Abstract
BACKGROUND
The effectiveness of Platelet-Rich Plasma (PRP) in the treatment of patients with Achilles tendon rupture (ATR) and Achilles tendinopathy (AT) has been controversial.
AIM
To assess PRP injections’ effectiveness in treating ATR and AT.
METHODS
A comprehensive review of relevant literature was conducted utilizing multiple databases such as Cochrane Library, PubMed, Web of Science, Chinese Science and Technology Journal, EMBASE, and China Biomedical CD-ROM. The present investigation integrated randomized controlled trials that assessed the effectiveness of platelet-rich plasma injections in managing individuals with Achilles tendon rupture and tendinopathy. The eligibility criteria for the trials encompassed publications that were published within the timeframe of January 1, 1966 to December 2022. The statistical analysis was performed utilizing the Review Manager 5.4.1, the visual analogue scale (VAS), Victorian Institute Ankle Function Scale (VISA-A), and Achilles Tendon Thickness were used to assess outcomes.
RESULTS
This meta-analysis included 13 randomized controlled trials, 8 of which were randomized controlled trials of PRP for AT and 5 of which were randomized controlled trials of PRP for ATR. PRP for AT at 6 wk [weighted mean difference (WMD) = 1.92, 95%CI: -0.54 to 4.38, I2 = 34%], at 3 mo [WMD = 0.20, 95%CI: -2.65 to 3.05, I2 = 60%], and 6 mo [WMD = 2.75, 95%CI: -2.76 to 8.26, I2 = 87%) after which there was no significant difference in VISA-A scores between the PRP and control groups. There was no significant difference in VAS scores between the PRP group and the control group after 6 wk [WMD = 6.75, 95%CI: -6.12 to 19.62, I2 = 69%] and 6 mo [WMD = 10.46, 95%CI: -2.44 to 23.37, I2 = 69%] of treatment, and at mid-treatment at 3 mo [WMD = 11.30, 95%CI: 7.33 to 15.27, I2 = 0%] after mid-treatment, the PRP group demonstrated better outcomes than the control group. Post-treatment patient satisfaction [WMD = 1.07, 95%CI: 0.84 to 1.35, I2 = 0%], Achilles tendon thickness [WMD = 0.34, 95%CI: -0.04 to 0.71, I2 = 61%] and return to sport [WMD = 1.11, 95%CI: 0.87 to 1.42, I2 = 0%] were not significantly different between the PRP and control groups. The study did not find any statistically significant distinction between the groups that received PRP treatment and those that did not, regarding the Victorian Institute of Sport Assessment - Achilles scores at 3 mo [WMD = -1.49, 95%CI: -5.24 to 2.25, I2 = 0%], 6 mo [WMD = -0.24, 95%CI: -3.80 to 3.32, I2 = 0%], and 12 mo [WMD = -2.02, 95%CI: -5.34 to 1.29, I2 = 87%] for ATR patients. Additionally, no significant difference was observed between the PRP and the control groups in improving Heel lift height respectively at 6 mo [WMD = -3.96, 95%CI: -8.61 to 0.69, I2 = 0%] and 12 mo [WMD = -1.66, 95%CI: -11.15 to 7.83, I2 = 0%] for ATR patients. There was no significant difference in calf circumference between the PRP group and the control group after 6 mo [WMD = 1.01, 95%CI: -0.78 to 2.80, I2 = 54%] and 12 mo [WMD = -0.55, 95%CI: -2.2 to 1.09, I2 = 0%] of treatment. There was no significant difference in ankle mobility between the PRP and control groups at 6 mo of treatment [WMD = -0.38, 95%CI: -2.34 to 1.58, I2 = 82%] and after 12 mo of treatment [WMD = -0.98, 95%CI: -1.41 to -0.56, I2 = 10%] there was a significant improvement in ankle mobility between the PRP and control groups. There was no significant difference in the rate of return to exercise after treatment [WMD = 1.20, 95%CI: 0.77 to 1.87, I2 = 0%] and the rate of adverse events [WMD = 0.85, 95%CI: 0.50 to 1.45, I2 = 0%] between the PRP group and the control group.
CONCLUSION
The use of PRP for AT improved the patient’s immediate VAS scores but not VISA-A scores, changes in Achilles tendon thickness, patient satisfaction, or return to sport. Treatment of ATR with PRP injections alone improved long-term ankle mobility but had no significant effect on VISA-A scores, single heel lift height, calf circumference or return to sport. Additional research employing more extensive sampling sizes, more strict experimental methods, and standard methodologies may be necessary to yield more dependable and precise findings.
Keywords: Platelet-Rich plasma, Achilles tendon rupture, Achilles tendinopathy, Systematic evaluation, Randomized controlled trial
Core Tip: Achilles tendon rupture (ATR) and Achilles tendinopathy (AT) are commonly seen in orthopaedic outpatient clinics. The effectiveness of Platelet-Rich Plasma in the treatment of patients with ATR and AT has been controversial. This study aims to inform the decisions of physicians faced with challenges when making treatment choices.
INTRODUCTION
Achilles tendon rupture (ATR) and Achilles tendinopathy (AT) are commonly seen in orthopaedic outpatient clinics. These conditions have serious complications and are treated clinically, mainly by conservative treatment. However, this treatment is ineffective and prone to recurrence[1]. The cause is regular unreasonable or excessive exercise, which leads to repeated friction or overstretching of the Achilles tendon and its surrounding tissues beyond the repair capacity of the tendon itself, caused inflammatory reactions in the tendon tissue. Chronic inflammation leads to the tendon’s collagen fibres and fatty tissue degeneration. This effect is diminished and can lead to spontaneous ATR[2].
The component of Achilles tendon tissue included tendon cells and fibrin collagen. Due to the lack of blood supply to the Achilles tendon, its healing rate is significantly lower than other damaged connective tissues[3].
AT and ATR are usually treated by surgical or non-surgical methods, which included non-steroidal anti-inflammatory drugs (NSAIDs), steroid hormone blocking treatment, low-frequency ultrasound stimulation, and hypothermia therapy. Steroid and lidocaine-blocking therapies brought excellent anti-inflammatory and analgesic results, which made it become the most popular treatments in physic therapist. However, collagen necrosis and decline in the Achilles tendon’s mechanical properties may caused by repeated injections[4-6]. Gastric ulcers is one of likely complications of prolonged use of NSAIDs. Therefore, the clinical use of these drugs in the treatment of Achilles tendon rupture and AT has been controversial[7]. Growth factors have been identified to play a vital role in ATR, Therefore Certain researchers suggested the utilization of platelet-rich plasma (PRP) as a potential treatment for conditions affecting the Achilles tendon[8,9].
PRP injections, which are increasingly used in clinical practice, are platelet concentrates that often contain various growth factors within them. In some cases, an increase on following growth factors were noticed, including epidermal growth factor, platelet-derived growth factor (PDGF), transforming growth factor-b1, basic fibroblast growth factor, vascular endothelial growth factor, insulin-like growth factor and hepatocyte growth factor[10]. Therefore, platelet-rich plasma has attracted the curiosity of numerous researchers as a tissue regeneration-inducing factor[11-14]. Several current investigations have demonstrated that PRP is useful in the treatment of orthopedic injuries, including syndrome of the carpal tunnel[15-17], Rotator cuff rupture[18-20], AT[21,22], Achilles tendon injury[23], etc. Animal experimentation has demonstrated that PRP injections speed up the histopathological recuperation of the Achilles tendon, boost the overall strength of the tendon, relieve the inflammatory response, and facilitate tendon regeneration[24,25].
