Abstract
Background
Anti-CD20 agents are commonly used in MS, NMOSD, and MOGAD. Few studies have compared strategies to address hypogammaglobulinemia.
Objective
To compare strategies to manage secondary hypogammaglobulinemia in neuroimmunology patients, including reducing anti-CD20 dose and dosing frequency, IVIG/SCIG, anti-CD20 cessation, and DMT switches.
Methods
All MS, NMOSD, and MOGAD patients at our institution with hypogammaglobulinemia on anti-CD20 agents from 2001 to 2022 were analyzed. The median change in IgG, infection frequency, and infection severity before and after the treatment was calculated.
Results
In total, 257 patients were screened, and 30 had a treatment for hypogammaglobulinemia. IVIG/SCIG yielded the largest increase in IgG per year (674.0 mg/dL), followed by B-cell therapy cessation (34.7 mg/dL), and DMT switch (5.9 mg/dL). Dose reduction had the largest decrease in yearly infection frequency (2.7 fewer infections), followed by IVIG/SCIG (2.5 fewer), DMT switch (2 fewer), and reduced dosing frequency (0.5 fewer). Infection grade decreased by 1.9 for reduced dosing frequency (less severe infections), by 1.3 for IVIG/SCIG, and by 0.6 for DMT switch.
Conclusion
This data suggests that IVIG/SCIG may yield the greatest recovery in IgG while also reducing infection frequency and severity. Stopping anti-CD20 therapy and/or switching DMTs also increase IgG and may lower infection risk.
Keywords: B cell depletion, anti-CD20 monoclonal antibodies, hypogammaglobulinemia, multiple sclerosis, neuromyelitis optica, neuroimmunology
Introduction
B-cell-depleting anti-CD20 monoclonal antibodies are increasingly used as therapies for autoimmune diseases of the CNS. Rituximab, a chimeric anti-CD20 monoclonal antibody, 1 is a commonly-used off-label therapy for aquaporin-4-positive neuromyelitis optica spectrum disorders (AQP4 IgG positive NMOSD).2,3 Rituximab is also used off-label in some patients with MS and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD).4,5 In addition, ocrelizumab and ofatumumab,6,7 which are humanized anti-CD20 monoclonal antibodies, are FDA-approved and effective therapies for relapsing MS. Ocrelizumab also reduces disability in primary progressive MS. 8
B-cell-depleting therapies often produce long-term sustained clinical responses in neuroinflammatory diseases; however, a subset of patients may develop secondary hypogammaglobulinemia and serious infections related to prolonged immunotherapy. 9 Variable definitions and cut-offs for hypogammaglobulinemia are employed in the literature, leading to wide variations in incidence estimates. Hypogammaglobulinemia of all subtypes – IgA, IgM, and IgG – can occur with B-cell depletion, but reduced levels of IgG have been particularly linked to increased infection risk.10–12 Hypogammaglobulinemia is therefore commonly defined as a deficiency in serum levels of IgG below 600–700 mg/dL.13–16 Studies investigating the incidence and consequences of hypogammaglobulinemia associated with anti-CD20 therapy in neuroinflammatory diseases have yielded conflicting results. A limited number of real-world observational studies have shown that long-term rituximab therapy in NMOSD9,14,17 and MOGAD 14 is associated with hypogammaglobulinemia (IgG < 600–700 mg/L) in 60%–70% of patients and serious infection in 10%–20% of those patients. One study of rituximab use in NMOSD further found that hypogammaglobulinemia was present prior to rituximab treatment in 36% of patients with low IgG in the setting of prior immunotherapy. 17
There is currently no consensus about the optimal approach to managing secondary hypogammaglobulinemia in patients treated with anti-CD20 agents. Conservative management strategies such as extended dosing intervals of anti-CD20 agents, dose reductions, or switching disease-modifying therapy (DMT) to an agent that does not cause low IgG levels are frequently utilized. However, intravenous immunoglobulin (IVIG) and subcutaneous immunoglobulin (SCIG) offer a potential strategy to proactively treat the secondary hypogammaglobulinemia. Although few studies have examined immunoglobulin replacement therapy for neuroinflammatory diseases, studies in other autoimmune diseases13,18 and lymphoma 16 have found that immunoglobulin replacement reduces infection risk in patients with symptomatic hypogammaglobulinemia. IVIG and SCIG can reduce severe infection risk, but high costs, need for administration at infusion centers for intravenous access, and risk of acute kidney injury and thromboembolism may limit the utility of these treatments. 19
This retrospective study aims to compare the efficacy of strategies to manage hypogammaglobulinemia in MS, NMOSD, and MOGAD patients treated with rituximab, ocrelizumab, or ofatumumab. Specifically, we examined the outcomes after anti-CD20 dose reduction, reduced dosing frequency, anti-CD20 therapy cessation, DMT switches, and immunoglobulin replacement therapy in patients with hypogammaglobulinemia. The impact of these strategies was compared in terms of the effect on immunoglobulin levels and infection risk.
