Abstract
Background
Prone positioning is routinely used among patients with COVID-19 requiring mechanical ventilation. However, its utility among spontaneously breathing patients is still debated.
Methods
In an open-label randomised controlled trial, we enrolled patients hospitalised with mild COVID-19 pneumonia, whose arterial oxygen tension to inspiratory oxygen fraction ratio (PaO2/FIO2) was >200 mmHg and who did not require mechanical ventilation or continuous positive airway pressure at hospital admission. Patients were randomised 1:1 to prone positioning on top of standard of care (intervention group) versus standard of care only (controls). The primary composite outcome included death, mechanical ventilation, continuous positive airway pressure and PaO2/FIO2 <200 mmHg; secondary outcomes were oxygen weaning and hospital discharge.
Results
A total of 61 subjects were enrolled, 29 adjudicated to prone positioning and 32 to the control group. By day 28, 24 out of 61 patients (39.3%) met the primary outcome: 16 because of a PaO2/FIO2 ratio <200 mmHg, five because of the need for continuous positive airway pressure and three because of the need for mechanical ventilation. Three patients died. Using an intention-to-treat approach, 15 out of 29 patients in the prone positioning group versus nine out of 32 controls met the primary outcome, corresponding to a significantly higher risk of progression among those randomised to prone positioning (HR 2.38, 95% CI 1.04–5.43; p=0.040). Using an as-treated approach, which included in the intervention group only patients who maintained prone positioning for ≥3 h·day−1, no significant differences were found between the two groups (HR 1.77, 95% CI 0.79–3.94; p=0.165). Also, we did not find any statistically significant difference in terms of time to oxygen weaning or hospital discharge between study arms in any of the analyses conducted.
Conclusions
We observed no clinical benefit from prone positioning among spontaneously breathing patients with COVID-19 pneumonia requiring conventional oxygen therapy.
Tweetable abstract
Awake prone positioning does not improve the outcome of patients with mild COVID-19 pneumonia. Because possible harm cannot be excluded, it should not be recommended in patients without severe respiratory failure due to COVID-19, outside clinical research. https://bit.ly/3MxTLkh
Introduction
Prone positioning (PP) is widely recognised as an effective treatment for acute respiratory distress syndrome (ARDS) requiring mechanical ventilation (MV) owing to its beneficial effects on respiratory mechanics, gas exchange and, ultimately, patients’ survival [1–4]. Thus, in the last 3 years, PP has been extensively used in intensive care units to treat COVID-19-related acute hypoxaemic respiratory failure requiring MV [5, 6]. Concurrently, researchers have started to explore the effects of PP in awake, non-intubated patients with COVID-19. Early observational studies suggested that PP could improve oxygenation in non-intubated patients with COVID-19 [7–9]. Following these preliminary results, awake PP was endorsed by several healthcare providers worldwide and proposed for hospitalised patients or even suggested for those treated at home or recently discharged [10–13].
However, evidence on clinically meaningful benefits from PP in patients spontaneously breathing is limited and often contradictory [14–20]. We therefore aimed to assess whether early PP in patients with initial COVID-19-related pneumonia, not requiring continuous positive airway pressure (CPAP) or MV, could improve patients’ outcomes.
Methods
Study design
The Early Pronation as COVID Treatment (EPCoT) study is a pragmatic, open-label, monocentric randomised controlled trial conducted on patients admitted to the infectious diseases ward of the San Gerardo Hospital (Monza, Italy) between 15 August 2021 and 31 May 2022.
The study protocol was approved by the local independent ethics committee (Comitato Etico Brianza, San Gerardo Hospital, ASST Monza) before the beginning of the trial and registered on ClinicalTrials.gov (registration number NCT05008380). For each patient, informed consent was obtained prior to enrolment.
Patients
Patients hospitalised with COVID-19 were eligible for enrolment if they were aged ≥18 years, had a positive PCR test for SARS-CoV-2 RNA on a respiratory sample within 7 days of enrolment and presented at least one of the following conditions: 1) radiological evidence of pneumonia or 2) clinical evidence of respiratory disease, defined as either room air arterial oxygen tension (PaO2) <80 mmHg or peripheral oxygen saturation (SpO2) <94% or need for oxygen supplementation in order to maintain SpO2 >93%. Patients were ineligible if they had already undergone PP, if their arterial oxygen tension to inspiratory oxygen fraction ratio (PaO2/FIO2) was <200 mmHg, if they required supplementary oxygen by high-flow nasal cannula (HFNC) or CPAP and if they had any contraindications or conditions that could hinder PP (including, but not limited to, unstable fractures, deep venous thrombosis, late pregnancy and altered mental status).
Randomisation and study procedures
Using permuted blocks of variable sizes (four, six or eight), participants were randomised 1:1 to awake PP on top of standard of care (intervention group) versus standard of care only (control group). The randomisation was stratified by symptoms duration before enrolment (≤10 or >10 days) and need for oxygen therapy (yes or no). The nature of the intervention precluded blinding for patients and for clinical staff. Study participant allocation was concealed using sealed opaque envelopes, prepared by separate people not involved in patient enrolment and care.
