Abstract
The SARS-CoV-2 pandemic highlighted the need for novel tools to promote health equity. There has been a historical legacy around the location and allocation of public facilities (such as health care) focused on efficiency, which is not attainable in rural, low-density, United States areas. Differences in the spread of the disease and outcomes of infections have been observed between urban and rural populations throughout the COVID-19 pandemic. The purpose of this article was to review rural health disparities related to the SARS-CoV-2 pandemic while using evidence to support wastewater surveillance as a potentially innovative tool to address these disparities more widely. The successful implementation of wastewater surveillance in resource-limited settings in South Africa demonstrates the ability to monitor disease in underserved areas. A better surveillance model of disease detection among rural residents will overcome issues around the interactions of a disease and social determinants of health. Wastewater surveillance can be used to promote health equity, particularly in rural and resource-limited areas, and has the potential to identify future global outbreaks of endemic and pandemic viruses.
Keywords: Africa, Disease surveillance, Health disparities, SARS-CoV-2, Vulnerable communities, Wastewater surveillance
1. Introduction
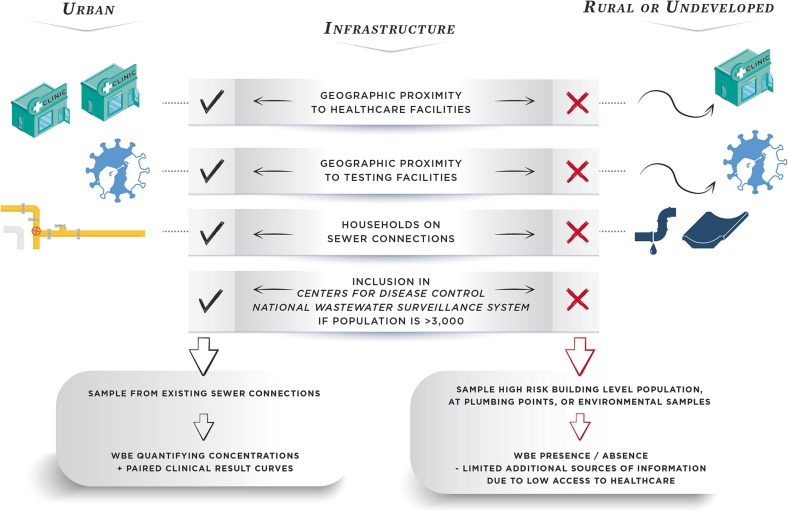
Health disparities among rural United States residents have been well documented (Summers-Gabr, 2020, Ashburn et al., 2021, Cross et al., 2021, Jackson et al., 2021, Dani et al., 2022). Many factors contribute to this, including poorer social determinants of health metrics such as poverty, lack of education, and life expectancy (Table 1 ; Rural Health Information Hub, 2022). Moreover, rural populations have critical barriers to healthcare; there are approximately three M.D.’s in metropolitan areas for every one in non-metropolitan locations on a per capita basis. There is also a historical legacy around the location and allocation of public facilities (such as health care), with a focus on spatial extent and coverage of human services to promote efficiency (DeVerteuil, 2000), a model which leaves behind rural, low-density areas. Further, rural areas are more likely to be served by critical access hospitals, which account for over 50% of hospitals in predominately rural states such as South Dakota. These hospitals, by federal regulations: 1) must be in a rural county at least 35 miles from another acute care facility; 2) may at most have 25 beds; 3) average an annual inpatient length of stay less than 96 h; and 4) have an emergency room or department with 24-hour availability (Centers for Medicare and Medicaid Services, 2021). These hospitals are designed to provide primary care to patients for common health emergencies. However, they often transfer patients to larger facilities for more complex or serious cases. This is important for public health surveillance activities, as federal regulations limit the role of these hospitals in treating large outbreaks of diseases in rural locations, given the limited number of beds, hospital stay capacity, and reduced medical resources. Although these hospitals provide a critical lifeline for basic care for many rural residents, frontier residents must still travel long distances, some of whom must travel over 80 miles each way to receive care (South Dakota Department of Health, 2020). In Kentucky, even in rural areas with geographical access to care, this travel could also include the mountainous Appalachian region, which is prone to flooding (Kentucky Cabinet for Health and Family Services, 2022). As access to equitable healthcare remains a constant struggle in rural and frontier locations, innovation in public health surveillance approaches is needed to maintain the health of residents and capacity at healthcare facilities (Fig. 1 ).
Table 1.
Rural health disparities in select United States locations.
| Disparity | Measure | Location | Metro | Non-Metro |
|---|---|---|---|---|
| Access to Healthcare | M.D.'s per 10,000 People | SD | 36 | 11 |
| KY | 32 | 11 | ||
| National | 32 | 11 | ||
| Social Determinates of Health | Poverty | SD | 8.6% | 14.4% |
| KY | 12.1% | 19.1% | ||
| National | 11.5% | 14.4% | ||
| Population Without a High School Diploma | SD | 6.4% | 9.1% | |
| KY | 9.8% | 17.2% | ||
| National | 11.2% | 12.9% | ||
| Outcomes | Life Expectancy at Birth (years) | SD | 80.3 | 78.9 |
| KY | 77.3 | 75 | ||
| National | 79.3 | 77.4 |
Fig. 1.
Framework for adapting rural wastewater surveillance to overcome health disparities.
