Abstract
The primary objective of this work was to characterize the pharmacokinetics (PK) of systemic clofarabine (clo-fara) in pediatric allogeneic hematopoietic cell transplantation (HCT) recipients receiving either nucleoside monotherapy or a dual nucleoside analogue preparative regimen. Fifty-one children (median age 4.9 years, range 0.25–14.9) undergoing allogeneic HCT for a variety of malignant and nonmalignant disorders underwent PK assessments. Plasma samples were collected over the 4–5 days of clofarabine treatment and quantified for clo-fara, using a validated liquid chromatography/tandem mass spectrometry assay. Nonlinear mixed effects modeling was used to develop the population PK model, including identification of covariates that influenced drug disposition. In agreement with previously published models, a two-compartment PK model with first-order elimination best described the PK of clo-fara. Final parameter estimates for clo-fara were consistent with previous reports and were as follows: clearance (CL), 23 L/h/15kg, volume of the central compartment, 42 L/15kg, volume of peripheral compartment, 47 L/15kg, and intercompartmental CL, 9.8 L/h/15kg. Unexplained variability was acceptable at 33% and the additive residual error (reflective of the assay) was estimated to be 0.36 ng/mL. Patient-specific factors significantly impacting clo-fara CL included actual body weight and age. The covariate model was able to estimate clo-fara CL with good precision in children spanning a wide range of ages from infancy to early adulthood and demonstrates the need for variable dosing in children of different ages. For example, the dose required for a 6-month and 1-year old was approximately 43% and 17% lower than the 40 mg/m2 typical dose to achieve the median AUC0–24 (1.04mg•hr/L) in the studied population. Despite the known renal elimination of clo-fara, no significant clinical parameters for renal function were retained in the final model (p>0.05). Co-administration of fludarabine with clofarabine did not alter the CL of clo-fara (p>0.05). These results will help to inform individualized dosing strategies for clofarabine to improve clinical outcomes and limit drug-related adverse events in children undergoing HCT.
Keywords: Clofarabine, fludarabine, pharmacokinetics, pediatric, allogeneic, hematopoietic cell transplantation
INTRODUCTION
Although major advancements have been made in recent years through improvements in supportive care, high rates of engraftment failure and disease relapse remain prominent clinical problems in allogeneic hematopoietic cell transplantation (HCT). Historically, fludarabine has been included in most reduced intensity allogeneic HCT conditioning regimens in combination with an alkylating agent, such as busulfan (BuFlu). More recently, combining clofarabine with BuFlu has been evaluated in an attempt to improve efficacy in children undergoing alloHCT for the treatment of leukemia and various non-malignant disorders.1
Clofarabine (clo-fara) is a newer generation nucleoside analogue with enhanced anti-tumor activity and an improved safety profile compared to fludarabine.2 Evidence for dual nucleoside analogue therapy as part of pre-transplant combination conditioning for high-risk malignancies is supported by both in vitro and in vivo research. Valdez et al. investigated the in vitro cytotoxic properties of clofarabine alone and in combination with fludarabine and busulfan in a human cell-line model of busulfan-resistant acute myelogenous leukemia.3 In these models the inhibitory concentrations of clofarabine and fludarabine were 0.06 μM and 3.0 μM, respectively, suggesting approximately a 50-fold difference in their cytotoxic activity. At low concentrations, the combination of clofarabine, fludarabine, and busulfan showed a higher degree of synergistic cytotoxicity when compared with either nucleoside alone in combination with busulfan.4 A similar response was observed in other AML cell lines and in the isolated peripheral blood mononuclear cells of leukemia patients. Andersson et al. reported the results of a 4-arm clinical trial in which escalating doses of clofarabine were combined with de-escalating doses of fludarabine, plus standard busulfan in primarily adult patients with high-risk myeloid malignancies.4 The doses of clofarabine utilized were 10 to 40 mg/m2/day for 4 to 5 consecutive days. All 51 patients engrafted and there was no significant difference between the four arms in overall or progression-free survival. However, an early trend for improved overall and event free survival was observed for AML patients in the arms treated with higher clofarabine doses. In abstract form, the combination of Bu/Flu/Clo has demonstrated good response, included low rates of relapse and toxicity in children treated for myeloid and lymphoblastic disease as compared to historic TBI containing regimens.1 Unfortunately, no concentration-response data was reported with either clinical trial.1,4
To date there has only been a single study published describing the PK of clofarabine in the setting of pediatric allogeneic HCT.5 Sixteen patients received a single nucleoside analogue conditioning regimen consisted of clofarabine, alemtuzumab, melphalan, and total body irradiation for the treatment of high-risk inherited metabolic disorders. Clofarabine (40 mg/m2/day) was administered daily and variability of 2–3-fold was observed in clo-fara PK parameters including clearance (CL), area-under-the-curve (AUC), and volume of distribution. PK variability was not sufficiently explained by markers of renal function and body size and due to the small number of subjects, and relationships between PK parameters and clinical outcomes were not evaluated.
Currently, there is no PK data available for a combination nucleoside analogue regimen consisting of both clofarabine and fludarabine. Given that both clofarabine and fludarabine share a similar metabolic pathway, drug disposition may be altered through several mechanisms, including altered drug CL. Moreover, PK guided dosing of nucleoside analogues has the potential to improve survival and reduce toxicity in children at high risk for graft rejection and disease relapse.6 Our primary objective of this work was to characterize the PK of systemic clo-fara in pediatric allogeneic HCT recipients receiving either nucleoside monotherapy or a dual nucleoside analogue preparative regimen.
PATIENTS AND METHODS
Study Population
This was a multi-center PK study of clofarabine in children who underwent allogeneic HCT for a variety of malignant and nonmalignant pediatric disorders. Patients were eligible to participate in PK analysis if they were between 0–18 years of age, met institutional and protocol specific eligibility criteria for HCT, and were to undergo an allogeneic HCT which included receiving single nucleoside therapy with clofarabine or dual nucleoside analogue therapy (fludarabine plus clofarabine) as part of their conditioning regimen. Patients that received clofarabine alone or in combination with fludarabine over 4 to 5 days were eligible to participate. Clofarabine PK data were collected between 2012 and 2018 at the University of California San Francisco Benioff Children’s Hospital and the University of Minnesota Masonic Children’s Hospital. Table 1 described the patient demographics, preparative regimen, and PK assessment strategies for both sites. All local institutional review boards approved this study and written informed consent/assent to undergo PK studies was obtained from all patients and/or guardians. This study was registered at ClinicalTrials.gov as NCT03609814.
