Abstract
Objective
While twin gestations are at increased risk of severe maternal morbidity (SMM), there is limited information about timing and causes of SMM in twins. Furthermore, existing data rely on screening definitions of SMM because a gold standard approach requires chart review. We sought to determine the timing and cause of SMM in twins using a gold standard definition outlined by the American College of Obstetricians and Gynecologists (ACOG).
Study Design
We used a perinatal database to identify all twin deliveries from 1998 to 2013 at a single academic medical center (n = 2,367). Deliveries were classified as screen positive for SMM if they met any of the following criteria: (1) one of the Centers for Disease Control and Prevention (CDC) International Classification of Diseases Ninth Revision diagnosis and procedure codes for SMM; (2) a prolonged postpartum length of stay (>3 standard deviations beyond mean length of stay by mode of delivery); or (3) maternal intensive care unit admission. We identified true cases of SMM through medical record review of all screen-positive deliveries using the definition of SMM outlined in the ACOG Obstetric Care Consensus. We also determined cause and timing of SMM.
Results
A total of 165 (7%) of twin deliveries screened positive for SMM. After chart review of all screen-positive cases, 2.4% (n = 56) were classified as a true case of SMM using the ACOG definition for a positive predictive value of 34%. The majority of SMM occurred postpartum (65%). Hemorrhage was the most common cause of SMM, followed by hypertensive and pulmonary etiologies.
Conclusion
Commonly used approaches to screen for SMM perform poorly in twins. This has important implications for quality initiatives and epidemiologic studies that rely on screening definitions of maternal morbidity. Our study demonstrates that the immediate postpartum period is a critical time for maternal health among women with twin pregnancies.
Keywords: severe maternal morbidity, twin gestations, postpartum period
Women in the United States die during pregnancy, childbirth, and the postpartum period at a rate higher than any other high-income country.1–3 While pregnancy-related mortality is a tragic outcome, it remains relatively rare and is therefore challenging to study.4 In contrast, many more pregnant women in the United States experience severe morbidity, or maternal near miss.5–8 Severe maternal morbidity (SMM) is broadly defined as any unintended outcome of the process of labor and delivery that results in significant short-term or long-term consequences to a woman’s health.7 These events result in significant health care costs, prolonged hospitalizations, emotional and physical sequelae, and separation of the mother from her newborn.5
To identify opportunities for improvement in maternity care, professional societies, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal Fetal Medicine (SMFM), and the American College of Nurse-Midwives, have called on hospitals to screen for cases of SMM.9–12 Currently, however, there is not a universally agreed upon way to screen for SMM. ACOG and SMFM recommended that institutions use two criteria to screen for SMM—transfusion of 4 or more units of blood OR admission to the intensive care unit (ICU). Another approach hospitals can use to identify SMM is the Centers for Disease Control and Prevention (CDC) list of diagnostic and procedure codes for SMM.7,13,14 Studies, however, have demonstrated the CDC approach has only a moderate positive predictive value (PPV; 0.4–0.5) for true cases of SMM. This is in part driven by the inclusion of a procedure code for transfusion in the CDC list—receipt of 1 unit of blood is generally not viewed as a SMM. Given this, investigators have added criteria including prolonged length of stay and ICU admission to the CDC criteria to improve the PPV.15,16
ACOG and SMFM also acknowledge that there is no consensus definition of a true case of SMM. In an effort to standardize and provide guidance to institutions in identifying cases of true SMM, they published a list of conditions that hospitals could utilize in their review process.7,9,13,14 Their list includes 35 different diagnoses and complications organized in 9 broad categories (see Supplementary Table S1, available in the online version). To use, this list requires careful chart review by an experienced provider and is very time-intensive.
