Abstract
Context:
Over half of American adults are diagnosed with a chronic condition, with an increasing prevalence being diagnosed with multiple chronic conditions. These adults are at higher risk for having unrelieved, co-occurring symptoms, known as symptom clusters.
Objectives:
To identify symptom phenotypes of patients diagnosed with four common chronic conditions, specifically, cancer, chronic obstructive pulmonary disease, heart failure, and/or type 2 diabetes mellitus, and to understand factors that predict membership in symptomatic phenotypes.
Methods:
We conducted a retrospective, cross-sectional analysis using participant responses (N=14,127) to All of Us Research Program, a National Institutes of Health biomedical database, survey questions. We performed hierarchical clustering to generate symptom phenotypes of fatigue, emotional distress, and pain and used multinomial regression to determine if demographic, healthcare access and utilization, and health-related variables predict symptom phenotype.
Results:
Four phenotypes, one asymptomatic or mildly symptomatic and three highly symptomatic (characterized by severe symptoms, severe pain, and severe emotional distress), were identified. The percentage of participants belonging to the severe symptoms phenotype increased with the number of chronic conditions. Most notably, foregoing or delaying medical care and rating mental health as poor or fair increased the odds of belonging to a highly symptomatic phenotype.
Conclusion:
We found meaningful relationships between demographic, healthcare access and utilization, and health-related factors and symptom phenotypes. With the increasing trends of American adults with one or more chronic conditions and a demand to individualize care in the precision health era, it is critical to understand the factors that lead to unrelieved symptoms.
Keywords: Signs and symptoms, Cancer, Chronic obstructive pulmonary disease, Heart failure, Type 2 diabetes mellitus, Surveys and questionnaires
INTRODUCTION
In the United States, over half of American adults are diagnosed with a chronic condition, with an increasing prevalence of individuals diagnosed with multiple chronic conditions (1). American adults living with one or more chronic conditions experience a decrease in health-related quality of life and higher overall health-related costs (1,2). Furthermore, these individuals are at higher risk for having unrelieved co-occurring symptoms, known as symptom clusters, related to the chronic condition(s) and/or its treatment that may negatively impact daily life and functional status (3,4). Research suggests that symptom clusters drive both symptom burden and the treatment course across several chronic diseases including breast cancer (5), chronic kidney disease (6), and chronic obstructive pulmonary disease (COPD; 7).
Current trends in disease prevention, management, and treatment take into consideration individual variability in genes, environment, and lifestyle to tailor care to achieve optimal health outcomes; this model of healthcare is defined as precision medicine (8). In line with this model, the goal of symptom science is to identify individuals at risk for symptoms or symptom clusters and develop targeted strategies for preventing symptom occurrence and mitigating symptom severity and burden (4). While the patient symptom experience is highly heterogenous (9–15), prior research specifically aimed a delineating predictors of severe symptom phenotypes is limited, with a small number of studies investigating any particular predictor almost exclusively in cancer populations (16–20). Moreover, research on symptom clusters in individuals diagnosed with multiple chronic conditions, in general, is sparse (17,21,22). It is critical to better understand susceptibilities to symptoms and symptom clusters in individuals diagnosed with one or more chronic conditions.
Large biomedical datasets, like the All of Us Research Program, offer a unique opportunity to study symptom clusters and predictors of symptoms, especially in individuals diagnosed with multiple chronic conditions, at scale (23). The All of Us Research Program includes diverse participants diagnosed with a wide range of conditions allowing for investigation of symptom experiences across various wellness, social, environmental, and economic contexts. We believe that we can leverage All of Us data to generate new knowledge to tailor and improve patient symptom management.
Therefore, the purpose of this study was to identify symptom phenotypes (i.e., characterization of subjective symptom experience) of patients diagnosed with one or more common chronic conditions, specifically cancer, COPD, heart failure, and/or type 2 diabetes mellitus, using All of Us Research Program survey data. Further, we aimed to understand the demographic, healthcare access and utilization, and health-related factors that predict membership to highly symptomatic phenotypes.
METHODS
We conducted a retrospective, cross-sectional analysis using the All of Us Research Program survey data to identify symptom (i.e., fatigue, emotional distress, and pain) phenotypes of patients diagnosed with common chronic conditions (i.e., cancer, COPD, heart failure, and type 2 diabetes) and demographic, healthcare access and utilization, and health-related factors that predict membership to symptomatic phenotypes. This study was exempt from human subjects’ approval as only deidentified data were analyzed.
All of Us Research Program
The All of Us Research Program (allofus.nih.gov) is an effort by the National Institutes of Health to gather health data from one million or more diverse individuals living in the United States to accelerate research discoveries that may improve human health. To date, over 315,000 participants have completed the initial steps of the program, which included agreeing to share electronic health records; completing The Basics, Overall Health, and Lifestyle surveys; providing physical measurements; and donating at least one biospecimen. Approved researchers can access the Registered Tier curated dataset of de-identified, individual level electronic health record, survey, physical measurement, and wearable data through the All of Us Research Hub (researchallofus.org).
Cohort Identification
First, we used the Cohort Builder within the All of Us Researcher Workbench to identify all eligible adult (≥18 years of age) participants (n=65,658) diagnosed with cancer (excluding skin and in situ cancer; n=23,983), COPD (including bronchitis and emphysema; n=18,154), heart failure (n=14,449), or type 2 diabetes mellitus (n=35,140) based on condition codes detailed in Appendix A. Then, we limited inclusion to participants who had two or more qualifying codes for a condition and complete responses for the three symptom-related (i.e., fatigue, emotional distress, and pain) items on the Overall Health survey on or after the earliest documented condition start date (n=45,907). We further limited inclusion to participants who answered one or more questions on each of The Basics, Overall Health (beyond the symptom items of interest), and Health Care Access & Utilization surveys (N=14,127).
Measures
We used participant responses to survey questions to generate symptom phenotypes and analyze potential predictors of symptomatic phenotypes. Three individual survey questions were used to evaluate participant symptoms of pain, fatigue, and emotional distress, respectively: 1) In the past 7 days, how would you rate your pain on average? Response – numeric scale, 0 (no pain) to 10 (worst imaginable pain); 2) In the past 7 days, how would you rate your fatigue? Response – 5-point Likert scale (none, mild, moderate, severe, very severe); and 3) In the past 7 days, how often have you been bothered by emotional problems such as feeling anxious, depressed or irritable? Response – 5-point Likert scale (never, rarely, sometimes, often, always) (24,25). We recoded responses for fatigue and emotional distress to a numeric 0–4 scale, with 4 indicating the worst symptom experience, for analysis purposes. Participants completed symptom questions between June 2016 and July 2020, with all dates systematically shifted backwards by a random number between 1 and 365 by the All of Us Research Program to minimize re-identification risks.
Predictors included demographic, healthcare access and utilization, and health-related variables. For demographics, we included age, gender identity, race/ethnicity, marital status, level of education, insurance status, employment status, and annual household income as potential predictors (26).