2. SARS-CoV-2 Rural Health Disparities
2.1. Cases, Hospitalizations, and Deaths
Health disparities have been devastating for rural residents during this pandemic, with access to healthcare being one of the social determinants of health that influenced disease outcomes in rural locations. Testing and disease surveillance was limited in rural populations, making it difficult to track the spread of the disease through cases, hospitalizations, and deaths (Chillag and Lee, 2020). Although the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic started in urban centers, the overall picture is that non-metropolitan residents have had a higher rate of deaths per capita than metropolitan residents (Cuadros et al., 2021, Dobis et al., 2021). Infection-induced seroprevalence among blood donors was lower in rural populations during the first few months of the pandemic but rapidly and consistently exceeded that of urban populations by the fall of 2020 (Li et al., 2022). Lower rates of vaccine-induced seroprevalence were also found among rural blood donors than among urban donors (Li et al., 2022), indicating an increased risk of infection in rural populations. Even within large counties, from August 2020 to February 2021, the overall positive seroprevalence for SARS-CoV-2 spike protein-specific IgG antibodies was lower in the urban core than in the lower-density county edges, indicating that the scale of the geographic zone matters (Smith et al., 2022). While cases and deaths were higher among the racially diverse urban poor during the first few months of the pandemic, they transitioned to rural areas, especially those with higher levels of poverty (Li et al., 2021). Barriers to testing remained a limitation to understanding the rate of infection reported by health facilities in rural populations throughout the pandemic. Rader and colleagues reported greater travel times to testing centers in rural counties (Rader et al., 2020). Given these barriers to testing, other metrics should be considered as indicators of infectious disease surveillance in rural populations.
The risk of serious illness is higher among rural populations; accordingly, the hospitalization of coronavirus disease 2019 (COVID-19) cases in rural populations was estimated to be 10% higher than in urban populations with similar levels of exposure (Kaufman et al., 2020). This increased risk is due to preexisting comorbidities and older age in rural populations (Kaufman et al., 2020). A large longitudinal study of health records found the adjusted odds ratios for hospitalization to be 1.18 and 1.29 times higher among urban-adjacent rural and non-urban-adjacent rural populations, respectively, compared to urban populations (Anzalone et al., 2023). Rural patients are also more likely to require invasive mechanical ventilation, vasopressor support, and extracorporeal membrane oxygenation than urban patients (Anzalone et al., 2023). Finally, the odds of death or transfer to hospice care were approximately 1.36 times higher in rural patients than in urban patients (Anzalone et al., 2023). Similarly, the cumulative death rate per capita from COVID-19 was nearly 50% higher in rural populations (446.12 deaths per 100,000 people) compared to large central metropolitan areas (302.25 deaths per 100,000 people) (CDC, 2022a). During this pandemic, poorer health outcomes were observed in rural patients than in their urban counterparts. Many factors contribute to these outcomes, including vaccination rates, comorbidities, and a reduction in community mitigation measures (Jackson et al., 2021).
2.2. Vaccination
Vaccination rates against COVID-19 differ between urban and rural populations. In a study on Arkansas residents, rural respondents reported lower trust in vaccines than urban populations (McElfish et al., 2021). In a study on Tennessee populations, access to testing and vaccination was greater in urban areas than in rural areas (Alcendor, 2021). Furthermore, Tennessee non-metropolitan residents were significantly more likely to resist vaccination (Gatwood et al., 2021). A national survey found that non-metropolitan populations were less likely to report intending to receive the COVID-19 vaccine (Salmon et al., 2021). Rural Oklahoma residents reported hesitation toward getting vaccinated as well, with skepticism and limited knowledge about the vaccine being commonly cited reasons (Hubach et al., 2022). Current vaccination rates suggest that these hesitations may have reduced the population receiving the vaccine. According to the Centers for Disease Control (2022b), in large central metropolitan areas, 70.3% of the population completed their primary COVID-19 vaccination series versus 50.1% of rural populations. The reduction in vaccine recipients among rural populations may have contributed to an increase in both mortality and case fatality rates in rural populations during the Omicron wave (Cuadros et al., 2022). Unvaccinated populations are significantly more likely to require hospitalization for COVID-19 than vaccinated populations (Winkelman et al., 2022). Lower vaccination rates in rural populations may contribute to greater vulnerability toward severe outcomes.
2.3. Access to treatment
Early experimental treatments and clinical trials were conducted primarily in a few urban centers (Sharma et al., 2020, Dandachi et al., 2021). Rural populations, on average, would need to travel 85.2 min to the nearest clinical trial site compared to 18.7 min for urban populations (Khazanchi et al., 2021). Furthermore, rural healthcare providers tend to have limited resources, such as intensive care unit beds, ventilators, and key staff to support patient care (Lakhani et al., 2020). In many cases, rural areas served by critical-access hospitals must transfer patients to urban hospitals for more complex care (Diaz et al., 2020, Henning-Smith, 2020). Visitor restrictions observed early in the pandemic, along with the need to travel away from their community of support, may have led rural patients to hesitate in seeking care (Henning-Smith, 2020). Recent studies have reported that rural patients had to travel a median time of 69.6 min to a test and treatment site compared to urban populations, where the median travel time was 11 min (Khazanchi et al., 2022). Since these sites provide access to treatments such as nirmatrelvir-ritonavir and molnupiravir, which need to be initiated within 5 days of symptom onset for maximum efficacy, long travel distances to treatment centers may be a barrier for patients residing in rural areas. This is especially problematic for individuals with limited means of transportation. Overall, barriers to healthcare, including access to testing that allows timely treatment, are likely to contribute to the observed rural health disparities.
