Abstract
Background
Percutaneous coronary intervention (PCI) is one of the most performed well-succeeded therapeutic procedures worldwide, reducing symptoms and improving quality of life. Neutrophil Gelatinase-associated Lipocalin (NGAL) is a biomarker of acute kidney injury (AKI) produced early after an ischemic renal insult. Osmotic diuresis and the vasoconstriction of the afferent arteriole promoted by Sodium-glucose Cotransporter-2 Inhibitors (SGLT2i) generate a concern regarding the possibility of dehydration and consequent AKI. There is no consensus on the maintenance or discontinuation of SGTL2i in patients who will undergo PCI. This study aimed to evaluate the safety of empagliflozin in diabetic patients submitted to elective PCI regarding kidney function.
Methods
SAFE-PCI trial is a prospective, open-label, randomized (1:1), single-center pilot study and a follow-up of 30 days. The SGLT2i empagliflozin 25 mg daily was initiated at least 15 days before PCI in the intervention group and maintained until the end of the follow-up period. Serum NGAL was collected 6 h after PCI and creatinine before PCI, 24 h, and 48 h after the procedure. As per protocol, both groups received optimal medical treatment and standard protocol of nephroprotection.
Results
A total of 42 patients were randomized (22 patients in the iSGLT-2 group and 20 patients in the control group). There was no difference between-group baseline data. The primary outcome (NGAL and creatinine values post PCI) did not differ in both groups: the mean NGAL value was 199 ng/dL in the empagliflozin group and 150 ng/dL in the control group (p = 0.249). Although there was an initial increase in creatinine in the SGLT-2i group compared to the control group between baseline creatinine and pre-PCI and 24 h post-PCI creatinine, no difference was detected in creatinine 48 h post-PCI (p = 0.065). The incidence of CI-AKI, determined by KDIGO criteria, in the iSGLT2-group was 13.6% and 10.0% in the control group without statistical difference.
Conclusion
The present study showed that the use of empagliflozin is safe regarding kidney function during elective PCI in patients with T2D when compared with no use of SGLT2i.
Trial registration Our clinical study is registered on ClinicalTrials.gov with the following number: NCT05037695.
Keywords: SGLT2 inhibitors, Coronary artery disease, Percutaneous coronary intervention, Acute kidney injury, Contrast-induced nephropathy
Keypoints
Question: Should we maintain or discontinue SGTL2 inhibitors in diabetic patients who will undergo percutaneous coronary intervention?
Findings: The present study showed that the use of SGLT2i (empagliflozin) is safe regarding kidney function during elective PCI in patients with T2D when compared with no use of SGLT2i.
Meaning: With published data on renal safety of empagliflozin pre-PCI in a pilot study, clinical outcomes such as CI-AKI can now be evaluated in a larger randomized clinical trial based on an adequate sample size calculation.
Background
There is established benefit between PCI and stable coronary artery disease (CAD) patients, by reducing symptoms and improving quality of life [1]. Still, type 2 diabetes (T2D) is present in 25% of patients undergoing elective PCI and represents the main risk factor for the onset of acute kidney injury (AKI) after PCI [1, 2]—mainly due to the use of contrast medium [1, 2]. Contrast-induced AKI (CI-AKI) is considered as a new-onset or an exacerbation of chronic renal dysfunction following the administration of contrast media dye, without other potential causes [2].
Neutrophil Gelatinase-associated Lipocalin (NGAL) is a biomarker of AKI produced early after an ischemic renal insult. The practice of determining the biomarker levels has been validated in previous publications, either in experimental or clinical studies [3, 4]. This includes the detection of the development of CI-AKI at least 24 h before the increase in serum creatinine. CI-AKI is related to increased death, progression of kidney disease, need for dialysis, and increased health-related costs [2, 5]. Although, a myriad of strategies were previously tested aiming to reduce CI AKI with inconsistent results, such as statins, n-acetylcysteine, and sodium bicarbonate. Up to now, the only effective therapy for CI AKI prevention, is intravenous hydration, with isotonic saline solution and the use of low- or iso-osmolar contrast media [5].
