Abstract
Purpose
To investigate ocular abnormalities in Fabry disease (FD).
Methods
Forty-five patients with FD diagnosed by genetic analysis were enrolled in a single medical centre. The following ocular examinations were performed: slit-lamp examination, ophthalmic fundus imaging, in vivo confocal microscopy (IVCM) and optical coherence tomography (OCT). The prevalences of typical abnormalities in the cornea, conjunctiva and retina were recorded; their differences between hemizygote and heterozygote were compared.
Results
In this study, the prevalence of corneal verticillata was 97.8% (44/45). Corneal examination with IVCM demonstrated hyper-reflective intracellular inclusions located within basal epithelial cells. Conjunctival vessel malformations were observed in 64.4% (29/45) of patients, and retinal vessel tortuosity was observed in 62.2% (28/45) of patients. OCT revealed many strong hyper-reflective foci (HRF) in the inner retinal layer (in 66.7% [30/45] of patients); these foci may represent retinal vascular plexi. The prevalences of conjunctival vessel malformation, retinal vessel tortuosity and HRF were higher in hemizygote than in heterozygote.
Conclusions
Corneal verticillata, HRF on OCT, conjunctival vessel malformation and retinal vessel tortuosity exhibit high prevalence in patients with FD. These ocular manifestations are characteristic and easily accessible; thus, they should be considered diagnostic criteria for FD.
Keywords: Fabry disease, corneal verticillata, retinal vessel tortuosity, optical coherence tomography
Key messages
The main ocular features of the patients with FD are corneal verticillata, HRF on OCT, conjunctival vessel malformation and retinal vessel tortuosity.
These ocular manifestations should be considered as the diagnostic criteria of FD.
Introduction
Fabry disease (FD) is an X-linked lysosomal storage disease that is caused by deficient activity of the lysosomal enzyme α-galactosidase A. This deficiency results in progressive lysosomal accumulation of glycosphingolipids with α-galactosyl residues, particularly globotriaosylceramide, which accumulates in epithelial cells of various organs [1]. Accumulations in renal, cardiac and nervous tissue lead to progressive kidney failure, cardiomyopathy and Fabry-associated pain or stroke, thus contributing to substantial morbidity and mortality [2–4]. Sensory systems (e.g. ocular, auditory and cochleovestibular) are also involved; the results include ocular manifestations, hearing loss, tinnitus and vertigo [5,6]
The incidence rate of FD was previously described as 1:40,000 to 1:117,000, but recent studies suggest a much higher occurrence rate related to population and ethnicity. Hemizygous males typically experience more severe effects and earlier onset of the disease, while disease-related complications may develop later and more easily go unnoticed in heterozygous females [5,7]. Enzyme replacement therapy (ERT) is the prevailing method of treating FD. Administering ERT bi-monthly can alleviate symptoms, mitigate cardiac, renal and neuropathic damage and enhance the quality of life by replacing the deficient α-galactosidase A (AGAL-A) [8,9].
Ocular manifestations are considered distinctive features of FD. Corneal verticillata, also known as vortex keratopathy, is the most commonly reported ocular manifestation [10]. Tortuous conjunctival and retinal vessels, as well as spoke-like lens opacities, are also present in multiple patients; they are usually referred to as Fabry-related ocular lesions [11]. Most Fabry-related ocular lesions do not affect vision, although they are often early and unique signs of disease that can be detected in a routine, non-contact eye examination [5]. This detection may be useful for the diagnosis of FD because this disease is rare and its early symptoms are subtle or non-specific [12,13].
In this report, we describe the features of common ocular manifestations of FD, detected by slit-lamp examination and novel ocular imaging devices. Some of these manifestations noticeably differ from the findings in earlier studies, and we hope that the novel ocular characteristics can aid in the prompt and accurate diagnosis of FD.
Methods
Patients
Forty-five FD patients (33 hemizygote/males and 12 heterozygote/females) attended the Department of Ophthalmology, Peking University and were included in this study between January 2014 and December 2021. All patients had their diagnosis confirmed by DNA testing that revealed the presence of an α-galactosidase A mutation. The mutation types included substitutions, deletions and duplications. Among them, 33 patients had substitution mutations, accounting for 73.3%, 10 patients had deletion mutations, accounting for 22.2% and 2 patients had duplication mutations, accounting for 4.4%. Informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of Peking University First Hospital; the study was performed in accordance with the tenets of the Declaration of Helsinki.