Patients allocated to awake PP were encouraged to adopt PP for at least three consecutive hours (up to 6 h according to tolerability) twice a day; patients in the control group were free to adopt and maintain any position during the day.
Patients from both groups received standard of care treatment for COVID-19 pneumonia according to the evidence available at the time.
Data collection
Demographic, clinical and anthropometric data were collected at enrolment. Vital signs, type of oxygen support, inspiratory oxygen fraction (FIO2), clinical status according to the Ordinal Scale for Clinical Improvement (OSCI) from the World Health Organization [21] and blood gas analysis were recorded at baseline and 1, 3, 7, 14, 21 and 28 days after randomisation. Patients discharged before day 28 received additional telephonic follow-up on the 28th day. During follow-up visits, we also assessed the time spent in the prone position in the previous 24 h according to patients’ self-reports. Occurrence of adverse events and therapy administered to patients were also recorded. In the event of death, the day and cause of death were recorded.
Outcome measure
The primary outcome measure was the incidence rate of a composite outcome, including death, MV, use of CPAP or HFNC, or PaO2/FIO2 ratio <200 mmHg (whichever came first), across 28 days of observation from hospital admission. Secondary outcome measures were time to oxygen weaning (defined as clinical status of 1, 2 or 3 on the OSCI scale), time to hospital discharge, change in clinical status and in PaO2/FIO2 ratio during follow-up, and rate of adverse events.
Statistical analysis
Data were checked for completeness and consistency. Characteristics of the two groups, as defined by the randomisation arm, were described using relative frequencies, means and standard deviations, as appropriate. Survival times were calculated starting from the enrolment date for all the end-points of interest except for time to hospital discharge, which was calculated starting from the date of hospital admission. The related event indicators were set equal to 1 if observed during the observation period and 0 otherwise. The end-points of interest were analysed by drawing the cumulative incidence/survival curves over time using the Kaplan–Meier method. The survival times in the two treatment groups, as defined by the treatment allocated at randomisation (intention-to-treat analysis) or by the actual treatment received (as-treated analysis), were compared using the exponential regression model. Multivariable models, adjusted for patient age and baseline PaO2/FIO2 ratio, were also conducted.
Patient clinical status across time was described using a bar plot by randomisation arm. Changes in PaO2/FIO2 ratio over time were analysed using a general linear mixed model including interactions between treatment arm and time points.
Assuming a 60% risk of clinical progression to the composite outcome and a 10% loss at follow-up and with the goal of detecting a relative difference of 50% between the intervention and control group (two-sided α of 0.05 and β of 0.8), a sample size of 96 patients (48 for each group) was originally planned. However, owing to the reduction in COVID-19 pneumonia incidence and to an overall low enrolment rate, patient enrolment was terminated earlier, not reaching the planned sample size. Final follow-up of the last patient enrolled was completed on 20 June 2022.
All analyses were conducted using Stata (StataCorp, release 17.0) and used two-sided p-values and an α of 0.05.
Results
Patient characteristics
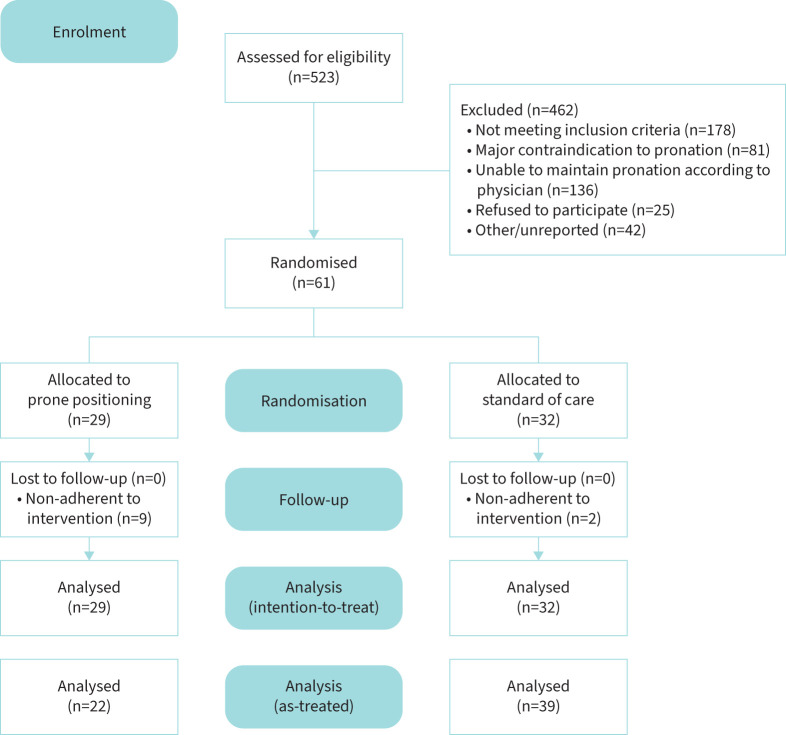
A total of 61 patients were enrolled and randomised. During the randomisation process, two participants were incorrectly randomised because they belonged to the stratum of patients with symptoms for ≤10 days although they had symptoms for 11 and 12 days at the time of randomisation. Among the 61 patients enrolled, 29 were adjudicated to PP and 32 to the control group (figure 1).