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduced the rate of hyperglycemia in patients with T2D by decreasing renal glucose reabsorption, thus increasing urinary glucose excretion [6]. SGLT2i have demonstrated a protective clinical effect in T2D patients with high cardiovascular risk, by reducing mortality rate, hospitalization due to heart failure, and a decrease in the progression of kidney disease [6–8]. On the other hand, the osmotic diuresis and the vasoconstriction of the afferent arteriole promoted by SGLT2i generate a concern regarding the possibility of dehydration and consequent AKI [9].
Real-world evidence studies suggest that patients using SGLT2i, including empagliflozin, developed less AKI and displayed less overall renal decline when compared to other glucose-lowering agents [6]. This discloses the likely renoprotective properties of SGLT2i counter to AKI for patients with T2D. The role of SGLT2i in reducing the risk of CI-AKI in T2D undergoing elective PCI is to be seen.
In SAFE-PCI trial, we evaluated if SGLT2i (empagliflozin) prevents CI-AKI among T2D patients undergoing elective PCI, as expressed by blood levels of biomarkers NGAL and creatinine. Our study intented to explore the specific effects of empagliflozin on the extent of CI-AKI among T2D patients with stable CAD undergoing PCI.
Methods
Trial design
SAFE-PCI trial is a pilot, single-center, prospective, open-label, interventional controlled randomized (1:1) study aiming to assess AKI up to 48 h after PCI. NGAL and creatinine changes were measured after using oral empagliflozin 25 m/day for at least 15 days before the procedure. The SGLTi group was compared to a control group, of stable CAD and T2D patients on optimal medical therapy and without SGLT2i.
The exclusion criteria were: estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2 or dialysis therapy; acute coronary syndrome in the last 30 days; need for urgent or emergency PCI; use of non-steroidal anti-inflammatory drugs in the last 30 days before randomization; known pregnancy; and inability to sign the consent form.
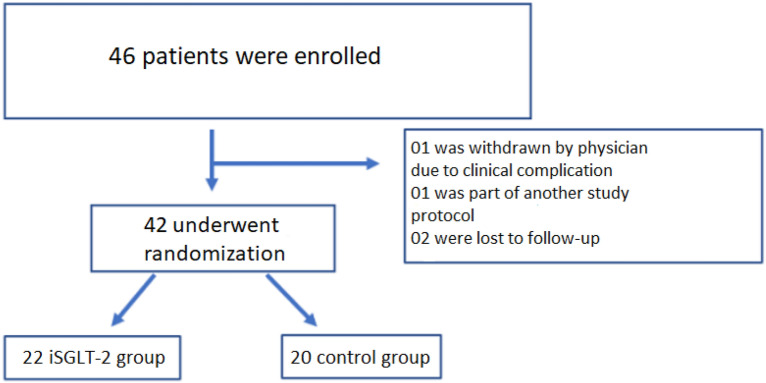
The patients were all admitted to the general cardiology ward of our institution and underwent coronary angiography (CAG) and PCI from August, 2021 to June, 2022. The study flow diagram presenting the enrolment and randomization data is seen in Fig. 1. For this study, those patients with a diagnosis of T2D and CAD and available serum creatinine measurements during hospitalization were included. As a pilot study, there were no preliminary data of CI-AKI incidence in patients submitted to PCI, and the sample size was estimated in 46 patients evaluated for inclusion. After excluding 4 patients, 42 patients were enrolled and randomized to the SAFE-PCI study, either the SGLT2i group (n = 22) or to the control group (n = 20). All 42 patients were followed until the end of the study.
Fig. 1.
Study flow showing patient enrollment and randomization
This study was approved by the institutional review board of the Instituto do Coracao (InCor) of the Faculty of Medicine of the University of Sao Paulo and patients needed to sign the consent form before recruitment.
Procedural aspects
PCI consisted of the use of drug-eluting stents only, due to its already established benefits for the T2D population [10].
The standard dose of acetylsalicylic acid 100 mg/day was maintained. All patients received a second antiplatelet drug on pre-PCI at the hospital admission in a loading dose for those who were already not on dual antiplatelet therapy, maintaining the following maintenance dosis either: clopidogrel 75 mg/day, ticagrelor 90 mg/twice a day or prasugrel 10 mg/day, according to institutional availability.