Healthcare access and utilization predictors included foregoing and/or delaying medical care, having a usual place for medical care, and time since last seen a health care professional (About how long has it been since you last saw or talked to a doctor or other health care provider about your own health?). We defined foregoing and/or delaying medical care as answering “yes” to any of the following three questions: 1) Have you delayed getting care for any of the following reasons in the past 12 months?; 2) During the past 12 months, was there any time when you needed any of the following, but didn’t get it because you couldn’t afford it?; and 3) During the past 12 months, were any of the following true for you? (27–29). We defined not having a usual place for medical care as answering “no” or “don’t know” to the question Is there a place that you usually go to when you are sick or need advice about your health? or answering “yes” but indicating going to any location other than a doctor’s office, clinic, or health center most often. Participants who endorsed having “more than one place” were coded as having a usual place for medical care (27–30).
Further, we included three 4-point Likert survey questions that assessed ease of understanding health information (How often did your doctors or health care providers tell or give you information about your health and health care that was easy to understand?), being treated with respect by the provider (How often were you treated with respect by your doctors or health care providers?), and being asked about opinions or beliefs by the provider (How often did your doctors or health care providers ask for your opinions or beliefs about your medical care or treatment?) (27,29). Response options were always, most of the time, some of the time, or none of the time.
Three 5-point Likert scale survey questions were used to evaluate participant general, physical, and mental health, respectively: In general, 1) would you say your health is:; 2) how would you rate your physical health?; and 3) how would you rate your mental health, including your mood and your ability to think? (24,25). Response options were excellent, very good, good, fair, or poor.
Data Analysis
All analyses were performed on All of Us data release version 4.0 within the secure Researcher Workbench platform using R (version 4.1.1). First, we generated descriptive statistics and data visualizations to characterize all variables. Because of the substantial number of participants who did not provide responses for the Health Care Access & Utilization survey, we compared demographic and health-related variables between participants with and without these data, using a t-test for age and chi-square tests for all other variables.
Then, we performed hierarchical clustering with Gower’s distance (due to ordinality of the symptom survey items) on participant fatigue, emotional distress, and pain scores to create symptom phenotypes. The optimal number of clusters was determined based on the dendrogram. We calculated the percentage of participants that belong to a particular symptom phenotype by chronic condition diagnosis. Finally, we used multinomial regression to determine if demographic, healthcare access and utilization, and health-related variables predict symptom phenotype. Please note that “skipped” is distinct from missing and was included as a response category for many variables. Coefficients were analyzed at a 0.05 significance level.
RESULTS
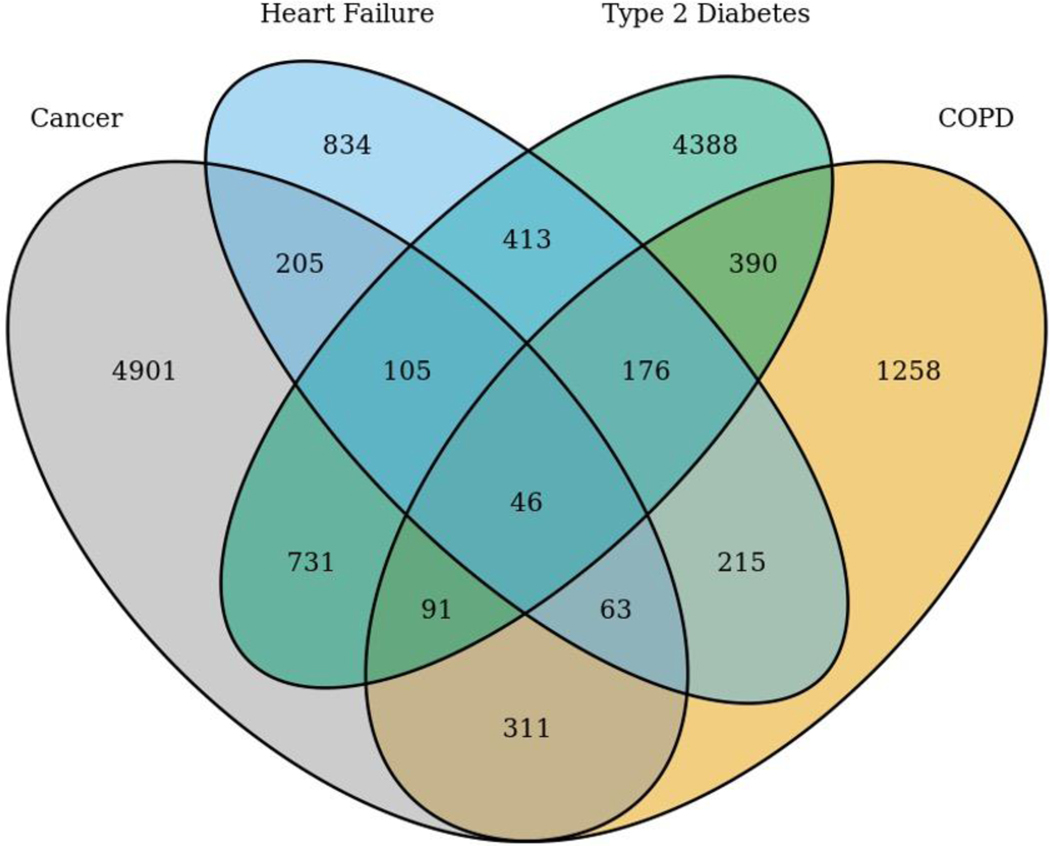
Participants (N=14,127; Figure 1) were approximately 62 years of age and primarily women, White non-Hispanic, married, highly educated, not employed, and insured (Table 1). Compared to participants who completed the Health Care Access & Utilization survey, participants who did not complete this survey (n=31,780) were younger; were less likely to be White non-Hispanic, married, graduate from college or high school, and employed; have lower household income; and reported poorer health (Table 1). The time between the earliest recorded condition start date for a participant’s earliest recorded condition and response to symptom survey items was positively skewed with a mean of 5.41 (SD=5.20) years and median of 3.66 (minimum=0; Q1=1.82, Q3=7.48, maximum=36.98) years.
Figure 1.
Chronic condition diagnoses in study participant cohort (N=14,127).
Table 1.