Clinical examination
Medical histories were collected for all patients. Complete ophthalmic examinations were then performed, comprising visual acuity assessment, intraocular pressure measurement, anterior segment slit-lamp examination, colour fundus photography and optical coherence tomography (OCT). Intraocular pressure was measured with a non-contact tonometer (CT800, Topcon, Tokyo, Japan). Undilated digital fundus photographs were collected using a 45° digital retinal camera (CR-2, Canon, Tokyo, Japan). A retinal image of each eye was obtained, with images centred at the fovea. Branching vessels within the macula lutea region were evaluated. The distribution of retinal vascular tortuosity was also recorded.
OCT examination
All patients underwent high-definition OCT (Spectralis, Heidelberg Engineering, Germany) scanning of the macular area without pupil dilation. A trained technician performed the scans after all other ophthalmic examination components had been completed. A one-line 6-mm horizontal scan was performed with a high-definition protocol focused on the fovea. Scans with quality >6 were selected. Hyper-reflective foci (HRF) were defined as discrete and well-circumscribed dots of increased reflectivity, compared with the retinal nerve fibre layer (RNFL) and a diameter >10 µm.
In vivo corneal confocal microscopy (IVCM) examination
Further corneal-contact assessment comprising IVCM (Heidelberg Tomography 3, Heidelberg Engineering, Germany) examination under topical anaesthesia was recommended for all patients, but only one-third of these patients consented to the examination.
The eyes were anesthetized by instillation of 4% oxybuprocaine hydrochloride (Santen Pharmaceutical Co., Ltd.) in the lower conjunctival cul-de-sac. The patient was positioned in front of the microscope with their chin placed in the chin rest and their forehead placed against the forehead support. A sterile single-use contact element (TomoCap) was placed between the microscope and the cornea. The confocal image plane inside the cornea was moved manually at the level of the microscope lens. Images of the corneal layers were taken from the central cornea. The IVCM collected images measuring 400 × 400 µm. The images were recorded digitally and stored within the tomography device to select the highest-quality pictures and analyse corneal morphology.
Statistical analyses
Statistical analyses were performed with SPSS Statistics (Version 20.0, IBM-SPSS, Chicago, IL, USA). The chi-squared test was used to analyse sex-related differences in the cornea, conjunctiva, retina and HRF. Differences were considered statistically significant when p < .05.
Results
The patients in this study included 33 hemizygote and 12 heterozygote with a mean age of 31.6 ± 16.4 years (range, 9–60 years) and normal intraocular pressure (mean, 14.25 ± 3.15 mmHg).
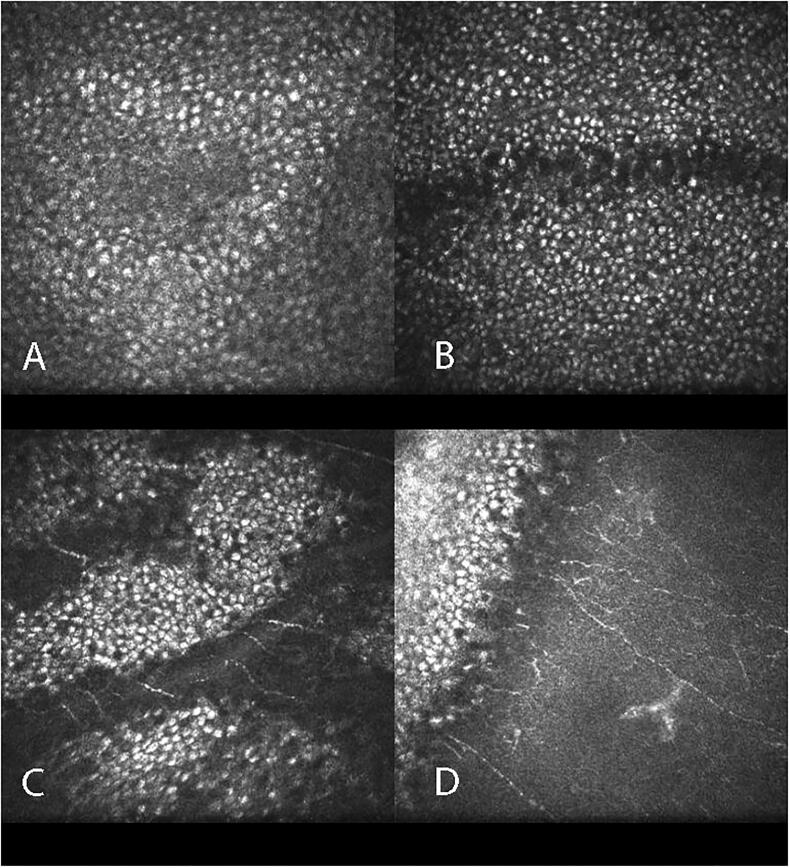
Cornea verticillata, the most common and distinctive finding visible by slit-lamp microscopy, was present in nearly all patients (44 patients, 97.8%) (Figure 1(A)). Only one patient had no obvious cornea verticillata, although some brown lines were evident on the cornea. In eyes examined by IVCM, hyper-reflective cells were noted in the entire epithelial layer at the location of cornea verticillata (Figure 2). However, the Bowman’s, stromal and endothelial layers appeared normal in IVCM.