FIGURE 1.
CONSORT diagram showing subject disposition in the trial.
The patients enrolled were predominantly male (62.3%) with a mean±sd age of 59.2±15.7 years. The most common comorbidities were hypertension (29.5%), drug-induced immunosuppression (25.6%) and history of solid or haematological malignancy (16.4% and 11.5%). Half of the patients (50.8%) were vaccinated against COVID-19 with at least one dose. Patients assigned to PP tended to have slightly more severe disease, as demonstrated by lower mean PaO2/FIO2 ratio (280 mmHg versus 309.8 mmHg). At the time of enrolment, all patients were on oxygen treatment but one, who required it later on during the follow-up. Approximately 89% and 64% of the patients received treatment with corticosteroids (mainly dexamethasone) and remdesivir, respectively. These and other characteristics are shown in table 1.
TABLE 1.
Patient characteristics
| Characteristics | PP group | Control group | Total |
| Subjects, n | 29 | 32 | 61 |
| Age, years | 61.0±16.7 | 57.5±14.8 | 59.2±15.7 |
| Male gender | 18 (62.1) | 20 (62.5) | 38 (62.3) |
| BMI, kg·m−2 | 26.9±4.1 | 26.1±3.9 | 26.5±4 |
| Comorbidities | |||
| Cerebrovascular disease | 2 (6.9) | 1 (3.1) | 3 (4.9) |
| Hypertension | 12 (41.4) | 6 (18.7) | 18 (29.5) |
| Cardiovascular disease | 1 (3.4) | 2 (6.2) | 3 (4.9) |
| Diabetes | 3 (10.3) | 2 (6.2) | 5 (8.2) |
| Chronic renal failure | 2 (6.9) | 1 (3.1) | 3 (4.9) |
| Obesity (BMI ≥34 kg·m−2) | 2 (6.9) | 2 (6.2) | 4 (6.6) |
| COPD | 1 (3.4) | 1 (3.1) | 2 (3.3) |
| Other chronic pulmonary disease | 3 (10.3) | 2 (6.2) | 5 (8.2) |
| History of solid malignancy | 5 (16.4) | 5 (15.6) | 10 (16.4) |
| History of haematological malignancy | 2 (6.9) | 5 (15.6) | 7 (11.5) |
| Vaccinated for SARS-CoV-2 | 16 (55.2) | 15 (46.9) | 31 (50.8) |
| Symptom duration before enrolment | |||
| ≤10 days | 22 (75.9) | 25 (78.1) | 47 (77) |
| >10 days | 7 (24.1) | 7 (21.9) | 14 (23) |
| Baseline respiratory rate, breaths·min−1 | 19.5±4 | 18.2±3 | 19.2±4 |
| PaO2/FIO2 , mmHg | 280±50 | 310±46 | 296±50 |
| Baseline oxygen support | |||
| None | 0 | 1 (3.1) | 1 (1.6) |
| Low-flow nasal cannula | 16 (55.2) | 16 (50) | 32 (52.5) |
| Venturi mask | 11 (37.9) | 12 (37.5) | 23 (37.7) |
| Non-rebreather mask | 2 (6.9) | 3 (9.4) | 5 (8.2) |
| Treatment with corticosteroids | 27 (93.1) | 27 (84.4) | 54 (88.5) |
| Treatment with remdesivir | 17 (58.6) | 22 (68.7) | 39 (63.9) |
| Treatment with monoclonal antibodies | 7 (25.0) | 8 (24.2) | 15 (24.6) |
|
Daily time in prone position, h·day−1,
median (IQR) |
|||
| Day 1 of follow-up | 3 (0–6) | 0 (0–0) | – |
| Day 3 of follow-up | 4 (0–7) | 0 (0–0) | – |
| Required CPAP helmet | 7 (24.1) | 4 (12.5) | 11 (18.0) |
| Intubated and mechanically ventilated | 4 (13.8) | 0 (0) | 4 (6.5) |
| Death | 2 (7.1) | 1 (3.0) | 3 (4.9) |
| Length of hospital stay, days | 15.2±11.0 | 12.7±7.2 | 13.9±9.2 |
Data are presented as mean±sd or n (%), unless otherwise indicated. BMI: body mass index; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; IQR: interquartile range; PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; PP: prone positioning.