Comparison of demographic- and health-related variables between participants with and without Health Care Access & Utilization survey information
| With information | Without information | |
|---|---|---|
| (N=14,127) | (N=31,780) | |
|
|
||
| Mean (SD) or n (%) | Mean (SD) or n (%) | |
|
| ||
| Age (years) | 62.32 (12.22) | 60.24 (13.01) |
| Gender identity | ||
| Woman | 8,280 (58.61) | 17,877 (56.25) |
| Man | 5,692 (40.29) | 13,301 (41.85) |
| Other/skipped | 155 (1.10) | 602 (1.89) |
| Race/ethnicity | ||
| Asian/non-Hispanic | 238 (1.71) | 588 (1.88) |
| Black/non-Hispanic | 1,588 (11.39) | 8,535 (27.29) |
| White/non-Hispanic | 10,586 (75.92) | 14,289 (45.69) |
| Hispanic/any race | 956 (6.86) | 6,262 (20.02) |
| Other | 352 (2.52) | 925 (2.96) |
| Skipped | 223 (1.60) | 675 (2.16) |
| Marital status | ||
| Married | 7,813 (55.31) | 12,039 (37.88) |
| Divorced | 2,254 (15.96) | 6,436 (20.25) |
| Cohabiting | 524 (3.84) | 1,248 (3.93) |
| Never married | 1,957 (13.85) | 6,413 (20.18) |
| Separated | 297 (2.10) | 1,549 (4.87) |
| Widowed | 1,125 (7.96) | 3,206 (10.09) |
| Skipped | 139 (0.98) | 889 (2.80) |
| Education level | ||
| College graduate or > | 7,708 (54.56) | 8,712 (27.41) |
| 1–3 years of college | 3,950 (27.96) | 9,053 (28.49) |
| High school or GED | 1,770 (12.53) | 8,162 (25.68) |
| < High school degree | 589 (4.17) | 5,001 (15.74) |
| Skipped | 110 (0.78) | 852 (2.68) |
| Insurance | ||
| Yes | 13,685 (97.78) | 28,575 (95.83) |
| No | 311 (2.22) | 1,244 (4.17) |
| Employment status | ||
| Employed | 4,966 (35.52) | 7,301 (24.62) |
| Not employed | 9,016 (64.48) | 22,357 (75.38) |
| Annual household income | ||
| < $10,000 | 975 (6.90) | 5,877 (18.49) |
| $10,000 - $25,000 | 1,717 (12.15) | 5,691 (17.91) |
| $25,000 - $35,000 | 1,045 (7.40) | 2,381 (7.49) |
| $35,000 - $50,000 | 1,291 (9.14) | 2,218 (6.98) |
| $50,000 - $75,000 | 1,968 (13.93) | 2,548 (8.02) |
| $75,000 - $100,000 | 1,576 (11.16) | 1,584 (4.98) |
| $100,000 - $150,000 | 1,933 (13.68) | 1,700 (5.35) |
| $150,000 - $200,000 | 857 (6.07) | 616 (1.94) |
| > $200,000 | 1,213 (8.59) | 848 (2.67) |
| Skipped | 1,552 (10.99) | 8,317 (26.17) |
| General health | ||
| Poor | 790 (5.59) | 3,892 (12.25) |
| Fair | 3,365 (23.82) | 10,671 (33.58) |
| Good | 5,217 (36.93) | 10,765 (33.87) |
| Very good | 3,885 (27.50) | 4,964 (15.62) |
| Excellent | 798 (5.65) | 1,179 (3.71) |
| Skipped | 72 (0.51) | 309 (0.97) |
| Physical health | ||
| Poor | 909 (6.43) | 3,737 (11.76) |
| Fair | 3,563 (25.22) | 10,855 (34.16) |
| Good | 5,241 (37.10) | 10,913 (34.34) |
| Very good | 3,603 (25.50) | 4,580 (14.41) |
| Excellent | 716 (5.07) | 1,121 (3.53) |
| Skipped | 95 (0.67) | 574 (1.81) |
| Mental health | ||
| Poor | 286 (2.02) | 1,301 (4.09) |
| Fair | 1,584 (11.21) | 5,668 (17.84) |
| Good | 3,586 (25.38) | 10,094 (31.76) |
| Very good | 5,049 (35.74) | 8,367 (26.33) |
| Excellent | 3,540 (25.06) | 5,951 (18.73) |
| Skipped | 82 (0.58) | 399 (1.26) |
| Cancer | ||
| Yes | 6,453 (45.68) | 9,606 (30.23) |
| No | 7,674 (54.32) | 22,174 (69.77) |
| COPD | ||
| Yes | 2,550 (18.05) | 7,943 (24.99) |
| No | 11,577 (81.95) | 23,837 (75.01) |
| Heart Failure | ||
| Yes | 2,057 (14.56) | 6,813 (21.44) |
| No | 12,070 (85.44) | 24,967 (78.56) |
| Type 2 Diabetes Mellitus | ||
| Yes | 6,340 (44.88) | 18.040 (56.77) |
| No | 7,787 (55.12) | 13,740 (43.23) |
| Number of conditions | ||
| 1 | 11,381 (80.56) | 23,276 (73.24) |
| 2 | 2,265 (16.03) | 6,606 (20.79) |
| 3 | 435 (3.08) | 1,678 (5.28) |
| 4 | 46 (0.33) | 220 (0.69) |
Note. All p-values <0.001. COPD=chronic obstructive pulmonary disease.
Symptom Phenotypes
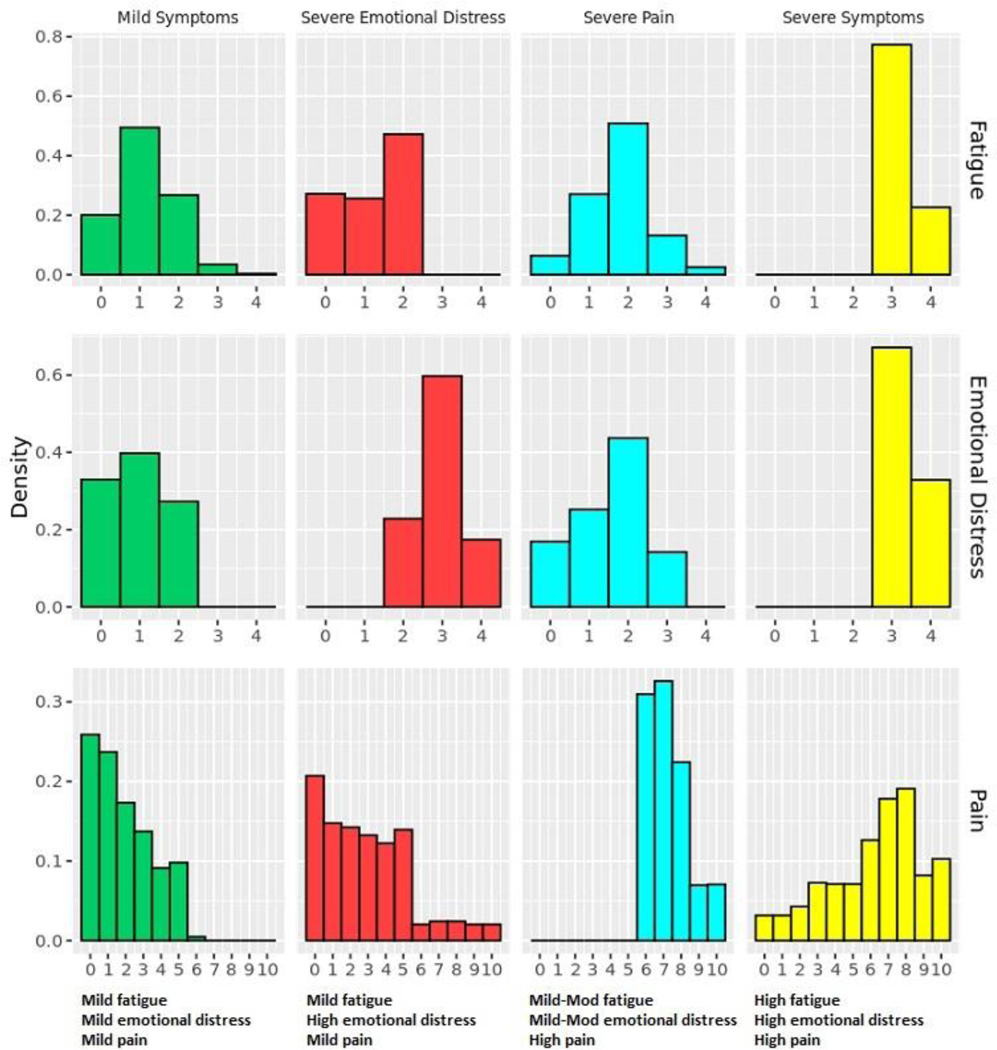
Four phenotypes, one asymptomatic or mildly symptomatic and three highly symptomatic, were identified (Figure 2). The “mild symptoms” phenotype contains n=9,961 participants and is characterized by minimal fatigue, infrequent emotional distress, and mild pain. Within the “mild symptoms” phenotype, approximately 7% (n=659) of participants denied all three symptoms. In contrast, the “severe symptoms” phenotype, which contains n=618 participants, is characterized by moderate to severe pain, severe fatigue, and frequent emotional distress. The “severe emotional distress” phenotype (n=972) is characterized by frequent emotional distress, no to moderate fatigue, and mild pain. The “severe pain” phenotype (n=2,576) is characterized by severe pain, mild to moderate fatigue, and infrequent emotional distress. By individual chronic condition (Table 2), a higher percentage of participants diagnosed with cancer (78%) belong to the “mild symptoms” phenotype than the other three conditions (62– 69%). Likewise, a lower percentage of participants diagnosed with cancer belong to the “severe pain” (12%) and “severe symptoms” (3%) phenotypes than the other three conditions (22–25% and 5–6%, respectively). The percentage of participants that belong to the “severe symptoms” phenotype increases with the number of chronic conditions. Approximately 3.96% of participants diagnosed with one condition belong to this phenotype, compared to 5.83% of participants with two conditions, 7.13% of participants with three conditions, and 8.70% of participants with all four conditions of interest.