TABLE 1.
Overview of the clinical studies included in the population PK analysis.
| Study Identifier | Number of Subjects | Pre-transplant Combination Therapy | PK Sampling Strategy |
|---|---|---|---|
| MN-Clo40 5 | 15 | Alemtuzumab 0.3mg/kg/day intravenously (IV) on days −12 to −8; clofarabine 40mg/m2/day IV on days −7 to −3; melphalan 140mg/m2 IV on day −2; and total body irradiation 200 cGy single fraction on day −1 | Day 1: 0 (pre-infusion), then 2, 3, 4, 6, 8, 24 hours post start of infusion Day 5: 0 (pre-infusion), then 4, 8, 72 hours post start of infusion |
| SF-Flu40/Clo10 | 18 | Model-based busulfan IV infusion over 2 hours administered every 6 hours on days −5 to −2; clofarabine 10 mg/m2/day IV over 2 hours on days −5 to −2; fludarabine 40mg/m2/day IV on days −5 to −2; and serotherapy | Limited sampling following any of 4 single daily doses of clofarabine (Doses 1–4), at 2, 3, 6 and 24 hours post start of infusion |
| SF-Clo30/Flu10 | 18 | Model-based busulfan once daily IV infusion over 3 hours on days −9 to −6; clofarabine 30mg/m2/day IV over 2 hours on days −5 to −2; fludarabine 10mg/m2 IV over 1 hour on days −5 to −2; +/− rATG mg/kg on days −5 to −2 | Limited sampling following a single dose of clofarabine (Doses 1– 4), at 2, 3, 6, and 24 hours post start of infusion |
Pre-Transplant Conditioning Regimen
For subjects enrolled at the University of Minnesota (MN-Clo40) pre-transplant conditioning for high-risk metabolic disorders consisted of alemtuzumab 0.3mg/kg/day intravenously (IV) on days −12 to −8; clofarabine 40mg/m2/day IV on days −7 to −3; melphalan 140mg/m2 IV on day −2; and total body irradiation 200 cGy single fraction on day −1.5 Two protocols utilizing dual nucleoside analogue therapy with clofarabine and fludarabine were evaluated at the University of California. From 2012 to 2013 (SF-Flu40/Clo10), patients undergoing HCT for a variety of non-malignant and high-risk malignancies were conditioned with exposure-targeted model-based dosing of IV busulfan on days −5 to −2; clofarabine 10mg/m2/day IV on days −5 to −2; fludarabine 40mg/m2 IV on days −5 to −2; and serotherapy. Subjects undergoing PK assessment from 2014 to 2018 (SF-Flu10/Clo30) received pre-transplant conditioning for high-risk malignancies with exposure-targeted model-based dosing of IV busulfan on days −5 to −2; clofarabine 30mg/m2/day IV on days −5 to −2; and fludarabine 10mg/m2 IV on days −5 to −2; +/− rATG mg/kg on days −5 to −2. In the SF-Flu10/Clo30 group, to account for potential differences in drug clearance with age, children weighing less than 12kg or younger than one year of age, clofarabine was administered at 1mg/m2 per protocol.
Bioanalysis
For MN-Clo40, plasma samples were analyzed for clo-fara by MicroConstants (San Diego, California) using a validated reverse phase high performance liquid chromatography with mass spectrometry as previously described.5 The assay was linear in the range of 1 to 500ng/ml. Samples with clo-fara levels reported below the lower limit of quantification (1ng/ml) were entered into PK analysis as having a concentration of 0.5ng/ml (half the lower limit of quantification).7 Assay accuracy, intra-day, and inter-day variability were range 95–96.2%, 5.1–7.4%, and 6.7–14.4%, respectively.
Plasma samples for studies SF-Flu40/Clo10 and SF-Flu10/Clo30 were analyzed for clo-fara by the University of California San Francisco, Department of Clinical Pharmacy Drug Research Unit Laboratory using a validated reverse phase high performance LC-MS/MS methods as previously described.8 Clo-fara plasma concentrations that fell below the lower limit of quantification (0.5ng/mL) were reported by the lab and entered into the model as the true value. The assay was linear in the range of 0.5–80ng/mL. The mean accuracy (mean ± coefficient of variation) of the assay was (98.5%±7.0), (101.7%±6.6), and (92.8%±7.8), at low, medium, and high-quality control levels, respectively.
Population Pharmacokinetic Analysis
A nonlinear mixed effects modeling approach to describe the time course of clo-fara plasma concentrations was implemented using Phoenix v8 (Certara, Princeton, New Jersey). The freely available software R (v3.4.4) was used for graphical inspection in order to aid in the selection of the most appropriate model and for displaying the model-predicted results in visual form.9 Model selection was based on comparison of the objective function value (OFV) (, df = 1, ) and the goodness-of-fit plots.
Between-subject variability (BSV)10,11 was modeled based on the assumption of a log-normal distribution as shown below:
where, is the individual PK parameter for patient such as , is the typical value of that PK parameter such as , is the corresponding BSV for patient , which is assumed to follow a normal distribution with mean 0 and variance of .
Different residual error models10,11 were tested (e.g. combined error model: proportional + additive) as shown below:
Where and are the observed and individual predicted concentration in the central compartment for patient at time , respectively, is corresponding proportional error term, which is assumed to follow a normal distribution with mean 0 and variance of ; and is the corresponding additive error term for patient at time , which is assumed to follow a normal distribution with mean 0 and variance of .
Covariate Modeling
Clinical data were collected on each day of PK sampling prior to clofarabine administration to assess the influence of patient-specific factors on clo-fara CL. Covariate analysis was performed using a step-wise forward additive approach followed by a step-wise backward elimination approach12 with an alpha of 0.05 and 0.001, respectively. An improvement in the precision of the parameter estimates (relative standard error, RSE), along with a decrease in between subject variability, and residual variability were used to determine the importance the tested covariate as a significant predictor of drug CL. Age, sex, height, body surface area (BSA), serum creatinine (SCr), creatinine clearance (CRCL), blood urea nitrogen (BUN), albumin (ALB), white blood cell count (WBC), absolute neutrophil count (ANC), diagnosis, and preparative regimen (co-administration of fludarabine) were evaluated.