In all cases, renoprotection strategies were used to prevent CI-AKI, counting low-osmolar or iso-osmolar contrast media dye and hydration with intravenous isotonic crystalloid solutions (1 ml/kg/hour 6 h before PCI), as well as the restraint of nephrotoxic agents. Intravascular imaging was used, when available [11].
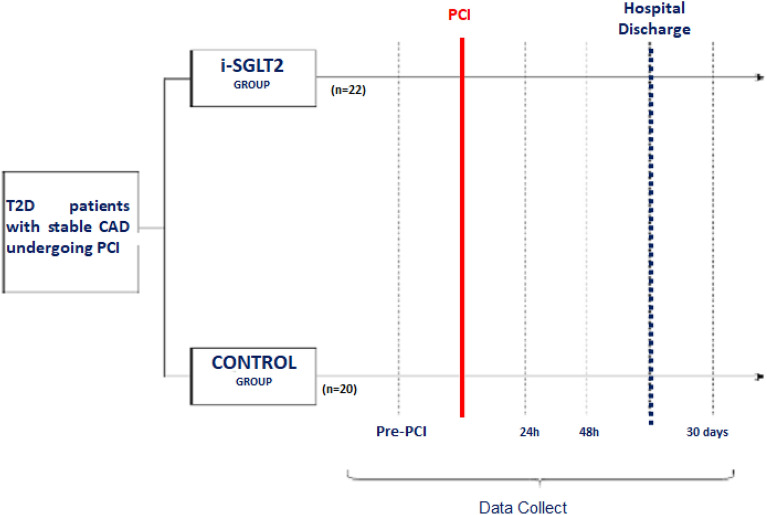
Blood sample collection
Serum NGAL was collected 6 h after the procedure. Serum creatinine was collected at the time of randomization, before and after PCI at pre-specified intervals of 6, 24, and 48 h, and at 30 days (see Fig. 2). Laboratory tests, including hemograms, myocardial necrosis markers, lipid profiles, kidney function tests and glycemic blood samples were collected before and after PCI.
Fig. 2.
Study protocol
Definitions
T2D was defined according to American Diabetes Association (ADA) guidelines [12]. Essentially, T2D was confirmed by fasting glucose levels (≥ 126 mg/dL) or by a previous diagnosis when the patient was on oral hypoglycemic agents or insulin [12].
CAD was defined as a narrowing of at least 50% luminal diameter in at least one major coronary artery, evaluated by two independent experienced interventional cardiologists.
A SYNTAX score value is the sum of the points assigned to each individual lesion identified in the coronary tree with > 50% diameter narrowing in vessels > 1.5 mm diameter evaluated by a coronary angiogram to determine the complexity of coronary artery disease. The higher the score, the greater the coronary artery disease complexity. All coronary angiographies were evaluated by an experienced interventional cardiologist and SYNTAX score was calculated and described in angiographic features [10].
CI-AKI is the third most frequent cause of acute kidney dysfunction and is defined as a 25.0% increase in baseline creatinine or an absolute increase of 0.5 mg/dL between 48 and 72 h after contrast use according to the Kidney Disease Improving Global Outcomes (KDIGO) classification [13].The individual risk of developing CI-AKI can be estimated using the Mehran Score, which consists of risk stratification. An increased Mehran Score confers an exponentially increased risk of CI-AKI, ranging from 8.4 to 55.9% [14].
Complications such as spontaneous myocardial infarction (MI) was defined by the 4th universal definition of myocardial infarction [15] and periprocedural MI (PMI) according to Society for Cardiovascular Angiography and Interventions (SCAI) definition [16]. Bleeding was defined by Bleeding Academy Research Consortium (BARC) definition [17].
Outcomes
The primary outcome of the study was serum NGAL measurements 6 h after the procedure.
Secondary outcomes considered the individual occurrences of: creatinine level changes (at the pre-specified time-points); CI-AKI; PMI; spontaneous MI; stroke; definite or probable stent thrombosis; major bleeding, namely BARC from 3 to 5; and all-cause mortality.
Statistical analysis
Quantitative variables were presented as average and standard deviation (SD), or median and interquartile ranges (IQR). Qualitative variables were expressed as absolute values and frequency of occurrences. The distribution of continuous variables was performed by the Kolmogorov–Smirnov test.