Figure 2.
Chronic condition symptom phenotypes. The x-axis represents the survey response scale for each symptom. The higher the value, the more severe the symptom. Mod=moderate.
Table 2.
Percentage of participants belonging to a symptom phenotype by chronic condition diagnosis
| Symptom Phenotype | ||||
|---|---|---|---|---|
|
|
||||
| % | Mild symptoms | Severe emotional distress | Severe pain | Severe symptoms |
|
| ||||
| Cancer | 78.0 | 6.7 | 12.4 | 2.9 |
| COPD | 61.9 | 6.7 | 25.2 | 6.2 |
| Heart failure | 68.6 | 4.7 | 21.8 | 4.9 |
| Type 2 diabetes mellitus | 64.0 | 7.1 | 22.8 | 6.0 |
Note. Percentages add to 100% row-wise across the chronic conditions. Participants can be diagnosed with multiple chronic conditions. COPD=chronic obstructive pulmonary disease.
Demographic, Healthcare Access and Utilization, and Health-related Factors
Results from the symptom phenotype multinominal regression model are presented in Table 3. Only participants with complete data (n=13,585) were included in the model. In all reported results, the “mild symptoms” phenotype serves as the reference phenotype.
Table 3.
Odds Ratios for Demographic, Healthcare Access and Utilization, and Health-related Factors From the Symptom Phenotype Multinomial Regression Model.
| Phenotypea |
||||||
|---|---|---|---|---|---|---|
| Severe Emotional Distress |
Severe Pain |
Severe Symptoms |
||||
| OR (95 CI) | P-value | OR (95 CI) | P-value | OR (95 CI) | p-value | |
|
| ||||||
| Demographic factors | ||||||
| Age (yrs) | 0.98 (0.97–0.98) | <0.001b | 0.99 (0.99–0.995) | <0.001b | 0.97 (0.96 – 0.98) | <0.001b |
| Gender identity | ||||||
| Woman | 1.08 (0.92–1.27) | 0.325 | 1.47 (1.32–1.64) | <0.001b | 1.64 (1.30 – 2.07) | <0.001b |
| Other/skipped | 1.51 (0.81–2.81) | 0.196 | 1.51 (0.94–2.43) | 0.091 | 0.72 (0.27 – 1.94) | 0.514 |
| Man (reference) | ||||||
| Race/ethnicity | ||||||
| Asian/non-Hispanic | 0.55 (0.29–1.06) | 0.074 | 1.23 (0.83–1.81) | 0.298 | 0.64 (0.25 – 1.63) | 0.351 |
| Black/non-Hispanic | 0.83 (0.64–1.07) | 0.152 | 1.42 (1.22–1.65) | <0.001b | 1.16 (0.87 – 1.56) | 0.305 |
| Hispanic/any race | 0.98 (0.73–1.32) | 0.905 | 1.39 (1.15–1.68) | 0.001b | 1.32 (0.95 – 1.85) | 0.103 |
| Other | 0.92 (0.57–1.49) | 0.747 | 1.37 (1.02–1.85) | 0.038b | 1.22 (0.67 – 2.22) | 0.517 |
| Skipped | 1.17 (0.65–2.14) | 0.599 | 1.05 (0.69–1.61) | 0.829 | 0.70 (0.23 – 2.10) | 0.519 |
| White/non-Hispanic (reference) | ||||||
| Marital status | ||||||
| Divorced | 1.25 (1.00–1.56) | 0.048b | 1.17 (1.01–1.35) | 0.039b | 1.15 (0.86 – 1.53) | 0.338 |
| Cohabiting | 0.99 (0.67–1.46) | 0.938 | 1.30 (1.01–1.66) | 0.041b | 0.73 (0.42 – 1.26) | 0.256 |
| Never married | 1.18 (0.94–1.47) | 0.156 | 1.07 (0.91–1.25) | 0.404 | 0.78 (0.58 – 1.06) | 0.118 |
| Separated | 0.88 (0.52–1.50) | 0.640 | 1.29 (0.94–1.77) | 0.113 | 1.11 (0.66 – 1.87) | 0.698 |
| Widowed | 1.36 (1.02–1.82) | 0.037b | 0.97 (0.80–1.18) | 0.788 | 1.06 (0.70 – 1.60) | 0.793 |
| Skipped | 1.08 (0.48–2.43) | 0.851 | 1.55 (0.95–2.51) | 0.077 | 1.49 (0.63 – 3.52) | 0.360 |
| Married (reference) | ||||||
| Education level | ||||||
| 1–3 years of college | 0.73 (0.60–0.88) | 0.001b | 1.36 (1.20–1.53) | <0.001b | 1.21 (0.94 – 1.55) | 0.146 |
| High school or GED | 0.94 (0.74–1.20) | 0.605 | 1.58 (1.36–1.85) | <0.001b | 1.09 (0.80 – 1.50) | 0.574 |
| < High school degree | 0.90 (0.62–1.32) | 0.600 | 1.37 (1.07–1.76) | 0.013b | 0.82 (0.52 – 1.28) | 0.378 |
| Skipped | 1.05 (0.45–2.47) | 0.915 | 1.17 (0.66–2.09) | 0.586 | 2.13 (0.85 – 5.35) | 0.107 |
| College graduate or > (reference) | ||||||
| Insurance | ||||||
| No | 1.45 (0.94–2.24) | 0.091 | 0.94 (0.69–1.29) | 0.712 | 1.43 (0.88 – 2.32) | 0.151 |
| Yes (reference) | ||||||
| Employment status | ||||||
| Not employed | 0.96 (0.80–1.14) | 0.619 | 1.53 (1.35–1.73) | <0.001b | 1.52 (1.17 – 1.97) | 0.002b |
| Employed (reference) | ||||||
| Annual household income | ||||||
| < $10,000 | 1.40 (0.98–2.02) | 0.067 | 2.19 (1.72–2.78) | <0.001b | 2.09 (1.32 – 3.32) | 0.002b |
| $10,000–$25,000 | 1.18 (0.88–1.59) | 0.276 | 1.59 (1.31–1.94) | <0.001b | 1.61 (1.07 – 2.44) | 0.024b |
| $25,000–$35,000 | 1.05 (0.76–1.47) | 0.759 | 1.32 (1.06–1.64) | 0.013b | 1.68 (1.07 – 2.63) | 0.025b |
| $35,000–$50,000 | 1.16 (0.85–1.59) | 0.339 | 1.18 (0.95–1.45) | 0.135 | 1.61 (1.01 – 2.56) | 0.045b |
| $75,000–$100,000 | 0.95 (0.69–1.31) | 0.758 | 1.23 (0.99–1.51) | 0.057 | 1.12 (0.66 – 1.90) | 0.680 |
| $100,000–$150,000 | 0.92 (0.69–1.25) | 0.600 | 0.93 (0.75–1.15) | 0.484 | 1.02 (0.61 – 1.71) | 0.941 |