In the intervention group, the median daily time spent in PP on day 1 was 3 h (IQR 0–6 h·day−1), compared to 0 h·day−1 among the controls; on day 3 we registered a median of 4 h·day−1 (IQR 0–7 h·day−1) and 0 h·day−1, respectively. Nonetheless, suboptimal adherence to the adjudicated treatment was observed in patients randomised to PP, given that 44.8% and 40.7% of them maintained pronation for <3 h·day−1 on day 1 and 3, respectively.
Patient outcome and disease progression
At study end, 56 patients (91.8%) had been discharged alive, three had died and two were still hospitalised (one in the infectious diseases ward and one in the intensive care unit). During hospitalisation, 11 patients (18%) required noninvasive ventilation with CPAP helmet and four (6.5%) orotracheal intubation and MV. The mean±sd length of hospital stay was 15.2±11.0 days for the PP group and 12.7±7.2 days for the control group.
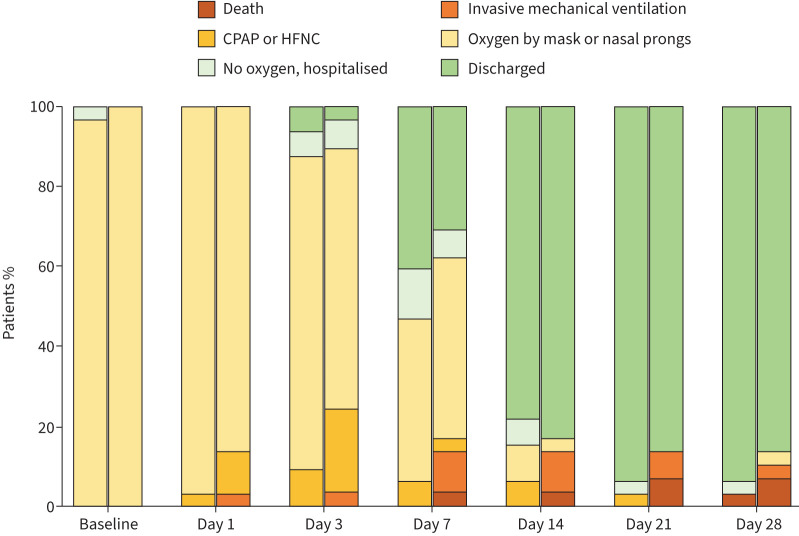
Figure 2 shows clinical status distribution across the follow-up in the two arms of the study.
FIGURE 2.
Clinical status distribution over time, grouped by randomisation arm. At each time point, the left column represents the control arm and the right column represents the awake prone positioning (intervention) arm. CPAP: continuous positive airway pressure; HFNC: high-flow nasal cannula.
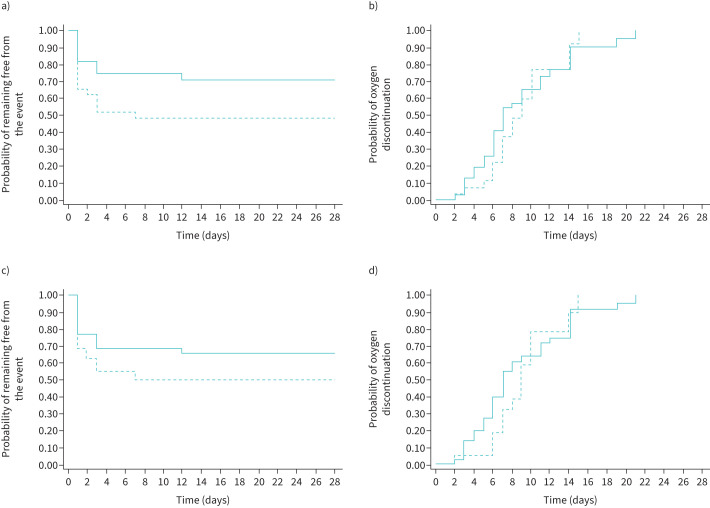
By day 28, 24 out of 61 patients (39.3%) had reached the primary outcome: 16 because they had a PaO2/FIO2 ratio <200 mmHg, five because they required CPAP and three because they needed mechanical ventilation. Using an intention-to-treat approach (figure 3a), the proportion of patients experiencing the primary outcome was 15 out of 29 (51.7%) in the PP group and nine out of 32 (28.1%) among controls. This resulted in a significantly higher event rate among the intervention group (25.0 (95% CI 15.1–41.5) events per 100 person-weeks of observation) than among controls (10.5 (95% CI 5.5–20.2) events per 100 person-weeks of observation), corresponding to a hazard ratio (HR) of 2.38 (95% CI 1.04–5.43; p=0.040). The association between PP and a worse outcome held in a multivariable model adjusted for age and PaO2/FIO2 ratio at enrolment. In this model, patients randomised to PP still had a higher risk of meeting the primary end-point than controls (adjusted HR 2.35, 95% CI 1.02–5.38; p=0.044), while higher PaO2/FIO2 ratio was protective (versus PaO2/FIO2 <250 mmHg; adjusted HR 0.4, 95% CI 0.12–1.34 for PaO2/FIO2 250–300 mmHg and HR 0.27, 95% CI 0.09–0.79 for PaO2/FIO2 >300 mmHg).