Allometric-scaling provides a mechanistic and physiological-based approach for describing the effects of organ size and blood flow on drug clearance.13,14 A bodyweight-based allometric model was added to all clearance and volume parameters for clo-fara with an exponent of 0.75 and 1, respectively.15 Although allometric scaling provided an improved model for describing drug clearance it was insufficient for describing changes in CL and PK variability, particularly in subjects under 2 years of age. The maturation of kidney function, which occurs over the first year of life, was found to best explain variations in PK attributed to age, in addition to weight, evaluated as a continuous covariate.
In pediatric patients CRCL was estimated by the Schwartz method and in young adults by the Cockcroft-Gault equation using ideal body weight and capping the maximum value at 150 ml/min/1.73m2 for covariate analysis.16,17 Continuous covariates were tested as various relationships including a power function and centered on the median population value, an function and an exponential function. Dichotomous covariates were tested in the model via binary indicator variables.
Model Evaluation
Model evaluation was based on various methods of evaluating the predictive ability of the final model on individual data. Normalized prediction distribution errors (NPDEs) were generated using 1000 simulations for each observation in the original dataset and used to identify trends for model misspecification.12 A nonparametric bootstrap resampling method was used to evaluate the robustness of the final PK model.18 Resampling with replacement generated 1000 bootstrap data sets and the final population PK model was fitted repeatedly to each of the data sets. The means and 95% confidence intervals of parameters obtained from this step were compared with the final parameter estimates. In addition, the prediction-corrected visual predictive check (pcVPC) with 1000 simulated datasets was also performed.19 Results from the pcVPC were assessed using graphical comparison of the appropriate 90% prediction intervals from simulated data and was visually explored in comparison with overlaid observed data from the original dataset.
Simulations to Demonstrate Covariate Effects on Clofarabine Dose
Based on our final population PK model, individual clo-fara doses were estimated using the final covariate model and compared to a typical dosing regimen of 40mg/m2 administered daily for 4 days. Clinical covariates (age and weight) for a typical patient were based on the 50th percentile estimates of weight per age as provided by the Center for Disease Control standard growth charts for infants and children.20 First, the AUC0–24 for each patient was derived from the empirical Bayes estimates of individual CL (AUC0–24 = Dose/CL). Then, individual AUC0–24 values were multiplied by the total number of doses of clofarabine received to derive the cumulative AUC (cAUC). For dose simulation purposes, we selected our target AUC0–24 to reflect the median value of patients receiving 40 mg/m2 as either monotherapy or with co-adminstration with fludarabine. The combination of clofarabine plus fludarabine is still relatively uncommon and we felt a four-day regimen of 40mg/m2 alone was most reflective of current HCT clinical practice (clofarabine used alone in combination with an alkylator). Doses of clo-fara administered once daily over 4 days of therapy were simulated to achieve the median AUC0–24 in patients receiving 40mg/m2 using the following equation:
RESULTS
Patient Demographics
A total of 51 subjects completed PK assessments and were used for PK model-building. Patient demographics for all subjects are presented in Table 2. Among the study subjects, the overall median age of patients was 4.9 years (range, 0.25–14.9), with 7.8% (n=4) of subjects 12 months or younger. Median actual body weight was 15.6 kg (range, 6.23–97.5) and included 16% (n=8) of children weighing less than or equal to 10 kg. All patients had normal renal function for age starting with the first dose of clofarabine with a median CRCL of 150mL/min (range, 96–150). Fifteen patients received single nucleoside therapy with clofarabine, while the remaining patients received dual nucleoside analogue therapy (fludarabine plus clofarabine).
TABLE 2.
Patient demographics and baseline characteristics1
| Median (range) / N (%) | |
|---|---|
| Number of subjects | 51 |
| Female/Male | 28 (55%) / 23 (45%) |
| Age (years) | 4.9 (0.25–14.9) |
| Weight (kg) | 15.6 (6.2–97.5) |
| Body surface area (m2) | 0.68 (0.31–1.89) |
| Serum creatinine (mg/dL) | 0.3 (0.1–0.5) |
| Creatinine clearance (mL/min/1.73m2)2 | 150 (96–150) |
| White Blood Cell Count (109 cells/L) | 2.9 (0.1–11.7) |
| Combination Pretransplant Conditioning Regimen | |
| Clofarabine 40mg/m2 / melphalan / total body irradiation | 15 (29.4%) |
| Clofarabine 10mg/m2 / fludarabine / busulfan | 18 (35.3%) |
| Clofarabine 30mg/m2 / fludarabine / busulfan | 18 (35.3%) |
Laboratory data was collected on the day of pharmacokinetic sampling, prior to drug administration.
Creatinine clearance was estimated in children using the Schwartz method and in young adults greater than 17 years of age by the Cockcroft-Gault equation using ideal body weight.