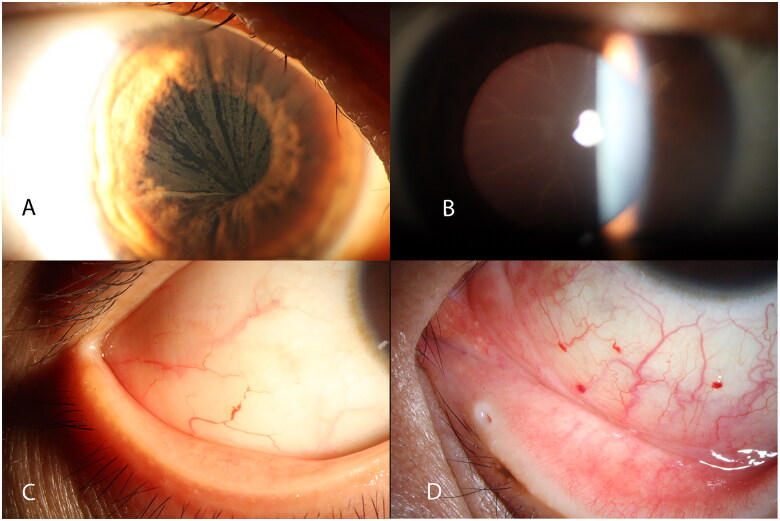
Figure 1.
Cornea verticillate (A), spoke-like appearance of lens (B) and conjunctival vessel malformation (C, D) of Fabry patient.
Figure 2.
Scans of cornea in in vivo confocal microscopy.
Fabry-related lens opacity is very unique but rare. Only four patients had such a spoke-like appearance on the lens capsule (Figure 1(B)). Two types of conjunctival vascular abnormalities were evident in this patient group: vascular tortuosity (often visible in the nasal and temporal bulbar conjunctiva, as well as in patients with other diseases) and vessel aneurysmal dilatation (often visible in the nasal-inferior quadrant of bulbar conjunctiva). This vessel malformation with dilatation and occlusion was more distinctive and present in 29 patients (64.4%) (Figure 1(C)). Notably, more than one aneurysmal dilatation was evident in the conjunctiva in some patients (Figure 1(D)).