The comparison of two means for the quantitative variables was performed using Student's t-test. When normality was rejected (non-parametric distribution), the Mann–Whitney U test was used. The Chi-square test was used to compare qualitative variables in groups. The Fisher exact test was used for categorical variables.
One-way ANOVA with repeated measures was used to compare creatinine levels in different times in each group.
Logistic regression analysis was used to establish the risk of occurrence of events between groups.
The tests were two-tailed and performed with a significance level of 5%. SPSS version 21.0 software for Macintosh (IBM® SPSS Statistics, Armonk, NY, USA) was used for statistical analysis. Randomization was performed using the REDCap—HCFMUSP program v11.0.3 (Vanderbilt University, Nashville, TN, USA).
Results
Baseline characteristics and medication use
Except for HDL and CKMB levels with a p-value of 0.037 and 0.016, respectively, even though their values remained in the range of normality (see Table 1), there were no differences in baseline clinical and laboratory characteristics between the groups. No differences were observed between the groups regarding insulin use, hypoglycemic and anti-ischaemic medications (see Table 2).
Table 1.
Baseline clinical and laboratory characteristics of the groups
| SGLT2i (n = 22) | Control (n = 20) | p-value | |
|---|---|---|---|
| Age (years) | 65 ± 10 | 64 ± 6 | 0.82 |
| Male sex | 14 (63%) | 15 (75%) | 0.42 |
| Arterial hypertension | 19 (86%) | 14 (70%) | 0.83 |
| Active smoker | 12 (54%) | 9 (45%) | 0.41 |
| Previous stroke | 5 (22%) | 2 (10%) | 0.26 |
| Peripheral arterial disease | 2 (9%) | 1 (5%) | 0.60 |
| eGFR (mL/min/1.73m2) | 62.10 ± 22.50 | 68.20 ± 17.70 | 0.10 |
| Mehran score | 6.70 ± 3.68 | 5.55 ± 2.09 | 0.44 |
| LVEF (%) | 50 ± 14% | 60.00 ± 9.16 | 0.18 |
| Prior PCI (%) | 5 (22%) | 3 (15%) | 0.52 |
| Prior CABG (%) | 4 (18%) | 2 (10%) | 0.44 |
| Hemoglobin (g/dL) | 11.8 ± 4.6 | 12.5 ± 4.0 | 0.50 |
| Platelets (mm3) | 220,000 ± 71,554 | 209,750 ± 41,079 | 0.23 |
| Troponin (ng/mL) | 56 ± 101 | 24 ± 43 | 0.18 |
| CKMB (ng/mL) | 2.32 ± 1.37 | 1.32 ± 0.75 | 0.016 |
| Glycated hemoglobin (%) | 6.3 ± 3.0 | 7.6 ± 2.0 | 0.16 |
| Urea (mg/mL) | 47.68 ± 17.00 | 45.10 ± 31.20 | 0.64 |
| Creatinine (mg/mL) | 1.24 ± 0.35 | 1.06 ± 0.26 | 0.24 |
| Total cholesterol (mg/mL) | 178 ± 55 | 160 ± 46 | 0.16 |
| HDL-cholesterol (mg/mL) | 47 ± 13 | 40 ± 9 | 0.037 |
| LDL-cholesterol (mg/mL) | 98 ± 41 | 96 ± 40 | 0.92 |
| Triglycerides (mg/mL) | 148 ± 63 | 144 ± 55 | 0.87 |
eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and, CABG, coronary artery bypass graft
T student was used to compare means between groups
Chi square test was used to compare proportion between groups
Table 2.