FIGURE 3.
Risk of clinical progression and probability of oxygen weaning by randomisation arm. a) Intention-to-treat analysis for risk of clinical progression, defined as death, mechanical ventilation, need for high-flow nasal cannula (HFNC) or noninvasive ventilation with continuous positive airway pressure (CPAP). b) Intention-to-treat analysis for probability of oxygen discontinuation by randomisation arm. c) As-treated analysis for risk of clinical progression, defined as death, mechanical ventilation, need for HFNC or noninvasive ventilation with CPAP. d) As-treated analysis for probability of oxygen discontinuation by randomisation arm. The continuous line represents the control group, the dashed line represents the prone positioning group.
Similarly, in an additional analysis only considering clinical end-points (death, endotracheal intubation or CPAP), we registered a significantly higher event rate in those randomised to PP than in the controls, with a HR of 4.04 (95% CI 1.28–12.68; p=0.017). This significant association also held in the multivariable model, adjusted for the same covariates mentioned above (adjusted HR 3.6, 95% CI 1.09–11.83; p=0.034).
PP was not associated with either accelerated oxygen weaning or hospital discharge. As shown in figure 3b, rates of oxygen discontinuation were similar between the two study groups (63.0 (95% CI 39.7–100.0) versus 75.3 (95% CI 51.6–109.8) events per 100 person-weeks of observation in PP and control group, respectively), accounting for a HR of 0.84 (95% CI 0.46–1.52; p=0.558). Moreover, no difference in time to hospital discharge was found between the two groups (36.5 (95% CI 23.8–55.9) events per 100 person-weeks of observation among the PP group versus 46.7 (95% CI 32.0–68.0) events per 100 person-weeks of observation among the controls; HR 0.78, 95% CI 0.44–1.38; p=0.397). Adjustment for possible other predictors of clinical progression, such as age and baseline PaO2/FIO2 ratio, did not change the results to a significant extent.
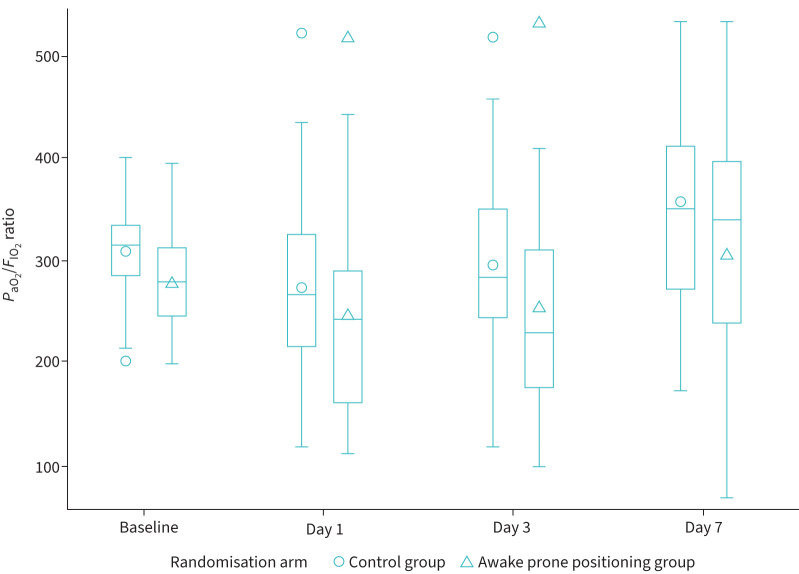
Using a linear mixed model, change in PaO2/FIO2 ratio (worst value measured on each day of follow-up) was compared between patients randomised to PP and those in the control group (figure 4). In both groups we observed, on average, an initial decline of the ratio during the first days of observation, followed by an improvement on day 7, which was slightly higher for the patients randomised to the control group (p=0.042 for the interaction term between time and study arm).
FIGURE 4.
Arterial oxygen tension to inspiratory oxygen fraction ratio (PaO2/FIO2) over time by randomisation arm. Horizontal lines represent medians, boxes represent interquartile ranges, whiskers represent minimum and maximum values (excluding outliers), the markers represent means and outliers.
As-treated analysis
Given the suboptimal adherence in a significant subgroup of patients randomised to PP, we conducted an as-treated analysis, in which we included in the intervention group only patients who maintained PP for ≥3 h·day−1. In this analysis, which assigned 22 patients to the intervention group and 39 to the control group, the primary outcome was met by 11 out of 22 patients (50%) in the PP group and 13 out of 39 (33.3%) in the control group (figure 3c). No statistically significant differences were found in the incidence of the primary composite outcome in the two groups (23.3 (95% CI 12.9–42.3) versus 13.2 (95% CI 7.7–22.7) events per 100 person-weeks of observation among PP and controls, respectively; HR 1.8, 95% CI 0.8–3.9; p=0.165).