2.4. Linking a medical model of disease and contagion with a social determinants of health model
A better surveillance model of health must still bump up against a lack of faith in the public health system among rural inhabitants, especially in terms of prevention. Rural residents are less likely to obtain recommended preventive healthcare services (Casey et al., 2001). Geospatial analyses on healthcare capacity have determined that local geography exacerbated the deployment of COVID-19 vaccines as far as access of residents to rural clinics, termed vaccination coldspots (Cuadros et al., 2023). The lack of faith in the public health system was also seen in an assessment of tweets for topics related to COVID-19 prevention (i.e., “vaccine” and “mandates”), which found rural social media users had a stronger negative sentiment than urban users (Liu et al., 2023). Linking a medical model of disease and contagion with a social determinants of health model is equally needed to overcome health disparities among rural residents.
Many studies have noted that rural populations with weaker social determinants of health have poorer outcomes. Rural minority populations are also more likely to experience poor COVID-19 outcomes (Henning-Smith et al., 2021, Wheeler et al., 2021). In North Carolina, the test positivity rate was the highest among minority populations living in rural areas (Brandt et al., 2021), and similar findings have been documented among rural and minority populations in Indiana (Dixon et al., 2021). A greater number of comorbid diseases was observed among hospitalized rural North Carolina patients early in the pandemic (Denslow et al., 2022), with similarly increased rates of comorbid diseases in rural populations in Georgia associated with higher COVID-19 mortality (Shah et al., 2020). Further complicating the clinical outcomes observed, rural populations are more likely to be older than urban populations (Chillag and Lee, 2020), which naturally puts these populations at a higher risk for severe COVID-19 disease. Persistent poverty is more common in rural settings than in urban settings, especially among minority populations (Chillag and Lee, 2020). Employers in the agricultural and food-processing industries are often in rural locations, working conditions that can foster the spread of the disease (Behrman et al., 2021). Finally, a combination of increased misinformation, lack of trust, and reduced health literacy in rural settings may further contribute to the barriers observed in rural populations (Lakhani et al., 2020). Trust in public health messages and the perception of a reduced risk of disease have been noted among rural populations (Ridenhour et al., 2022). These social factors might increase the probability of severe outcomes in rural populations.
Underpinning the SARS-CoV-2 rural health disparities is the larger issue of 'access' and density. Rural areas lack the density of both population and of health services - this is not surprising, but it does raise important issues around the location and allocation of public facilities (such as health care) and equity. DeVerteuil (2000) reviews the literature on access, density, and equity when relating to public facilities. This comes to attention where in rural areas, away from urban centers, efficiency according to facility distance, pattern, accessibility, impacts, and externalities are more sensitive to facility spacing. This is a case where wastewater surveillance may overcome health disparities among rural residents to circumvent a human services location legacy model.
3. Wastewater surveillance to improve rural health
Despite this, there is an opportunity for innovation in identifying the virus in rural communities as a step toward alleviating these health disparities. The current pandemic has enabled the development and utilization of temporary mobile clinical testing units and traveling healthcare professionals to aid rural communities experiencing surges in illness. However, early warning health surveillance systems are necessary to allow states to efficiently mobilize resources and healthcare workers to rural populations during health crises. The lack of effective surveillance in rural areas leads to an increased probability of the spread of COVID-19 among rural communities, which may lead to the spread of the virus to the surrounding urban areas (Souch and Cossman, 2021). Wastewater surveillance is a tool that can be used to provide surveillance in rural and remote locations (Medina et al., 2022). This surveillance has been well-received by the public for its use in tracking diseases, environmental toxins, and terrorist threats when large populations, such as those served by wastewater treatment plants, are included (La Joie et al., 2022). However, complicating surveillance, many rural populations use on-site sanitation systems rather than piped sewer connections. Approximately 15% of United States households are served by septic systems (World Health Organization, 2021). While this is true, rural populations often travel to adjacent larger communities for school, to work, and to shop, allowing regional wastewater treatment plants to still offer an opportunity for inclusive sampling. Working with local experts to understand travel patterns among rural populations is key to establishing surveillance systems that capture rural populations that are otherwise off the grid. Currently, the National Wastewater Surveillance System excludes communities with populations of less than 3,000 (Centers for Disease Control, 2022c). In situations where travel to regional urban centers exceeding 3,000 people is not well documented, surveillance efforts can be uniquely aligned to identify the relative risk of the disease in these rural populations.
Wastewater or non-sewered sanitation system surveillance is a flexible form of surveillance with the potential to provide information on many diseases circulating in a community with relatively few samples being collected. In the absence of wastewater treatment, septic tank emptying operations or building or factory-level plumbing access points can be sampled as representative of the community. Since the launch of widespread COVID-19 sanitation system surveillance in the United States, some jurisdictions have expanded their surveillance to include emerging diseases such as monkeypox, influenza, and polio (Ryerson et al., 2022, Mercier et al., 2022, Nelson, 2022, Tanne, 2022). Methods have been developed to include common circulating diseases, such as human adenovirus, measles, and norovirus (Kevill et al., 2022). Importantly, to evaluate many of these diseases, additional samples are not needed and can be assessed from the same sample volume collected to monitor SARS-CoV-2. This provides cost efficiency that cannot be matched using traditional clinical surveillance techniques. It is estimated that clinical surveillance costs approximately ten times more than wastewater surveillance (Manuel et al., 2022), and the cost savings are, in part, because wastewater represents a greater proportion of the population than clinical testing. Wastewater samples represent the pooled contribution of the community yet still provide the individual privacy of each community member. As well, wastewater surveillance may provide information on both symptomatic and asymptomatic populations (Cavany et al., 2022), including vaccinated individuals who may shed the virus during low-level infections. Furthermore, the samples of treatment-seeking and non-treatment-seeking infected individuals includes those who used at-home testing to make decisions regarding current and future healthcare visits. Given the barriers to healthcare and testing faced by rural patients, ensuring that the health surveillance system does not depend on clinical testing capacity is critical.