PRP has been shown to increase the histopathological recovery of the Achilles tendon in humans. However, the role of PRP in human Achilles tendon healing remains controversial[21,22,24-28]. Overall, additional research is required to investigate and uncover the efficacy of PRP treatment for individuals with AT to inform and guide clinical decision-making. This study aimed to determine whether PRP injection is viable for patients with AT. Treatment success depends on measuring Achilles tendon function scores, pain levels, return to sport rates, complication rates and the time taken to return to the pre-injury functional range. There are few studies on the effects of PRP injections on Achilles tendon disease. This study aims to inform the decisions of physicians faced with challenges when making treatment choices.
MATERIALS AND METHODS
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards, before initiating the investigation, we drafted a protocol delineating our objectives, criteria for eligibility literature search methodologies, statistical assessments, and outcome measures. The registration number for PROSPERO is CRD 42023388903.
Study design
This study employed meta-analysis to compile quantitative research on the efficacy of PRP for treating AT. The research was conducted in a structured manner, and the methodology employed in this study involved several steps. (1) A study strategy and criteria aligned with the study’s objectives were developed; (2) The primary databases of interest were searched using specific search terms and formulas; (3) The studies were screened for eligibility based on the predetermined criteria; (4) The quality of the literature was evaluated using the Jadad scale to eliminate those of inferior quality; (5) Involves using a data extraction form to extract the required data meticulously; (6) Entails the utilization of Review Manager 5.4.1 for data examination; and (7) Involves analyzing and interpreting the results and marking decisions.
Data collection
The inclusion criteria were restricted to the following conditions: (1) The investigation focused on patients with AT or ATR; (2) The study intervention involved administering a local PRP injection to the experimental group; (3) Various outcome indicators, including the Victorian Institute Ankle Function Scale (VISA-A) score, visual analogue scale (VAS) for pain, variations in Achilles tendon thickness, rate of returning to sports activities, patient satisfaction, return to sport, ankle flexion, return to pre-injury heel-rise height, and incidence of adverse events, were utilized in this study; and (4) The focus of this research is on randomized controlled trials (RCTs) published prior to December 2022.
Exclusion criteria
The study excluded trials with the following criteria: (1) Non-randomized trials; (2) A follow-up duration of less than 6 wk; (3) No control group; (4) Used PRP with other drugs; and (5) Did not report results.
Search method
Searches were conducted in Cochrane Library, PubMed, Web of Science, Chinese Science and Technology Journal, EMBASE, and China Biomedical CD-ROM without restriction on language. The search terms were “PRP”, “Platelet Concentrate”, “platelet gel”, “ATR”, “Achilles tendon injury”, “AT”, and “AT”, all the full text from 1966 to 2022 was download, carefully read.
Literature screening
At least two individuals conduct separate literature searches using predetermined keywords and formulas to access databases. The literature screening process outcomes were then consolidated through literature management tools, with duplicate papers being eliminated and insignificant literature being excluded based on title lists. Potentially appropriate research was downloaded and thoroughly read, with the final choice of literature determined by inclusion and exclusion criteria. In the event of divergent results, a third person was invited for Arbitration and selection.
Quality evaluation of the included studies
The present study conducted a methodological analysis of previous research about the research design protocol, randomization approach concealment, blinding implementation, and the incidence of missed visits. The Jadad scale was employed for this purpose. The total score achievable on this scale was seven. Research studies that obtained a cumulative score ranging from one to three were categorized as low-quality, while those that obtained a cumulative score ranging from four to seven were classified as high-quality. Studies with a score ≥ three or higher were deemed eligible for participation in this investigation[29-31].
Statistical analysis
Review Manager 5.4.1 (Revman 5.4) served as the statistical tool for this investigation. Following the evaluation criteria, all admissible data from the literature were extracted and inserted into Revman 5.4 for analysis. The risk difference or the odds ratio was used to describe count data (such as adverse events and return to sport rate).
All metrics (such as VISA-A score, ankle mobility, VAS score, etc.) were reported as weighted mean difference (WMD)[32]. The study assessed heterogeneity utilizing the I2 index, which served as an indicator of the degree of heterogeneity. Studies with I2 values below 31% were deemed homogeneous, while those with I2 values above 56% were considered more heterogeneous. Studies with I2 values between 56% and 70% could not be excluded from heterogeneity. When I2 values were below 50%, the fixed-effects model Peto method was employed to combine effect sizes. Conversely, when I2 values exceeded 50%, the random-effects model DerSimonian-Laird method was utilized for calculation. In cases where there was significant heterogeneity among groups, meta-analysis was abandoned in favor of descriptive analysis.
RESULTS
A total of 16 publications were screened, and 3 were excluded through further evaluation: 1 trial design protocol with no outcome indicators. One was a retrospective case-control study and not a true RCT trial. 1 had no primary outcome indicators. A total of 13 publications were included in the final results (Figure 1).
Figure 1.
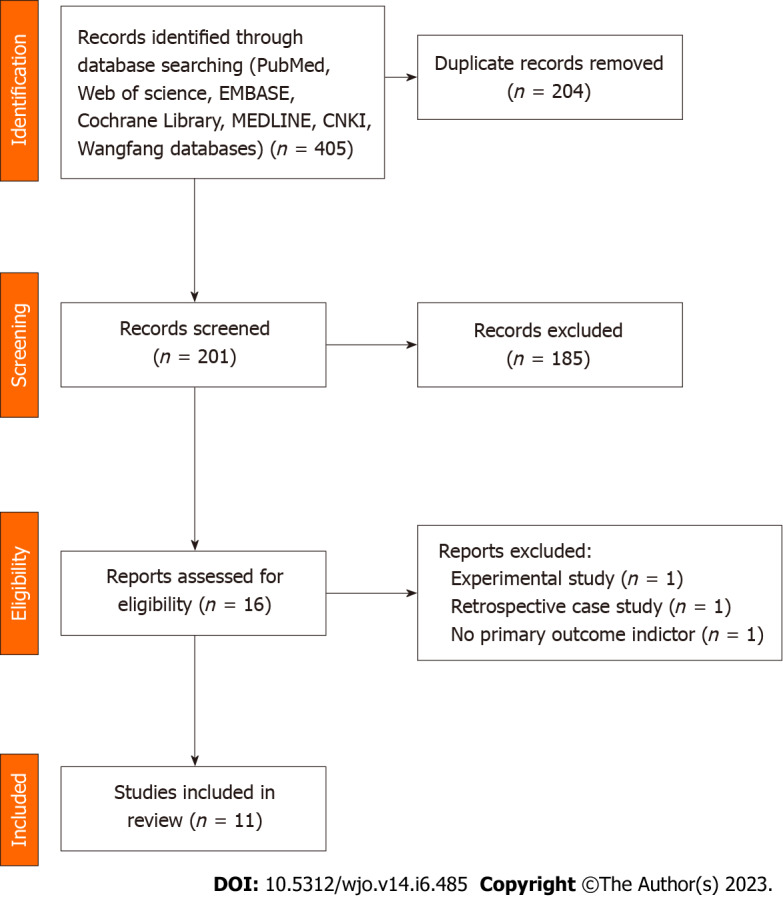
Flow diagram of study selection.