Materials and methods
All MS, NMOSD, and MOGAD patients within the Mass General Brigham system with hypogammaglobulinemia (IgG < 650 mg/dL) taking rituximab, ocrelizumab, or ofatumumab from 2001 to 2022 were identified using the Mass General Brigham Research Patient Data Registry (RPDR). 20 While 650 mg/dL is not particularly low, this cutoff was chosen based on the lower limit of reference interval (95%) and reflects thresholds commonly reported in the literature. 21 Patients who had comorbid cancer diagnoses on chemotherapy, patients with a history of hematopoietic stem cell transplant, and patients younger than 18 years were excluded. Ethics committee approval and waiver of informed consent was obtained (Mass General Brigham IRB #2019P003556).
Electronic medical records were used to determine the following in the identified patients: age, sex, and date of birth; other major comorbidities; CNS disease phenotype; date of CNS disease onset; dose and dosing interval of anti-CD20 therapy; date of initial hypogammaglobulinemia and subsequent IgG levels during anti-CD20 therapy (typically checked at 6-month intervals); use of IVIG/SCIG, including frequency and duration; cessation of anti-CD20 therapy and/or switch to other DMTs; number of clinical relapses per year; CD19+/CD20+ B-cell repopulation; number of infections per year while on anti-CD20 therapy; and severity of infections.
Relapses were defined as objective worsening of existing symptoms or new neurological symptoms lasting at least 24 h and preceded by at least 30 days of clinical stability. CD19+/CD20+ B-cell repopulation was defined as cell counts of 10/uL or higher or percentages of >0.2%. Infections were graded based on the Common Terminology Criteria for Adverse Events v4.0 as follows: grade 1: asymptomatic, pathologic, or radiographic findings only; grade 2: localized, local, or noninvasive intervention indicated; grade 3: IV antibiotic, antifungal, or antiviral intervention indicated, interventional radiology or operative intervention indicated; grade 4: life-threatening consequences (septic shock, hypotension, acidosis, and necrosis); and grade 5: death. 22 Infections were documented based on those mentioned in electronic medical records from neurology, infectious disease, and allergy/immunology providers (as available). Baseline IgG levels were documented as the first recorded IgG < 650mg/dL during B-cell therapy, as we aim to study the impact of treatments for hypogammaglobulinemia.
Patients were divided into the following groups according to the strategy used to address hypogammaglobulinemia: dose reduction, reduced dosing frequency, DMT switch, cessation of B-cell therapy, IVIG/SCIG, and an untreated group. Outcomes measured during the treatment included repopulation of CD19+/CD20+ B-cells, the number of clinical relapses at any point during treatment, and the median follow-up time between the initiation of the treatment for hypogammaglobulinemia and the last recorded time a patient was seen. Outcomes measured before and after the treatment included change in IgG per year; number of infections per year; and infection severity. While current guidelines recommend treating only symptomatic hypogammaglobulinemia, we included all patients treated for hypogammaglobulinemia regardless of their tendency toward infection to limit selection bias and better understand the impact of the studied treatments.
All analyses were performed using SAS software (Cary, NC). Medians and quartiles were reported for continuous variables and frequencies and relative frequencies were reported for categorical variables. The nonparametric Kruskal-Wallis test was used to compare groups with continuous variables and pairwise comparisons were performed using the Wilcoxon rank-sum test. Spearman's rank-order correlation was used to evaluate the association between two ranked variables. P-values <0.05 were considered significant.
Results
A total of 257 patients with hypogammaglobulinemia meeting inclusion criteria were identified, of which 39 had a documented strategy to address hypogammaglobulinemia and were included in the treated groups. A total of three patients had a dose reduction; eight had decreased dosing frequency; seven stopped B-cell therapy; seven switched DMTs; five received IVIG/SCIG; nine took extended treatment holidays out of caution during the COVID-19 pandemic. Dose reductions ranged from 25% to 50% reductions of standard doses (i.e., 500–750 mg of rituximab and 300 mg of ocrelizumab). Interval extensions ranged from receiving B-cell therapy every 7–14 months rather than the standard 6 months. Immunoglobulin levels and infections were monitored for these patients, and dosing intervals were adjusted as necessary according to clinical judgement. Dosing regimens for IVIG/SCIG ranged from 0.4 to 2 g/kg monthly. The median BMI of the five patients who received IVIG/SCIG was 23.1 kg/m2 (20.8, 28.3).
Table 1 summarizes baseline clinical characteristics before the treatment as well as outcomes during the treatment for these 39 patients. Of note, two patients experienced a combination of two strategies to address hypogammaglobulinemia and were not included in Table 1 or in the final analysis due to an inability to assess the differential impact of the two strategies. An additional 10 patients underwent a strategy to address hypogammaglobulinemia but were not included in the treated groups due to a lack of follow-up assessing infectious history and/or change in IgG. These patients were included in the untreated group in Table 1, which includes 216 patients.
Table 1.