Population PK Analysis
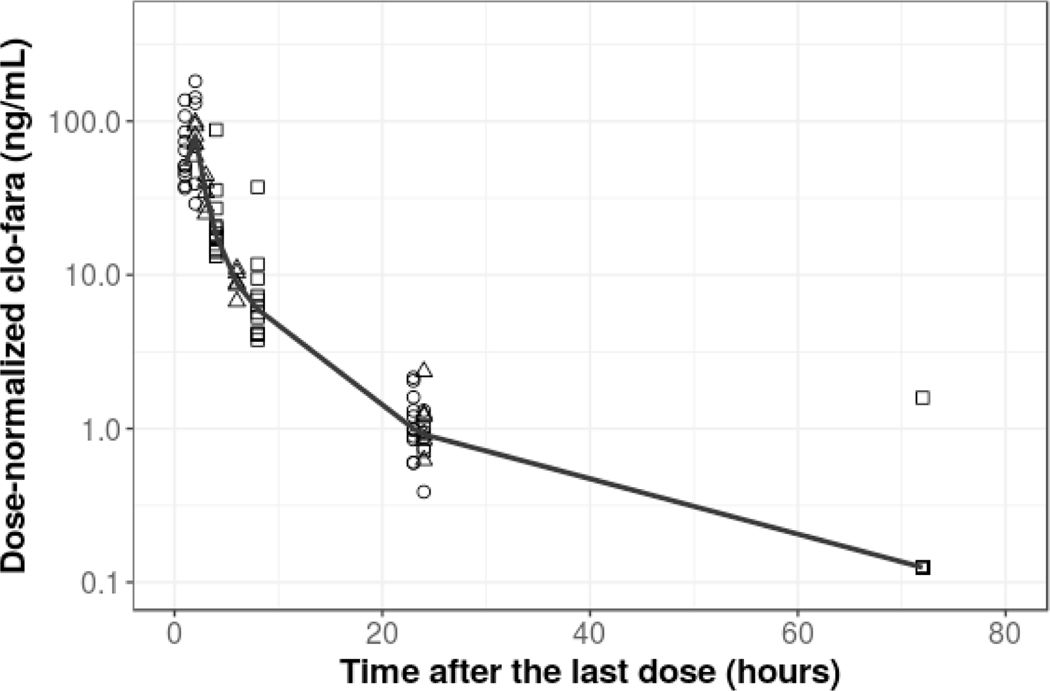
Following inspection of the data a total of 311 quantifiable concentrations clo-fara were available for population PK model building. Less than 5% (n=10) of the plasma samples collected from MN-Clo40 were reported as below the LLOQ. Figure 1 displays the dose-normalized observed clo-fara plasma concentrations (normalized to 10mg/m2). A two-compartment model provided the most reasonable fit of the data and was thus retained for subsequent covariate model development. A bodyweight based allometric model was added to all clearance and volume parameters for clo-fara with an exponent of 0.75 and 1, respectively.15 The model describes an increase in clo-fara CL with increasing body weight in children. For children less than 2 years of age, age-related developmental changes in physiological and elimination processes independent of body weight can lead to significantly altered drug disposition. Hence, we found that body weight alone was not sufficient to describe drug clearance in children, particularly in subjects less than 1 year of age. When we implemented an inverse exponential function, age was found to be significant covariate affecting clo-fara CL (independent of weight) and it was retained in the final model to best describe both the processes of renal maturation (P<0.001) and body weight on drug exposure and disposition. No other laboratory parameters or patient-specific clinical factors evaluated were found to significantly impact clo-fara CL including markers for renal function, diagnosis, or preparative regimen co-administration with fludarabine (p=0.2).
FIGURE 1.
Scatterplot of dose-normalized (by 10 mg/m2) plasma clo-fara concentration-time profiles. Black points represent the observed dose-normalized clo-fara concentrations from the different dose regimens: 10mg/m2(open circles), 30mg/m2(open triangles) and 40mg/m2(open squares). The black solid line represents the median values of the observed concentrations.
The population PK parameters estimates and their relative standard errors (%) from the final model are presented in Table 3. The final model for clo-fara CL incorporating age and weight were as follows:
where, 23.4 L/hr is the typical value of clo-fara for a child weighing 15kg and equal to 5-years. is the renal maturation half-life of age effect on CL which was estimated to be 0.41 years. The between-subject variability for clo-fara CL and volume of central compartment were 26% and 40%, respectively. A combination residual error model best described residual unexplained variability with proportional residual and additive residual error values of 33.2% and 0.36 ng/mL, respectively. The inclusion of inter-occasion variability did not improve the model and was therefore not included.