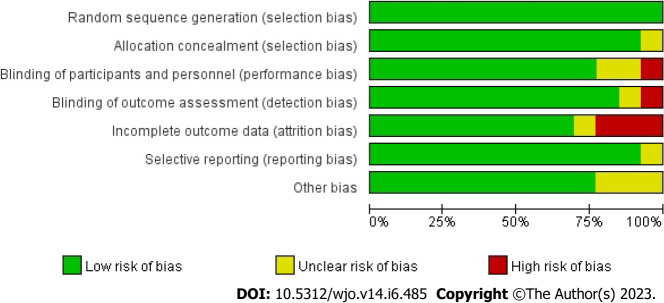
All 13 RCTs, comprising 857 cases, were included in this study. All trials were published in full text, with 13 being in English and none in Chinese. Eight trials included 526 subjects in a randomized controlled trial of PRP for AT, and five included 349 subjects in a randomized controlled trial of PRP for ART. All had modified Jadad scores greater than 3. Saline were used in eight studies as a control group, and blank controls were used in five studies; 12 were double-blind RCTs, and one was a single-blind RCT; the basic characteristics of the related literature are listed in Table 1. Every included studies were compared at baseline, and there found no significant differences in age, weight, gender and treatment between the two comparable groups. Regarding the risk of bias, Figures 2 and 3 shows the assessment results of the included studies.
Table 1.
Characteristics of randomized controlled studies included in this meta-analysis
|
Ref.
|
Sample size (I/C)
|
Achilles tendon lesion
|
Location
|
Control
|
Follow-up
|
PRP injection frequency/interval/dose (mL)
|
| de Vos et al[42], 2010 | 27/27 | C-AT (> 2 mo) | Netherlands | Saline | 6, 12, 24 wk | Once/-/4 |
| de Jonge et al[41], 2011 | 27/27 | C-AT (> 2 mo) | Netherlands | Saline | 6, 12, 24, 48 wk | Once/-/4 |
| Kearney et al[50], 2013 | 9/10 | C-AT (> 8 mo) | United Kingdom | Blank | 6 wk, 3 mo, 6 mo | Once/-/3 to 5 |
| Krogh et al[22], 2016 | 12/12 | C-AT (mean 33 mo) | Denmark | Saline | 3, 6, 12 mo | Once/-/6 |
| Boesen et al[21], 2017 | 19/19 | C-AT (> 3 mo) | Denmark | Saline | 6, 12, 24 wk | 4 times/2-wk/4 |
| van der Vlist et al[53], 2020 | 39/41 | C-AT (> 6 mo) | Netherlands | Saline | 2, 6, 12, 24 wk | Once/-/NR |
| Thermann et al[51], 2020 | 17/19 | C-AT (> 6 mo) | Italy | Blank | 6 wk, 3, 6, 12 mo | Once/-/NR |
| Kearney et al[52], 2021 | 121/119 | C-AT (> 3 mo) | United Kingdom | Blank | 2 wk, 3, 6 mo | Once/-/3 |
| Schepull et al[28], 2011 | 15/14 | A-ATR (< 3 d) | Sweden | Blank | 6, 12 mo | Once/-/10/4 |
| De Carli et al[49], 2015 | 15/15 | A-ATR | Italy | Blank | 1, 3, 6, 24 mo | 2 times/2-wk/4 |
| Zou et al[27], 2016 | 16 /20 | A-ATR (< 3 wk) | China | Placebo | 3 wk, 3, 6, 12, 24 mo | Once/-/NR |
| Keene et al[33], 2019 | 107/109 | A-ATR (< 12 d) | United Kingdom | Placebo | 4, 7, 13, 24 wk | Once/-/4 |
| Boesen et al[34], 2020 | 19/19 | A-ATR (< 3 d) | Denmark | Saline | 8 wk, 3, 6, 12 mo | Once/-/4 |
PRP: Platelet-Rich Plasma; AT: Achilles tendinopathy; ATR: Achilles tendon rupture.
Figure 2.
Proportional risk of bias graph: Judgement of the percentage of bias items arising from all included studies.
Figure 3.
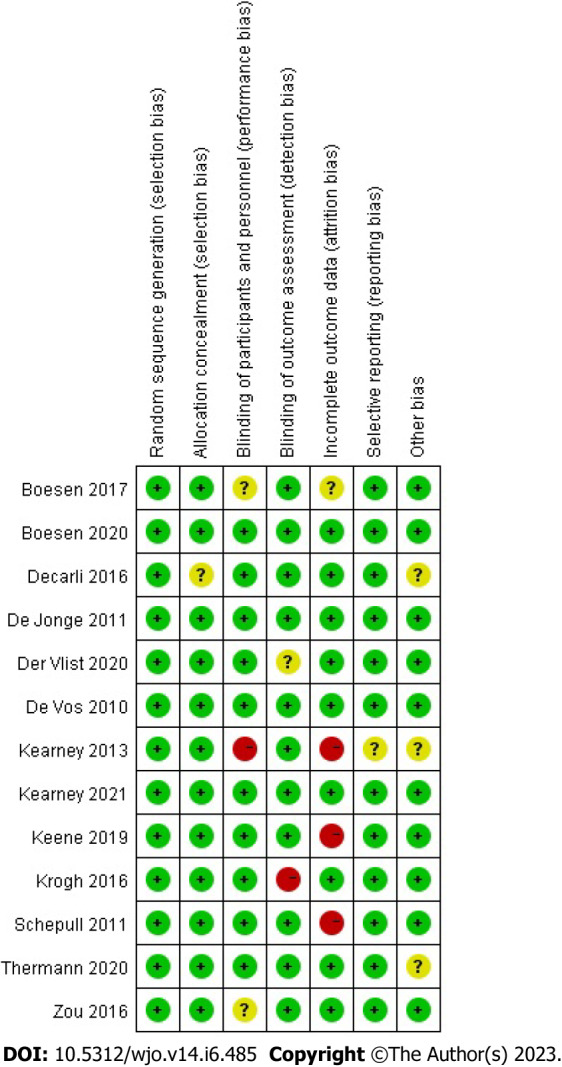
Risk of bias summary diagram: Assessment of specific items of bias arising from all included studies.
Results of the analysis of AT
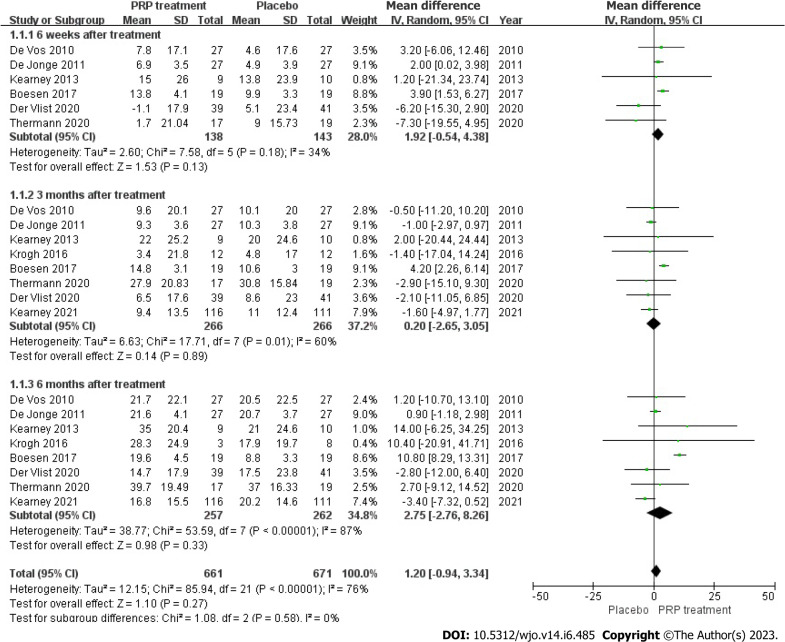
VISA-A rating: The VISA-A score changes were accurately and fairly stated in 8 articles, including 526 patients. The combined statistic was calculated using a random effects model due to statistical heterogeneity between studies (I2 = 76%, P = 0.00001), The study revealed that the VISA-A score was greater in the PRP group as compared to the placebo group [WMD = 1.20, 95%CI: -0.94 to 3.34, P = 0.27]. No important statistical improvement was observed in the VISA-A score among those who had PRP therapy (Figure 4).
Figure 4.