Similarly, using the as-treated approach, no significant differences were found between the PP and control groups in terms of time to hospital discharge (HR 0.7, 95% CI 0.4–1.3; p=0.300) or oxygen weaning (HR 0.8, 95% CI 0.4–1.6; p=0.582) (figure 3d).
Safety
No serious adverse events deemed to be caused by the study procedures were reported. At least one adverse event was reported by 13 patients from among a predefined list of relevant clinical events, including thrombosis or thromboembolism, gastrointestinal bleeding, cardiovascular event, renal failure, diabetes, infections and delirium (table 2). Among them, the most commonly reported were infections (7 out of 61 patients, 11.5%) and venous thromboembolisms (5 out of 61 patients, 8.2%). The rate of these events was comparable between patients randomised to PP and those to standard of care (p=0.544). 10 patients presented an adverse event or a laboratory abnormality (other than those correlated with respiratory failure) graded ≥3 on a 5-grade scale of severity. No statistically significant difference was found according to treatment group (p=0.682).
TABLE 2.
Adverse events
| Prone positioning group | Control group | |
| Subjects, n | 29 | 32 |
| Clinically relevant adverse events | ||
| Thromboembolic event | 2 (6.9) | 3 (9.4) |
| Gastrointestinal bleeding | 0 | 1 (3.1) |
| Cardiovascular events | 2 (6.9) | 1 (3.1) |
| Infection | 3 (10.3) | 4 (12.5) |
| Grade ≥3 adverse events # | 4 (13.8) | 6 (18.7) |
Data are presented as n (%), unless otherwise indicated. #: severity scale: 1=mild, 2=moderate, 3=serious, 4=life-threatening, 5=fatal.
Discussion
In this pragmatic, open-label, randomised controlled trial, we found that early PP had no benefit over standard of care in terms of survival, need for respiratory support, oxygen weaning or hospital discharge among patients hospitalised with mild COVID-19 pneumonia. By contrast, our data suggested a potential harmful effect associated with this procedure.
A previous randomised trial enrolling 400 patients hospitalised with acute respiratory failure due to COVID-19, the majority of whom were treated with high-flow oxygen or noninvasive ventilation, failed to demonstrate a statistically significant effect of PP on patient mortality or need for MV, although a numerically lower number of patients treated with PP underwent endotracheal intubation [14]. A subsequent meta-analysis of six open-label, randomised controlled trials suggested that awake PP may result in a decreased risk of intubation in patients with COVID-19 who require support with a HFNC [18]. Our data did not confirm these findings in a population of patients with less severe respiratory impairment at baseline and even suggested that too early PP could be associated with an increased risk of clinical progression. Consistent with our findings, in a non-randomised controlled trial including mostly patients receiving low-flow oxygen through a nasal cannula, those adjudicated to PP had a worse clinical status at 5 days than controls [16]. Moreover, in a randomised trial conducted in patients using high-flow nasal oxygen or noninvasive ventilation for respiratory support, PP did not reduce the rate of intubation [17]. Several observational studies conducted mostly on patients with severe respiratory failure showed no beneficial effects on mortality or orotracheal intubation in patients undergoing awake PP [7, 19, 20].
These apparent contradictions possibly reflect a lack of effectiveness of PP in patients with mild-to-moderate respiratory impairment. Of note, among MV non-COVID-19 patients, a clear clinical benefit of PP has been demonstrated only for those who require elevated positive end-expiratory pressure and have severe ARDS (i.e. PaO2/FIO2 ratio <150 mmHg) [4, 22]. Timing of PP, therefore, may be relevant, because it has been speculated that different phases and phenotypes of ARDS exist which may influence its response to pronation [23]. It has been suggested that the early phase of COVID-related ARDS is characterised by high lung compliance [24], a condition that is likely not to benefit from PP because it is characterised by a low amount of non-aerated lung tissue and, consequently, reduced alveolar recruitability through PP [25]. Consequently, radiology could be useful to identify lung phenotypes more likely to benefit from PP. Unfortunately, however, in our study computed tomography scans were not available for all patients, thus we cannot exclude that those with basal consolidations could have benefitted from awake PP more than others. Taken together, these findings suggest that awake PP could be futile in populations like ours, with mild COVID-related pneumonia, and that it should be reserved for patients with more advanced disease who require a high fraction of inspired oxygen or noninvasive ventilation. In those patients, PP might reduce the risk of MV, as observed in the already-mentioned meta-trial conducted on COVID-19 patients requiring HFNC [18]. However, when PP was used as a rescue therapy to avoid MV in patients with refractory hypoxaemia despite HFNC or noninvasive ventilation, it did not help to reduce the rate of orotracheal intubation [17]. Thus, the optimal timing for awake PP is yet to be identified, because an excessive delay may limit its usefulness. It is reasonable to think that a trial of awake PP could be tempting in patients who require HFNC or noninvasive ventilation, but not earlier, and that, in any case, awake PP should not be regarded as a substitute for intubation in patients who meet the indications for MV.