TABLE 3.
Final population PK model parameter estimates and bootstrap results
| Final Model Results | Bootstrap Results | |||
|---|---|---|---|---|
| Population PK Parameters | Parameter Estimates | RSE (% mean)1 | Mean Values | 95% Confidence Interval |
| Typical value for clo-fara CL (L/h/15kg) | 23.4 | 6.30 | 23.4 | 20.7–26.2 |
| Volume of the central compartment (, L/15kg) | 42.3 | 11.6 | 42.3 | 32.5–52.2 |
| Inter-compartmental CL (L//h/kg)2 | 9.82 | 9.88 | 9.82 | 7.91–11.8 |
| Volume of the peripheral compartment (,L/15kg) | 47.4 | 6.14 | 47.3 | 42.0–53.8 |
| Maturation half-time (, years) | 0.41 | 42.2 | 0.43 | 0.17–0.81 |
| Inter-individual variability on CL (%CV) | 25.7 | 39.0 | 24.2 | 10.2–32.7 |
| Inter-individual variability on Vc4 (%CV) | 40.3 | 26.5 | 40.1 | 22.7–52.0 |
| Proportional residual unexplained variability (%) | 33.2 | 8.89 | 32.7 | |
| Additive error (ng/mL) | 0.36 | 6.43 | 0.36 | |
Relative standard error (%)
First order rate constant for drug moving from the central compartment to the intracellular compartment
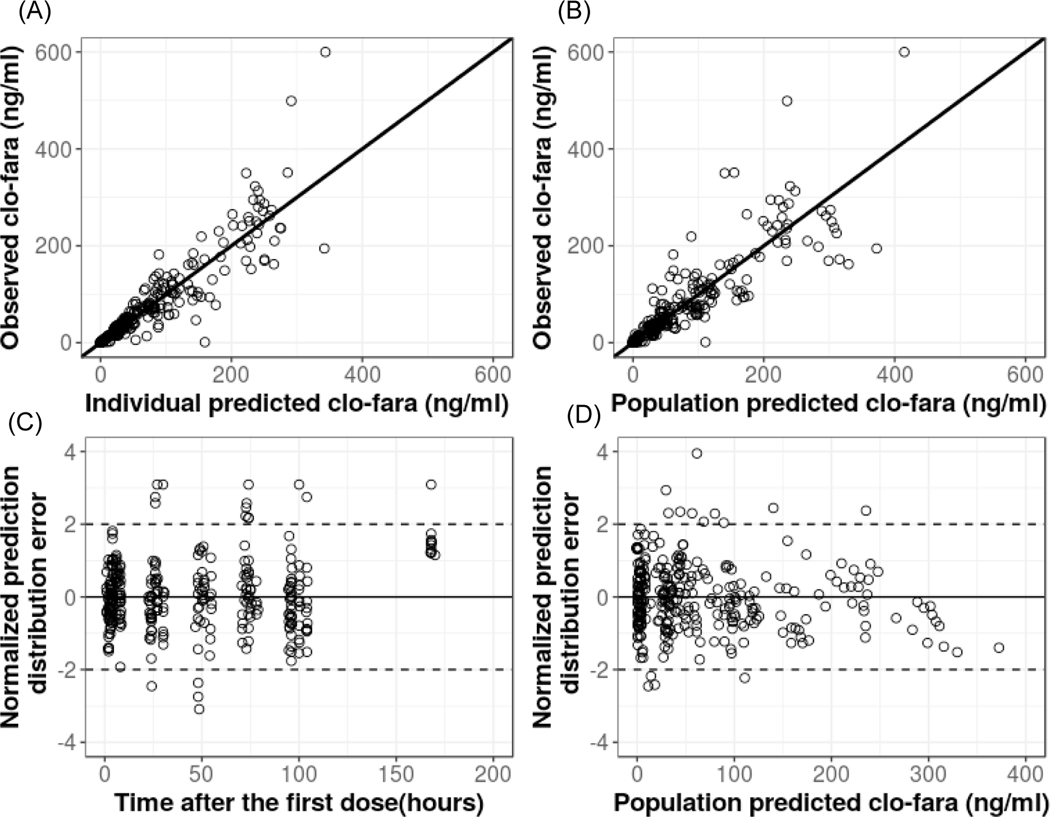
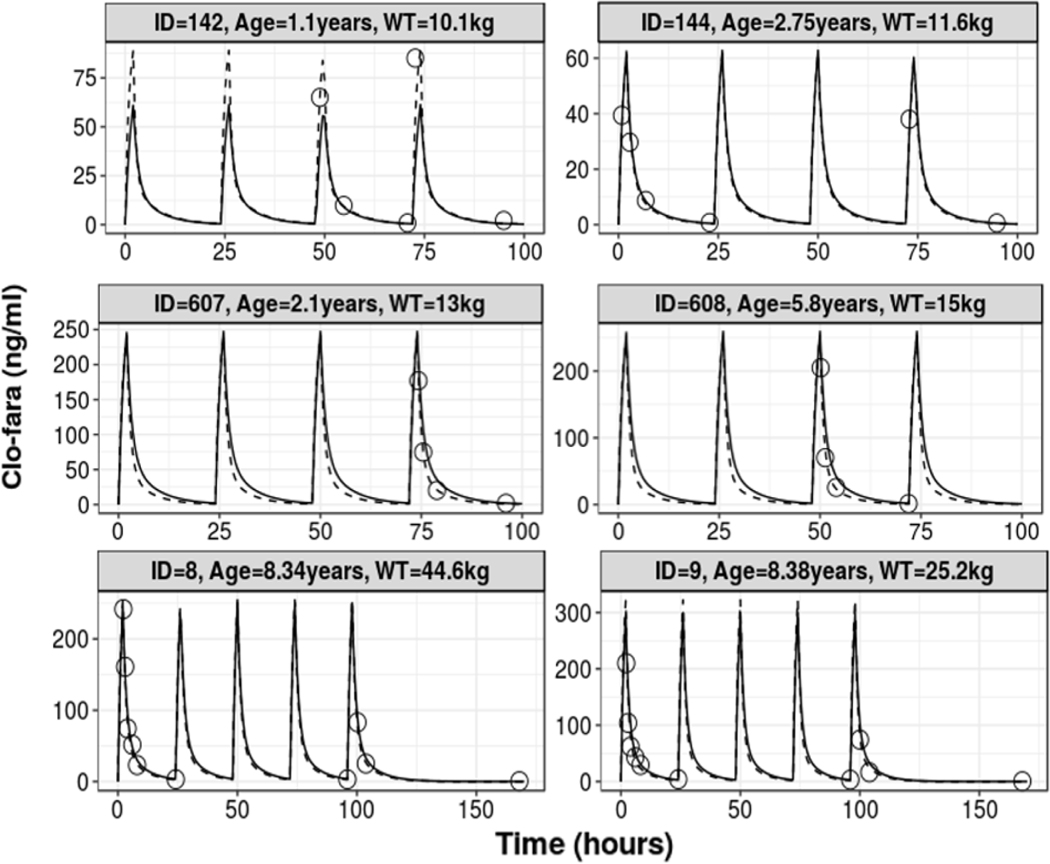
The goodness-of-fit plots for the base and final model demonstrated good improvement with adequate distribution of population-predicted concentrations around the line of unity and no obvious trends for model misspecification or bias (Figure 2). Standard diagnostic plots of the normalized standard predication error (Figure 2) and representative model fits (Figure 3) for individual PK profiles for clo-fara indicate that the model captured the data very well. The mean PK parameter estimates and 95% confidence intervals from the bootstrap analysis are presented in Table 3. Estimates of PK parameters, between-subject variability, and residual unexplained variability derived from the bootstrap analysis were comparable with the typical values derived from the original data set. Additionally, the pcVPC for the final covariate model shows that the variability in the data (quantified by 5th and 95th percentiles) was well predicted (Supplemental Figure S1).
FIGURE 2.
(A) Observed vs. individual-predicted clo-fara plasma concentrations. (B) Observed vs. population-predicted clo-fara plasma concentrations. (C) Normalized prediction distribution error vs. time after first dose. (D) Normalized prediction distribution error vs. population predicted clo-fara plasma concentrations.
FIGURE 3.
Representative individual fit plots of observed and predicted time-concentration data of for the different dosing regimens: 10mg/m2(top row), 30mg/m2(middle row) and 40mg/m2(bottom row). Open circles represent the observed concentrations, black solid line is the population prediction, and the dashed line is individual prediction.