While it is known that women with twin gestations are at increased risk for obstetric and neonatal complications, there are significant gaps in our knowledge about SMM among twin pregnancies.17,18 The largest studies of twin pregnancies that identified true cases of SMM based on detail chart data were done in the Netherlands and France, and thus may not be as such applicable to the United States.19,20 We are unaware of any U.S. studies of twin gestations that have collected detailed information on true cases of SMM. Thus, we lack high quality detailed clinical data on cause-specific morbidity as well as timing of SMM in twins.21
Given the limitations of previous research, our objective was two-fold. First, we aimed to determine the PPV of various criteria that hospitals might use to screen for SMM in twin pregnancies. We evaluated three screening criteria—(1) any CDC diagnostic and procedure codes, (2) prolonged postpartum length of stay, and (3) ICU admission. We used the list of diagnoses and complications outlined by ACOG and SMFM in the Obstetric Care Consensus on SMM to define true cases of SMM.9 Second, using the detailed clinical data gathered from identifying true cases of SMM, we determined causes and timing of all SMM among twins.
Materials and Methods
This study was performed at a single tertiary care maternity hospital with approximately 10,000 births annually. Detailed information on all deliveries is maintained in a perinatal delivery database that contains over 300 variables from admitting services, medical records abstraction, the birth record, and ultrasound. Data administrators maintain the database by coding, cleaning, and storing these data. We used this database to identify all twin deliveries from 1998 to 2013. We ascertained additional data from medical record review.
As outlined above, there is no one recommended approach to screen for SMM.15 For this study, deliveries were classified as screen positive for SMM if they had any of the following: (1) one of the CDC International Classification of Diseases Ninth Revision diagnosis and procedure codes for the identification of SMM (Supplementary Table S2, available in the online version); (2) prolonged postpartum length of stay (>3 standard deviations beyond the mean length of stay by mode of delivery); or (3) maternal ICU admission. We chose these criteria because they had the highest PPV in a previous validation study of singletons.15
The outcome of interest was SMM based on detailed clinical chart review using the guidelines outlined in the Obstetric Care Consensus on SMM from ACOG and SMFM.9 These guidelines were developed through a systematic Delphi consensus process by a team of researchers with expertise in SMM.10 The list of morbidities is categorized as hemorrhage, hypertension, neurologic, renal, sepsis, intensive care/intensive monitoring, surgical complications, anesthesia complications, and other (see Supplementary Table S1, available in the online version).
To determine true case status per the ACOG and SMFM guidelines, a maternal fetal medicine fellow (A.B.) performed detailed chart abstractions using a standardized protocol and an electronic database. She reviewed detailed data from clinical notes, vital signs, medication lists, operative notes, transfusion records, laboratory studies, and radiologic studies to jury cases according to the ACOG/SMFM consensus. We further designated the events as having occurred antepartum (before labor, induction, or delivery), intrapartum (during labor or delivery), or postpartum (from completed delivery to discharge from the hospital).
We calculated the PPV of different screening criteria—defined as the number of true positives divided by all screen positives. True positives were screen-positive deliveries that met criteria for a SMM as suggested by the diagnoses in the 2016 ACOG and SMFM Obstetric Care Consensus on SMM. We were not able to calculate sensitivity and specificity given we did not perform chart abstraction of screen-negative cases. This study was approved by the University of Pittsburgh Institutional Review Board (approval no.: STUDY19090261).
Results
The cohort of eligible twin deliveries was predominantly non-Hispanic white, married, and had private insurance (Table 1). A total of 7.0% (n = 165) of the cohort met screened positive for SMM (Fig. 1). Deliveries that screened positive for a SMM were more likely to be nulliparous, have used in vitro fertilization to conceive, and have pregestational hypertension or diabetes compared with deliveries that screened negative for SMM (Table 1). Maternal age, chorionicity, and type of insurance did not vary significantly between screen-negative and screen-positive women. Women who screened positive for SMM were more likely to have had preeclampsia (37 vs. 18%) compared with women who had no screening criteria.
Table 1.