Another possible explanation for our findings could lie in the short time spent by the patients in PP. Although the optimal duration of awake PP has not been established, a recent systematic review suggested that a threshold of 4 h of pronation per day was adequate to observe a beneficial effect on patient gas exchanges [26]. The scheduled and actual time spent in PP by our patients was close to this threshold and comparable to that of other studies [18, 22, 24]. Nonetheless, this time was lower than expected based on the recommendation given to the patients, and lower than the time generally recommended in patients undergoing MV, for whom the time suggested for PP is 12–16 h per cycle [14]. Although reduced adherence to PP could have hampered its effect, no statistically nor clinically significant benefit was associated with awake pronation even when the analysis was focused only on patients who were actually able to comply with PP recommendations. Whether maintaining PP for longer than proposed in our study (6 h·day−1) is associated with additional benefit among non-intubated patients merits further investigation. Additional efforts to improve patient comfort during pronation and patient adherence to the procedure should be pursued.
Why awake PP appears to be detrimental in our study is difficult to ascertain. Overall, PP was well tolerated while adverse events attributable to PP were rare and not significantly different in the study groups. We cannot exclude, however, that transient improvements in peripheral oxygenation or respiratory rate during PP could have delayed appropriate therapeutic interventions in patients whose lung function showed signs of deterioration. While previous studies on patients with COVID-19 pneumonia showed that improved oxygenation and reduced respiratory rate were observed during PP, these changes were transient in most cases and conditions reverted to baseline once the patient returned to the supine position [7, 27, 28]. However, delayed access to intubation or noninvasive ventilation could increase the risk of self-induced lung injury due to vigorous respiratory efforts during spontaneous breathing [29, 30].
Interestingly enough, we did not observe any beneficial effect of awake PP on gas exchange, as measured by PaO2/FIO2 ratios, in apparent contradiction with previous studies [7]. It should be noted, however, that in our study we reported the lowest PaO2/FIO2 ratios measured on each day of follow-up, while other studies considered the oxygenation improvement during or at the end of pronation. This suggests that the improvement of gas exchange after PP is likely to be temporary and lost once the patient re-supinates, as other authors have speculated before [27].
Our study has some limitations that should be acknowledged. First, given the unblinded nature of the trial, which was inevitable owing to the nature of the intervention, we cannot exclude the presence of ascertainment bias. As a matter of fact, indication for CPAP, which was used as a component of the primary composite outcome, may have been somehow subjective. Nonetheless, all the components of the outcome are relevant from a clinical perspective. Moreover, the inclusion of hard end-points, such as death, MV and even reduction of PaO2/FIO2, helped to capture all severe ARDS. Second, we failed to enrol the anticipated number of subjects and the outcome occurred in a lower proportion of patients than predicted, due to the changing characteristics of the COVID-19 epidemic (vaccination roll-out and decreasing pathogenicity of viral variants). The small sample size may thus limit the validity and the generalisability of our results. Third, adherence to awake PP was suboptimal. This reflects the difficulties in implementing this measure in patients with a good performance status and calls for further studies regarding strategies to increase the time spent in PP in this category of patients. In addition, future work should aim to assess whether subgroups of patients may still benefit from awake PP. Our study also presents some strengths. The randomised design allowed us to reduce the risk of bias and to obtain comparable groups. Its pragmatic nature reflected the real-world challenges of implementing awake PP. In addition, the selected outcomes (death, use of MV or CPAP, oxygen weaning and hospital length of stay) are clinically relevant compared to surrogate end-points such as changes in respiratory parameters. Last, the enrolment of patients with mild COVID-19 pneumonia allowed the study of a population less explored in the previous trials on the same subject.
In conclusion, we observed no evidence of any clinically significant effect of PP in awake patients with mild COVID-19 pneumonia; however, we cannot rule out that too early or inappropriate PP may cause harm to patients. Thus, awake PP should not be recommended as a routine treatment for patients with COVID-19 who do not have severe hypoxaemic respiratory failure. Further clinical research aimed at investigating if and in which population awake PP could be more beneficial is warranted.
Acknowledgments
We kindly thank Anna Coppo and Giacomo Bellani (Intensive Care, Fondazione IRCCS San Gerardo dei Tintori, University of Milano-Bicocca) for sharing with us their experience and for their useful advice on the implementation of awake prone positioning.
EPCoT Study Group: Eleonora Maria Beretta, Anna Cappelletti, Ilaria Caramma, Viola Cogliandro, Ilaria De Benedetto, Giulia Gustinetti, Marta Iannace, Valentina Orsini, Alice Ranzani and Anna Spolti.