| $150,000–$200,000 | 1.00 (0.69–1.46) | 0.998 | 0.87 (0.64–1.18) | 0.367 | 1.22 (0.59 – 2.54) | 0.595 |
| > $200,000 | 1.19 (0.85–1.68) | 0.318 | 0.88 (0.67–1.17) | 0.372 | 0.57 (0.22 – 1.45) | 0.235 |
| Skipped | 1.29 (0.95–1.77) | 0.103 | 1.54 (1.26–1.88) | <0.001b | 1.85 (1.17 – 2.92) | 0.008b |
| $50,000–$75,000 (reference) | ||||||
| Healthcare access and utilization factors | ||||||
| Foregoing or delaying medical care | ||||||
| Yes | 1.30 (1.10–1.53) | 0.002b | 1.32 (1.18–1.46) | <0.001b | 1.68 (1.31 – 2.16) | <0.001b |
| Skipped | 2.04 (0.24–17.12) | 0.512 | 4.33 (1.22–15.30) | 0.023b | 21.95 (2.24 – 215.30) | 0.008b |
| No (reference) | ||||||
| Usual place for medical care | ||||||
| No | 1.08 (0.86–1.37) | 0.502 | 1.15 (0.98–1.34) | 0.082 | 1.29 (0.97 – 1.72) | 0.080 |
| Skipped | 2.30 (0.41–12.97) | 0.344 | 1.63 (0.49–5.44) | 0.427 | 0.01 (0.01 – 0.01) | <0.001b |
| Yes (reference) | ||||||
| Last seen by health care professional | ||||||
| 6 months–1 year | 0.88 (0.63–1.24) | 0.474 | 0.83 (0.64–1.08) | 0.161 | 0.81 (0.46 – 1.44) | 0.473 |
| > 1 year | 0.002 (0.002–0.002) | <0.001b | 1.02 (0.36–2.90) | 0.975 | 3.24 (0.72 – 14.57) | 0.125 |
| Skipped/Don’t know | 0.66 (0.28–1.52) | 0.325 | 1.21 (0.75–1.93) | 0.435 | 1.45 (0.62 – 3.43) | 0.393 |
| ≤6 months (reference) | ||||||
| Ease of understanding health information | ||||||
| Most of the time | 0.86 (0.72–1.03) | 0.099 | 1.03 (0.91–1.17) | 0.612 | 0.97 (0.76 – 1.24) | 0.808 |
| Some of the time | 0.99 (0.69–1.40) | 0.937 | 1.37 (1.08–1.74) | 0.011b | 1.23 (0.81 – 1.87) | 0.333 |
| None of time | 0.80 (0.30–2.15) | 0.664 | 1.02 (0.52–1.98) | 0.964 | 1.11 (0.36 – 3.39) | 0.860 |
| Skipped/Don’t know | 0.94 (0.50–1.78) | 0.852 | 0.84 (0.54–1.31) | 0.445 | 0.70 (0.27 – 1.82) | 0.469 |
| Always (reference) | ||||||
| Treated with respect by the provider | ||||||
| Most of the time | 1.06 (0.87–1.30) | 0.572 | 1.13 (0.99–1.30) | 0.082 | 1.18 (0.91 – 1.53) | 0.210 |
| Some of the time | 1.01 (0.63–1.61) | 0.974 | 1.27 (0.92–1.75) | 0.150 | 1.17 (0.70 – 1.95) | 0.542 |
| None of time | 5.82 (1.06–31.90) | 0.042b | 1.56 (0.37–6.63) | 0.547 | 0.04 (0.04 – 0.04) | <0.001b |
| Skipped/Don’t know | 0.89 (0.43–1.84) | 0.745 | 1.16 (0.75–1.79) | 0.494 | 0.63 (0.24 – 1.62) | 0.336 |
| Always (reference) | ||||||
| Asked opinion/beliefs by provider | ||||||
| Most of the time | 0.86 (0.70–1.06) | 0.161 | 0.78 (0.68–0.89) | <0.001b | 0.90 (0.68 – 1.19) | 0.453 |
| Some of the time | 0.88 (0.70–1.09) | 0.242 | 0.69 (0.59–0.80) | <0.001b | 0.71 (0.52 – 0.97) | 0.029b |
| None of time | 1.19 (0.92–1.54) | 0.191 | 0.73 (0.60–0.87) | 0.001b | 0.87 (0.60 – 1.27) | 0.476 |
| Skipped/Don’t know | 1.56 (1.00–2.45) | 0.0497b | 0.99 (0.72–1.35) | 0.939 | 0.85 (0.43 – 1.68) | 0.644 |
| Always (reference) | ||||||
| Health-related factors | ||||||
| General health | ||||||
| Poor | 0.62 (0.39–0.99) | 0.045b | 1.62 (1.22–2.16) | 0.001b | 2.96 (1.90 – 4.62) | <0.001b |
| Fair | 0.98 (0.76–1.24) | 0.837 | 1.16 (1.00–1.36) | 0.052 | 1.51 (1.10 – 2.08) | 0.012b |
| Very good | 1.13 (0.84–1.52) | 0.414 | 0.88 (0.72–1.08) | 0.232 | 0.30 (0.12 – 0.78) | 0.013b |
| Excellent | 1.03 (0.56–1.91) | 0.924 | 0.64 (0.39–1.03) | 0.065 | 0 (0 – 0) | <0.001b |
| Skipped | 1.07 (0.38–3.00) | 0.893 | 0.94 (0.44–1.98) | 0.862 | 1.17 (0.24 – 5.73) | 0.850 |
| Good (reference) | ||||||
| Physical health | ||||||
| Poor | 1.92 (1.27–2.90) | 0.002b | 2.53 (1.92–3.33) | <0.001b | 4.18 (2.67 – 6.56) | <0.001b |
| Fair | 1.12 (0.88–1.42) | 0.374 | 1.68 (1.44–1.95) | <0.001b | 2.07 (1.48 – 2.91) | <0.001b |
| Very good | 1.76 (1.30–2.38) | <0.001b | 0.65 (0.52–0.80) | <0.001b | 0.83 (0.32 – 2.15) | 0.704 |
| Excellent | 2.11 (1.10–4.02) | 0.024b | 0.52 (0.32–0.87) | 0.013b | 1.74 (0.22 – 13.85) | 0.601 |
| Skipped | 0.82 (0.27–2.45) | 0.718 | 1.42 (0.82–2.45) | 0.214 | 1.43 (0.43 – 4.75) | 0.558 |
| Good (reference) | ||||||
| Mental health | ||||||
| Poor | 24.10 (14.05–41.34) | <0.001b | 3.64 (2.10–6.31) | <0.001b | 39.79 (22.86 – 69.24) | <0.001b |
| Fair | 4.73 (3.88–5.78) | <0.001b | 1.89 (1.60–2.23) | <0.001b | 6.44 (4.99 – 8.33) | <0.001b |
| Very good | 0.29 (0.24–0.36) | <0.001b | 0.80 (0.70–0.91) | 0.001b | 0.35 (0.24 – 0.52) | <0.001b |
| Excellent | 0.10 (0.07–0.13) | <0.001b | 0.86 (0.74–1.00) | 0.057 | 0.16 (0.08 – 0.33) | <0.001b |
| Skipped | 0.73 (0.28–1.87) | 0.505 | 1.27 (0.68–2.37) | 0.456 | 5.63 (2.35 – 13.53) | <0.001b |
| Good (reference) | ||||||
Note. OR=odds ratio; 95CI=95% confidence interval.