Clinical characteristics of the treated and untreated groups.
| Treated Groups (n = 39) | Untreated Group (n = 216) | |
|---|---|---|
| Median Age, (Q1, Q3) | 57.9 (49.6, 65.8) | 58.6 (53.2, 66.0) |
| Female Patients, n (%) | 27 (69.2) | 155 (71.8) |
| Patients w/ MS, n (%) | 30 (76.9) | 202 (93.5) |
| Patients w/ NMOSD, n (%) | 8 (20.5) | 8 (3.7) |
| Patients w/ MOGAD, n (%) | 1 (2.6) | 6 (2.8) |
| Patients w/ Other Major Comorbidities, n (%) | 33 (84.6) | 157 (72.7) |
| Patients w/ History of Rituximab Use, n (%) | 32 (82.1) | 100 (46.3) |
| Patients w/ History of Ocrelizumab Use, n (%) | 16 (41.0) | 156 (72.2) |
| Patients w/ History of Ofatumumab Use, n (%) | 0 | 7 (3.2) |
| Median Baseline Level of Hypogammaglobulinemia during B-cell Therapy (mg/dl) | 537 (438, 619) | 591 (534, 641) |
| Patients w/ Hypogammaglobulinemia Before Starting B-cell Therapy, n (%) | 7 (17.9) | 53 (24.5) |
| Patients w/ Infections During B-cell Therapy (before the treatment for hypogammaglobulinemia in treated groups), n (%) | 27 (69.2) | 53 (24.5) |
| Outcomes | ||
| Patients who Relapsed During Treatment for Hypogammaglobulinemia, n (%) | 1 (2.6) | N/A |
| Patients w/ B-cell Repopulation During Treatment for Hypogammaglobulinemia, n (%) | 4 (10.3) | N/A |
| Median Follow-up Time During Treatment for Hypogammaglobulinemia (months) | 22.4 (11.8, 31.1) | N/A |
Two patients had CD19+/CD20+ B-cell repopulation in the reduced dosing frequency group, and one patient relapsed in the IVIG/SCIG group (Table 1). The patient who relapsed had secondary progressive MS treated with rituximab but stopped rituximab and started IVIG due to recurrent infections. While off rituximab, the patient had two mild relapses that resolved spontaneously.
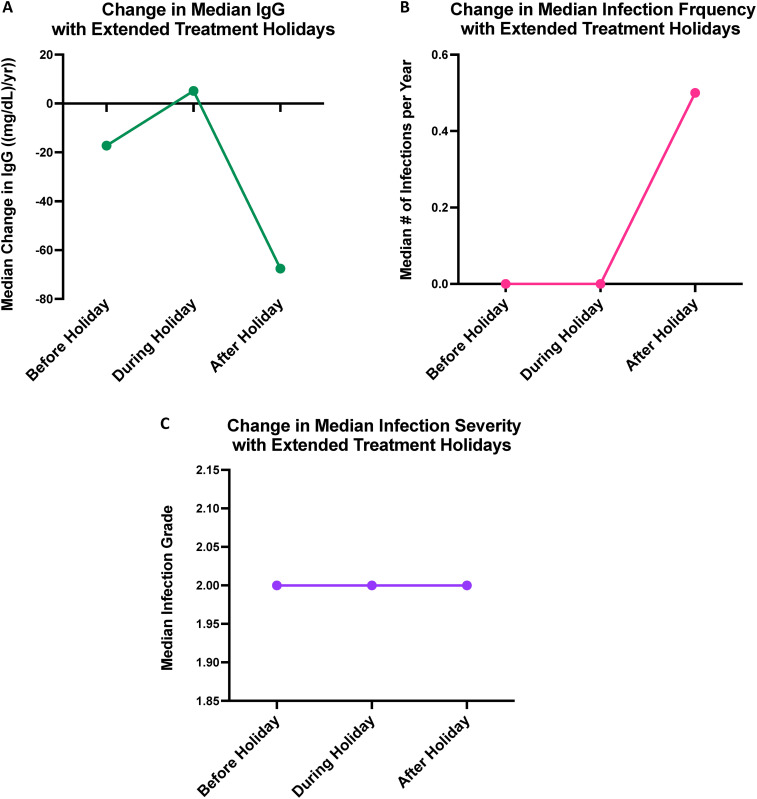
The nine patients who took extended treatment holidays during the COVID-19 pandemic were included as a separate “extended treatment holiday” group that was not compared with the other five treated groups, as treatment pauses were not one of the five strategies to address hypogammaglobulinemia that we intended to study. Overall, the median total treatment holiday was 18 months. Median IgG levels increased by 42.8 mg/dL per year during the treatment holiday and decreased by 45.9 mg/dL per year after the holiday. Also, the median change in the number of infections per year and infection severity were both 0 during and after the holiday. Importantly, no patients in this group relapsed during or after the holiday. However, two patients had repopulation of CD19+/CD20+ B-cells toward the end of their treatment holidays. Figure 4 shows the change in IgG (Figure 4(a)), infection frequency (Figure 4(b)), and infection severity (Figure 4(c)) in these patients before, during, and after the extended treatment holidays.
Figure 4.
Xy scatter plot depicting the change in median IgG per year (A), yearly infection frequency (B), and infection severity (C), before, during, and after extended treatment holidays.