Significantly, wastewater samples are collected by researchers, wastewater utility workers, or sanitation workers, and analyzed by science, technology, engineering, and mathematics (STEM) professionals, which avoids overtaxing healthcare workers in rural locations. This health surveillance system can then be linked to continuously (and within days) notify healthcare providers to prepare for an influx of sick patients, including requests to mobilize testing and increase the number of traveling healthcare workers in the region. As rural locations comprise approximately 97% of the land in the United States (Ratcliffe et al., 2016), finding surveillance methods that involve these widespread communities is important. Creating a more inclusive disease surveillance system increases the chances for early detection of emerging diseases and outbreaks. This allows mitigation and prevention efforts to be implemented, thereby reducing the probability of an outbreak becoming an epidemic or pandemic. A truly robust framework should also consider opportunities to connect this surveillance with conventional public health surveillance mechanisms. For example, seasonal influenza will most likely be detected in wastewater before conventional syndromic surveillance using electronic health records. Connecting geographic reporting systems from clinical settings with the associated data from environmental sampling will increase situational awareness. Wastewater surveillance also has the potential to help mitigate the economic impact of disease outbreaks by preventing their spread and proactively addressing potential ones, thus overcoming many barriers found in rural communities that contribute to health disparities.
Moreover, wastewater surveillance allows the participation of more community members. One common theme among leaders at rural health workshops is that building trust among rural communities is the key to addressing rural health disparities (Cacari Stone et al., 2021). Treating rural communities as small urban communities often results in poor outcomes and frustration among the rural participants. Investing in long-term partnerships and building research capacity are critical for improving rural health (Cacari Stone et al., 2021). Partnerships among rural-serving academic institutions, healthcare providers, and communities allow for trust and collaboration among rural stakeholders. Designing health surveillance programs to meet the unique needs of rural populations, while building the trust necessary to implement changes, is critical in rural communities.
4. Learning from advancements in underserved areas of other countries
Developing countries, which compare to some rural communities in the United States, also have lower sewer connection coverage. Despite this, they have been able to show usefulness of sanitation system surveillance for the health of the communities as part of their pandemic response. Only 43% of the global population has access to household toilets connected to sewers (WHO, 2021). Furthermore, approximately 6% of the world’s population does not have any access to sanitation facilities (WHO, 2021). However, a case in point is South Africa, where 61% of the population has access to toilets connected to sewer systems, although sewer infrastructure distribution is not uniform across provinces (WHO, 2021). Household toilets connected to sewers are common in more urbanized provinces, namely the Western Cape (87%) and Gauteng (84%) provinces, while in South African metropolitan areas, approximately 16.8% of the population lives in informal dwellings (Statistics South Africa, 2019).
The adaptation model of the wastewater surveillance approach for pathogen detection in non-sewered settlements in South Africa is highly beneficial for developing countries that do not have fully inclusive sewer connections. Almost 40% of South Africans would not be included in surveillance for the health of their communities with wastewater surveillance , given a lack of sewered connections. Those left behind in sanitation access also typically lack access to sufficient healthcare or financial resources. In communities lacking formal sewerage networks, poorly or partially treated human excreta and graywater are often disposed into the environment, which enters a nearby stream or water source that has the potential to enter nearby rivers. Alternatively, community-level data and hotspot detection have been used to overcome the burden of individual testing by including a framework for sampling and surveillance of gray and wastewater within non-sewered communities, ensuring a timely response to an upsurge in disease detection. In contrast to wastewater, where a largely homogenous influent sample can be taken from a facility in South Africa, non-sewered informal settlement sampling becomes more challenging owing to the variety of systems and waste products generated. Previous sampling of non-sewered communities for SARS-CoV-2 carried out by Pocock et al. (2020) found that rivers downstream of the community were the most reliable sample sources in densely populated, non-sewered, settlements. The deployment of low-cost passive sampling devices into the rivers during periods of high rainfall, a process pioneered by Schang et al. (2021), overcomes the challenges of a dilute sample matrix in these vulnerable communities.
Although this wastewater approach in non-sewered portions of South Africa cannot relate viral loads in surface water to a defined population or possible number of cases, river sampling still provides an alternative means to monitor the spread of respiratory and enteric pathogens within informal settlements by monitoring trends and presence or absence in viral loads. During the COVID-19 pandemic, this was evidenced in that it allowed the identification of possible spikes in infections within the communities upstream of the sample point. This presence or absence approach may provide an alternative method for early warning against infections in unsewered communities, which have a high risk of rapid spread and a low likelihood of convenient clinical testing to trigger the deployment of rapid response teams.
5. Limitations of rural wastewater surveillance programs
Innovative approaches to implement wastewater surveillance in low-resource settings, such as rural locations, can be learned from these international efforts but are still not without limitations. Calibration of wastewater and clinical data that can be performed in urban settings remains an unrealistic expectation in rural or resource-limited settings. In such sampling programs, well-defined limits of detection and specific metrics employed to interpret results below quantification boundaries will be needed. This may require different approaches toward reporting data to rural public health officials or to a larger (academic) audience.
Understanding the boundaries of rural wastewater surveillance programs is essential. From a public health perspective, it may be more important to focus on common health disparities rather than rare diseases. Similarly, sampling locations may not be easily accessible due to limitations in building-level locations, such as agricultural, food-processing industries, correctional or long-term care facilities, or limited environmental samples, such as surface water. Finally, not all households in the United States have complete plumbing facilities (Rural Community Assistance Partnership, 2015). It is estimated that 1.7 million people in the United States lack basic plumbing. Rural populations, especially minority populations living in rural locations, are more than twice as likely to not have basic plumbing. These populations cannot be served by wastewater surveillance because basic sanitation services are not available. Promoting equity among these populations is dire and challenging.