Forest plot of change values for Victorian Institute Ankle Function Scale scores. PRP: Platelet-Rich Plasma.
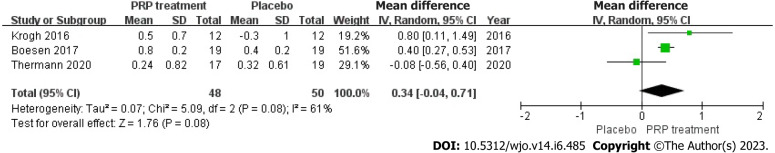
Change in Achilles tendon thickness: Changes in Achilles tendon thickness were accurately and appropriately reported by 98 individuals across 3 articles. Due to statistical heterogeneity among studies (I2 = 61%, P = 0.08), we applied a model with random effects to calculate the pooled statistic, which indicated that the PRP group had thicker Achilles tendons than the placebo group [WMD = 0.34, 95%CI: -0.04 to 0.71, P = 0.08], a difference not supported by statistical evidence. This indicates no significant improvement in AT thickness in the PRP group (Figure 5).
Figure 5.
Forest plot of Achilles tendon thickness variation. PRP: Platelet-Rich Plasma.
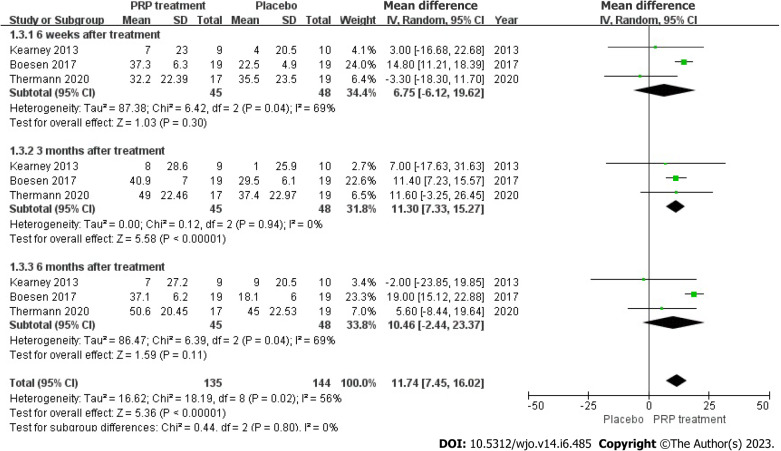
VAS score: Changes in VAS score were accurately and appropriately reported by 93 individuals across 3 articles. The result shows there was a statistical heterogeneity among those studies (I2 = 56%, P = 0.02), we used a random effects model to determine the pooled statistic, the result indicated that the VAS scores in PRP group was greater than the placebo group [WMD = 11.74, 95%CI: -7.45 to 16.02, P < 0.00001], with a significant statistical difference. According to this result, PRP treatment group brings significantly promotions on VAS scores compare to the placebo group (Figure 6).
Figure 6.
Forest plot of change in visual analogue scale scores. PRP: Platelet-Rich Plasma.
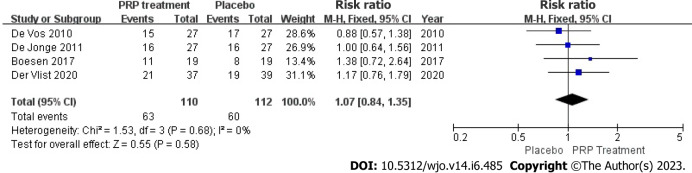
Patient satisfaction: Enhancements in patient satisfaction were accurately and appropriately reported by 222 patients across four studies. Because there was heterogeneity among due to the heterogeneity among the research papers (I2 = 0%, P = 0.68), the study employed a fixed-effects approach to determine the paired statistic. The findings indicate that the level of patient satisfaction was higher in the group that received platelet-rich plasma compared to the group that received a placebo [Relative risk (RR) = 1.07, 95%CI: 0.84 to 1.35, P = 0.58], but there was no statistically significant difference between the two groups. According to this information, the level of patient satisfaction did not substantially increase in the PRP group compared to the placebo group (Figure 7).
Figure 7.
Forest plot of change in patient satisfaction. PRP: Platelet-Rich Plasma.
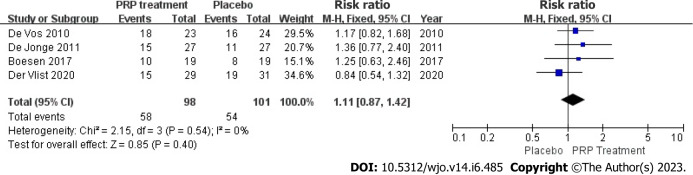
Return to sport rate: 199 patients accurately and reasonably described their exercise resumption rates across 4 publications. The results indicated no statistical heterogeneity among studies (I2 = 0%, P = 0.54), in present study we used a fixed-effects approach to determine the combined statistic, the result indicated that the ratio of return to exercise in the PRP-treated group (experimental group) was greater than in the placebo group (control group) [RR = 1.11, 95%CI: 0.87 to 1.42, P = 0.40]. This suggests that the PRP therapy group did not substantially increase the patient’s return to activity rate compared to the placebo group (Figure 8).
Figure 8.
Forest plot of patient return to exercise rate. PRP: Platelet-Rich Plasma.
Results of the analysis of the effectiveness of PRP in the treatment of ATR
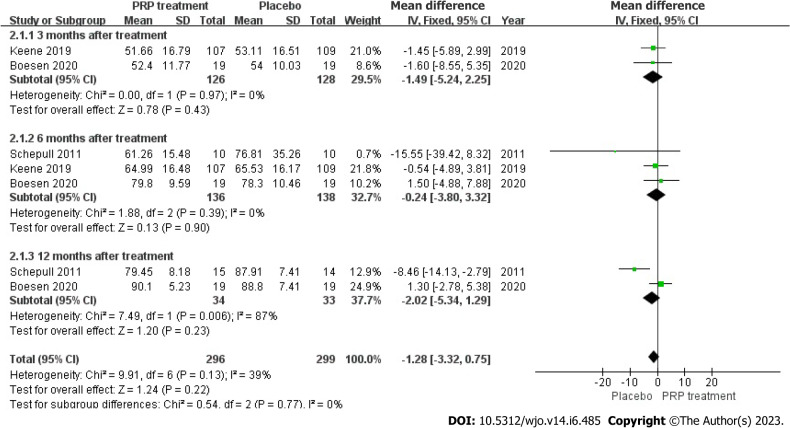
VISA-A Rating: In total, 274 patients from three different articles gave an accurate and credible description of the value of the change in their VISA-A score. Because the statistical result shows no heterogeneity among the studies (I2 = 39%, P = 0.13), we used a fixed-effects approach to generate the paired statistic. This result indicated that the PRP group had smaller VISA-A Scale scores compared to the placebo group [WMD = -1.28, 95%CI: -3.32 to 0.75, P = 0.22]; there was also no significant difference among both groups statistically (Figure 9).
Figure 9.
Forest plot of Victorian Institute Ankle Function Scale scores in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
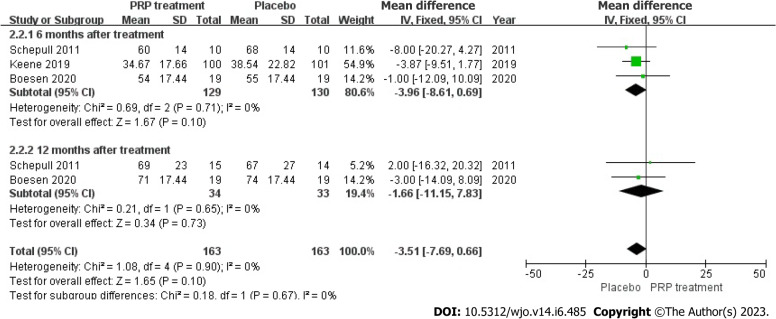
Single-foot heel height: In three articles, 259 patients accurately and adequately defined the height of a single heel lift. Because the statistical result shows no heterogeneity among those studies (I2 = 0%, P = 0.90), we used a fixed effects approach to determine the overall statistic, the results revealed that the PRP group had a smaller single heel lift height compared to the placebo group. [WMD = -3.51, 95%CI: -7.69 to 0.66, P = 0.10], there was no statistical difference. This result suggested that the PRP group did not substantially enhance the single heel lift height among individuals with ATR (Figure 10).