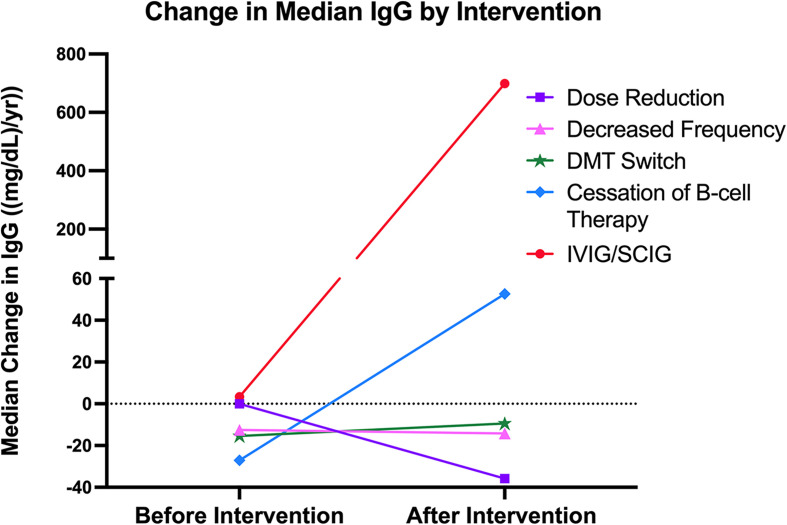
The median annual change in IgG among the five treated groups and the untreated group is summarized in Table 2. As expected, IVIG/SCIG supplementation yielded the largest increase in IgG per year (674.0 mg/dL), followed by cessation of B-cell therapy (34.7 mg/dL), and DMT switch (5.9 mg/dL). IgG decreased by 2.3 mg/dL per year in the reduced dosing frequency group, by 18.2 mg/dL in the untreated group, and by 35.8 mg/dL in the dose reduction group. For the statistical analysis comparing IgG levels between treated groups, the IVIG/SCIG group was excluded due to the significant and artificial increase in IgG. Among the four remaining treated groups, no significant differences were observed in terms of the median change in IgG per year. Likewise, no significant differences in the change in IgG were observed between the four treated groups and the untreated group. Figure 1 illustrates the annual change in IgG among the five treated groups before and after treatment.
Table 2.
Change in median IgG per year for treated groups and untreated group.
| Change in IgG (mg/dL)/year) | Pairwise Difference* | Any Difference** | |||
|---|---|---|---|---|---|
| Group | n | Median [Q1, Q3] | Wilcoxon Rank Sum p-value | Kruskal-Wallis p-value | Kruskal-Wallis p-value |
| Dose Reduction | 3 | −35.8 [−90.6, −16.4] | 0.22 | 0.23 | 0.57 |
| Reduced Dosing Frequency | 8 | −2.3 [−38.9, 50.6] | 0.47 | ||
| DMT Switch | 7 | 5.9 [−21.4, 152.4] | 0.13 | ||
| B-cell Therapy Cessation | 7 | 34.7 [−92.4, 238.5] | 0.22 | ||
| IVIG/SCIG | 5 | 674.0 [114.0, 750.5] | 0.0003 | ||
| Untreated | 216 | −18.2 [−36.6, −3.6] | Ref | ||
* Testing pairwise differences, if an individual treated group is different from untreated group (Ref).
** Testing overall difference, if at least one individual group is different among the unshaded groups.
Figure 1.
Xy scatter plot depicting the change in median IgG per year before and after strategies were implemented to address hypogammaglobulinemia.
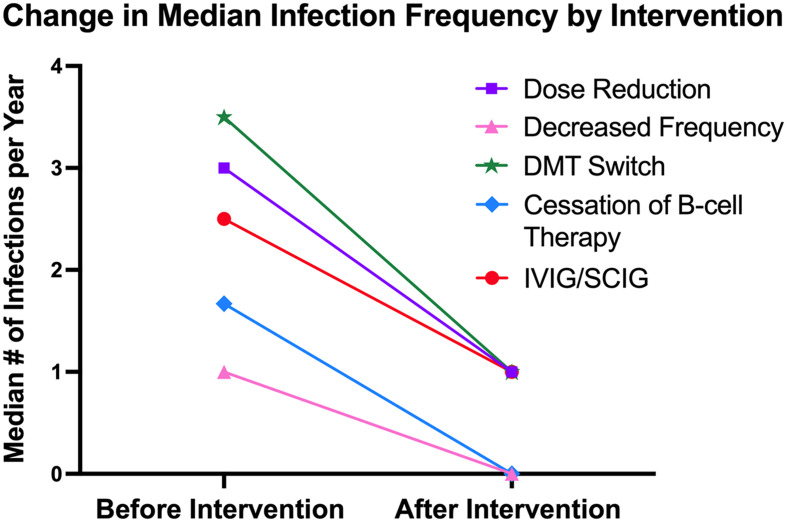
Table 3 summarizes the change in yearly infection number among the five treated groups and the untreated group. The dose reduction group had the largest decrease in the number of infections per year (2.7 fewer infections), followed by IVIG/SCIG (2.5 fewer), DMT switch (2 fewer), and reduced dosing frequency (0.5 fewer). Among the five treated groups, no significant differences were observed in terms of the median change in the number of infections per year. Pairwise comparisons between the treated groups and the untreated revealed that the dose reduction, DMT switch, cessation of B-cell therapy, and IVIG/SCIG groups all had significantly fewer infections per year compared to the untreated group. Figure 2 illustrates the change in yearly infection number among the five treated groups before and after treatment.
Table 3.
