Abstract
Importance:
Improvements in risk factor control in the US have stalled, remain badly sub-optimal. The benefit of continue improving goal achievement was not evaluated.
Objective:
To quantify potential gains in life expectancy (LE) from lowering glycated hemoglobin (A1c), systolic blood pressure (SBP), low-density lipoprotein (LDL) cholesterol, and body mass index (BMI) toward optimal levels.
Design:
We calibrated the Building, Relating, Assessing, and Validating Outcomes (BRAVO) diabetes microsimulation model to a nationally representative sample with type 2 diabetes (T2D) using their linked short-term mortality data from the National Death Index. We then used the model to conduct the simulation experiment on the study population over a lifetime.
Setting:
United States (US).
Participants:
We used data from the National Health and Nutrition Examination Survey (2009–2016) to generate a nationally representative US sample with T2D.
Intervention:
We grouped the study population into quartiles based on levels of A1c, SBP, LDL, and BMI. We then estimate the LE gains resulting from achieving better control by moving people with T2D from the current quartile of each biomarker to the lower quartiles.
Main Outcome:
life expectancy.
Results:
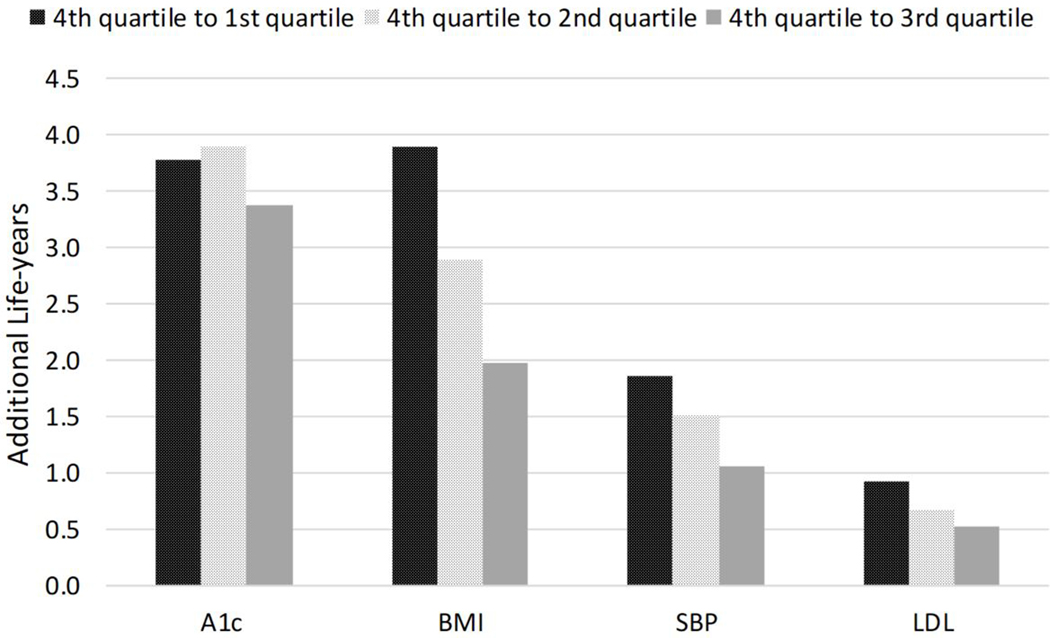
Compared to a BMI level of 41.4 kg/m2 (mean of the 4th quartile (4th)), a lower BMI of 24.3 kg/m2 (1st), 28.6 kg/m2 (2nd ), and 33.0 kg/m2 (3rd) were associated with 3.9, 2.9 and 2.0 additional life-years in people with T2D, respectively. Compared to SBP of 160.4 mmHg (4th), a lower SBP level of 114.1 mmHg (1st), 128.2 mmHg (2nd ), and 139.1 mmHg (3rd) were associated with 1.9, 1.5, and 1.1 years gained in LE in people with T2D, respectively. A lower LDL level of 59 mg/dl (1st), 84.0 mg/dl (2nd), and 107.0 mg/dl (3rd) were associated with 0.94, 0.68, and 0.53 years gain in LE, compared to LDL of 146.2 mg/dl (4th). Reducing A1c from 9.9% (4th) to 7.7% (3rd) was associated with 3.4 years gain in LE. However, a further reduction to 6.8% (2nd) was associated with only an average of 0.5 years gain in LE, and from 6.8% to 5.9% (1st) resulted in no benefit in prolonging LE.