Figure 10.
Forest plot of single heel lift height in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
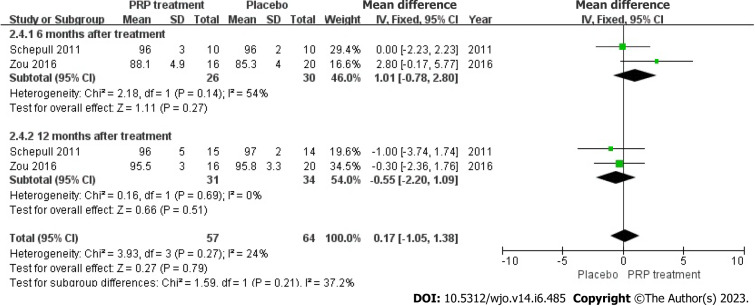
Calf circumference: Two papers accurately and reasonably reported the calf circumference measurements of 75 patients. The statistical result shows no heterogeneity among those studies (I2 = 24%, P = 0.27), we used fixed-effects approach to determine the paired statistic, the result indicated that calf circumference in the PRP group was greater compared to the placebo group [WMD = 0.17, 95%CI: -1.05 to 1.38, P = 0.79], there was no statistical difference. This suggests that the PRP therapy did not substantially improve calf circumference length in patients with ATR (Figure 11).
Figure 11.
Forest plot of calf circumference in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
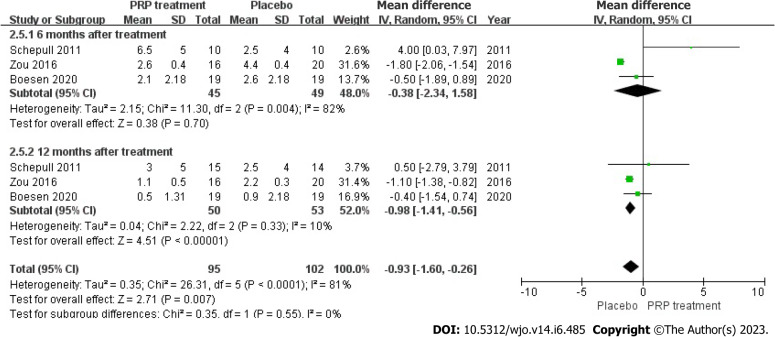
Ankle mobility: Ankle mobility was accurately and fairly assessed in 103 patients across 3 articles. The statistical result shows no heterogeneity among each study (I2 = 81%, P < 0.0001), we used a random effects approach to determine the paired statistic, the result demonstrated that ankle mobility in the PRP group was lower compared to the placebo group [WMD = 0.93, 95%CI: -1.60 to -0.26, P = 0.007], the statistically difference was significant. This indicates that the PRP therapy did not substantially increase ankle mobility in individuals with ATR (Figure 12).
Figure 12.
Forest plot of ankle mobility in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
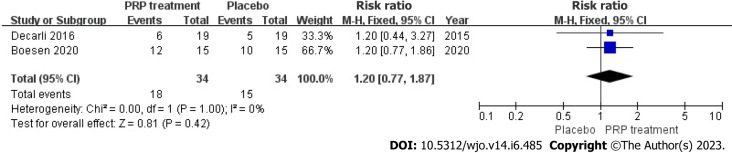
Return to sport rate: Two studies included 68 patients who accurately and sufficiently reported the ratio of their return to exercise. The statistical result shows no heterogeneity among those studies (I2 = 0%, P = 1.00), we used a fixed-effects approach to determine the paired statistic, the outcome showed that there was no statistically significant difference in the ratio of return to sport in the PRP group and the placebo group [RR = 1.20, 95%CI: 0.77 to 1.87, P = 0.42]. This suggests that the PRP the therapy did not substantially increase the return-to-sport rate among individuals with ATR (Figure 13).
Figure 13.
Forest plot of return to sport rates in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
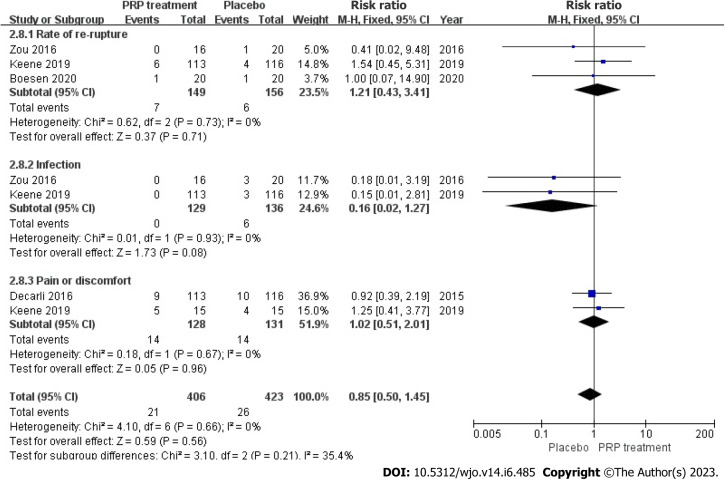
Incidence of adverse events: A total of 2 publications with 305 patients correctly and reasonably described the incidence of adverse events. The statistical result shows no heterogeneity among the studies (I2 = 0%, P = 0.66), we used a fixed-effects approach to determine the paired statistic, the PRP group’s Achilles tendon re-rupture was greater than the placebo group [RR = 1.21, 95%CI: 0.43 to 3.47, P = 0.71]; no statistical difference existed. The prevalence of pain and distress in the PRP-treated group was greater compared to the placebo group [RR = 1.02, 95%CI: 0.51 to 2.01, P = 0.96]; however, the difference between the groups was not statistically significant. The results showed no statistically significant difference between the PRP-treated group and the control group in terms of re-rupture rate of the Achilles tendon, infection, and incidence of pain and discomfort (Figure 14).
Figure 14.
Forest plot of the incidence of adverse events in patients with Achilles tendon rupture. PRP: Platelet-Rich Plasma.
DISCUSSION
PRP is a platelet-rich plasma obtained by centrifugation of whole blood from animals or humans, to which thrombin is added to turn it into a gel, and the platelet concentration of PRP increases on average by a factor of two to six. Platelets contain high levels of growth factors such as growth-like insulin growth factor, PDGF, epidermal growth factor (EGF). Transforming growth factor-β, vascular EGF, etc. Theoretically, when platelet concentrates are activated, PRP injections can release supraphy siological concentrations of various autologous growth factors with healing and regenerative abilities for treating musculoskeletal disorders[33,34]. Theoretical In vitro and in vivo experiments have also demonstrated the mechanobiological properties of PRP to promote the healing of tendon injuries[24,35-37]. Li et al[38] demonstrated that PRP heals tendon tissue by reducing the number of cells, decreasing the number of blood vessels, promoting type I collagen deposition and increasing the glycosaminoglycan content. PRP is also derived from the patient’s own body. Furthermore, PRP is derived from the patient himself and is administered after a short period of in vitro centrifugation. PRP therapies have achieved satisfactory clinical results; however, there are no consistent results on their overall efficacy and inconsistencies in patient outcomes, and new insights challenge the usefulness of PRP for clinical use[39,40].