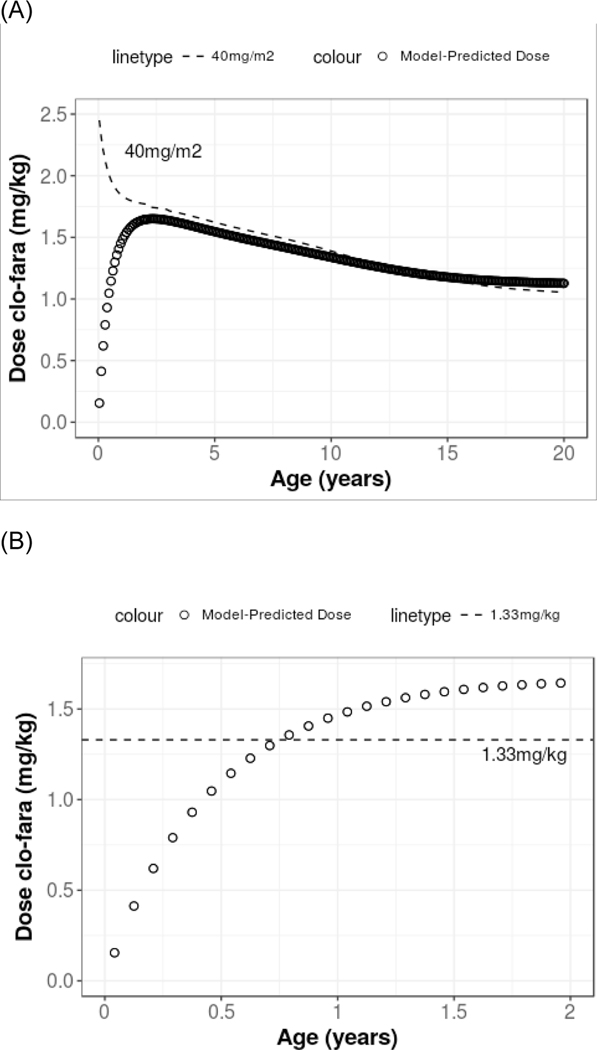
Table 4 displays the derived clo-fara AUC0–24 values from the observed data at different dose regimens for a single 24-hour dosing interval. The median daily clo-fara AUC0–24 for all patients included in the analysis, irrespective of the protocol specific dose (e.g. 10, 30, or 40 mg/m2), was 0.78mg•hr/L (range, 0.22–4.0). For those patients receiving a daily dose of 40 mg/m2 (n=15), the median AUC0–24 was 1.04 mg•hr/L (range, 0.87–4.0), which is equal to a cumulative AUC of 4.2 mg•hr/L for 4 doses. One subject, a 6-month old with diagnosis of osteopetrosis, receiving 40mg/m2 has an outlier value of AUC0–24 (4 mg•hr/L) as shown in Table 4. Notably, this child developed acute renal failure with dose 2 of clofarabine, required the initiation of peritoneal dialysis on Day 0, and unfortunately died of multi-organ failure shortly post-transplant. This is the only subject who has an AUC0–24 greater than 1.2 mg•hr/L in patients receiving a daily dose of 40mg/m2. However, other subjects have an AUC0–24 of 1.17 mg•hr/L or less. Important patient-specific covariates found to significantly impact clo-fara CL were actual bodyweight and age. Figure 4 demonstrates the change in model-predicted dose of clofarabine with age compared with the common dosing strategies of 40mg/m2 and 1.33mg/kg. The model demonstrates that for subjects under 2 years of age, dose modifications are needed to avoid elevated drug exposure. The model-predicted dose increases from birth (0.011 mg/kg) to 2-year-old (1.7mg/kg) and then begins to decrease until around 18-year-old (1.2 mg/kg). Simulated CL values suggest that for young children, particularly those less than 6 months old of age, a decrease in the dose is required to provide exposure comparable to that of an older child (Figure 4). For example, the model-predicted dose for a 6 month-old child weighing 6.3 kg would be 7.2 mg per dose, which is a 45% dose decrease as compared to a conventional dose of 40mg/m2/dose (13mg) and a 14% decrease when compared to the modified regimen of 1.33mg/kg (8.4mg) which is often performed in young children to prospectively prevent drug-related toxicity. In contrast, the model-predicted dose for a 6 month-old weighing 10 kg would be 10.2 mg per dose, which reflects a dose decrease of 44% and 23%, respectively when compared to a conventional dose of 40mg/m2 /dose (18.4mg) or a modified regimen of 1.33mg/kg (13.3 mg).
TABLE 4.
Comparison of daily and cumulative exposure of clo-fara in the observed data presented by clofarabine daily regimen.
| Clofarabine Regimen | Number of Subjects | Total Number of Doses | AUC0–24 (mg•hr/L)1 | cumulative AUC (mg•hr/L) |
|---|---|---|---|---|
| All dose regimens | 0.78 (0.22 – 4.0) | 3.3 (0.87 – 20.0) | ||
| 10 mg/m2/dose | 18 | 4 | 0.30 (0.22 – 0.36) | 1.2 (0.87 – 1.44) |
| 30 mg/m2/dose | 18 | 4 | 0.82 (0.37 – 1.39) | 3.3 (1.5 – 5.5) |
| 40 mg/m2/dose | 15 | 5 | 1.04 (0.87 – 4.0) | 5.2 (4.4 – 20.0) |
Presented as median (range)
FIGURE 4.
Model-predicted dose of clofarabine in mg/kg aimed to achieve a daily AUC0–24 of 1.04mg•hr/L for (A) 0–20 years of age and (B) 0–2 years of age. The dashed line in plot (A) represents the doses at corresponding ages based on the conventional dose 40mg/m2. The dashed line in plot (B) represents the modified regimen of 1.33 kg in young children.
DISCUSSION
The primary objective of this work was to characterize the PK of systemic clo-fara in pediatric allogeneic HCT recipients receiving either nucleoside monotherapy or a dual nucleoside analogue preparative regimen. Prior to these results there was no PK data available for a combination nucleoside analogue regimen consisting of both clofarabine and fludarabine and limited data characterizing the PK of clofarabine alone in a pediatric HCT population. Given that both clo-fara and fludarabine share a similar metabolic pathway for drug disposition we hypothesized fludarabine may alter the PK of clofarabine with co-administration and provide insight into the potential contribution of enhanced synergy of the combination reported in vitro. Clofarabine and fludarabine are primarily excreted unchanged via the kidneys through a combination of glomerular filtration and active tubular secretion via transporters.2,21 On both the basolateral and brush border membrane, the proximal tubule contains numerous drug transport systems, including equilibrative and concentrative nucleoside transporters (ENTs and CNTs).22,23 These transporters have been shown to influence distribution and accumulation of fludarabine and clofarabine in vitro and are potential sites for significant drug-drug interaction in vivo.21,24–26 Co-administration of these two drugs could potentially lead to significantly altered drug clearance and systemic exposure via competitive inhibition of transporters. However, based on our model, co-administration of fludarabine in the presence of clofarabine was not found to have a significant impact on clo-fara CL. Subtle differences in the transporters required for intracellular influx, efflux, or conversion to the active triphosphate species or timing of the infusion may contribute to such findings. Going forward studies evaluating the intracellular concentrations of clo-fara may provide more insight and should be pursued.