Change in median infection frequency per year for treated groups and untreated group.
| Change in Infection Rate (# infections/ year) | Pairwise Difference* | Any Difference** | |||
|---|---|---|---|---|---|
| Group | n | Median [Q1, Q3] | Wilcoxon Rank Sum p-value | Kruskal-Wallis p-value | Kruskal-Wallis p-value |
| Dose Reduction | 3 | −2.7 [−6.0, 0] | 0.004 | 0.001 | 0.5 |
| Reduced Dosing Frequency | 8 | −0.5 [−2.8, 1.0] | 0.099 | ||
| DMT Switch | 7 | −2.0 [−2.5, −1.5] | <0.001 | ||
| B-cell Therapy Cessation | 7 | 0 [−4.0, 0] | 0.007 | ||
| IVIG/SCIG | 5 | −2.5 [−4.0, −2.0] | <0.001 | ||
| Untreated | 216 | 0 [0, 0] | Ref | ||
* Testing pairwise differences, if an individual treated group is different from untreated group (Ref).
** Testing overall difference, if at least 1 individual group is different among the unshaded groups.
Figure 2.
Xy scatter plot depicting the change in the median number of infections per year before and after strategies were implemented to address hypogammaglobulinemia.
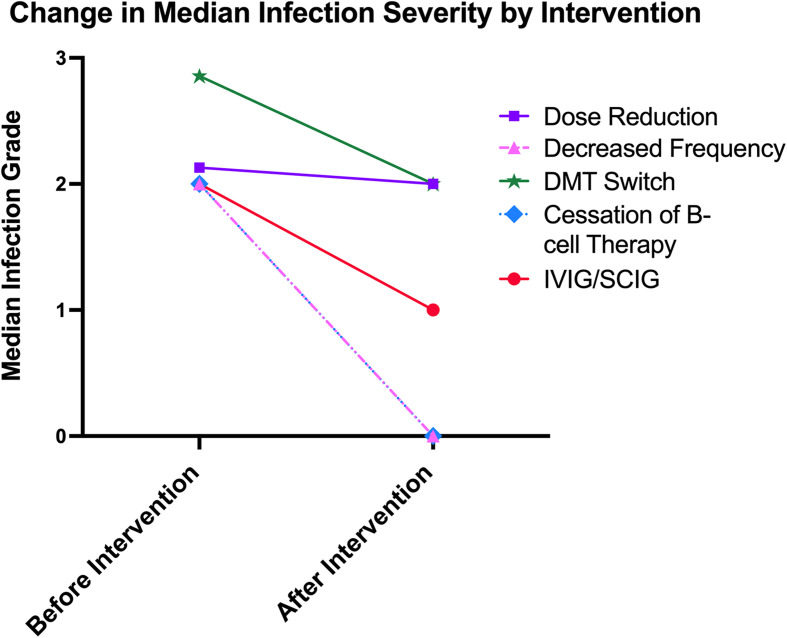
Median infection grade decreased by 1.9 in the reduced dosing frequency group (less severe infections), by 1.3 in the IVIG/SCIG group, and by 0.6 in the DMT switch group (see Table 4). Median change in infection severity was 0 in the dose reduction, cessation of B-cell therapy, and untreated groups. Among the five treated groups, no significant differences were observed in terms of the median change in infection severity. Pairwise comparisons between the treated and untreated groups revealed that the reduced dosing frequency, DMT switch, and IVIG/SCIG groups had significantly fewer infections compared to the untreated group. Figure 3 illustrates the median infection severity among the five treated groups before and after the treatment.
Table 4.
Change in median infection severity for treated groups and untreated group.
| Infection Severity | Pairwise Difference* | Any Difference** | |||
|---|---|---|---|---|---|
| Group | n | Median [Q1, Q3] | Wilcoxon Rank Sum p-value | Kruskal-Wallis p-value | Kruskal-Wallis p-value |
| Dose Reduction | 3 | 0 [−2.1, 0] | 0.05 | 0.001 | 0.6 |
| Reduced Dosing Frequency | 8 | −1.9 [−2.2, 0.5] | 0.003 | ||
| DMT Switch | 7 | −0.6 [−1.0, 0.5] | 0.008 | ||
| B-cell Therapy Cessation | 7 | 0 [−2.0, 1.0] | 0.19 | ||
| IVIG/SCIG | 5 | −1.3 [−1.3, -1.0] | <0.001 | ||
| Untreated | 216 | 0 [0, 0] | Ref | ||
* Testing pairwise differences, if an individual treated group is different from untreated group (Ref).
** Testing overall difference, if at least 1 individual group is different among the unshaded groups.
Figure 3.
Xy scatter plot depicting the change in median infection severity before and after strategies were implemented to address hypogammaglobulinemia.
Table 5 demonstrates the strength and direction of the association between changes in IgG levels and infection frequency and severity for the treated and untreated groups. Among the treated patients, the spearman's rank correlation between change in IgG levels and infection frequency and severity was −0.23 and −0.11, respectively, meaning that increases in IgG were associated with reduced infection frequency and severity. However, these associations were not statistically significant. For the 216 untreated patients, there was a smaller negative correlation between change in IgG levels and infection frequency (r = −0.05) and severity (r = −0.06), with no significant associations found.
Table 5.