AT develops from many factors, and this disorder’s exact mechanism is unknown. Most studies suggest that AT is primarily caused by anatomical abnormalities resulting from overexertion, incorrect exercise training, stiffness and weakness of the limbs. Various factors first lead to local inflammation of the Achilles tendon. These effects lead to degenerative changes and eventually to partial or complete Achilles tendon rupture. This meta-analysis found that the use of PRP for AT did not significantly change patients’ VISA-A scores, patient satisfaction, return to sport rates, or Achilles tendon thickness compared to controls, and interestingly, patients’ VAS scores at 3 mo were significantly improved compared to controls but did not significantly change long-term clinical outcomes. (This is similar to De Jonge’s findings.) The randomized controlled trial by de Jonge et al[41] showed that PRP injections did not significantly improve pain and function in patients with tendonitis compared to placebo injections in patients with chronic Achilles tendonitis after 1 year of PRP treatment, and there was no advantage in ultrasound findings. deVos et al[42] conducted a randomized controlled trial utilizing a double-blind method. The study involved 54 patients with chronic inflammatory Achilles tendon disease, all receiving PRP treatment. The study demonstrated that the administration of PRP therapy did not result in significant alterations in the ultrasound echo structure or neovascularisation scores associated with Achilles tendon injuries[42]. Neither study described a clinical benefit of PRP injection over placebo. The study by Krogh et al[22] also concluded that PRP injection had no additional value in treating AT. However, this study raises concerns about the quality of the method. Patients were blinded, but the outcome assessors were aware of the interventions implemented. Furthermore, they reported a 54% decline rate three months after PRP, making it almost impossible to observe a potential late effect of PRP. Interestingly Boesen et al[21] reported a significant improvement in VISA-A scores in the PRP group. This difference may be because Boesen et al[21] considered tendon healing a slow process, so they repeated PRP injections to achieve a prolonged effect of the growth factor on the tendon. Another possibility is that there were differences in the rehabilitation protocols of these studies; for example, Boesen et al[21] prescribed running to start 10 d after PRP injection, while other studies ranged from 4 d (Krogh) to 28 d (deVos)[22,42]. Thirdly, there were differences in the effects of PRP on vascular tendon distribution and tendon thickness, with Boesen et al[21] concluding that PRP administration reduced vascular distribution and tendon thickness within the tendon. de Jonge et al[41] reported an improvement in tendon structure in the PRP and placebo groups, but no significant difference was found between the two groups. These results did not confirm an association between PRP and changes in tendon structure. The results did not confirm an association between PRP and changes in the tendon structure. Finally, it is possible that the location of PRP injections was not uniform, with Krogh et al[22] injecting at the very back of the tendon and de Vos et al[42] injecting at the most severely affected site.
The Achilles tendon is a relatively blood-deprived tissue, with the blood supply to the Achilles tendon coming mainly from the posterior tibial artery return branch[43]. The surface skin blood supply system is fragile. As a result, healing after an injury is slower than in other tissues. Some studies have shown that growth factors can promote tendon repair[44]. Some studies have shown that growth factors can promote tendon repair. Some in vitro experiments have demonstrated that instantaneous injection of plasma-rich protein for tendon injury enhances tendon recovery in rats[45]. Zhang et al[46] found that the premature injection of plasma-rich protein into a tendon injury may have provided sufficient growth factors to promote angiogenesis, which ultimately shortened tendon healing time. However, Zou et al[27] found that leukocyte-rich platelets may be detrimental to treating tendon injuries in rabbits. Leukocyte-rich platelets caused catabolic gene expression, increased protein production, and promoted an increase in inflammatory mediators. This inflammatory mediator induces non-tendon cell differentiation[47,48].
PRP injections in the treatment of ATR as a new treatment method with no consistent results on its overall efficacy. Therefore, we performed a meta-analysis to provide evidence-based information on using PRP injections for ATR. The results showed that the PRP-treated group (experimental group) was not statistically significantly different from the control group in terms of VISA-A score, single heel lift height, return to motion rate, Achilles tendon re-rupture rate, infection, and incidence of pain and discomfort. There was no significant improvement in ankle mobility in the PRP-treated group at 6 mo, and after 12 mo, the results showed that PRP injection improved ankle mobility. Overall, our findings do not support using PRP for ATR; however, this meta-analysis has some limitations, and the findings need to be considered cautiously. This is because differences in PRP preparation methods can lead to differences in the biological characteristics of the final product. The frequency and dosage of PRP injections in the experimental groups varied in many studies, which may lead to uncertainty in clinical outcomes. A study by Keene et al[33] described each component of PRP injections, as well as several representative growth factors; two studies by Schepull et al[28] and Zou et al[27] reported only the platelet concentrations of their PRP injections. Zou’s study showed that PRP positively affected ankle function in short to medium term[27]. However, in a study by De Carli et al[49], there was no difference between the PRP and control groups after acute Achilles tendon rupture surgery as measured by the VISA-A score and the Foot and Ankle Outcome Score. Similar inconsistent results may be due to the inconsistencies in PRP concentrations, preparation protocols and injection sites described above.
Hence, additional research is required to determine the optimal concentration, injection duration, and injection location for PRP for ART and AT. The study results indicate a lack of statistical significance in the impact of PRP on ankle plantarflexion angle, ankle plantar flexion strength, and pain.
Limitations
Although clear inclusion and exclusion criteria have been established for this study, the small total sample size and a limited number of trials limit the strength of the evidence. Moreover, there remains a high degree of heterogeneity in the quantitative analysis of these randomized controlled trial studies. Heterogeneity may stem from the degree of tendinopathy, the method used to produce PRP, the cellular composition of PRP, the conditions in which PRP was stored, the method of injection, the dose and frequency of injection and the setting of the control group. The scoring criteria and methods used in the included studies also differed.
This study was unable to analyze PRP in different subgroups due to the heterogeneous nature of PRP production and application techniques and the absence of an standardized classification system. Consequently, a comprehensive investigation into the effectiveness and safety of a specific form of PRP therapy in treating tendinopathies was not feasible.
CONCLUSION
This study showed no significant efficacy of PRP injection alone in patients with ATR and AT. Thirteen high-quality RCT articles were reviewed to reach this conclusion. Future studies should focus on completing RCTs with large sample sizes and standardizing the preparation/procedure of PRP injections. As well as exploring the clinical efficacy of PRP injections combined with minimally invasive AT techniques.
ARTICLE HIGHLIGHTS
Research background
Achilles tendon rupture (ATR) and Achilles tendinopathy (AT) are commonly seen in orthopaedic outpatient clinics. These conditions have serious complications and are treated clinically, mainly by conservative treatment. However, this treatment is ineffective and prone to recurrence. Along with the deep investigation of research, it is found that growth factors promoted Achilles tendon repair. The use of Platelet-Rich Plasma (PRP) was indicated to treat Achilles tendon diseases.
Research motivation
But the effectiveness of PRP in the treatment of patients with ATR and AT has been controversial.
Research objectives
To determine whether PRP injection is viable for patients with AT and to inform the decisions of physicians faced with challenges when making treatment choices.
Research methods
This study conducted a comprehensive review of relevant literature was conducted utilizing multiple databases such as Cochrane Library, PubMed, Web of Science, Chinese Science and Technology Journal, EMBASE, and China Biomedical CD-ROM. The present investigation integrated randomized controlled trials that assessed the effectiveness of platelet-rich plasma injections in managing individuals with ATR and AT. The eligibility criteria for the trials encompassed publications that were published within the timeframe of January 1, 1966 to December 2022. The statistical analysis was performed utilizing the Review Manager 5.4.1. The Victorian Institute Ankle Function Scale (VISA-A), Visual Analogue Scale (VAS) and Achilles tendon thickness were used to assess outcomes.