Maternal characteristics and perinatal outcomes of twin pregnancies by severe maternal morbidity screening statusa
| Screen negative for severe maternal morbidity (n = 2,202) | Screen positive for severe maternal morbidity (n = 165) | |
|---|---|---|
| Maternal age (y), mean (SD) | 30 (5.9) | 31 (6.2) |
| Maternal age >35 y | 537 (24) | 47 (29) |
| Maternal race/ethnlclty | ||
| Non-Hlspanlc White | 1,754 (80) | 124 (76) |
| Non-Hlspanlc Black | 374 (17) | 34 (21) |
| Hispanic | 14 (1.0) | 2(1.2) |
| Other | 55 (2.5) | 4 (2.4) |
| Insurance | ||
| Private | 1,252 (56) | 88 (53) |
| Medicaid | 941 (43) | 76 (46) |
| Other | 9 (0.4) | 1 (1.0) |
| Parity | ||
| Nulliparous | 997 (45) | 97 (59) |
| Multiparous | 1,205 (55) | 68 (41) |
| Assisted reproductive technology | ||
| None | 1,657 (75) | 105 (64) |
| In vitro fertilization | 351 (16) | 42 (26) |
| Other | 194 (9) | 18 (10) |
| Chorionicity | ||
| Dichorionic | 1,754 (80) | 135 (82) |
| Monochorionic | 448 (20) | 30 (18) |
| Prepregnancy hypertension | 104 (4.7) | 14 (8.5) |
| Prepregnancy diabetes | 55 (2.5) | 7 (4.2) |
| Preterm birth <32 wk | 369 (17) | 40 (24) |
| Preeclampsia | 393 (18) | 61 (37) |
| Birthweight Twin A (g), mean (SD) | 2,283 (721) | 2,155 (782) |
| Birthweight Twin B (g), mean (SD) | 2,263 (712) | 2,154 (772) |
Abbreviation: SD, standard deviation.
Screen-positive deliveries met one of the following criteria: one of the Centers for Disease Control and Prevention (CDC) International Classification of Diseases 9th Revision diagnosis and procedure codes for the identification of severe maternal morbidity; prolonged postpartum length of stay (>3 standard deviations beyond the mean length of stay by mode of delivery); OR maternal intensive care unit (ICU) admission.
Fig. 1.
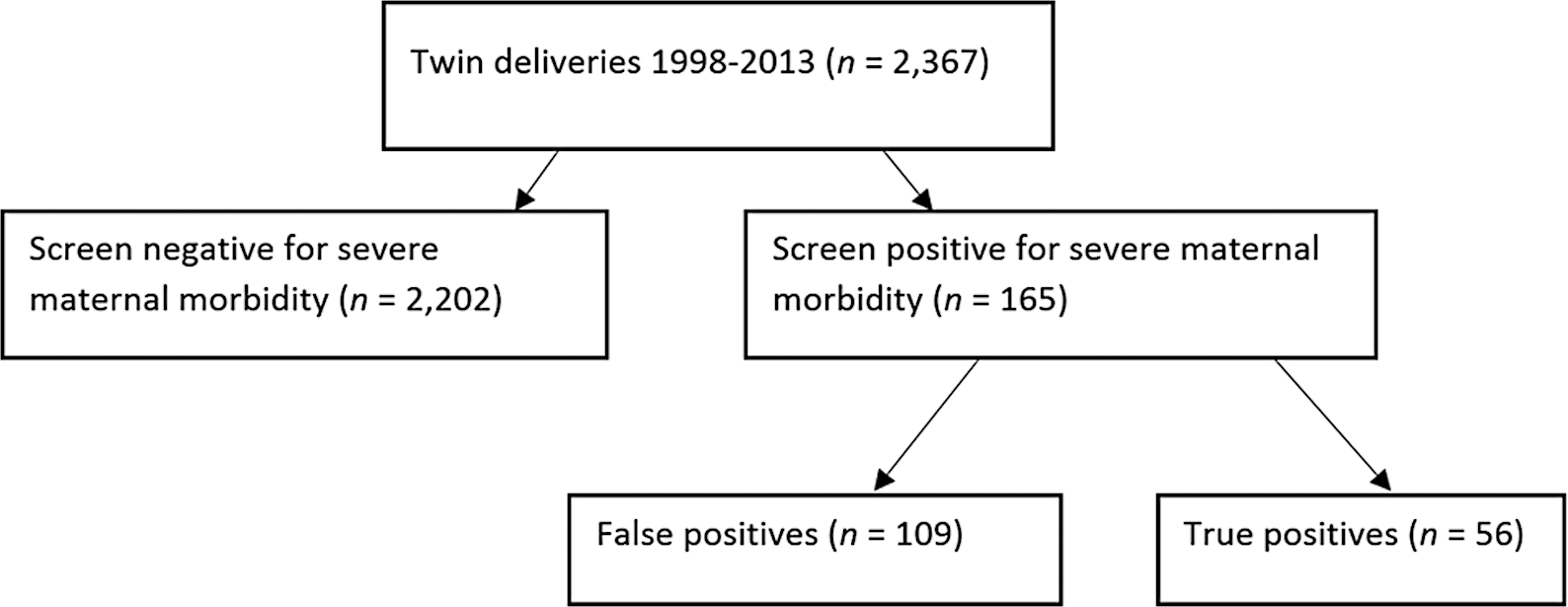
Screen and case identification of severe maternal morbidity among twins, Magee Womens Hospital, Pittsburgh PA.