Association between IgG levels and infection risk.
| Group | n | Spearman Correlation Coefficient for Infection Frequency | p-value | Spearman Correlation Coefficient for Infection Severity | p-value |
|---|---|---|---|---|---|
| Treated | 30 | −0.23 | 0.23 | −0.11 | 0.59 |
| Untreated | 216 | −0.05 | 0.49 | −0.06 | 0.41 |
Discussion
Few studies have examined the impact and efficacy of strategies other than immunoglobulin replacement therapy to address secondary hypogammaglobulinemia in neuroinflammatory disease. Our study offers preliminary insight into the differential impact of other strategies on IgG recovery, infection frequency, infection severity, B-cell repopulation, and clinical relapses. Only one patient (IVIG/SCIG group) experienced mild relapses and two patients (reduced dosing frequency) had early B-cell repopulation, although an extended follow-up period is necessary to adequately assess the risk of relapse and waning efficacy of treatment in the therapy cessation group.
As expected, immunoglobulin replacement therapy yields the greatest recovery in IgG levels. While no significant differences were observed between the other treated groups and the untreated group, B-cell therapy cessation and switching DMTs to agents other than anti-CD20 monoclonal antibodies may also increase IgG over time, although our follow-up time was limited. Reducing the dose and dosing frequency of anti-CD20 agents may not be sufficient to boost IgG levels over time. One study in NMOSD similarly found no association between hypogammaglobinemia and the number of rituximab cycles or the duration of rituximab treatment, although their small sample size limited robust statistical analysis. 17 Interestingly, a retrospective study of rituximab-treated NMOSD patients found that a low annual rituximab dose was associated with increased hypogammaglobulinemia risk, suggesting that patients with delayed memory B-cell reconstitution may be at higher risk for hypogammaglobulinemia. 23 Another study in rituximab-treated granulomatosis with polyangiitis patients found that 1 gram biannual treatment increased the risk of hypogammaglobulinemia compared to a 2 gram annual regimen, suggesting that intermittent B-cell recovery may protect against hypogammaglobulinemia. 15 The patients in our study with decreased IgG levels following anti-CD20 dose reduction or reducing dosing frequency may similarly have delayed memory B-cell repopulation and were thus more prone to develop hypogammaglobulinemia.
Our results provide interesting observations on the impact of hypogammaglobulinemia management strategies on infection risk. No significant differences were found among the five treated groups in terms of infection frequency and severity; however, IVIG/SCIG, switching DMTs, and reducing the dosing frequency trended towards decreasing both the infection frequency and severity. The dose reduction group also had a trend toward decreased infection frequency. Neither the B-cell therapy cessation nor untreated groups appeared to have any change on infection frequency or severity. Moreover, when compared to the untreated group, IVIG/SCIG and DMT switch were associated with a lower infection frequency and severity. Also, compared to the untreated group, dose reduction and B-cell therapy cessation were associated with lower infection frequency, and reduced dosing frequency was associated with lower infection severity. The overall proportion of patients on anti-CD20 therapies with infections in our cohort (69%) is in line with that from a similar small study conducted at a tertiary neuroimmunology clinic (73%), 14 although the incidence of infections is known to vary with patient disability and follow-up time.14,23–25 The proportion of patients with infections on B-cell therapy was greater in the treated groups than the untreated group, although about 30% of patients underwent a treatment for hypogammaglobulinemia only because of low IgG and not due to a significant infectious history, which limits selection bias.
Our results on infection frequency and severity are largely in line with those from previous studies assessing the impact of dose adjustments on lowering infection risk while still maintaining efficacy. Indeed, shorter rituximab dosing intervals in some NMOSD and MOGAD patients with earlier B-cell repopulation has been linked to higher infection risk that is presumably related to lower IgG levels. 14 Studies in rituximab-treated MS patients have also shown that extended dosing intervals may be associated with a low MS relapse risk.26–28 These findings suggest that extending the dosing interval of anti-CD20 agents may improve safety concerns related to hypogammaglobulinemia in neuroimmunology patients without necessarily compromising efficacy. While we observed no relapses in the three NMOSD patients treated with extended dosing intervals, it is important to acknowledge that this strategy may pose safety concerns in NMOSD, as even minimal B-cell repopulation may provoke relapses causing further neurologic disability.14,29 A study of rituximab-treated rheumatoid arthritis patients found that reduced doses decreased the rate of severe infection while maintaining adequate disease control at 5 years. 30
Interestingly, although IVIG/SCIG supplementation may yield the greatest recovery in IgG levels, it did not seem to lower infection susceptibility as much as other strategies in our cohort. While IVIG/SCIG supplementation has been shown to reduce infection frequency in rituximab-treated lymphoma 16 and autoimmune vasculitis 31 patients, other reports have found conflicting results. One study of rituximab-treated patients with autoimmune diseases found no benefit of immunoglobulin replacement therapy in preventing severe infections, particularly of the respiratory tract. 13 Immunoglobulin replacement therapy may not benefit patients with certain comorbidities and chronic airway damage. 13 Another study found that reductions in infection frequency and severity seen in rituximab-treated vasculitis patients were not sustained in patients with severe respiratory disease. 31
Finally, no studies have discussed the impact of switching DMTs in neuroimmunology patients with hypogammaglobulinemia secondary to anti-CD20 agents. In our study, seven patients switched from anti-CD20 agents to other DMTs such as mycophenolate mofetil, glatiramer acetate, fingolimod, and teriflunomide. DMT switch appeared to increase IgG levels over time while also lowering infection risk in our cohort, and while none of these patients relapsed, it is important to recognize that these DMTs are less efficacious than anti-CD20 therapies and thus patients may eventually relapse. Nevertheless, switching to other DMTs may be an option for patients who experience severe hypogammaglobulinemia and significant infections while on B-cell therapy.