The “mild symptoms” phenotype is the reference phenotype.
P<0.05
Demographic Factors
In terms of demographic variables, increasing age was associated with lower odds of being in any one of the three highly symptomatic phenotypes (OR=0.97–0.99; all p<0.001). The odds of belonging to the “severe symptoms” or “severe pain” phenotypes were higher for women (OR=1.64, p<0.001; OR=1.47, p<0.001), participants who are not employed (OR=1.52, p=0.002; OR=1.53, p<0.001), and those reporting lower annual household incomes (see Table 3). In addition, describing oneself as Black race/non-Hispanic ethnicity (OR=1.42, p<0.001), Hispanic ethnicity (OR=1.39, p=0.001), or other race/ethnicity (OR=1.37, p=0.038) compared to White race/non-Hispanic ethnicity; being divorced (OR=1.17, p=0.039) or cohabitating (OR=1.30, p=0.041) compared to being married; and earning less than a college degree (see Table 3) increased the odds of belonging to the “severe pain” phenotype. Marital status (divorced vs. married: OR=1.25, p=0.048; widowed vs. married: OR=1.36, p=0.037) and level of education (1–3 years of college vs. college graduate or greater: OR=0.73, p=0.001) were also associated with membership in the “severe emotional distress” phenotype.
Healthcare Access and Utilization Factors
For healthcare access and utilization variables, the odds of belonging to a highly symptomatic phenotype were greater for participants who had foregone or delayed medical care (OR=1.30–1.68, p<0.001–0.002). The odds of belonging to the “severe pain” (OR=4.33, p=0.023) or “severe symptoms” (OR=21.95, p=0.008) phenotypes were also higher for participants who skipped this question. The odds of belonging to the “severe emotional distress” phenotype were lower for participants who reported that they had not seen a health care professional in over a year compared to the past six months (OR=0.002, p<0.001). Having a usual place for medical care was not associated with symptom phenotype membership; however, the odds of belonging to the “severe symptoms” phenotype were lower for participants who skipped this question compared to participants who positively endorsed having a usual place for medical care (OR=0.01, p<0.001).
Being asked about opinions or beliefs about medical care by the provider most of the time (OR=0.78, p<0.001), some of the time (OR=0.69, p<0.001), or none of the time (OR=0.73, p=0.001) decreased the odds of belonging to the “severe pain” phenotype compared to always being asked. Being asked about opinions or beliefs some of the time also decreased the odds of belonging to the “severe symptoms” phenotype (OR=0.71, p=0.029). Being treated with respect by the provider none of the time increased the odds of belonging to the “severe emotional distress” phenotype (OR=5.82, p=0.042) and decreased the odds of belonging to the “severe symptoms” phenotype (OR=0.04, p<0.001). In addition, being told or given information about health that was easy to understand some of the time increased the odds of belonging to the “severe pain” phenotype (OR=1.37, p=0.011).
Health-related Factors
Notably, the odds of belonging to any of the highly symptomatic phenotypes were higher for participants who rated their mental health as poor (OR=3.64–39.79, all p<0.001) or fair (OR=1.89–6.44, all p<0.001) and lower for participants who rated their mental health as very good (OR=0.29–0.80, p<0.001–0.001) or excellent (OR=0.10–0.86, p<0.001–0.057; please note, rating mental health as excellent for the “severe pain” phenotype did not reach statistical significance but trended in the lower odds direction) compared to good. Similarly, the odds of belonging to the “severe symptoms” or “severe pain” phenotypes were higher for participants who rated their general or physical health as poor (OR=1.62–4.18, p<0.001–0.001) or fair (OR=1.16–2.07, p<0.001–0.052; please note, rating general health as fair for the “severe pain” phenotype did not reach statistical significance but trended in the higher odds direction) compared to good. Moreover, very good (OR=0.30, p=0.013) and excellent (OR=0, p<0.001) general health lowered the odds of belonging to the “severe symptoms” phenotype, while very good (OR=0.65, p<0.001) and excellent (OR=0.52, p=0.013) physical health lowered the odds of belonging to the “severe pain” phenotype. The influence of general health and physical health ratings on the “severe emotional distress” phenotype was remarkably different, with poor general health (OR=0.62, p=0.045) decreasing and very good (OR=1.76, p<0.001) and excellent (OR=2.11, p=0.024) physical health increasing the odds of membership.
DISCUSSION
Our analysis illustrates the utility of the All of Us Research Program dataset to advance symptom science research in individuals diagnosed with one or more chronic conditions. Using survey responses for fatigue, emotional distress, and pain, we identified four distinct symptom phenotypes – asymptomatic or mild symptoms, severe emotional distress, severe pain, and severe symptoms – in participants diagnosed with cancer, COPD, heart failure, and/or diabetes. The number of participants that belong to the “severe symptoms” phenotype increases in a direct relationship with the number of chronic conditions. We, further, discovered meaningful relationships between the identified symptom phenotypes and demographic, healthcare access and utilization, and health-related factors.
Most notably, we found that individuals who rated their mental health as poor or fair had significantly higher odds of belonging to one of the highly symptomatic phenotypes compared to those who rated their mental health as good. Likewise, individuals who rated their mental health as very good or excellent had lower odds of belonging to a highly symptomatic phenotype. While poor mental health may be related to emotional distress, relationships were not limited to the severe emotional distress phenotype. These findings support a growing body of literature which suggests a complex, bidirectional relationship between psychological and physical symptoms in chronic conditions (13,31–33). Given this relationship of mental health with not only the severe emotional distress phenotype but the severe pain and severe symptoms phenotypes as well, mental health interventions such as cognitive behavioral strategies (13,34,35), collaborative care models for mental health and primary care providers (36), and use of telehealth to expand access to mental health services (37,38) should be explored as methods of symptom management in individuals with chronic conditions.
Consistent with previous research (28), we found that foregoing or delaying medical care was a key factor in unmet health care needs, increasing the likelihood of an individual belonging to one of the phenotypes characterized by severe, unrelieved symptoms. While our analysis did not include information on individuals’ reasons for delaying or foregoing medical care, identifying and remedying systemic or interpersonal barriers to receiving care may be a beneficial next step in addressing severe symptom phenotypes. Our analysis also revealed thought-provoking findings related to patient-provider interaction and communication. Specifically, individuals who reported never being treated with respect by a provider had increased odds of belonging to the severe emotional distress phenotype but decreased odds of belonging to the severe symptoms phenotype compared to individuals who reported always being treated with respect. However, caution must be exercised in interpretation of results generated from small numbers of participants. Furthermore, being asked for opinions or beliefs at any frequency other than always decreased the odds of belonging to the severe pain phenotype. This may be because participants who feel ignored may be less likely to feel comfortable reporting the true experience of their symptoms.