After comprehensive case review of all 165 screen-positive deliveries, 56 were classified as true cases for a SMM rate of 2.4% (Fig. 1). Roughly 43% of the true cases had 1 morbid event, 32% had 2 events, and 25% had 3 or more qualifying morbidities. The demographic and perinatal outcomes between false positive (screen positive but not a true case of SMM) and true positive were similar; however, true positives were more likely to be multiparous and have a Medicaid product compared with false positives (Table 2). Additionally, true positives were more likely to have pregestational hypertension and be diagnosed with preeclampsia (7 vs. 11% and 33 vs. 45%, respectively).
Table 2.
Maternal characteristics and perinatal outcomes of false positive versus true positive severe maternal morbidity casesa
| False positive (n = 109) | True positive (n = 56) | |
|---|---|---|
| Maternal age (y), mean (SD) | 31 (6.2) | 31 (6.2) |
| Maternal age >35 (y) | 33 (30) | 14(25) |
| Maternal race/ethnicity | ||
| Non-Hispanic white | 83 (76) | 43 (77) |
| Non-Hispanic black | 22 (20) | 10 (18) |
| Hispanic | 1 (1.0) | 1 (1.8) |
| Other | 3 (3.0) | 2 (3.2) |
| Insurance | ||
| Private | 63 (58) | 25 (45) |
| Medicaid | 46 (42) | 30 (54) |
| Other | 0(0) | 1 (1.0) |
| Parity | ||
| Nulliparous | 72 (66) | 25 (45) |
| Multiparous | 37 (34) | 31 (55) |
| Assisted reproductive technology | ||
| None | 68 (62) | 37 (66) |
| In vitro fertilization | 28 (26) | 14(25) |
| Other | 13 (12) | 5 (9.0) |
| Chorlonlclty | ||
| Dichorionic | 88 (81) | 47 (84) |
| Monochorionic | 21 (19) | 9(16) |
| Prepregnancy hypertension | 8 (7) | 6(11) |
| Prepregnancy diabetes | 5 (4.6) | 2 (3.6) |
| Preterm birth <32 wk | 29 (27) | 10 (18) |
| Preeclampsia | 36 (33) | 25 (45) |
| Birthweight Twin A (g), mean (SD) | 2,127 (846) | 2,209 (641) |
| Birthweight Twin B (g), mean (SD) | 2,121 (821) | 2,217 (668) |
Abbreviation: SD, standard deviation.
True cases of severe maternal morbidity were those that met criteria from the ACOG and SMFM guidelines.