Conclusion:
Our findings can be used by clinicians to motivate patients in achieving the recommended treatment goals and to help prioritize interventions and programs to improve diabetes care in the US.
Introduction
People with type 2 diabetes (T2D) have increased risks of macrovascular and microvascular complications1, which lead to an escalated risk of premature death.1 Compared to people without T2D at the age of 50, having T2D is associated with a life expectancy (LE) loss of 6 years.2 Better control of blood pressure, glucose and cholesterol levels, and body weight in people with T2D can potentially reduce the risk of diabetes-related complications and mortality, thus extending LE.3–5 Several studies have shown that higher body weight was associated with a significant loss in LE.6–8 Other studies also found that lowering blood pressure among individuals with T2D could lead to a longer LE.9–11 The benefit from better management of glucose, blood pressure, cholesterol, and body weight is also influenced by age and health conditions. A reduction of A1c from 8% to 6% was estimated to increase 1.2 life-years in women aged 55, but this benefit was much smaller in women in their 70s (0.8 life-years).3
Quantifying life-years gained from better diabetes care is imperative in clinical practice and designing public health interventions. Clinicians can use this information in the shared decision-making process with their patients, emphasizing the benefit of diabetes care in prolonging life expectancy. Policymakers can also use this information to prioritize and design public health interventions /programs. For the European population, LE associated with changes in glucose, blood pressure, cholesterol level, and body weight were estimated from the United Kingdom Prospective Diabetes Study (UKPDS)3 and the Framingham risk charts.3,12,13 Similar estimates are not available for the US population, a distinctive population from Europe in terms of racial demographics, healthcare systems, and environment. Data have been lacking from long-term trials in the US to conduct the lifetime projection of individuals with T2D.
The completion of several long-term US-based trials (e.g., the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial14) and recent innovation in the US-based diabetes simulation modeling provide essential data and methods needed for quantifying the long-term benefit of improving diabetes care in the US. In this study, we used the Building, Relating, Assessing, and Validating Outcomes (BRAVO) diabetes model to quantify the potential gains in LE from achieving different levels of A1c, SBP, LDL, and BMI in a US representative population with T2D.
Methods
The overall goal of the study was to to evaluate the impact of modifiable biomarker control on LE in patients with T2D using microsimulation experiment. To achieve this goal we: 1) Calibrated the validated BRAVO simulation model to a US nationaly representative sampe and 2) Use the calibrated BRAVO model to evaluate LE in patients wit T2D.Model Calibration
The BRAVO diabetes model is a discrete-time patient-level microsimulation model recently developed using data from the ACCORD trial.14 This model utilizes patients’ current risk profile to project long-term health outcomes, including diabetes-related complications, hypoglycemia, mortality, and the progression of modifiable biomarkers.14,15 Unlike most mainstream diabetes simulation models using the UKPDS risk equations, the BRAVO model uses novel risk equations developed from the ACCORD trial. The BRAVO diabetes model has been extensively validated and calibrated against international trials15–17 and used in several studies for program evaluation.15,18,19
Study participants enrolled in the ACCORD trial (2001–2009) were persons with T2D with elevated risks for cardiovascular complications. We performed model calibration to bridge the differences between ACCORD participants and the general T2D population in the US. To calibrate the BRAVO model for the general US population, we built a calibration sample from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) and linked mortality records from the National Death Index (NDI).20 We then re-validated and re-calibrated the BRAVO model using the NHANES calibration sample. We used relative bias (RB), defined as the ratio of the difference between predicted and observed mortality to the observed mortality, to measure the prediction accuracy of the model. Detailed methods are provided in Appendix 1.
Study Population
We used the 2015–2016 NHANES survey cycle and corresponding survey weights to construct a US-representative study population of individuals with diabetes. The study included individuals aged 51–80 years with self-reported diagnosed diabetes by a health care provider with data on demographic characteristics (age, race and ethnicity, duration of diagnosed diabetes, sex, education attainment, current smoking status (yes/no)), and measured biomarkers for four biomarkers (A1c, SBP, LDL, and BMI). We excluded those with a self-reported history of CVD as treatment goals and management strategies are likely to differ. Details regarding how information was collected in NHANES have been published previously.21
Gain in LE associated with biomarker control at the population level
The BRAVO diabetes model utilizes the patients’ risk profile to populate the simulation of the diabetes progression and estimate the corresponding LE. As we focused on evaluating the potential life-year gain from multi-faceted diabetes care in the US T2D population, we first grouped all persons with T2D into quartiles based on the distributions of the four biomarkers examined in our study. We then estimated the LE for patients from each quartile and the LE if patients from each quartile improved one of their biomarker levels to the next lower quartile while keeping other biomarkers constant. This allowed us to estimate the potential gains in LE associated with improvement of each biomarker from 4th to 3rd, 2nd, and 1st quartile, respectively.