Baseline medications
| SGLT2i (n = 22) | Control (n = 20) | p-value | |
|---|---|---|---|
| Insulin | 9 (40.9%) | 5 (25%) | 0.27 |
| Metformin | 20 (90.9%) | 18 (90%) | 0.92 |
| Sulfonylurea | 5 (22.7%) | 9 (45%) | 0.12 |
| DPP-4 inhibitor | 0 (0%) | 1 (5%) | 0.28 |
| Glitazone | 0 (0%) | 0 (0%) | – |
| Oral anticoagulant | 4 (18.2%) | 1 (5%) | 0.18 |
| Statin | 21 (95.5%) | 20 (100%) | 0.33 |
| Beta-blocker | 19 (86.4%) | 16 (80%) | 0.58 |
| Angiotensin II receptor blocker | 4 (18.2%) | 9 (45%) | 0.06 |
| ACE inhibitors | 15 (68.2%) | 9 (45%) | 0.12 |
DDP, dipeptidyl peptidase 4; ACE, angiotensin-converting enzyme. Chi square test was used to compare proportion between groups
Angiographic characteristics
Baseline angiographic characteristics are shown in Table 3. There was no difference between groups regarding the SYNTAX score (p = 0.11), the number of implanted stents (p = 0.20), stent diameter (p = 0.55), and contrast dye volume (p = 0.23).
Table 3.
Angiographic features
| SGLT2i (n = 22) | Control (n = 20) | p-value | |
|---|---|---|---|
| Location of lesions | |||
| LMCA | 0 | 1 | 0.28 |
| LAD | 12 | 14 | 0.08 |
| LCx | 11 | 4 | 0.043 |
| RCA | 5 | 7 | 0.10 |
| VG | 0 | 1 | 0.28 |
| Bifurcation lesion | 14 | 7 | 0.054 |
| Stent diameter (mm) | 40 ± 23 | 61 ± 30 | 0.551 |
| Number of stents placed | 28 | 27 | 0.20 |
| SYNTAX score | 16.60 ± 7 | 14.05 ± 9 | 0.11 |
| Contrast medium volume (mL) | 144 ± 66 | 176 ± 54 | 0.23 |
| Procedural time (min) | 60 ± 30 | 77 ± 40 | 0.46 |
| ACC/AHA Lesion classification | A-5 | A-2 | 0.08 |
| B1–6 | B1–6 | ||
| B2–5 | B2–9 | ||
| C-6 | C-4 |
ACC, American College of Cardiology; AHA, American Heart Association; LMCA, left main coronary artery; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery; VG, vein graft. T student was used to compare means between groups
One patient in the SGLT2i group underwent balloon angioplasty only to due unsuccessful stent implantation.
Outcomes
There was no difference in the primary endpoint of the study (see Fig. 3). Mean serum NGAL 6 h after PCI was 199 ng/dL in the SGLT2i group, and 150 ng/dL in the control group (p = 0.249).
Fig. 3.
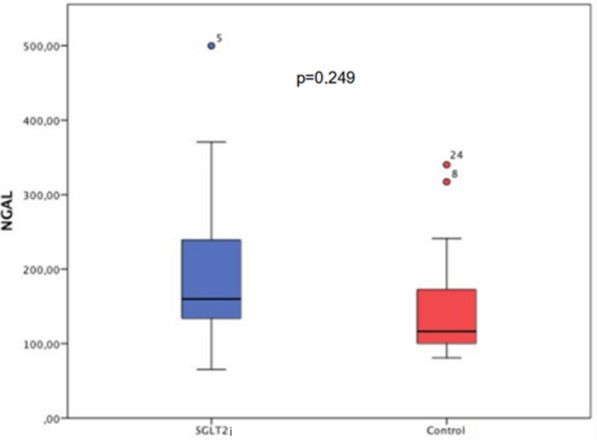
NGAL value (ng/dL) 6 h after percutaneous coronary intervention. There was no difference in the primary endpoint of the study. Mean serum NGAL 6 h after PCI was 199 ng/dL in the SGLT2i group and 150 ng/dL in the control group (p = 0.249). *Test-t used to compare two different groups
As seen in Fig. 4, there was no difference in baseline creatinine levels (p = 0.061) between groups. In the SGLT2i group, an absolute increase in creatinine from baseline to creatinine levels at pre-PCI and 24 h after PCI was observed when compared to the Control group (p = 0.009 and 0.016, respectively). However, the difference between groups after 48 h of PCI is no longer statistically significant (p = 0.065).
Fig. 4.