6. Conclusions
A better surveillance model of disease detection among rural residents will overcome issues around the interactions of a disease and social determinants of health (Fig. 1). The SARS-CoV-2 pandemic has highlighted rural health disparities across the United States, but solutions require transdisciplinary approaches. Barriers to healthcare, comorbid diseases, poverty, and hesitation in prevention messaging from outside entities are some of the factors contributing to poor outcomes among rural patients infected with the disease. Wastewater surveillance has the potential to reduce rural health disparities by providing a flexible tool for assessing diseases in a wider community. Importantly, this form of surveillance does not rely on rural healthcare providers, who may be limited and overworked. Yet, this tool could provide the necessary warnings of potential outbreaks that may require coordinated responses from healthcare providers and community officials, as well as rural employers such as the agricultural and food-processing industries. The case study of South Africa, which demonstrates the ability to innovatively sample vulnerable communities, provides an alternative approach for monitoring rural communities in the United States. Furthermore, community participation and partnerships with local researchers involved in wastewater surveillance may help provide the boots-on-the-ground approach needed to encourage trust and the willingness to engage in mitigation measures that reduce the spread of diseases within the community. Building this infrastructure and trust will be key to improving not only health disparities related to COVID-19 but also a variety of other diseases. Wastewater surveillance is a critical tool that can be readily implemented to reduce future disparities in rural health.
Funding
This work was supported by the National Institutes of General Medical Sciences (grant numbers GM103443 and GM121341) and grants from the James Graham Brown Foundation, Owsley Brown II Family Foundation, Jon Rieger Seed Grant, and the Water Research Commission in South Africa. The content, study design, collection, analysis and interpretation of data, writing of the article, and decision to submit the article for publication are solely the responsibility of the authors and do not necessarily represent the views of the funding sources.
CRediT authorship contribution statement
Rochelle H. Holm: Writing – original draft, Writing – review & editing. Gina Pocock: Writing – original draft, Writing – review & editing. Marie A. Severson: Writing – original draft, Writing – review & editing. Victor C. Huber: Funding acquisition, Writing – review & editing. Ted Smith: Funding acquisition, Writing – review & editing. Lisa M. McFadden: Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Alcendor D.J. Targeting COVID vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID-19 pandemic in the south. Vaccines (Basel) 2021;9(11):1279. doi: 10.3390/vaccines9111279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A.J., Horswell R., Hendricks B.M., Chu S., Hillegass W.B., Beasley W.H., Harper J.R., Kimble W., Rosen C.J., Miele L., McClay J.C., Santangelo S.L., Hodder S.L. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J. Rural Health. 2023;39(1):39–54. doi: 10.1111/jrh.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn N.P., Snavely A.C., Angi R.M., Scheidler J.F., Crowe R.P., McGinnis H.D., Hiestand B.C., Miller C.D., Mahler S.A., Stopyra J.P. Prehospital time for patients with acute cardiac complaints: A rural health disparity. Am. J. Emerg. Med. 2021;52:64–68. doi: 10.1016/j.ajem.2021.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman P., Fitzgibbon M.L., Dulin A., Wang M.L., Baskin M. Society of behavioral medicine statement on COVID-19 and rural health. Transl. Behav. Med. 2021;11(2):625–630. doi: 10.1093/tbm/ibaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K., Goel V., Keeler C., Bell G.J., Aiello A.E., Corbie-Smith G., Wilson E., Fleischauer A., Emch M., Boyce R.M. SARS-CoV-2 testing in North Carolina: Racial, ethnic, and geographic disparities. Health Place. 2021;69 doi: 10.1016/j.healthplace.2021.102576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacari Stone L., Roary M.C., Diana A., Grady P.A. State health disparities research in Rural America: Gaps and future directions in an era of COVID-19. J. Rural Health. 2021;37(3):460–466. doi: 10.1111/jrh.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M.M., Call K.T., Klingner J.M. Are rural residents less likely to obtain recommended preventive healthcare services? Am. J. Prev. Med. 2001;21(3):182–188. doi: 10.1016/S0749-3797(01)00349-X. [DOI] [PubMed] [Google Scholar]
- Cavany S., Bivins A., Wu Z., North D., Bibby K., Perkins T.A. Inferring SARS-CoV-2 RNA shedding into wastewater relative to the time of infection. Epidemiol. Infect. 2022;150:e21. doi: 10.1017/S0950268821002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control, 2022A. Trends in COVID-19 Cases and Deaths in the United States, by County-level Population Factors. https://covid.cdc.gov/covid-data-tracker/#pop-factors_totaldeaths.
- Centers for Disease Control, 2022B. COVID-19 Vaccination Equity. https://covid.cdc.gov/covid-data-tracker/#vaccination-equity.
- Centers for Disease Control, 2022C. National Wastewater Surveillance System (NWSS). https://www.cdc.gov/nwss/wastewater-surveillance/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhealthywater%2Fsurveillance%2Fwastewater-surveillance%2Fwastewater-surveillance.html.
- Centers for Medicare and Medicaid Services, 2021. Critical Access Hospitals. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/CAHs.