The PPVs of different screening approaches are outlined in Table 3. The most expansive screening approach of CDC criteria, ICU admission, or prolonged postpartum stay had a PPV of 34%. Using CDC criteria alone resulted in a similar PPV of 32%. Removing the blood transfusion procedural code from the CDC criteria resulted in a PPV of 87%; however, with this screening approach, 17 of the previously identified true positives would have been classified as screen negative. The performances of the different CDC diagnosis and procedure codes are outlined in Supplementary Table S2 (available in the online version). Only half (54%) of women with a procedure code for a blood transfusion were classified as true cases on chart review. Other indicators that had a low percent of true cases after chart review included sepsis, venous thrombotic emboli, and severe anesthesia complications.
Table 3.
Positive predictive value of different screening approaches to identify severe maternal morbidity in twin gestations
| Screening approach | Screen positives identified (n) | Gold standard definition | Positive predictive value (%) | True positive cases missed with screening approach | |
|---|---|---|---|---|---|
| True positives | False positives | ||||
| CDC, ICU, or LOS | 165 | 56 | 109 | 34 | 0 |
| CDC | 149 | 48 | 101 | 32 | 8 |
| CDC minus blood transfusion | 44 | 39 | 5 | 87 | 17 |
Abbreviations: CDC, Centers for Disease Control and Prevention; ICU, intensive care unit; LOS, length of stay.
Severe maternal morbidities were most likely to occur during the postpartum period, with a total of 69 (65%) events occurring after delivery (Table 4). Hemorrhage was the most common cause of SMM among true cases. Two-thirds of these hemorrhages occurred during delivery and the remaining one-third occurred postpartum. Other frequent causes of maternal morbidity in this cohort were hypertension, pulmonary etiologies, and neurologic complications. Interestingly, hypertensive morbidities were evenly split between antepartum events and postpartum events. Neurologic complications, including eclampsia, were more common antepartum than postpartum. Cardiac complications were the least common morbidities, and all occurred postpartum.
Table 4.
Cause and timing of true cases of severe maternal morbidity (n = 56 women with 106 events)a
| Cause of severe maternal morbidity (AGOG/SMFM Criteria) | Total events (n = 106) | Antepartum (n = 16) | Intrapartum (n = 21) | Postpartum (n = 69) |
|---|---|---|---|---|
| Hemorrhage (i.e., ≥4 U PRBCs, unplanned hysterectomy) | 29 (27) | 0 | 19 (90) | 10(15) |
| Hypertension (i.e., liver capsular rupture, continuous infusion of antihypertensive medication) | 12(11) | 5 | 0 | 7(10) |
| Neurologic (i.e., eclampsia, stroke) | 9 (8.5) | 6 | 0 | 2 (2.9) |
| Renal (i.e., Cr ≥ 2.0 with no pre-existing renal disease, dialysis) | 4(3.8) | 0 | 0 | 4(5.8) |
| Sepsis (i.e., infection with pressor support) | 3 (2.8) | 0 | 0 | 3 (4.3) |
| Pulmonary (i.e., use of a ventilator, venous thromboembolism) | 13 (12) | 2 | 0 | 11 (16) |
| Cardiac (i.e., arrhythmia requiring medication) | 2(1.9) | 0 | 0 | 2 (2.9) |
| ICU for treatment | 27 (25) | 2 | 0 | 25 (36) |
| Surgical complications (i.e., bowel or bladder injury beyond serosal tear) | 4(3.8) | 0 | 2 (9.5) | 2 (2.9) |
| Anesthesia complications (i.e., total spinal anesthesia, aspiration pneumonia) | 3 (2.8) | 0 | 2 (9.5) | 1 |
Abbreviation: Cr, creatinine; ICU, intensive care unit; PRBCs, packed red blood cells.
Women had more than one morbidity and thus there are a total of 106 events.