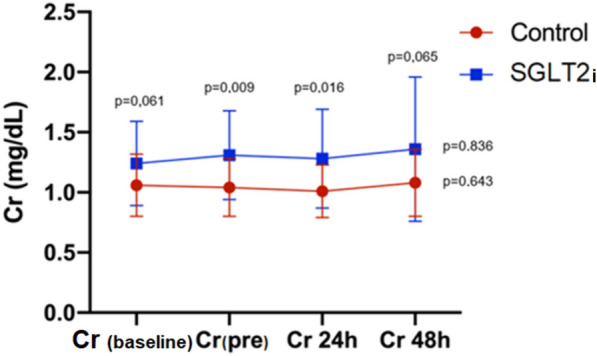
Creatinine levels at pre-specified time-points. There was no difference in baseline creatinine levels (p = 0.061) between groups. In the SGLT2i group, an absolute increase in creatinine from baseline to creatinine levels at pre-PCI and 24 h after PCI was observed when compared to the Control group. However, the difference between groups after 48 h of PCI was not statistically significant. *Test-t used to compare two creatinine levels in different groups. **One-way ANOVA with repeated measures was used to compare creatinine levels in different times in each group
The ratio of creatinine at 48 h and creatinine before PCI did not show a difference between groups (p = 0.888) (see Fig. 5).
Fig. 5.
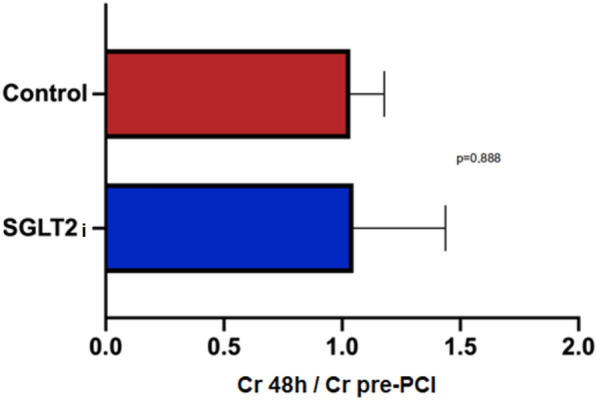
There was no difference between the groups in the ratio of creatinine at 48 h and creatinine before PCI. PCI, percutaneous coronary intervention. *Test-t used to compare two creatinine levels in different groups
The incidence of CI-AKI was similar in both groups, occurring in 3 patients in the SGLT2i group (13.6%) and 2 patients in the control group (10%) (p = 0.71). The the same result was observed regarding the occurrence of PMI with 26% in the SGLT2i group vs. 30% in the Control group (p = 0.59).
A multivariate sensitivity analysis of the predictors of CI-AKI was performed. The Mehran score was found as the only independent variable to predict the occurrence of CI-AKI (Harzard Ratio: 1.37; 1.02–1.85; p = 0.033). The isolated serum value of NGAL 6 h after PCI did not correlate with the occurrence of CI-AKI.
One patient in the SGLT2i group had a hemorrhagic stroke as well as an AKI that required hemodialysis. In the 30-day analysis, there were no episodes of major bleeding, stent thrombosis or death. Other patient in the SGLT2i group presented a coronary perforation without hemodynamic instability during and after the procedure.
Discussion
In our trial, the use of empagliflozin before PCI did not increase the occurrence of AKI in patients with T2D and stable CAD undergoing PCI through serum NGAL value and creatinine curve. In addition, there was similar rates of CI-AKI between groups.
These observations are consistent with previously reported data from non-randomized clinical trials. Despite the kidney injury after the use of contrast media, CI-AKI rates did not raise after PCI when associated with the use of SGLT2i. In addition, to the best of our knowledge, SAFE-PCI was the first trial to evaluate kidney injury in the context of PCI through assessment of NGAL, as an early predictor of CI-AKI, which is a biomarker more accurate than serum creatinine.
Part of the benefits of SGLT2i is attributed to the reduction in blood pressure, weight, and albuminuria [18–20]. A recently published meta-analysis, including data from the three largest randomized studies with this therapeutic class (DECLARE, EMPA REG, and CANVAS), pointed to a 44% reduction in the incidence of AKI in the population using SGLT2i compared to the control group [21]. Although, SAFE-PCI is a pilot study, it is the first randomized trial evaluating the impact of SGLT2i in peri-procedural status of stable CAD and T2D patients undergoing to PCI.