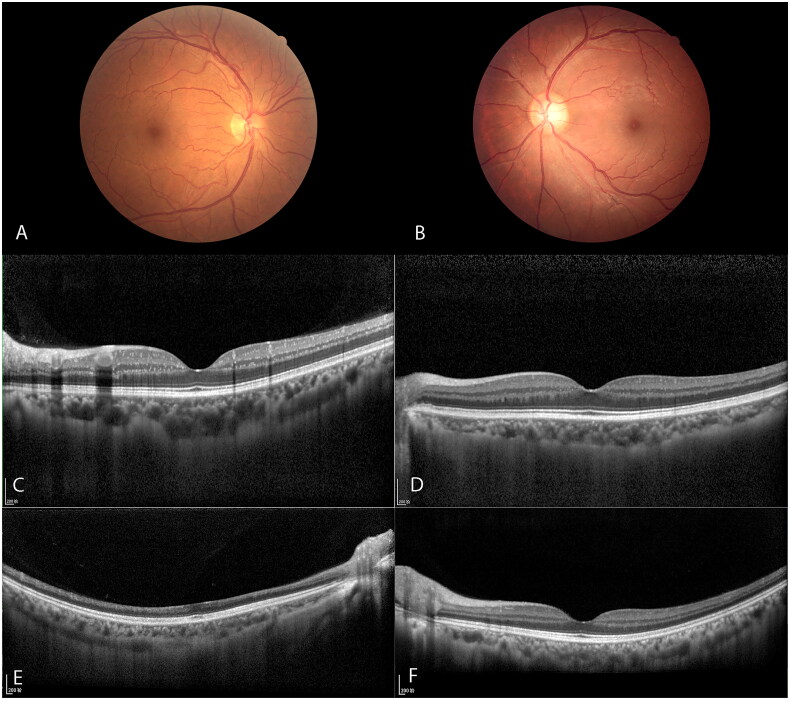
Retinal vessel tortuosity was observed in 28 patients (62.2%). Venular tortuosity was more obvious than arteriolar tortuosity. Nineteen patients (42.2%) had tortuosity involving both primary and secondary branch vessels (Figure 3(A)). Nine patients (20%) had tortuosity involving only on secondary branch vessels (Figure 3(B)). All eyes were scanned by OCT and distinct HRF; foci diameters of 10–30 µm were scattered in retinal layers between the inner nuclear layer (INL) and RNFL. Such foci could be divided into two layers (Figure 3(C)). The outer foci layer was visible mainly within the INL, which presumably comprised particles in the cells of the deeper plexus wall. The inner layer was visible mainly within the RNFL, which may comprise materials of the superficial plexus vessel wall. Such foci also could be divided into two types: strong HRF and weak HRF (i.e. near normal). Strong HRF had similar reflectivity, compared with the retinal pigment epithelium (RPE) band (Figure 3(C)); weak HRF had similar reflectivity, compared with the inner plexiform layer (IPL) or RNFL (Figure 3(D)). In this study, 30 patients (66.7%) had strong HRF and 15 patients (33.3%) had weak HRF. One patient had a history of sudden vision loss in the right eye and had been diagnosed with central retinal artery occlusion (CRAO) many years prior. OCT scanning revealed partial loss and thinning of inner retinal layers, while outer retinal layers remained intact (Figure 3(E)). The contralateral eye of the patient was normal, with minimal weak HRF in the inner retinal layer (Figure 3(F)).
Figure 3.
Venular tortuosity in retina (A, B), strong HRF and weak HRF in OCT scans (C, D), OCT scans of an old central retinal artery occlusion of Fabry patient (E) and her normal contralateral eye (F).
Finally, the prevalences of conjunctival and retinal abnormalities, as well as strong HRF, were greater in hemizygote than in heterozygote (Table 1).
Table 1.
The different percentage of conjunctival, retinal abnormalities and strong HRF between hemizygotes and heterozygotes.
| Abnormalities | Hemizygotes (n = 33) | Heterozygotes (n = 12) | Chi-square | p |
|---|---|---|---|---|
| Cornea verticillata | 97.0% | 100% | 0.372 | 1 |
| Conjunctival vessel malformation | 75.8% | 33.3% | 6.912 | .01 |
| Retinal vessels tortuosity | 75.8% | 25% | 9.645 | .00 |
| Strong HRF in retina | 78.7% | 33% | 8.182 | .01 |
Discussion
FD is a rare disease with diverse signs. Ocular manifestations were first reported by Spaeth in 1965 [14]. Subsequently, corneal opacities (cornea verticillata), cataract and conjunctival and retinal vascular abnormalities were considered typical findings in patients with FD [5,14,15]. In our study, unique whorl-like corneal opacities were evident in almost all patients. The prevalence of cornea verticillata was 97.8% in this study, similar to the results of prior enzymatic and genetic research involving FD patients [11]. Notably, some other studies reported a lower prevalences of cornea verticillata, potentially because of selection and observer errors. In our study, the patients were mainly referred from neurology, and a higher proportion of classic FD patients were included. This may explain why the corneal signs had a high positivity rate in our study. In some studies, the presence of FD was confirmed on the basis of typical symptoms or through partial molecular genetic testing [16]. Importantly, cornea verticillata is difficult to identify in some patients without using the sclerotic scatter technique. The resulting missed diagnoses may have contributed to the large difference in corneal opacity prevalence. In our study, we found hyper-reflective intracellular inclusions from superficial to basal corneal epithelium, using IVCM examination. Bright hyper-reflective dots in superficial stroma and complex basal-Bowman’s membrane irregularities have also been reported in some studies [17,18]. Importantly, we did not observe any stromal abnormalities. For this study, we conducted IVCM on the initial third of the patients and observed a notable similarity between corneal verticillate identified under the slit lamp and the corresponding IVCM findings. Based on this, we conclude that IVCM is likely to yield positive results in patients with corneal verticillate and we suggest that it be considered as an optional test for such patients. As a result, we did not perform IVCM on every subsequent patient.