Discussion
In this cohort of twin gestations, common screening approaches to identify cases of SMM performed poorly. Only 34% of deliveries with a CDC indicator, ICU admission or a prolonged postpartum length of stay, were classified as true positives after chart review using the list of diagnoses suggested in the Obstetric Care Consensus. CDC criteria alone had a similar PPV of 32%. When we removed the transfusion procedure code from the CDC criteria, the PPV improved to 87%. However, with this screening approach 17 true cases would have been missed as they met no other CDC criteria. Importantly, almost half of SMM cases were in deliveries with a concomitant diagnosis of preeclampsia, highlighting the significance of this diagnosis in twins. We found that more than half of severely morbid events occurred after delivery. Finally, hemorrhage was the most common cause of SMM in twins. Other common causes of SMM in this population were hypertensive, neurologic, and pulmonary complications.
Data on SMM using a case definition of SMM derived from chart review in twins, particularly from the United States, are limited.17,22 Our rate of SMM in this cohort (2.4%) is similar to the incidence (28 per 1,000) of SMM in a study of twins from the Netherlands that used a slightly different case definition for SMM.19 A large international study that used the World Health Organization definition of maternal near miss demonstrated rates of maternal near miss ranging from 1.2 to 1.9% in twins.18 It is important to note that our rate of 2.4% does not represent the true incidence of SMM in our cohort as we did not review the charts of all screen-negative deliveries. Prior studies indicate that due to coding errors and other data-related issues, the number of false negatives in any given cohort may be significant. The rate of SMM (2.4%) is significantly higher than the rate of SMM in singletons and is important to discuss with women carrying twins. Women with pregestational hypertension were more likely to have SMM.
Clinical and Research Implications
We are unaware of other studies that have examined the PPV of screening criteria for SMM in twins. Similar to studies in singletons, the PPV improved significantly when the transfusion indicator was removed from the CDC criteria; however, the improvement was much more notable than what is seen in singletons as transfusion of 1 unit of blood is relatively common in twins.23 This suggests that using the CDC criteria as a criterion to screen for SMM in twins has significant limitations. The poor PPV also places a significant burden on hospitals’ review boards as more than half of reviewed cases will be false positive and chart review is time-intensive and laborious. Finally, risk factors (i.e., parity, hypertension) between screen-positive cases and true cases differ, demonstrating the limitations of using screening definitions in epidemiologic studies.
Our cause and timing analysis highlights the importance of the postpartum period—specifically the first 24 hours after delivery—for morbidity in twins. While hemorrhage was an important cause of these morbidities, women also had hypertensive events, pulmonary events including venous thromboembolism, sepsis, and renal complications. It is imperative that clinicians are aware of this high-risk window so that adequate postpartum care can be provided to women. Dedicated high-risk postpartum units with lower nurse-to-patient ratios are one approach to address this.
Limitations
Our study has several limitations. While we are able to determine the PPV of common screening criteria, we did not review screen-negative cases for SMM. As mentioned above, a previous study that examined screen-negative cases had a negative predictive value of 99%.23 This suggests that we could have as many as 22 false negatives. Considering that SMM is rare, this would represent a sizable proportion of cases. Additionally, this is a delivery database and thus it does not accurately account for morbidities associated with postpartum readmissions. Given that women remain at risk for SMM after delivery—particularly hypertensive and embolic events—the total burden of morbidity in twin pregnancies is likely underestimated.
Conclusion
Our study adds to the body of literature that highlights the need for better ways to identify and track cases of SMM.10,15,16 It confirms that twins are a high-risk group with a rate of SMM that is likely twice that of singletons. Finally, it highlights that the postpartum period is a critical time for women’s health. Obstetrical teams must stay alert for complications in this period—particularly hemorrhage and progression of hypertensive disorders.
Supplementary Material
Key Points.
Screening approaches for SMM have low positive predictive value in twins.
Hemorrhage, hypertensive, and pulmonary complications were the most common morbidities.
SMM was most common postpartum.
Acknowledgments
Funding
This work was supported by NIH Grant (grant no.: R01 NR014245) to L.M.B. (PI) and K.P.H. (Co-I). The funding source had no role in the design of this study or manuscript preparation.
Footnotes
Conflict of Interest
None declared.