The reduction of Na+ reabsorption by the kidney is associated with SGLT2 inhibition with consequent reduction of Na+/K+ ATPase activity in the proximal tubule seems to be related to greater perfusion and oxygenation in the renal cortex, as well as a greater tolerance to ischemia and reperfusion injury [22]. Part of a potential benefit in preventing AKI could be explained by these mechanisms. Moreover, an anti-inflammatory mechanism of dapagliflozin is also postulated from the reduction of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-Kβ), reducing AKI [21].
The tubuloglomerular feedback system is an adaptive mechanism through which the reabsorption of sodium and chloride in the macula densa promotes the release of adenosine, contributing to the vasoconstriction of the afferent arteriole [20]. In diabetes, as a result of increased reabsorption of sodium and chloride in the proximal tubule, delivery to the macula densa is decreased, leading to less reabsorption of solutes and a consequent decrease in adenosine production. By promoting relative afferent arteriolar vasodilation, this mechanism contributes to glomerular hyperperfusion, hypertension and hyperfiltration in diabetes [20, 23, 24].
By blocking the reabsorption of NaCl in the proximal tubule, inhibition of SGLT2 restores solute release to the macula densa, restoring normal tubuloglomerular feedback, reversing afferent vasodilation, and normalizing glomerular hemodynamics [24].
Kidokoro et al. [25], studying renal hemodynamic alterations promoted by the use of empagliflozin in diabetic rats, demonstrated adenosine-dependent afferent arteriolar vasoconstriction with reduction of hyperfiltration within a few hours after drug administration.
On the other hand, the osmotic diuresis and the vasoconstriction of the afferent arteriole promoted by SGLT2i generate a concern regarding the possibility of dehydration and consequent AKI [9]. Although the exact mechanisms of acute kidney injury secondary to the use of iSGLT2 are not fully known, it is postulated that some factors related to the mechanism of action of this class may contribute to the acute worsening of renal function, such as, osmotic diuresis and glycosuria which can cause hyperosmolarity and dehydration. Increased levels of glucose in the urine can also be reabsorbed by the glucose transporter GLUT9b, located in the apical membrane of proximal tubular cells, in exchange for uric acid. Consequently, an increase in urinary uric acid level has also been proposed as a risk factor for AKI by crystal-dependent or crystal-independent mechanisms [21].
Food and Drug Administration (FDA) recommendation is for withdrawal of this class of drugs from 3 to days before a surgical procedure and it is based on these mechanisms [26]. Up to this date, there is no consensus on the maintenance or discontinuation of SGTL2i in patients who will undergo PCI.
With published data on the renal safety of empagliflozin pre-PCI in a pilot study, clinical outcomes such as CI-AKI can now be evaluated in a larger randomized clinical trial based on an adequate sample size calculation.
Study limitations
This study has some limitations.
SAFE-PCI is a pilot study, open label, with a relatively small sample size and a single-center trial which was not powered to assess clinical outcomes. CI-AKI was evaluated by a surrogate endpoint (serum NGAL) and not using a clinical definition for AKI. Other laboratory biomarkers such as L-FABP, NAG, albuminuria and urinary output were not measured due to early hospital discharge after elective and uncomplicated PCIs.
Conclusion
The present study showed that the use of empagliflozin is safe regarding to kidney function during elective PCI in patients with T2D when compared to ones without SGLT2i. We did not observe significant changes after 6 h of PCI regarding serum NGAL levels, a biomarker of CI-AKI in this setting.
Acknowledgements
Not applicable.
Abbreviations
- ADA
American diabetes association
- AKI
Acute kidney injury
- BARC
Bleeding Academy Research Consortium
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- CI-AKI
Contrast-induced acute kidney injury
- eGFR
Estimated glomerular filtration rate
- IQR
Interquartile ranges
- KDIGO
Kidney Disease Improving Global Outcomes LVEF: left ventricular ejection fraction
- MI
Myocardial infarction
- NF-Kβ
Nuclear factor kappa-light-chain-enhancer of activated B cells, NGAL: Neutrophil Gelatinase-associated Lipocalin
- PCI
Percutaneous coronary intervention
- PMI
Periprocedural myocardial infarction
- SCAI
Society for Cardiovascular Angiography and Interventions
- SD
Standard deviation
- SGLT2i
Sodium-glucose cotransporter 2 inhibitors
- T2D
Type 2 diabetes
Author contributions
All authors reviewed the manuscript.