The observed relationships between healthcare access and utilization and the presence of highly symptomatic phenotypes have the potential to guide intervention. While expansion of health insurance and programs to support cost-saving are important to increasing healthcare utilization, simple interventions aimed at clinicians could support decreased symptom burden. Existing literature suggests that breakdowns in patient-provider communication, including feeling dismissed or the lack of an open relationship, can negatively impact the management of chronic conditions (39,40). How these aspects of patient-provider interaction and communication impact symptom management and subsequent outcomes requires further investigation.
Characterization of the relationship between symptom phenotypes and the patient-provider relationship may be a critical step in addressing racial disparities in symptom burden. In line with prior literature (41), we found that those who report their race/ethnicity as Black/non-Hispanic, Hispanic/any race, or other have a higher risk of experiencing more severe symptom phenotypes compared to individuals who report their race/ethnicity as White/non-Hispanic. Given our results relating foregoing or delaying medical care and patient-provider interaction and communication to symptomatic phenotypes, lack of care and/or suboptimal patient-provider interaction and communication may be contributing to disparities in symptom outcomes. Some research shows that racial concordance between the patient and physician can impact communication (42). Additionally, evidence suggests that failure to attend to culturally specific and informational needs in patients who belong to historically marginalized groups can lead to lower trust and harm the patient-provider relationship (43). While research in this area is sparse, it has the potential to move symptom science from the characterization of disparities to potential interventions targeting patient-provider interaction and communication. This line of investigation would complement research on the reduction of systemic inequities to address racism in healthcare at both a systemic and interpersonal level. Interpretation of these results in a way that identifies actionable targets is supported by Critical Race Theory, which emphasizes a movement from characterization of disparities to identifying potential mechanisms of eliminating them (44). Incorporation of additional surveys within the All of Us Research Program capturing more encompassing social determinants of health may allow for future analysis addressing these issues more thoroughly.
There are several limitations to our analysis using the All of Us Research Program data. First, we focused on four common chronic conditions. Generalizability of our findings to other chronic conditions is unknown. Second, because the Health Care Access & Utilization survey is not required for enrollment, there may be differences in participants who completed the survey and those who did not, again, potentially impacting the generalizability of results. In addition, geographical factors (i.e., urban versus rural, proximity to academic medical center) were not available for participants. Third, the analysis focused on the three symptoms evaluated with All of Us surveys. Given that these three symptoms are often clustered with other symptoms, such as sleep disturbance, nausea, anxiety, and depression in chronic conditions (11,45,46), identified symptom phenotypes may be characterized by additional symptoms. Likewise, evaluated predictors may be associated with additional symptoms.
Building on these limitations for future research, we encourage the addition of more symptom information to the All of Us Research Program dataset and other large biomedical databases. Incoming genomic information will allow for the exploration of an additional dimension of symptom science precision medicine, the biological underpinnings of symptoms and symptom clusters with shared etiology (47). The results of this study, as well as future analyses of symptom clusters using All of Us data, may identify targets for symptom management interventions at the biological, interpersonal, and systemic levels.
Conclusion
The objective of this study was to understand the factors that contribute to unrelieved symptoms in All of Us participants diagnosed with one or more common chronic conditions. We report relationships between demographic, healthcare access and utilization, and health-related factors and four symptom phenotypes. All of Us Research Program data offers a unique opportunity to study symptoms in individuals diagnosed with one or more chronic conditions and inform targeted interventions to mitigate symptom burden.
Supplementary Material
KEY MESSAGE.
This article describes demographic, healthcare access and utilization, and health-related factors that predict symptom phenotypes in the All of Us Research Program, a National Institutes of Health biomedical database, dataset. Our results indicate distinct symptom phenotypes that can be leveraged for future intervention.
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health [P30 NR016587, R00 NR017651]. The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. In addition, the All of Us Research Program would not be possible without the partnership of its participants.
Footnotes
DATA STATEMENT
Data used in this study are from the All of Us Research Hub through the National Institutes of Health. Data was accessed through the Registered Tier and is available to authorized users who have registered with the All of Us Research Program.
DISCLOSURES
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boersma P, Black LI, Ward BW. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis 2020;17:200130, 10.5888/pcd17.200130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Health-related quality of life and healthcare utilization in multimorbidity: results of a cross-sectional survey. Qual Life Res 2013;22(4):791–9, 10.1007/s11136-012-0214-7 [DOI] [PubMed] [Google Scholar]
- 3.Baker TA, Clay OJ, Johnson-Lawrence V, et al. Association of multiple chronic conditions and pain among older black and white adults with diabetes mellitus. BMC Geriatr 2017. 30;17(1):255, 10.1186/s12877-017-0652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst 2017;109(4):djw253, 10.1093/jnci/djw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browall M, Brandberg Y, Nasic S, et al. A prospective exploration of symptom burden clusters in women with breast cancer during chemotherapy treatment. Support Care Cancer 2017;25(5):1423–1429, 10.1007/s00520-016-3527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore C, Santhakumaran S, Martin GP, et al. Symptom clusters in chronic kidney disease and their association with people’s ability to perform usual activities. PLoS One 2022;17(3):e0264312, 10.1371/journal.pone.0264312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.7. Fei F, Siegert RJ, Zhang X, Gao W, Koffman J. Symptom clusters, associated factors and health-related quality of life in patients with chronic obstructive pulmonary disease: a structural equation modelling analysis. J Clin Nurs 2022, 10.1111/jocn.16234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. The Promise of Precision Medicine. 2020. Available from: https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/promise-precision-medicine. Accessed February 10, 2020.