Gain in LE associated with improved biomarkers at the individual level, according to age and sex subgroups
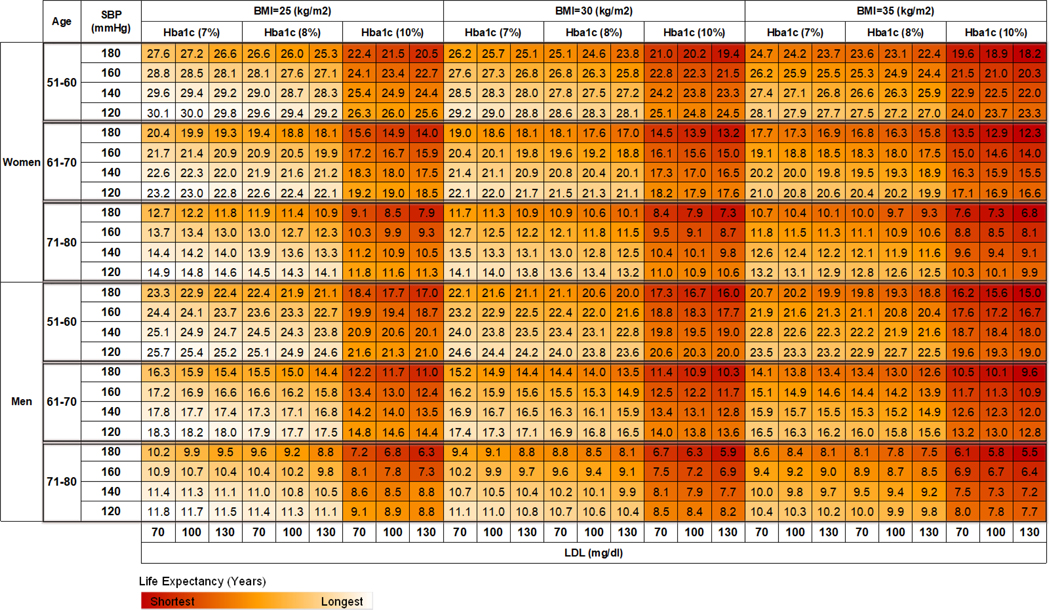
We further examined how an improvement in each biomarker affects LE at the individual level by age, sex, and levels of biomarkers. We grouped our simulation sample into six subgroups based on age (51 to 60 years, 61 to 70 years, and 71 to 80 years) and sex (male or female). Multiple alternative scenarios with different levels of each biomarker were simulated for each age-sex subgroup. Three BMI levels were tested: 25 kg/m2, 30 kg/m2, and 35 kg/m2. A1c was tested in three levels: 7%, 8%, and 10%. Four SBP levels (120 mmHg, 140 mmHg, 160 mmHg, and 180 mmHg) and three LDL levels (70 mg/dl, 100 mg/dl, and 130 mg/dl) were also examined according to the relevance of these risk levels in clinical practice.3,22,23 A heatmap was created to summarize the results from these simulation scenarios.
Results
Detailed demographics and risk profiles of the target population are provided in eTable 3 (Appendix 2). We grouped A1c into quartiles (<6.4%, 6.4–7.2%,7.3–8.2%, and >8.2%) with a mean of 5.9%, 6.8%,7.7%, and 9.9% for each corresponding quartile. We grouped SBP into quartiles (<122 mmHg, 122–132 mmHg, 133–144 mmHg, >144mmHg) with a mean of 114.1 mmHg, 128.2 mmHg, 139.1 mmHg, and 160.4 mmHg for each quartile respectively. We grouped LDL into quartiles (<73 mg/dl, 73–96 mg/dl, 97–122 mg/dl, >122 mg/dl) with a mean of 59 mg/dl, 84 mg/dl, 107 mg/dl, and 146.2 mg/dl for each quartile. BMI quartiles (<27 kg/m2, 27–31 kg/m2, 32–36 kg/m2, >36 kg/m2) had means of 24.3 kg/m2, 28.6 kg/m2, 33.0 kg/m2, and 41.4 kg/m2, respectively.