- Chillag K.L., Lee L.M. Synergistic disparities and public health mitigation of COVID-19 in the rural United States. J. Bioeth. Inq. 2020;17(4):649–656. doi: 10.1007/s11673-020-10049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S.H., Califf R.M., Warraich H.J. Rural-urban disparity in mortality in the US from 1999 to 2019. J. Am. Med. Assoc. 2021;325(22):2312–2314. doi: 10.1001/jama.2021.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros D.F., Branscum A.J., Mukandavire Z., Miller F.D., MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann. Epidemiol. 2021;59:16–20. doi: 10.1016/j.annepidem.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros D.F., Moreno C.M., Musuka G., Miller F.D., Coule P., MacKinnon N.J. Association between vaccination coverage disparity and the dynamics of the COVID-19 Delta and Omicron waves in the US. Front. Med. (Lausanne) 2022;9 doi: 10.3389/fmed.2022.898101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros D.F., Gutierrez J.D., Moreno C.M., Escobar S., Miller F.D., Musuka G., Omori R., Coule P., MacKinnon N.J. Impact of healthcare capacity disparities on the COVID-19 vaccination coverage in the United States: A cross-sectional study. Lancet Regional Health-Am. 2023;18 doi: 10.1016/j.lana.2022.100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandachi D., Reece R., Wang E.W., Nelson T., Rojas-Moreno C., Shoemaker D.M. Treating COVID-19 in rural America. J. Rural Health. 2021;37(1):205–206. doi: 10.1111/jrh.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani S.S., Lone A.N., Javed Z., Khan M.S., Zia Khan M., Kaluski E., Virani S.S., Shapiro M.D., Cainzos-Achirica M., Nasir K., Khan S.U. Trends in premature mortality from acute myocardial infarction in the United States, 1999 to 2019. J. Am. Heart Assoc. 2022;11(1):e021682. doi: 10.1161/JAHA.121.021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow S., Wingert J.R., Hanchate A.D., Rote A., Westreich D., Sexton L., Cheng K., Curtis J., Jones W.S., Lanou A.J., Halladay J.R., Chen R.J. Rural-urban outcome differences associated with COVID-19 hospitalizations in North Carolina. PLoS One. 2022;17(8):e0271755. doi: 10.1371/journal.pone.0271755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVerteuil G. Reconsidering the legacy of urban public facility location theory in human geography. Prog. Hum. Geog. 2000;24(1):47–69. doi: 10.1191/030913200668094045. [DOI] [Google Scholar]
- Diaz A., Chhabra K.R., Scott J.W. The COVID-19 pandemic and rural hospitals —Adding insult to injury. Health Affairs Blog. 2020 https://www.healthaffairs.org/do/10.1377/hblog20200429.583513/full/ [Google Scholar]
- Dixon B.E., Grannis S.J., Lembcke L.R., Valvi N., Roberts A.R., Embi P.J., Shaman J. The synchronicity of COVID-19 disparities: statewide epidemiologic trends in SARS-CoV-2 morbidity, hospitalization, and mortality among racial minorities and in rural America. PLoS One. 2021;16(7):e0255063. doi: 10.1371/journal.pone.0255063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobis, E.A., Krumel, T.P., Cromartie, J., Conley, K.L., Sanders, A., Ortiz, R., 2021. Rural America at a glance. https://www.ers.usda.gov/webdocs/publications/102576/eib-230.pdf?v=7224.1.
- Gatwood J., McKnight M., Fiscus M., Hohmeier K.C., Chisholm-Burns M. Factors influencing likelihood of COVID-19 vaccination: A survey of Tennessee adults. Am. J. Health Syst. Pharm. 2021;78(10):879–889. doi: 10.1093/ajhp/zxab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning-Smith C. The Unique impact of COVID-19 on older adults in rural areas. J. Aging Soc. Policy. 2020;32(4–5):396–402. doi: 10.1080/08959420.2020.1770036. [DOI] [PubMed] [Google Scholar]
- Henning-Smith C., Tuttle M., Kozhimannil K.B. Unequal distribution of COVID-19 risk among rural residents by race and ethnicity. J. Rural Health. 2021;37(1):224–226. doi: 10.1111/jrh.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rural Health Information Hub, 2022. Rural Data Explorer. https://www.ruralhealthinfo.org/data-explorer.
- Hubach R.D., Shannon B., Morgan K.D., Alexander C., O'Neil A.M., Ernst C., Giano Z. COVID-19 vaccine hesitancy among rural Oklahomans. Rural Remote Health. 2022;22(2):7128. doi: 10.22605/RRH7128. [DOI] [PubMed] [Google Scholar]
- Jackson S.L., Derakhshan S., Blackwood L., Lee L., Huang Q., Habets M., Cutter S.L. Spatial disparities of COVID-19 cases and fatalities in United States counties. Int. J. Env. Res. Pub. He. 2021;18(16):8259. doi: 10.3390/ijerph18168259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B.G., Whitaker R., Pink G., Holmes G.M. Half of rural residents at high risk of serious illness due to COVID-19, creating stress on rural hospitals. J. Rural Health. 2020;36(4):584–590. doi: 10.1111/jrh.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevill J.L., Lambert-Slosarska K., Pellett C., Woodhall N., Richardson-O'Neill I., Pântea I., Alex-Sanders N., Farkas K., Jones D.L. Assessment of two types of passive sampler for the efficient recovery of SARS-CoV-2 and other viruses from wastewater. Sci. Total Environ. 2022;838(Pt 4) doi: 10.1016/j.scitotenv.2022.156580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazanchi, R., Powers, S.D., Rogawski, McQuade, E.T., McManus, K.A., 2021. Inequities in the geographic accessibility of COVID-19 biomedical therapeutic Trials in the United States. J. Gen. Intern. Med., 36, 11, 3650–3653. https://doi.org/10.1007/s11606-021-07081-0. [DOI] [PMC free article] [PubMed]