In some studies, conjunctival vessel tortuosity has been regarded as a characteristic sign of FD [11]. In contrast, our results indicated that conjunctival vessel tortuosity was a less specific finding, which could also be observed in many older patients. Vessel malformation with dilatation, or aneurysm, was a more characteristic sign of FD. Retinal vessel tortuosity is an obvious sign of FD, but its prevalence was approximately 60%. Similar to conjunctival vessel tortuosity, retinal vessel tortuosity can also occur in patients with other diseases. Some patients’ ocular manifestations, such as cornea verticillate, conjunctival vessel malformation and retinal vascular tortuosity are not symmetrical. If both eyes of the patient have an asymmetric phenotype, we record the eye with the more severe lesion as ocular manifestation.
HRF in the retina affected most patients in this study. Importantly, this is not the first report of OCT findings in FD patients. Albert et al. reported normal macula findings in OCT scans of 23 FD patients [11]. Such foci may be neglected because they do not affect retinal morphology. To our knowledge, Yevgeniya et al. were the first to describe intraretinal HRF in FD patients [19]. They graded intraretinal HRF on a scale of 0 to 3 (none, mild, moderate, or severe) for easy use in clinical practice. However, this classification system is rather subjective; mild to moderate HRF could also be observed in some healthy individuals, including younger people. We suggest using a dichotomous classification system to evaluate such changes. Similar reflectivity relative to the RPE band or RNFL band could be used to define HRF types (e.g. strong and weak); in our study, only strong HRF comprised a key ocular feature of FD. Small foci with diameter of 10–30 µm spread from INL to RNFL. Based on this spread, we presumed that foci in the RNFL and ganglion cell layer (GCL) represented vessel wall images of superficial vascular plexi. Furthermore, foci in the INL and IPL may represent vessel wall images of deep vascular plexi.
Unique spoke-like lens opacity was evident in our patients. Fabry disease can affect the lens and cause the lens opacities into two entities: anterior subcapsular and posterior subcapsular. In this study, we did not perform pupil dilation exams on every patient, which may have resulted in some cases of anterior lens opacities being missed. Therefore, the incidence of lens opacity in this study may have been underestimated. However, the main objective of this study was to identify eye signs that can be used for rapid screening of FD. Anterior lens opacity is not suitable for rapid screening of FD because it requires pupil dilation, which requires more time investment. The prevalence of retinal vessel obstruction was very low, and we do not suggest regarding it as a characteristic sign of FD.
Among the patients in this study, 73.3% were hemizygote and 37.7% were heterozygote. Approximately 75% of the hemizygote and 20%–30% of the heterozygote had typical conjunctival signs, retinal signs and OCT findings. Hemizygote had more severe ocular involvement, compared with heterozygote, despite similar systemic severity. Recently, enzyme-replacement treatment has become available, which increases the importance of early diagnosis to facilitate treatment initiation before irreversible organ damage. The ocular findings from slit-lamp and imaging devices are sufficiently distinctive to support eventual diagnosis with FD.
In recent reports, it has been observed that vessel tortuosity be linked to the upper outer eyelid [10,20]. However, in this study group, pathological changes involving the skin were not given much attention. It is known that FD can affect the skin and result in angiokeratoma typically [1]. Therefore, further monitoring observation is needed to observe the angiokeratoma and telangiectasia manifest in the skin of eyelid.
In conclusion, ophthalmic evaluation is a suitable clinical screening tool for FD, potentially enabling early and rapid diagnosis as an alternative to pathology and genetic analyses. Ophthalmologists can identify patients with early disease. In particular, patients with cornea verticillata, conjunctival vessel aneurysmal dilatation, retinal vessel tortuosity, or HRF on OCT are recommended to undergo a thorough review of symptoms and family history to exclude a diagnosis of FD.
Acknowledgments
The previous version of this manuscript had been preprinted in Research Square last year (https://www.researchsquare.com/article/rs-16983/v1) but the data of two versions was different. More FD patients had been added in the new version. By the way, we thank Ryan Chastain-Gross, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Funding Statement
This work was supported by ULM-PUHSC Joint Institute for Translational and Clinical Research (No: PKU2017ZC001-5) and the National Natural Science Foundation (China) (No: 81100840).