Funding
Funding was provided by a research grant from the department of Atherosclerosis-InCor HCFMUSP, São Paulo, Brazil.
Availability of data and materials
The data of this trial is stored in REDCap–HCFMUSP program v11.0.3 (Vanderbilt University, Nashville, TN, USA).
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the Instituto do Coracao (InCor) of the Faculty of Medicine of the University of Sao Paulo and the need for informed consent was obtained as part of the study approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mateus Paiva Marques Feitosa, Email: mateusfeitosa@gmail.com.
Eduardo Gomes Lima, Email: eduglima@yahoo.com.br.
Alexandre Antônio Cunha Abizaid, Email: aabizaid@uol.com.br.
Roxana Mehran, Email: roxana.mehran@mountsinai.org.
Neuza Helena Moreira Lopes, Email: neuzalopes1@gmail.com.
Thiago de Assis Fischer Ramos, Email: fischerthiago87@gmail.com.
Alexandre Hideo-Kajita, Email: ahkajita@gmail.com.
Roberto Kalil Filho, Email: roberto.kalil@usp.br.
Carlos Vicente Serrano Junior, Email: cvserranojr@gmail.com.
References
- 1.Feres F, et al. Guideline of the Brazilian society of cardiology and the brazilian society of hemodynamics and interventional cardiology on percutaneous coronary intervention. Braz Arch Cardiol. 2017;109(1):1–81. [Google Scholar]
- 2.James MT, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 3.Devarajan P. Lipocalin associated with neutrophil gelatinase: a troponin-like biomarker for human acute kidney injury. Nephrology. 2010;15(4):419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 4.Ling W, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108(3):c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 5.Ozkok S, Ozkok A. Contrast-induced acute kidney injury: a review of practical points. World J Nephrol. 2017;6(3):86. doi: 10.5527/wjn.v6.i3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wanner C, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 9.Hahn K, et al. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711–712. doi: 10.1038/nrneph.2016.159. [DOI] [PubMed] [Google Scholar]
- 10.Knuuti J, Wijns W. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(5):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 11.Almendarez M, et al. Procedural strategies to reduce the incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. J Am Coll Cardiol Cardiovasc Interv. 2019;12(19):1877–1888. doi: 10.1016/j.jcin.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 12.Petersmann A, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127(S01):S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 13.Levin A, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for assessment and management of chronic kidney disease. Kidney Suppl Int. 2013;3(1):1–150. [Google Scholar]
- 14.Mehran R, et al. A simple risk score for predicting contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 16.Moussa ID, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI) J Am Coll Cardiol. 2013;62(17):1563–1570. doi: 10.1016/j.jacc.2013.08.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndrepepa G, et al. Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2012;125(11):1424–1431. doi: 10.1161/CIRCULATIONAHA.111.060871. [DOI] [PubMed] [Google Scholar]
- 18.Solini A. Extraglycemic properties of empagliflozin. Diabetes Metab Res Rev. 2016;32(3):230–237. doi: 10.1002/dmrr.2666. [DOI] [PubMed] [Google Scholar]
- 19.Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 20.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al. The SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Physiol Renal. 2014;306(2):F194–204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose cotransporter-2 inhibitors: a meta-analysis of cardiovascular outcome studies. Diabetes Obes Metab. 2019;21(8):1996–2000. doi: 10.1111/dom.13754. [DOI] [PubMed] [Google Scholar]
- 22.Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS ONE. 2016;11(7):e0158810. doi: 10.1371/journal.pone.0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43(5):629–633. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 24.Alicic RZ, et al. Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019;68(2):248–257. doi: 10.2337/dbi18-0007. [DOI] [PubMed] [Google Scholar]
- 25.Kidokoro K, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140(4):303–315. doi: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 26.FDA US. FDA approves label changes to SGLT2 inhibitors regarding temporary discontinuation of medication before scheduled surgery. FDA approves label update to sodium glucose co-transporter-2 (SGLT2) inhibitors. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this trial is stored in REDCap–HCFMUSP program v11.0.3 (Vanderbilt University, Nashville, TN, USA).