Research results
This meta-analysis included 13 randomized controlled trials, 8 of which were randomized controlled trials of PRP for AT and 5 of which were randomized controlled trials of PRP for ATR. PRP for AT at 6 wk, at 3 mo, and 6 mo after which there was no significant difference in VISA-A scores between the PRP and control groups. There was no significant difference in VAS scores between the PRP group and the control group after 6 wk and 6 mo of treatment, and at mid-treatment at 3 mo after mid-treatment, the PRP group demonstrated better outcomes than the control group. Post-treatment patient satisfaction, Achilles tendon thickness and return to sport were not significantly different between the PRP and control groups. There was no significant difference between the PRP and control groups for VISA-A score improvement at 3 mo, 6 mo, and 12 mo for ATR patients. Additionally, no significant difference was observed between the PRP and the control groups in improving heel lift height respectively at 6 mo and 12 mo for ATR patients. There was no significant difference in calf circumference between the PRP group and the control group after 6 mo and 12 mo of treatment. There was no significant difference in ankle mobility between the PRP and control groups at 6 mo of treatment and after 12 mo of treatment there was a significant improvement in ankle mobility between the PRP and control groups. There was no significant difference in the rate of return to exercise after treatment and the rate of adverse events between the PRP group and the control group.
Research conclusions
This study showed no significant efficacy of PRP injection alone in patients with ATR and AT. Thirteen high-quality RCT articles were reviewed to reach this conclusion. Future studies should focus on completing randomized controlled trials with large sample sizes and standardizing the preparation/procedure of PRP injections. As well as exploring the clinical efficacy of PRP injections combined with minimally invasive AT techniques.
Research perspectives
This meta-analysis reviewed 13 high-quality randomized controlled trials articles and the result suggested that there is no significant efficacy of PRP injection alone in patients with ATR and AT.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 24, 2022
First decision: March 28, 2023
Article in press: April 20, 2023
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rahmati M, Iran; Tavan H, Iran S-Editor: Liu XF L-Editor: A P-Editor: Yuan YY
Contributor Information
Dan Huang, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China.
Djandan Tadum Arthur Vithran, Department of Orthopaedics, Xiangya Hospital of Central South University, Changsha 410013, Hunan Province, China.
Hao-Li Gong, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China.
Ming Zeng, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China.
Zhong-Wen Tang, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China.
Zhou-Zhou Rao, Department of Physiology, Hunan Normal University School of Medicine, Changsha 410005, Hunan Province, China.
Jie Wen, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China; Department of Anatomy, Hunan Normal University School of Medicine, Changsha 410005, Hunan Province, China. cashwj@qq.com.
Sheng Xiao, Department of Pediatric Orthopedics, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410013, Hunan Province, China.
References
- 1.McClinton S, Luedke L, Clewley D. Nonsurgical Management of Midsubstance Achilles Tendinopathy. Clin Podiatr Med Surg. 2017;34:137–160. doi: 10.1016/j.cpm.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Abbassian A, Khan R. Achilles tendinopathy: pathology and management strategies. Br J Hosp Med (Lond) 2009;70:519–523. doi: 10.12968/hmed.2009.70.9.43869. [DOI] [PubMed] [Google Scholar]
- 3.Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3:29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- 4.Wetke E, Johannsen F, Langberg H. Achilles tendinopathy: A prospective study on the effect of active rehabilitation and steroid injections in a clinical setting. Scand J Med Sci Sports. 2015;25:e392–e399. doi: 10.1111/sms.12326. [DOI] [PubMed] [Google Scholar]
- 5.Speed CA. Fortnightly review: Corticosteroid injections in tendon lesions. BMJ. 2001;323:382–386. doi: 10.1136/bmj.323.7309.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava P, Aggarwal A. Ultrasound-guided retro-calcaneal bursa corticosteroid injection for refractory Achilles tendinitis in patients with seronegative spondyloarthropathy: efficacy and follow-up study. Rheumatol Int. 2016;36:875–880. doi: 10.1007/s00296-016-3440-4. [DOI] [PubMed] [Google Scholar]
- 7.Caudell GM. Insertional Achilles Tendinopathy. Clin Podiatr Med Surg. 2017;34:195–205. doi: 10.1016/j.cpm.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Najafbeygi A, Fatemi MJ, Lebaschi AH, Mousavi SJ, Husseini SA, Niazi M. Effect of Basic Fibroblast Growth Factor on Achilles Tendon Healing in Rabbit. World J Plast Surg. 2017;6:26–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Ai G, Shao X, Meng M, Song L, Qiu J, Wu Y, Zhou J, Cheng J, Tong X. Epidermal growth factor promotes proliferation and maintains multipotency of continuous cultured adipose stem cells via activating STAT signal pathway in vitro. Medicine (Baltimore) 2017;96:e7607. doi: 10.1097/MD.0000000000007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanco D, Vigano' M, Croiset SJ, De Girolamo L. Applications and limits of platelet-rich plasma in sports related injuries. J Biol Regul Homeost Agents. 2012;26:53S–61S. [PubMed] [Google Scholar]
- 11.Liu CJ, Yu KL, Bai JB, Tian DH, Liu GL. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: A meta-analysis. Medicine (Baltimore) 2019;98:e15278. doi: 10.1097/MD.0000000000015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65:1951–1957. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Dong SW, Liu JP, Tao X, Tang KL, Xu JZ. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res. 2012;30:991–997. doi: 10.1002/jor.22033. [DOI] [PubMed] [Google Scholar]
- 14.Mifune Y, Matsumoto T, Takayama K, Ota S, Li H, Meszaros LB, Usas A, Nagamune K, Gharaibeh B, Fu FH, Huard J. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage. 2013;21:175–185. doi: 10.1016/j.joca.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Wu YT, Ho TY, Chou YC, Ke MJ, Li TY, Huang GS, Chen LC. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: A prospective randomized, single-blind controlled trial. Sci Rep. 2017;7:94. doi: 10.1038/s41598-017-00224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med. 2018;12:e1480–e1488. doi: 10.1002/term.2566. [DOI] [PubMed] [Google Scholar]
- 17.Uzun H, Bitik O, Uzun Ö, Ersoy US, Aktaş E. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome. J Plast Surg Hand Surg. 2017;51:301–305. doi: 10.1080/2000656X.2016.1260025. [DOI] [PubMed] [Google Scholar]
- 18.Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil. 2020;33:387–396. doi: 10.3233/BMR-191519. [DOI] [PubMed] [Google Scholar]
- 19.Han L, Fang WL, Jin B, Xu SC, Zheng X, Hu YG. Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur Rev Med Pharmacol Sci. 2019;23:9075–9084. doi: 10.26355/eurrev_201910_19310. [DOI] [PubMed] [Google Scholar]
- 20.Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative Effectiveness of Injection Therapies in Rotator Cuff Tendinopathy: A Systematic Review, Pairwise and Network Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil. 2019;100:336–349.e15. doi: 10.1016/j.apmr.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of High-Volume Injection, Platelet-Rich Plasma, and Sham Treatment in Chronic Midportion Achilles Tendinopathy: A Randomized Double-Blinded Prospective Study. Am J Sports Med. 2017;45:2034–2043. doi: 10.1177/0363546517702862. [DOI] [PubMed] [Google Scholar]