The relationship between hypogammaglobulinemia and infection risk remains controversial. Interestingly, the treated and untreated groups in our cohort had similar baseline levels of hypogammaglobulinemia, suggesting that factors in addition to IgG levels impact the decision to treat hypogammaglobulinemia. Tendency toward infection likely played a role in our cohort, as a higher percentage of the treated groups had infections documented during B-cell therapy compared to the untreated group. While we found no significant association between increasing IgG levels and lowering infection frequency or severity, we observed a small but negative correlation between these variables in the treated and untreated groups. Furthermore, the strength of the correlation appears larger in the treated groups, perhaps suggesting an additional protective effect provided by the treatments for hypogammaglobulinemia. Given our small cohort sizes for the treated groups, however, we were unable to assess the impact of individual treatment strategies on the association between IgG recovery and infection risk. Nonetheless, certain strategies such as IVIG/SCIG and DMT switches appear to increase IgG while also lowering both infection frequency and severity.
Because B-cell-depleting therapies modulate the immune system in several ways, infection risk may be related to treatment effects beyond hypogammaglobulinemia. 32 A retrospective study of rituximab-treated NMOSD patients found no association between hypogammaglobulinemia and infection risk and instead found that high EDSS scores at rituximab initiation and relapses during rituximab treatment increased infection risk, likely due to increased neurologic disability and need for steroid administration. 23 In other studies, however, hypogammaglobulinemia significantly increased infection risk in rituximab-treated MS, 25 NMOSD,14,33 and MOGAD 14 patients. Several other factors, such as prior immunosuppression exposure, hypogammaglobulinemia prior to B-cell therapy initiation, and male sex have also been shown to increase infection risk. 33 The variable definitions and cutoffs for hypogammaglobulinemia reported in the literature may also obscure the relationship between low IgG levels and infection risk. The association between hypogammaglobulinemia and infection risk may be attenuated in some studies if patients stopped anti-CD20 therapy due to low IgG before any infections occurred. Nonetheless, it is possible that low IgG may not necessarily impact infection risk given that rituximab only partially depletes B cells in the bone marrow and lymphoid organs and thus may spare enough B-cells in other tissues to maintain adequate humoral immunity.23,32
Observations from the cohort of patients who took extended treatment holidays during the COVID-19 pandemic suggest that anti-CD20 therapy withdrawal during a median period of 18 months was associated with increasing IgG levels and no change in infection frequency or severity. No patients in our cohort relapsed during the treatment holiday, and only two patients experienced early B-cell repopulation. Although our results suggest that extended treatment holidays may lead to IgG recovery without greatly impacting disease activity, the short follow-period limits our ability to draw robust conclusions about the safety and efficacy of this strategy.
Major limitations to our study include the small number of patients with treated hypogammaglobulinemia and lack of long-term follow-up, which limit assessment of the differential efficacy of management strategies, including specific dosing regimens of B-cell therapies. Furthermore, while evaluating the number of clinical relapses per year captures disease activity and reflects DMT efficacy, it fails to assess important aspects of neurological functioning and progression. Higher EDSS scores are associated with infection risk, 23 but insufficient data was available in our medical records to consistently assess neurological status as measured by the EDSS. Also, the number of yearly infections was potentially underestimated, as some infections were likely not documented in the records. These undocumented infections were likely mild, so the overall infection grade may be artificially-inflated. Nevertheless, severe infections were readily documented in the records and it is clinically relevant to focus on these when evaluating the efficacy of strategies to address hypogammaglobulinemia. Lastly, imbalances in baseline characteristics between the treated and untreated groups, such as higher rates of NMOSD, prior rituximab use, and baseline infections amongst the treated groups compared to untreated may contribute to confounding of the results. Along those lines, our small number of NMOSD and MOGAD patients limited our ability to analyze the safety and efficacy of treatments for hypogammaglobulinemia within different disease categories, which likely makes our results more relevant to the MS population. It is important to acknowledge that decisions surrounding hypogammaglobulinemia treatment should depend on disease type. For example, extended dosing intervals are likely less safe in NMOSD14,29 than MS,26–28 and IVIG supplementation may treat hypogammaglobulinemia while also protecting against relapses in MOGAD. 34
Given the increased utilization of B-cell-depleting therapies in CNS inflammatory diseases, it is important to further explore the management of hypogammaglobulinemia in neuroinflammatory disease. Overall, this study offers important preliminary data that expands the discussion of strategies to address hypogammaglobulinemia secondary to anti-CD20 therapies in neuroimmunology patients. Despite our small sample size and limited follow-up time, our results suggest that cost-effective and less-invasive strategies such as dose adjustments and DMT switches may offer safe and effective alternatives to IVIG/SCIG in certain patients. Large-scale prospective studies are needed to fully assess the safety, efficacy, and feasibility of such strategies in neuroimmunology patients on B-cell-depleting therapies.