References
- 1.Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am 2018;45(02):175–186 [DOI] [PubMed] [Google Scholar]
- 2.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol 2017;130(02):366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. Accessed March 28, 2022 at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm
- 4.Say L, Souza JP, Pattinson RCWHO working group on Maternal Mortality and Morbidity classifications. Maternal near miss–towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009;23(03):287–296 [DOI] [PubMed] [Google Scholar]
- 5.Chen HY, Chauhan SP, Blackwell SC. Severe maternal morbidity and hospital cost among hospitalized deliveries in the United States. Am J Perinatol 2018;35(13):1287–1296 [DOI] [PubMed] [Google Scholar]
- 6.Campbell KH, Savitz D, Werner EF, et al. Maternal morbidity and risk of death at delivery hospitalization. Obstet Gynecol 2013;122(03):627–633 [DOI] [PubMed] [Google Scholar]
- 7.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120(05):1029–1036 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. Accessed March 1, 2022 at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html
- 9.Obstetric Care Consensus No 5 Summary: severe maternal morbidity: screening and review. Obstet Gynecol 2016;128(03):670–671 [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick SJ, Berg C, Bernstein P, et al. Standardized severe maternal morbidity review: rationale and process. Obstet Gynecol 2014;124(2, pt. 1):361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick SK, Ecker JL American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine. Severe maternal morbidity: screening and review. Am J Obstet Gynecol 2016;215(03):B17–B22 [DOI] [PubMed] [Google Scholar]
- 12.Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol 2014;123(05):978–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe A, Sheikh M, Hetherington E. Impact of the ICD-9-CM to ICD-10-CM transition on the incidence of severe maternal morbidity among delivery hospitalizations in the United States. Am J Obstet Gynecol 2021;225(04):422.e1–422.e11 [DOI] [PubMed] [Google Scholar]
- 14.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol 2008;199(02):133. e1–133.e8 [DOI] [PubMed] [Google Scholar]
- 15.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol 2016;214(05):643.e1–643.e10 [DOI] [PubMed] [Google Scholar]
- 16.Ozimek JA, Eddins RM, Greene N, et al. Opportunities for improvement in care among women with severe maternal morbidity. Am J Obstet Gynecol 2016;215(04):509.e1–509.e6 [DOI] [PubMed] [Google Scholar]
- 17.Wang ET, Ozimek JA, Greene N, et al. Impact of fertility treatment on severe maternal morbidity. Fertil Steril 2016;106(02):423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santana DS, Silveira C, Costa ML, et al. ; WHO Multi-Country Survey on Maternal and Newborn Health Research Network. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: evidence from the WHO Multicountry Survey on Maternal and Newborn Health. BMC Pregnancy Childbirth 2018;18(01):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witteveen T, Van Den Akker T, Zwart JJ, Bloemenkamp KW, Van Roosmalen J. Severe acute maternal morbidity in multiple pregnancies: a nationwide cohort study. Am J Obstet Gynecol 2016;214(05):641.e1–641.e10 [DOI] [PubMed] [Google Scholar]
- 20.Madar H, Goffinet F, Seco A, Rozenberg P, Dupont C, Deneux-Tharaux CEPIMOMS (EPIdémiologie de la MOrbidité Maternelle Sévère) Study Group. Severe acute maternal morbidity in twin compared with singleton pregnancies. Obstet Gynecol 2019;133(06):1141–1150 [DOI] [PubMed] [Google Scholar]
- 21.Luke B, Brown MB, Wantman E, et al. Risk of severe maternal morbidity by maternal fertility status: a US study in 8 states. Am J Obstet Gynecol 2019;220(02):195.e1–195.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belanoff C, Declercq ER, Diop H, et al. Severe maternal morbidity and the use of assisted reproductive technology in Massachusetts. Obstet Gynecol 2016;127(03):527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himes KP, Bodnar LM. Validation of criteria to identify severe maternal morbidity. Paediatr Perinat Epidemiol 2020;34(04):408–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


