. 2015;53(7):629–632. doi: 10.3109/15563650.2015.1054500. [DOI] [PubMed] [Google Scholar]
- 14.Pichamuthu K, Jerobin J, Nair A, John G, Kamalesh J, Thomas K, et al. Bioscavenger therapy for organophosphate poisoning – An open-labeled pilot randomized trial comparing fresh frozen plasma or albumin with saline in acute organophosphate poisoning in humans. Clin Toxicol. 2010;48(8):813–819. doi: 10.3109/15563650.2010.518970. [DOI] [PubMed] [Google Scholar]
- 15.Chillistone S, Hardman JG. Factors affecting drug absorption and distribution. Anaesth Intensive Care Med. 2014;15(7):309–313. doi: 10.1016/j.mpaic.2014.04.004. [DOI] [Google Scholar]
- 16.Chlorpyrifos technical fact sheet [Internet] [cited 2020 Jan 11]. Available from: http://npic.orst.edu/factsheets/archive/chlorptech.html.
- 17. PubChem. Triazophos [Internet] [cited 2020 Jan 11]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/32184.