Footnotes
Declaration of competing interests: Ms. Hannah Kelly, Dr. Lori Chibnik, and Dr. Anastasia Vishnevetsky declare that there is no conflict of interest. Michael Levy has received fees for participation on advisory boards for Alexion (now AstraZeneca), Horizon, Genentech/Roche/Chugai, Mitusbishi Pharma, UCB and Sanofi.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Sylvia Lawry Physician Fellowship from the National Multiple Sclerosis Society, (grant number NA).
ORCID iDs: Hannah Kelly https://orcid.org/0000-0001-5818-1914
Michael Levy https://orcid.org/0000-0002-7969-8346
References
- 1.Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997; 90: 2188–2195. [PubMed] [Google Scholar]
- 2.Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol 2014; 71: 324–330. [DOI] [PubMed] [Google Scholar]
- 3.Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol 2016; 73: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 4.Chisari CG, Sgarlata E, Arena S, et al. Rituximab for the treatment of multiple sclerosis: a review. J Neurol 2021; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nepal G, Kharel S, Coghlan MA, et al. Safety and efficacy of rituximab for relapse prevention in myelin oligodendrocyte glycoprotein immunoglobulin G (MOG-IgG)-associated disorders (MOGAD): a systematic review and meta-analysis. J Neuroimmunol 2022; 364. doi: 10.1016/j.jneuroim.2022.577812 [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011; 378: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383: 546–557. [DOI] [PubMed] [Google Scholar]
- 8.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 30–31. [DOI] [PubMed] [Google Scholar]
- 9.Tallantyre EC, Whittam DH, Jolles S, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol 2018; 265: 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barmettler S, Ong MS, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Md Yusof MY, Vital EM, McElvenny DM, et al. Predicting Severe Infection and Effects of Hypogammaglobulinemia During Therapy with Rituximab in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol (Hoboken, NJ) 2019; 71: 1812–1823. [DOI] [PubMed] [Google Scholar]
- 12.Boleto G, Avouac J, Wipff J, et al. Predictors of hypogammaglobulinemia during rituximab maintenance therapy in rheumatoid arthritis: a 12-year longitudinal multi-center study. Semin Arthritis Rheum 2018; 48: 149–154. [DOI] [PubMed] [Google Scholar]
- 13.Tieu J, Smith RM, Gopaluni S, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avouac A, Maarouf A, Stellmann JP, et al. Rituximab-Induced hypogammaglobulinemia and infections in AQP4 and MOG antibody–associated diseases. Neurol Neuroimmunol Neuroinflammation 2021; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besada E, Koldingsnes W, Nossent JC. Serum immunoglobulin levels and risk factors for hypogammaglobulinaemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology 2014; 53: 1818–1824. [DOI] [PubMed] [Google Scholar]
- 16.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk 2013; 13: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcinno A, Marnetto F, Valentino P, et al. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflammation 2018; 5: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DM, Jones RB, Smith RM, et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun 2015; 57: 24–29. [DOI] [PubMed] [Google Scholar]
- 19.Katz U, Achiron A, Sherer Yet al. et al. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev 2007; 6: 257–259. [DOI] [PubMed] [Google Scholar]
- 20.Nalichowski R, Keogh D, Chueh HCet al. et al. Calculating the benefits of a research patient data repository. AMIA Annu Symp Proc 2006; 2006: 1044. [PMC free article] [PubMed] [Google Scholar]
- 21.Jolliff CR, Cost KM, Stivrins PC, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem 1982; 28: 126–128. [PubMed] [Google Scholar]
- 22.Anon. Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP.
- 23.Kim SH, Park NY, Kim KH, et al. Rituximab-Induced hypogammaglobulinemia and risk of infection in neuromyelitis optica spectrum disorders. Neurol - Neuroimmunol Neuroinflammation 2022; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oksbjerg NR, Nielsen SD, Blinkenberg M, et al. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult Scler Relat Disord 2021; 52. [DOI] [PubMed] [Google Scholar]
- 25.Vollmer BL, Wallach AI, Corboy JR, et al. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol 2020; 7(9): 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juto A, Fink K, Al Nimer Fet al. et al. Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord 2020; 37. [DOI] [PubMed] [Google Scholar]
- 27.Maarouf A, Rico A, Boutiere C, et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflammation 2020; 7: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker D, Pryce G, James LK, et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020; 44: 102279. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011; 68: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 30.Henry J, Gottenberg JE, Rouanet S, et al. Doses of rituximab for retreatment in rheumatoid arthritis: influence on maintenance and risk of serious infection. Rheumatology 2018; 57: 538–547. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DM, Jones RB, Smith RM, et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun 2015; 57: 24–29. [DOI] [PubMed] [Google Scholar]
- 32.Ramwadhdoebe TH, Van Baarsen LGM, Boumans MJH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019; 58: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmer BL, Wallach AI, Corboy JR, et al. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol 2020; 7: 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and MRI features, diagnosis, and management. Front Neurol 2022; 13: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]