Organ function maturation and allometric scaling play an important role in predicting the PK of many drugs in infants and young children, whereas the latter is often sufficient in children >2 years of age with normal renal function. Prior to our results, very little information was available regarding the PK of clo-fara in children less than 2 years of age to guide dose selection in clinical trials and individualized dose regimens in clinical therapy. Our analysis identified both weight and age as significant independent patient-specific factors impacting clo-fara CL. Clo-fara CL increased with age in our analysis, however, Bonate et al. reported that age has a decreasing relationship with clo-fara CL in pediatric and adult patients.27 The decreasing relationship between CL and age in Bonate et al. was driven by an older population (>40years of age). While an increasing relationship between CL and age was observed in the population younger than 20 years of age (2.8–21-year-old), the age range in our study was 0.25 to 15-year-old which makes the comparison difficult. For each drug, one model across the continuum of physiological changes (age) should be the goal of population PK modeling. However, seldom do we get access to such comprehensive datasets. The inclusion of both allometric scaling and a maturation function in our model enhances the ability to estimate drug CL with relative accuracy in a pediatric population of variable ages. Particularly for children ages <6 months, the model suggests significant dose reductions are necessary to achieve comparable exposure to older children when using the traditional dosing strategy based on mg per body surface area (mg/m2). Additionally, clinicians often struggle with the decision to prospectively modify the dosing strategy in children less than 12kg or younger than 1-year of age in their attempts to limit the potential for drug related toxicity. By accounting for both age and weight, our model provides an improved strategy as compared to the empiric 1.33mg/kg conversion that is often applied in the clinical setting for young children. Based on our model, the predicted CL of clo-fara in a child of 6 months and 1-year of age was predicted to be was around 57% and 27% lower than the expected CL of a 2-year-old.
Clofarabine PK parameters were estimated by non-compartmental analysis (vs. nonlinear mixed effect modeling in our study) reported by Long-Boyle et al..5 Non-compartment methods are much less sensitive for evaluating clinical covariates that may impact drug exposure, particularly in children. Additionally, the sample size of 16 was very limited. In the prior analysis clofarabine clearance was weakly correlated with weight () or BSA (). Given the current analysis was conducted using non-linear mixed effects modeling and has a much larger sample size, including a wider range of ages and weights, it would be statistically better powered to determine a significant weight effect on clearance.
Additional other significant covariates found to affect clo-fara CL have been identified in prior reports primarily based in the pediatric leukemic population. Bonate et al. reported CRCL (range, 33.1 – 200 ml/min/1.73m2) as a significant covariate on clo-fara CL.27 This is to be expected considering clo-fara undergoes extensive renal elimination through a combination of both glomerular filtration and active tubular secretion. This is further supported by the fact that clo-fara undergoes limited hepatic and non-hepatic metabolic conversion (0.2%) and 24-hour urine collections in pediatric studies describe that approximately 49–60% of the dose is excreted in the urine unchanged as per the drug’s label.28 Time dependent maturation of transporters in the kidneys likely contributes to an increase in drug exposure in children under 2 years of age. However, the range of CRCL observed in the current study did not support CRCL as predictor for clo-fara CL. This is likely the result of all subjects having normal renal function for age in this study. Further, there was no trend observed between empirical Bayes estimates of individual clo-fara CL and other relevant biomarkers for renal function (such as SCr or BUN) in our analysis. This relationship of CRCL on CL warrants further investigations in a more heterogenous population, particularly in older children with pre-existing renal impairment. However, the evaluation of such a population may prove challenging given the criteria for adequate renal function as part of the pre-HCT assessment process.
Similarly, in the previous study, white blood cell count (WBC) was found to be a significant covariate with the predicted volume of the central compartment of clo-fara increasing 2.38 fold between a WBC of 0.3 × 103 /μL and 259 × 103 /μL.29 The narrow range of WBC counts in this study (0.1 × 103 to 11.7 × 103 /μL), along with inherent differences between patient populations, may account for the lack of a relationship between ANC and clo-fara exposure in our study. This study includes a mix of indications for transplant (malignant and nonmalignant) and, in general, patients present to transplant with normal to low WBC counts. This is significantly different from the leukemic population that routinely initiate clofarabine therapy with an expected elevated WBC. In the covariate analysis preparative regimen (including busulfan) or underlying disease were not found to be significant predictors of clofarabine drug clearance. These factors may be important for clinical outcomes and will be evaluated again in future, planned analyses, once a larger sample size is achieved to adequately evaluate them.
The primary purpose of this study was to better understand the PK of systemic clo-fara in pediatric allogeneic HCT recipients receiving either nucleoside monotherapy or a dual nucleoside analogue preparative regimen. This analysis was not designed to determine if the combination of Bu/Flu/Clo is more safe or efficacious than Bu/Flu or Bu/Clo. Subjects included in this data set widely vary in several areas, including indication for transplant, comorbidities, combination pre-transplant conditioning (cPTC) and immunosuppressive strategies. Thus, due to the sample size of the current analysis, no exposure-response analysis was performed. We continue to collect additional PK and outcomes data at both centers such that sufficient numbers for subjects to adequately inform drug-response relationships will be available for analysis. Additionally, at our center we are now able to quantify drug levels for all agents used in a single individual as part of combination cPTC. Univariate PK-PD associations have been identified for several commonly used agents in pediatric HCT including busulfan, fludarabine, and antithymocyte globulin. However, the analysis of single agents is likely insufficient to describe the overall effect of cPTC on clinical outcomes and, to date, no comprehensive evaluation of PK-PD relationships describing the relative contributions of individual agents when used in combination for cPTC has been performed. Therefore, our current, ongoing studies move to a more comprehensive investigation of cPTC and exposure-response relationships in pediatric HCT with the goal of identifying optimal immunosuppression and prevention of severe toxicity.