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT05008380. Aggregated or de-identified patient data and all the relevant documents related to the study (e.g. study protocol, statistical analysis and informed consent form) will be available upon request to the corresponding author.
Conflict of interest: The authors declare that they have no conflicts of interest for the present study.
References
- 1.Gattinoni L, D'Andrea L, Pelosi P, et al. Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 1993; 269: 2122–2127. doi: 10.1001/jama.1993.03500160092039 [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Pelosi P, Vitale G, et al. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 1991; 74: 15–23. doi: 10.1097/00000542-199101000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Lamm WJE, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med 1994; 150: 184–193. doi: 10.1164/ajrccm.150.1.8025748 [DOI] [PubMed] [Google Scholar]
- 4.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–2168. doi: 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 5.Weiss TT, Cerda F, Scott JB, et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth 2021; 126: 48–55. doi: 10.1016/j.bja.2020.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med 2021; 49: E219–E234. doi: 10.1097/CCM.0000000000004899 [DOI] [PubMed] [Google Scholar]
- 7.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020; 8: 765–774. doi: 10.1016/S2213-2600(20)30268-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 2020; 323: 2338–2340. doi: 10.1001/jama.2020.7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AE, Ranard BL, Wei Y, et al. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Intern Med 2020; 180: 1537–1539. doi: 10.1001/jamainternmed.2020.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo N, Strayer R, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med 2020; 27: 375–378. doi: 10.1111/acem.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health & Family Welfare Government of India . COVID-19 Proning for Self-Care. www.mohfw.gov.in/pdf/COVID19ProningforSelfcare3.pdf Date last updated: 22 April 2022. Date last accessed: 26 September 2022.
- 12.Bentley SK, Iavicoli L, Cherkas D, et al. Guidance and patient instructions for proning and repositioning of awake, nonintubated COVID-19 patients. Acad Emerg Med 2020; 27: 787–791. doi: 10.1111/acem.14067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIH . COVID-19 Treatment Guidelines. Oxygenation and Ventilation for Adults. www.covid19treatmentguidelines.nih.gov/management/critical-care-for-adults/oxygenation-and-ventilation-for-adults/ Date last updated: 26 September 2022. Date last accessed: 26 September 2022.
- 14.Alhazzani W, Parhar KKS, Weatherald J, et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure. JAMA 2022; 327: 2104–2113. doi: 10.1001/jama.2022.7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fralick M, Colacci M, Munshi L, et al. Prone positioning of patients with moderate hypoxaemia due to COVID-19: multicentre pragmatic randomised trial (COVID-PRONE). BMJ 2022; 376: e068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian ET, Gatto CL, Amusina O, et al. Assessment of awake prone positioning in hospitalized adults with COVID-19: a nonrandomized controlled trial. JAMA Intern Med 2022; 182: 612–621. doi: 10.1001/jamainternmed.2022.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosén J, Von Oelreich E, Fors D, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care 2021; 25: 209. doi: 10.1186/s13054-021-03602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 2021; 9: 1387–1395 doi: 10.1016/S2213-2600(21)00356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrando C, Mellado-Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care 2020; 24: 597. doi: 10.1186/s13054-020-03314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padrão EMH, Valente FS, Besen BAMP, et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: exploratory findings in a single-center retrospective cohort study. Acad Emerg Med 2020; 27: 1249–1259. doi: 10.1111/acem.14160 [DOI] [PubMed] [Google Scholar]
- 21.WHO . COVID-19 Therapeutic Trial Synopsis. www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis Date last updated: 18 February 2020. Date last accessed: 26 September 2022.
- 22.Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36: 585–599. doi: 10.1007/s00134-009-1748-1 [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Zhang Y, Li Y, et al. The application of awake-prone positioning among non-intubated patients with COVID-19-related ARDS: a narrative review. Front Med (Lausanne) 2022; 9: 817689. doi: 10.3389/fmed.2022.817689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354: 1775–1786. doi: 10.1056/NEJMoa052052 [DOI] [PubMed] [Google Scholar]
- 26.Fazzini B, Page A, Pearse R, et al. Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Br J Anaesth 2022; 128: 352–362. doi: 10.1016/j.bja.2021.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elharrar X, Trigui Y, Dols A, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 2020; 323: 2336–2338. doi: 10.1001/jama.2020.8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Retucci M, Aliberti S, Ceruti C, et al. Prone and lateral positioning in spontaneously breathing patients with COVID-19 pneumonia undergoing noninvasive helmet CPAP treatment. Chest 2020; 158: 2431–2435. doi: 10.1016/j.chest.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battaglini D, Robba C, Ball L, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth 2021; 127: 353–364. doi: 10.1016/j.bja.2021.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González J, Benítez ID, De Gonzalo-Calvo D, et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: a prospective cohort study. Crit Care 2022; 26: 18. doi: 10.1186/s13054-021-03882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]