- 22.Krogh TP, Ellingsen T, Christensen R, Jensen P, Fredberg U. Ultrasound-Guided Injection Therapy of Achilles Tendinopathy With Platelet-Rich Plasma or Saline: A Randomized, Blinded, Placebo-Controlled Trial. Am J Sports Med. 2016;44:1990–1997. doi: 10.1177/0363546516647958. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Fan H, Li Y, Yun Z, Zhang Z, Zhu Q. Effectiveness of platelet-rich plasma injections for the treatment of acute Achilles tendon rupture: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e27526. doi: 10.1097/MD.0000000000027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuksel S, Guleç MA, Gultekin MZ, Adanır O, Caglar A, Beytemur O, Onur Küçükyıldırım B, Avcı A, Subaşı C, İnci Ç, Karaoz E. Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect Tissue Res. 2016;57:360–373. doi: 10.1080/03008207.2016.1189909. [DOI] [PubMed] [Google Scholar]
- 25.Yüksel S, Adanır O, Gültekin MZ, Çağlar A, Küçükyıldırım BO, Güleç MA, Alagöz E. Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthop Traumatol Turc. 2015;49:544–551. doi: 10.3944/AOTT.2015.15.0028. [DOI] [PubMed] [Google Scholar]
- 26.Takamura M, Yasuda T, Nakano A, Shima H, Neo M. The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop Traumatol Turc. 2017;51:65–72. doi: 10.1016/j.aott.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou J, Mo X, Shi Z, Li T, Xue J, Mei G, Li X. A Prospective Study of Platelet-Rich Plasma as Biological Augmentation for Acute Achilles Tendon Rupture Repair. Biomed Res Int. 2016;2016:9364170. doi: 10.1155/2016/9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39:38–47. doi: 10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- 29.Rahmati M, Koyanagi A, Banitalebi E, Yon DK, Lee SW, Il Shin J, Smith L. The effect of SARS-CoV-2 infection on cardiac function in post-COVID-19 survivors: A systematic review and meta-analysis. J Med Virol. 2023;95:e28325. doi: 10.1002/jmv.28325. [DOI] [PubMed] [Google Scholar]
- 30.Rahmati M, Fatemi R, Yon DK, Lee SW, Koyanagi A, Il Shin J, Smith L. The effect of adherence to high-quality dietary pattern on COVID-19 outcomes: A systematic review and meta-analysis. J Med Virol. 2023;95:e28298. doi: 10.1002/jmv.28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahmati M, Gondin J, Malakoutinia F. Effects of Neuromuscular Electrical Stimulation on Quadriceps Muscle Strength and Mass in Healthy Young and Older Adults: A Scoping Review. Phys Ther. 2021;101 doi: 10.1093/ptj/pzab144. [DOI] [PubMed] [Google Scholar]
- 32.Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Physiotherapy. 2021;113:12–28. doi: 10.1016/j.physio.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Keene DJ, Alsousou J, Harrison P, Hulley P, Wagland S, Parsons SR, Thompson JY, O'Connor HM, Schlüssel MM, Dutton SJ, Lamb SE, Willett K PATH-2 trial group. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ. 2019;367:l6132. doi: 10.1136/bmj.l6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boesen AP, Boesen MI, Hansen R, Barfod KW, Lenskjold A, Malliaras P, Langberg H. Effect of Platelet-Rich Plasma on Nonsurgically Treated Acute Achilles Tendon Ruptures: A Randomized, Double-Blinded Prospective Study. Am J Sports Med. 2020;48:2268–2276. doi: 10.1177/0363546520922541. [DOI] [PubMed] [Google Scholar]
- 35.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med. 2018;11:624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connell B, Wragg NM, Wilson SL. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019;376:143–152. doi: 10.1007/s00441-019-02996-x. [DOI] [PubMed] [Google Scholar]
- 37.Jiang G, Wu Y, Meng J, Wu F, Li S, Lin M, Gao X, Hong J, Chen W, Yan S, Yan R, Feng G, Cheng Z. Comparison of Leukocyte-Rich Platelet-Rich Plasma and Leukocyte-Poor Platelet-Rich Plasma on Achilles Tendinopathy at an Early Stage in a Rabbit Model. Am J Sports Med. 2020;48:1189–1199. doi: 10.1177/0363546520906142. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Wu Y, Jiang G, Tian X, Hong J, Chen S, Yan R, Feng G, Cheng Z. Intratendon delivery of leukocyte-rich platelet-rich plasma at early stage promotes tendon repair in a rabbit Achilles tendinopathy model. J Tissue Eng Regen Med. 2020;14:452–463. doi: 10.1002/term.3006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Yu J, Zhang J, Hua Y. Simvastatin With PRP Promotes Chondrogenesis of Bone Marrow Stem Cells In Vitro and Wounded Rat Achilles Tendon-Bone Interface Healing In Vivo. Am J Sports Med. 2019;47:729–739. doi: 10.1177/0363546518819108. [DOI] [PubMed] [Google Scholar]
- 40.Alsousou J, Thompson M, Harrison P, Willett K, Franklin S. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. Lancet. 2015;385 Suppl 1:S19. doi: 10.1016/S0140-6736(15)60334-8. [DOI] [PubMed] [Google Scholar]
- 41.de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 42.de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 43.Browning SR, Weiser AM, Woolf N, Golish SR, SanGiovanni TP, Scuderi GJ, Carballo C, Hanna LS. Platelet-rich plasma increases matrix metalloproteinases in cultures of human synovial fibroblasts. J Bone Joint Surg Am. 2012;94:e1721–e1727. doi: 10.2106/JBJS.K.01501. [DOI] [PubMed] [Google Scholar]
- 44.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 45.Doral MN, Alam M, Bozkurt M, Turhan E, Atay OA, Dönmez G, Maffulli N. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc. 2010;18:638–643. doi: 10.1007/s00167-010-1083-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, Beck J, Dorsett-Martin W, Lineaweaver WC. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112:1613–1619. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- 47.Çirci E, Akman YE, Şükür E, Bozkurt ER, Tüzüner T, Öztürkmen Y. Impact of platelet-rich plasma injection timing on healing of Achilles tendon injury in a rat model. Acta Orthop Traumatol Turc. 2016;50:366–372. doi: 10.3944/AOTT.2015.15.0271. [DOI] [PubMed] [Google Scholar]
- 48.Lyras DN, Kazakos K, Verettas D, Botaitis S, Agrogiannis G, Kokka A, Pitiakoudis M, Kotzakaris A. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129:1577–1582. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 49.De Carli A, Lanzetti RM, Ciompi A, Lupariello D, Vadalà A, Argento G, Ferretti A, Vulpiani MC, Vetrano M. Can platelet-rich plasma have a role in Achilles tendon surgical repair? Knee Surg Sports Traumatol Arthrosc. 2016;24:2231–2237. doi: 10.1007/s00167-015-3580-1. [DOI] [PubMed] [Google Scholar]
- 50.Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-richplasma injection with an eccentric loading programme. Bone Joint Res. 2013;2:227–232. doi: 10.1302/2046-3758.210.2000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thermann H, Fischer R, Gougoulias N, Cipollaro L, Maffulli N. Endoscopic debridement for non-insertional Achilles tendinopathy with and without platelet-rich plasma. J Sport Health Sci. 2023;12:275–280. doi: 10.1016/j.jshs.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearney RS, Ji C, Warwick J, Parsons N, Brown J, Harrison P, Young J, Costa ML ATM Trial Collaborators. Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial. JAMA. 2021;326:137–144. doi: 10.1001/jama.2021.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Vlist AC, van Oosterom RF, van Veldhoven PLJ, Bierma-Zeinstra SMA, Waarsing JH, Verhaar JAN, de Vos RJ. Effectiveness of a high volume injection as treatment for chronic Achilles tendinopathy: randomised controlled trial. BMJ. 2020;370:m3027. doi: 10.1136/bmj.m3027. [DOI] [PMC free article] [PubMed] [Google Scholar]