- 9.Park J, Moser DK, Griffith K, Harring JR, Johantgen M. Exploring symptom clusters in people with heart failure. Clin Nurs Res 2019;28(2):165–81, 10.1177/1054773817729606 [DOI] [PubMed] [Google Scholar]
- 10.Nagamine T, Gillette B, Pakhomov A, et al. Multiscale classification of heart failure phenotypes by unsupervised clustering of unstructured electronic medical record data. Sci Rep 2020;10(1):21340, 10.1038/s41598-020-77286-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Ji M, Scott P, Dunbar-Jacob JM. The effect of symptom clusters on quality of life among patients with type 2 diabetes. Diabetes Educ 2019;45(3):287–94, 10.1177/0145721719837902 [DOI] [PubMed] [Google Scholar]
- 12.Hertzog MA, Pozehl B, Duncan K. Cluster analysis of symptom occurrence to identify subgroups of heart failure patients: a pilot study. J Cardiovasc Nurs 2010;25(4):273–83, 10.1097/JCN.0b013e3181cfbb6c [DOI] [PubMed] [Google Scholar]
- 13.Christensen VL, Rustøen T, Cooper BA, et al. Distinct symptom experiences in subgroups of patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11(1):1801–9, 10.2147/COPD.S105299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69(10):2086–93, 10.2337/dbi20-0001 [DOI] [PubMed] [Google Scholar]
- 15.Ahmad T, Pencina MJ, Schulte PJ, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol 2014;64(17):1765–74, 10.1016/j.jacc.2014.07.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo X, Gandhi P, Storey S, Zhang Z, Han Z, Huang K. A computational framework to analyze the associations between symptoms and cancer patient attributes post chemotherapy using EHR data. IEEE J Biomed Health Inform 2021;25(11):4098–109, 10.1109/JBHI.2021.3117238 [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Storey S, Gandhi P, Zhang Z, Metzger M, Huang K. Analyzing the symptoms in colorectal and breast cancer patients with or without type 2 diabetes using EHR data. Health Informatics J 2021;27(1):14604582211000784, 10.1177/14604582211000785 [DOI] [PubMed] [Google Scholar]
- 18.Lee LJ, Wehrlen L, Wallen GR, Ding Y, Ross A. Symptom clusters and influencing factors in family caregivers of individuals with cancer. Cancer Nurs 2021;44(6):E547–55, 10.1097/NCC.0000000000000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethares KA, Chin E. Age and gender differences in physical heart failure symptom clusters. Heart Lung. 2021;50(6):832–7., 10.1016/j.hrtlng.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Wu F, Yi H, et al. Gender differences in chronic obstructive pulmonary disease symptom clusters. Int J Chron Obstruct Pulmon Dis 2021;16:1101–7, 10.2147/COPD.S302877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corwin EJ, Brewster G, Dunbar SB, et al. The metabolomic underpinnings of symptom burden in patients with multiple chronic conditions. Biol Res Nurs 2021;23(2):270–9, 10.1177/1099800420958196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeidzadeh S, Perkhounkova Y, Gilbertson-White S, Cherwin CH. The influence of multiple chronic conditions on symptom clusters in people with solid tumor cancers. Cancer Nurs 2022;45(1):E279–90, 10.1097/NCC.0000000000000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakken S, Koleck TA, Dreisbach C, Hickey KT. Enabling precision health approaches for symptom science through big data and data science. In: Dorsey SG, Starkweather AR, eds. Genomics of pain and co-morbid symptoms. Cham: Springer International Publishing, 2020:239–53. [Google Scholar]
- 24.National Institutes of Health All of Us Research Program All of Us Research Hub. Overall Health Survey. 2018. Available from: https://databrowser.researchallofus.org/survey/overall-health. Accessed February 9, 2022.
- 25.HealthMeasures. Patient Reported Outcomes Measurement Information System (PROMIS). 2022. Available from: https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed January 19, 2022.
- 26.National Institutes of Health All of Us Research Program All of Us Research Hub. The Basics Survey. 2019. Available from: https://databrowser.researchallofus.org/survey/the-basics. Accessed February 9, 2022.
- 27.Centers for Disease Control and Prevention National Center for Health Statistics. National Health Interview Survey. 2021. Available from: https://www.cdc.gov/nchs/nhis/index.htm. Accessed February 9, 2022.
- 28.Caraballo C, Massey D, Mahajan S, et al. Racial and ethnic disparities in access to health care among adults in the United States: a 20-year National Health Interview Survey analysis, 1999–2018. medRxiv 2020;2020.10.30.20223420, 10.1101/2020.10.30.20223420 [DOI] [Google Scholar]
- 29.National Institutes of Health All of Us Research Program All of Us Research Hub. Health Care Access & Utilization Survey. 2018. Available from: https://databrowser.researchallofus.org/survey/health-care-access-&-utilization. Accessed February 9, 2022.
- 30.Martinez ME, Ward BW, Adams PF. Health care access and utilization among adults aged 18–64, by race and hispanic origin: United States, 2013 and 2014. NCHS Data Brief 2015;(208):1–8. [PubMed] [Google Scholar]
- 31.Faller H, Strahl A, Richard M, Niehues C, Meng K. Symptoms of depression and anxiety as predictors of physical functioning in breast cancer patients. A prospective study using path analysis. Acta Oncol 2017;56(12):1677–81, 10.1080/0284186X.2017.1333630 [DOI] [PubMed] [Google Scholar]
- 32.Park SS. Caregivers’ mental health and somatic symptoms during COVID-19. J Gerontol B Psychol Sci Soc Sci 2021;76(4):e235–40, 10.1093/geronb/gbaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillis C, Ilie G, Mason R, et al. Personality traits and urinary symptoms are associated with mental health distress in patients with a diagnosis of prostate cancer. Curr Oncol 2021;28(4):2993–3002, 10.3390/curroncol28040262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med 2015;175(11):1773–82, 10.1001/jamainternmed.2015.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwekkeboom K, Zhang Y, Campbell T, et al. Randomized controlled trial of a brief cognitive-behavioral strategies intervention for the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Psychooncology 2018;27(12):2761–9, 10.1002/pon.4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali MK, Chwastiak L, Poongothai S, et al. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT randomized clinical trial. JAMA 2020;324(7):651–62, 10.1001/jama.2020.11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldwin PA, Sanatkar S, Clarke J, et al. A web-based mental health intervention to improve social and occupational functioning in adults with type 2 diabetes (The Springboard Trial): 12-month outcomes of a randomized controlled trial. J Med Internet Res 2020;22(12):e16729, 10.2196/16729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo Y, Wang H, Huang G, Chu M. Effectiveness of nurse-led program on mental health status and quality of life in patients with chronic heart failure. Medicine (Baltimore) 2020;99(33):e21746, 10.1097/MD.0000000000021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivynian SE, Newton PJ, DiGiacomo M. Patient preferences for heart failure education and perceptions of patient–provider communication. Scand J Caring Sci 2020;34(4):1094–101, 10.1111/scs.12820 [DOI] [PubMed] [Google Scholar]
- 40.Jones G, Gollish M, Trudel G, Rutkowski N, Brunet J, Lebel S. A perfect storm and patient-provider breakdown in communication: two mechanisms underlying practice gaps in cancer-related fatigue guidelines implementation. Support Care Cancer 2021;29(4):1873–81, 10.1007/s00520-020-05676-7 [DOI] [PubMed] [Google Scholar]
- 41.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer 2014;120(15):2371–8, 10.1002/cncr.28699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities 2018;5(1):117–40, 10.1007/s40615-017-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly EP, Mcgee J, Obeng-Gyasi S, et al. Marginalized patient identities and the patient-physician relationship in the cancer care context: a systematic scoping review. Supportive Care Cancer 2021;29(12):7195–207, 10.1007/s00520-021-06382-8 [DOI] [PubMed] [Google Scholar]
- 44.Ford CL, Airhihenbuwa CO. The public health critical race methodology: praxis for antiracism research. Soc Sci Med 2010;71(8):1390–8, 10.1016/j.socscimed.2010.07.030 [DOI] [PubMed] [Google Scholar]
- 45.Koleck TA, Topaz M, Tatonetti NP, et al. Characterizing shared and distinct symptom clusters in common chronic conditions through natural language processing of nursing notes. Res Nurs Health 2021;44(6):906–19, 10.1002/nur.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Sereika SM, Marsland AL, Conley YP, Bender CM. Symptom clusters in women with breast cancer during the first 18 months of adjuvant therapy. J Pain Symptom Manage 2020;59(2):233–41, 10.1016/j.jpainsymman.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 47.McCall MK, Stanfill AG, Skrovanek E, Pforr JR, Wesmiller SW, Conley YP. Symptom science: omics supports common biological underpinnings across symptoms. Biol Res Nurs 2018;20(2):183–91, 10.1177/1099800417751069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.