Data availability statement
All data supporting the results reported in this study are available from the corresponding author upon request.
Authors contributions
Yuan Wu, Wenbo Zhang, Yun Yuan and Wei Zhang conceived and designed the study. Yuan Wu, Xuyang Yao, Wenjing Song and Yawen Zhao performed the observations and recorded the data. Yuan Wu, Wenbo Zhang and Wei Zhang wrote the paper. Yuan Wu, Wenbo Zhang and Yun Yuan reviewed and edited the manuscript. All authors reviewed and approved the final manuscript and agree to be held accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Chan B, Adam DN.. A review of Fabry disease. Skin Therapy Lett. 2018;23:1–7. [PubMed] [Google Scholar]
- 2.Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur J Clin Invest. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 3.Linhart A, Kampmann C, Zamorano JL, et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28(10):1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 4.Mehta A, Ginsberg L.. Natural history of the cerebrovascular complications of Fabry disease. Acta Paediatr Int J Paediatr Suppl. 2005;94(0):24–27. doi: 10.1080/08035320510028076. [DOI] [PubMed] [Google Scholar]
- 5.Sodi A, Ioannidis AS, Mehta A, et al. Ocular manifestations of Fabry’s disease: data from the Fabry outcome survey. Br J Ophthalmol. 2007;91(2):210–214. doi: 10.1136/bjo.2006.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germain DP, Avan P, Chassaing A, et al. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet. 2022;3(1):10. doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köping M, Shehata-Dieler W, Schneider D, et al. Characterization of vertigo and hearing loss in patients with Fabry disease. Orphanet J Rare Dis. 2018;13(1):137. doi: 10.1186/s13023-018-0882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Dib R, Gomaa H, Carvalho RP, et al. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev. 2016;7(7):CD006663. doi: 10.1002/14651858.CD006663.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Embrett M, Mackinnon NJ.. Qualitative evaluation of the Canadian Fabry disease initiative. Can Pharm J. 2012;145(3):136–141.e3. doi: 10.3821/145.3.cpj136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaud L. Longitudinal study on ocular manifestations in a cohort of patients with Fabry disease. PLoS One. 2019;14(6):e0213329. doi: 10.1371/journal.pone.0213329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morier AM, Minteer J, Tyszko R, et al. Ocular manifestations of Fabry disease within in a single kindred. Optometry. 2010;81(9):437–449. doi: 10.1016/j.optm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138(4):338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann B, Mayatepek E.. Morbus Fabry - Oft gesehen, selten erkannt. Dtsch Arztebl. 2009;106:440–447. doi: 10.3238/arztebl.2009.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam T, Alexandrescu L, Voinea F, et al. Fabry’s disease. Rom J Intern Med. 2006;44(4):455–464. doi: 10.1001/archopht.1965.00970040762005. [DOI] [PubMed] [Google Scholar]
- 15.Sher NA, Letson RD, Desnick RJ.. The ocular manifestations in Fabry’s disease. Arch Ophthalmol. 1979;97(4):671–676. doi: 10.1001/archopht.1979.01020010327008. [DOI] [PubMed] [Google Scholar]
- 16.Pitz S, Kalkum G, Arash L, et al. Ocular signs correlate well with disease severity and genotype in Fabry disease. PLoS One. 2015;10(3):e0120814. doi: 10.1371/journal.pone.0120814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastropasqua L, Nubile M, Lanzini M, et al. Corneal and conjunctival manifestations in Fabry disease: in vivo confocal microscopy study. Am J Ophthalmol. 2006;141(4):709–709.e11. doi: 10.1016/j.ajo.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Wasielica-Poslednik J, Pfeiffer N, Reinke J, et al. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1689–1696. doi: 10.1007/s00417-011-1726-5. [DOI] [PubMed] [Google Scholar]
- 19.Atiskova Y, Rassuli R, Koehn AF, et al. Retinal hyperreflective foci in Fabry disease. Orphanet J Rare Dis. 2019;14(1):8. doi: 10.1186/s13023-019-1267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud L. Vascular tortuosities of the upper eyelid: a new clinical finding in Fabry patient screening. J Ophthalmol. 2013;2013:207573. doi: 10.1155/2013/207573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the results reported in this study are available from the corresponding author upon request.