- Khazanchi R., Strumpf A., Essien U.R., Powers S.D., McManus K.A. Geographic accessibility of COVID-19 test to treat sites by race, ethnicity, age, and rurality. JAMA Netw. Open. 2022;5(11):e2241144. doi: 10.1001/jamanetworkopen.2022.41144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJoie A.S., Holm R.H., Anderson L.B., Ness H.D., Smith T., Aslan A. Nationwide public perceptions regarding the acceptance of using wastewater for community health monitoring in the United States. PLoS One. 2022;17(10):e0275075. doi: 10.1371/journal.pone.0275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani H.V., Pillai S.S., Zehra M., Sharma I., Sodhi K. Systematic review of clinical insights into novel coronavirus (COVID-19) pandemic: Persisting challenges in U.S. rural population. Int. J. Environ. Res. Public Health. 2020;17(12):4279. doi: 10.3390/ijerph17124279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Gaynor S.M., Quick C., Chen J.T., Stephenson B.J.K., Coull B.A., Lin X. Identifying US county-level characteristics associated with high COVID-19 burden. BMC Public Health. 2021;21(1):1007. doi: 10.1186/s12889-021-11060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lewis B, Berney K, et al. Social Vulnerability and Rurality Associated With Higher Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection-Induced Seroprevalence: A Nationwide Blood Donor Study-United States, July 2020-June 2021. Clin Infect Dis. 2022;75(1):e133–e143. doi: 10.1093/cid/ciac105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yin Z., Ni C., Yan C., Wan Z., Malin B. Examining rural and urban sentiment difference in COVID-19–related topics on Twitter: Word embedding–based retrospective study. J. Med. Internet Res. 2023;25:e42985. doi: 10.2196/42985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel, D., Amadei, C.A., Campbell, J.R., Brault, J.M., Zierler, A., 2022. Strengthening public health surveillance through wastewater testing: An essential investment for the COVID-19 pandemic and future health threats. Washington, DC: World Bank. Strengthening-Public-Health-Surveillance-Through-Wastewater-Testing-An-Essential-Investment-for-the-COVID-19-Pandemic-and-Future-Health-Threats (2).pdf.
- McElfish P.A., Willis D.E., Shah S.K., Bryant-Moore K., Rojo M.O., Selig J.P. Sociodemographic determinants of COVID-19 vaccine hesitancy, fear of infection, and protection self-efficacy. J. Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211040746. 21501327211040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C.Y., Kadonsky K.F., Roman F.A., Jr., Tariqi A.Q., Sinclair R.G., D'Aoust P.M., Delatolla R., Bischel H.N., Naughton C.C. The need of an environmental justice approach for wastewater-based epidemiology for rural and disadvantaged communities: A review in California. Curr. Opin. Environ. Sci. Health. 2022;27 doi: 10.1016/j.coesh.2022.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier E., D'Aoust P.M., Thakali O., Hegazy N., Jia J.J., Zhang Z., Eid W., Plaza-Diaz J., Kabir M.P., Fang W., Cowan A., Stephenson S.E., Pisharody L., MacKenzie A.E., Graber T.E., Wan S., Delatolla R. Municipal and neighbourhood level wastewater surveillance and subtyping of an influenza virus outbreak. Sci. Rep. 2022;12(1):15777. doi: 10.1038/s41598-022-20076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio. BMJ. 2022;378 doi: 10.1136/bmj.o1869. [DOI] [PubMed] [Google Scholar]
- Rural Community Assistance Partnership, 2015. Still living without the basics in the 21st century: Analyzing the availability of water and sanitation services in the United States. http://opportunitylinkmt.org/wp-content/uploads/2015/07/Still-Living-Without-the-Basics-Water.pdf.
- Pocock G., Coetzee L., Mans J., Taylor M., Genthe B. Proof of Concept Study: Application of wastewater-based surveillance to monitor SARS-CoV-2 prevalence in South African Communities. WRC Report TT832/20. 2020 [Google Scholar]
- Rader B., Astley C.M., Sy K.T.L., Sewalk K., Hswen Y., Brownstein J.S., Kraemer M.U.G. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. J. Travel Med. 2020;27(7):taaa076. doi: 10.1093/jtm/taaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe M., Burd C., Holder K., Fields A. Defining Rural at the U.S. Census Bureau. 2016 https://www.census.gov/content/dam/Census/library/publications/2016/acs/acsgeo-1.pdf [Google Scholar]
- Ridenhour B.J., Sarathchandra D., Seamon E., Brown H., Leung F.