CONCLUSION
To date, this work represents the largest and most comprehensive study of clofarabine pharmacology in pediatric patients undergoing HCT. We found no significant impact on clo-fara PK when co-administered with fludarabine. Our covariate analysis revealed actual body weight and age to be significant patient-specific factors affecting clo-fara CL, demonstrating the application of model-based dosing can ensure equivalent exposure across different age and weights for children requiring clofarabine as part of cPTC in HCT. We suggest that each individual child, especially the very young or small children should be administered a personalized dose based on the patients specific age and body weight. Dose reductions of clofarabine based on our model suggested the current dose alternative dose 1.33 mg/kg may be sufficient for some children under 6 months of age and largely insufficient for many children <12kg or under 2 years of age, where it is often applied. Finally, moving away from the traditional dosing intensity strategies to model-based dosing in this setting has the potential to limit drug-related toxicity while maintaining efficacy. Future work will involve the identification of exposure-response relationships between clofarabine and clinical outcomes to enhance drug efficacy, when applied with a combination of model-based dosing and Bayesian-driven TDM.
Supplementary Material
Highlights.
Evaluation of clofarabine pharmacokinetics was performed in a pediatric population.
Body weight and age influence clofarabine clearance.
Young children less than 2-year-of-age require dose modifications of clofarabine.
Model-based dosing of clofarabine has the potential to limit drug-related toxicity while maintaining efficacy in children undergoing hematopoietic cell transplantation.
ACKNOWLEDGEMENTS
We would like to thank all the patients, families, nursing and support staff for their contributions to this study.
FINANCIAL DISCLOSURE STATEMENTS
This work was supported by the Thrasher Research Fund and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2 TR000143 (JLB).
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boelens J-J et al. Combining Clofarabine and Fludarabine with Exposure Targeted Busulfan for Pediatric Leukemia: An Effective, Low Toxicity TBI-Free Conditioning Regimen. Biol. Blood Marrow Transplant 22, S99–S100 (2016). [Google Scholar]
- 2.Bonate PL et al. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat. Rev. Drug Discov 5, 855–863 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Valdez BC, Li Y, Murray D, Champlin RE & Andersson BS The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochem. Pharmacol 81, 222–232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson BS et al. Clofarabine ± Fludarabine with Once Daily i.v. Busulfan as Pretransplant Conditioning Therapy for Advanced Myeloid Leukemia and MDS. Biol. Blood Marrow Transplant 17, 893–900 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long Boyle J. et al. Pharmacokinetics of Clofarabine in Patients With High-Risk Inherited Metabolic Disorders Undergoing Brain-Sparing Hematopoietic Cell Transplantation. J. Clin. Pharmacol 51, 679–686 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Ivaturi V. et al. Pharmacokinetics and Model-Based Dosing to Optimize Fludarabine Therapy in Pediatric Hematopoietic Cell Transplant Recipients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant 23, 1701–1713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byon W, Fletcher CV & Brundage RC Impact of censoring data below an arbitrary quantification limit on structural model misspecification. J. Pharmacokinet. Pharmacodyn 35, 101–116 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Lizak P, Dvorak CC, Aweeka F. & Long-Boyle J. Simultaneous determination of fludarabine and clofarabine in human plasma by LC–MS/MS. J. Chromatogr. B 960, 194–199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R Development Core Team. R: A language and enviroment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing. 2010. [Google Scholar]
- 10.Mould DR & Upton RN Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacomet. Syst. Pharmacol 1, e6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mould DR & Upton RN Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development—Part 2: Introduction to Pharmacokinetic Modeling Methods. CPT Pharmacomet. Syst. Pharmacol 2, e38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wählby U, Jonsson EN & Karlsson MO Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci 4, 68–79 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BJ & Holford NHG Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet 24, 25–36 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Ghafoori P. & Gobburu JVS Allometry Is a Reasonable Choice in Pediatric Drug Development. J. Clin. Pharmacol 57, 469–475 (2017). [DOI] [PubMed] [Google Scholar]
- 15.West GB A General Model for the Origin of Allometric Scaling Laws in Biology. Science 276, 122–126 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ & Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J. Pediatr 106, 522–526 (1985). [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW & Gault MH Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976). [DOI] [PubMed] [Google Scholar]
- 18.Ette EI & Onyiah LC Estimating inestimable standard errors in population pharmacokinetic studies: The bootstrap with winsorization. Eur. J. Drug Metab. Pharmacokinet 27, 213–224 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Bergstrand M, Hooker AC, Wallin JE & Karlsson MO Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 13, 143–151 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Growth Charts - Clinical Growth Charts. (2019). Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. (Accessed: 4th March 2019)
- 21.King KM et al. A Comparison of the Transportability, and Its Role in Cytotoxicity, of Clofarabine, Cladribine, and Fludarabine by Recombinant Human Nucleoside Transporters Produced in Three Model Expression Systems. Mol. Pharmacol 69, 346–353 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Mangravite LM, Badagnani I. & Giacomini KM Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney. Eur. J. Pharmacol 479, 269–281 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Mangravite LM, Xiao G. & Giacomini KM Localization of human equilibrative nucleoside transporters, hENT1 and hENT2, in renal epithelial cells. Am. J. Physiol.-Ren. Physiol 284, F902–F910 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Elwi AN et al. Transepithelial fluxes of adenosine and 2′-deoxyadenosine across human renal proximal tubule cells: roles of nucleoside transporters hENT1, hENT2, and hCNT3. Am. J. Physiol.-Ren. Physiol 296, F1439–F1451 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Ritzel MWL et al. Molecular Identification and Characterization of Novel Human and Mouse Concentrative Na + −Nucleoside Cotransporter Proteins (hCNT3 and mCNT3) Broadly Selective for Purine and Pyrimidine Nucleosides (System cib ). J. Biol. Chem 276, 2914–2927 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Ajavon AD, Bonate PL & Taft DR Renal excretion of clofarabine: Assessment of dose-linearity and role of renal transport systems on drug excretion. Eur. J. Pharm. Sci 40, 209–216 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Bonate PL et al. Population pharmacokinetics of clofarabine and its metabolite 6-ketoclofarabine in adult and pediatric patients with cancer. Cancer Chemother. Pharmacol 67, 875–890 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Clolar FDA label. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021673s023lbl.pdf. (Accessed: 1st November 2018)
- 29.Bonate PL et al. Population Pharmacokinetics of Clofarabine, a Second-Generation Nucleoside Analog, in Pediatric Patients With Acute Leukemia. J. Clin. Pharmacol 44, 1309–1322 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.