-Y., Johnson-Leon M., Megheib M., Miller C.R., Johnson-Leung J., Molina J.A. Effects of trust, risk perception, and health behavior on COVID-19 disease burden: Evidence from a multi-state US survey. PLoS One. 2022;17(5):e0268302. doi: 10.1371/journal.pone.0268302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson A.B., Lang D., Alazawi M.A., Neyra M., Hill D.T., St. George K., Fuschino M., Lutterloh E., Backenson B., Rulli S., Ruppert P.S., Lawler J., McGraw N., Knecht A., Gelman I., Zucker J.R., Omoregie E., Kidd S., Sugerman D.E., Jorba J., Gerloff N., Ng T.F.F., Lopez A., Masters N.B., Leung J., Burns C.C., Routh J., Bialek S.R., Oberste M.S., Rosenberg E.S., Anderson B.J., Anderson N., Augustine J.A., Baldwin M., Barrett K., Bauer U., Beck A., Belgasmi H., Bennett L.J., Bhatt A., Blog D., Boss H., Brenner I.R., Brister B., Brown T.W., Buchman T., Bullows J., Connelly K., Cassano B., Castro C.J., Cirillo C., Cone G.E., Cory J., Dasin A., de Coteau A., DeSimone A., Chauvin F., Dixey C., Dooling K., Doss S., Duggar C., Dunham C.N., Easton D., Egan C., Emery B.D., English R., Faraci N., Fast H., Feumba G.S., Fischer N., Flores S., Frolov A.D., Getachew H., Gianetti B., Godinez A., Gray T., Gregg W., Gulotta C., Hamid S., Hammette T., Harpaz R., Smith L.H., Hanson B., Henderson E., Heslin E., Hess A., Hoefer D., Hoffman J., Hoyt L., Hughes S., Hutcheson A.R., Insaf T., Ionta C., Miles S.J., Kambhampati A., Kappus-Kron H.R., Keys G.N., Kharfen M., Kim G., Knox J., Kovacs S., Krauchuk J., Krow-Lucal E.R., Lamson D., Laplante J., Larsen D.A., Link-Gelles R., Liu H., Lueken J., Ma K., Marine R.L., Mason K.A., McDonald J., McDonough K., McKay K., McLanahan E., Medina E., Meek H., Mustafa G.M., Meldrum M., Mello E., Mercante J.W., Mhatre M., Miller S., Migliore N., Mita-Mendoza N.K., Moghe A., Momin N., Morales T., Moran E.J., Nabakooza G., Neigel D., Ogbamikael S., O’Mara J., Ostrowski S., Patel M., Paul P., Paziraei A., Peacock G., Pearson L., Plitnick J., Pointer A., Popowich M., Punjabi C., Ramani R., Raymond S.J., Rickerman L., Rist E., Robertson A.C., Rogers S.L., Rosen J.B., Sanders C., Santoli J., Sayyad L., Schoultz L., Shudt M., Smith J., Smith T.L., Souto M., Staine A., Stokley S., Sun H., Terranella A.J., Tippins A., Tobolowsky F., Wallace M., Wassilak S., Wolfe A., Yee E. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case - New York, March 9-October 11, 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71(44):1418–1424. doi: 10.15585/mmwr.mm7144e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D.A., Dudley M.Z., Brewer J., Kan L., Gerber J.E., Budigan H., Proveaux T.M., Bernier R., Rimal R., Schwartz B. COVID-19 vaccination attitudes, values and intentions among United States adults prior to emergency use authorization. Vaccine. 2021;39(19):2698–2711. doi: 10.1016/j.vaccine.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., John N., Baker L., Scales P., Schmidt J., Thorley B.R., Hill K., Zamyadi A., Tseng C.W., Henry R., Kolotelo P., Langeveld J., Schilperoort R., Shi B., Einsiedel S., Thomas M., Black J., Wilson S., McCarthy D.T. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ. Sci. Tech. 2021;55(15):10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
- Shah P., Owens J., Franklin J., Mehta A., Heymann W., Sewell W., Hill J., Barfield K., Doshi R. Demographics, comorbidities and outcomes in hospitalized COVID-19 patients in rural southwest Georgia. Ann. Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Cain J., Sakhuja A., Schaefer G., Krupica T., Sarwari A. Guidance for Healthcare Providers Managing COVID-19 in Rural and Underserved Areas. J. Racial Ethn. Health Disparities. 2020;7(5):817–821. doi: 10.1007/s40615-020-00820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Holm R.H., Keith R.J., Amraotkar A.R., Alvarado C.R., Banecki K., Choi B., Santisteban I.C., Bushau-Sprinkle A.M., Kitterman K.T., Fuqua J., Hamorsky K.T., Palmer K.E., Brick J.M., Rempala G.A., Bhatnagar A. Quantifying the relationship between sub-population wastewater samples and community-wide SARS-CoV-2 seroprevalence. Sci. Total Environ. 2022;853 doi: 10.1016/j.scitotenv.2022.158567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souch J.M., Cossman J.S. A commentary on rural-urban disparities in COVID-19 testing rates per 100,000 and risk factors. J. Rural Health. 2021;37(1):188–190. doi: 10.1111/jrh.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Dakota Department of Health, 2020. South Dakota Primary Care Needs Assessment. https://doh.sd.gov/documents/Providers/RuralHealth/2016-2020_PrimaryCareNeedsAssessment.pdf.
- Statistics South Africa. (2019). Toilet facilities. (E. S. SA, Compiler) Pretoria.
- Summers-Gabr N.M. Rural-urban mental health disparities in the United States during COVID-19. Psychol Trauma-US. 2020;12(S1) doi: 10.1037/tra0000871. S222–S224. [DOI] [PubMed] [Google Scholar]
- Tanne J.H. Polio emergency declared in New York State over virus found in wastewater. BMJ. 2022;378 doi: 10.1136/bmj.o2211. [DOI] [PubMed] [Google Scholar]
- Wheeler P.H., Patten C.A., Wi C.I., Bublitz J.T., Ryu E., Ristagno E.H., Juhn Y.J. Role of geographic risk factors and social determinants of health in COVID-19 epidemiology: Longitudinal geospatial analysis in a midwest rural region. J. Clin. Transl. Sci. 2021;6(1):e51. doi: 10.1017/cts.2021.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman T.N.A., Rai N.K., Bodurtha P.J., Chamberlain A.M., DeSilva M., Jeruzal J., Johnson S.G., Kharbanda A., Klyn N., Mink P.J., Muscoplat M., Waring S., Yu Y., Drawz P.E. Trends in COVID-19 vaccine administration and effectiveness through October 2021. JAMA Netw. Open. 2022;5(3):e225018. doi: 10.1001/jamanetworkopen.2022.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF), 2021. Progress on household drinking water, sanitation and hygiene 2000-2020: Five years into the SDGs. Geneva, 2021. https://data.unicef.org/resources/progress-on-household-drinking-water-sanitation-and-hygiene-2000-2020/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.