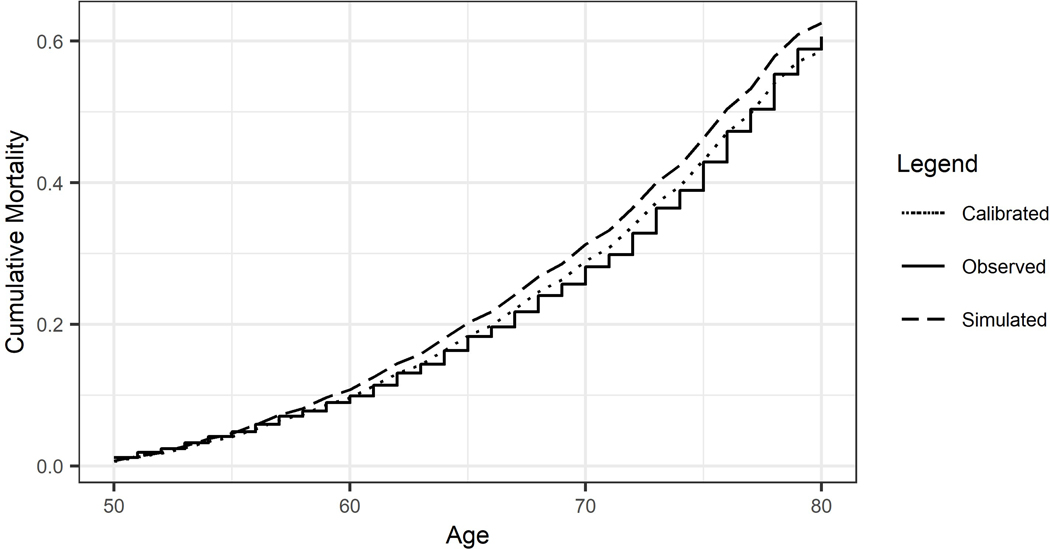
Figure 1 presents the cumulative mortality over time following the 51–55 age group. The solid line denotes the Kaplan–Meier curve for the observed cumulative mortality, serving as the benchmark for the calibration. The dashed line shows the predicted cumulative mortality generated from the un-calibrated BRAVO model. And the dotted line denotes the cumulative mortality predicted using the calibrated BRAVO model. The BRAVO diabetes model produced predictions with an average 5-year RB of 13.3% over the six age groups before the calibration. The average RB was reduced to 2.9% as a result of the calibration. Detailed measurements for model performance and interpretation were provided in eTable 2 (appendix 1).
Figure 1:
Cumulative mortality over 30 years in individuals with type 2 diabetes at 51–55 years old.
Figure 2 presents gains in LE associated with improving biomarkers in people with T2D at a population level. For glucose control, reducing A1c from 9.9% (mean of 4th quartile) to 7.7% (mean of 3rd quartile) was associated with an average of 3.4 years gain in LE. However, a further reduction from 7.7% to 6.8% (mean of 2nd quartile) was associated with only an average of 0.5 years gain in LE, and from 6.8% to 5.9% (mean of 1st quartile) resulted in no benefit in prolonging LE (0.1 years shorter in LE). Compared to individuals with a BMI level of 41.4 kg/m2 (mean of 4th quartile), a lower BMI level of 24.3 kg/m2 (mean of 1st quartile), 28.6 kg/m2 (mean of 2nd quartile), and 33.0 kg/m2 (mean of 3rd quartile), were associated with an average of 3.9, 2.9, and 2.0 additional life-years, respectively. Compared to individuals with an SBP level of 160.4 mmHg (mean of 4th quartile), having a lower SBP level of 114.1 mmHg (mean of 1st quartile), 128.2 mmHg (mean of 2nd quartile), and 139.1 mmHg (mean of 3rd quartile) were associated with 1.9, 1.5, and 1.1 years gain in LE, respectively. A lower LDL level of 59 mg/dl (mean of 1st quartile), 84.0 mg/dl (mean of 2nd quartile), and 107.0 mg/dl (mean of 3rd quartile) was associated with an average of 0.9, 0.7, and 0.5 additional life-year, compared to individuals with LDL of 146.2 mg/dl (mean of 4th quartile) to, respectively. Due to the public health relevance, we also examined the benefit of smoking cessation, which ranged from 0.7 years for women aged 50–60 to 1.1 years for men aged 70–80 (efigure 1).
Figure 2.
Gains in life-years associated with different levels of modifiable risk factors in individuals with type 2 diabetes.
Note: the mean values of biomarkers for the 1st, 2nd, 3rd, and 4th quartile were as follows: A1c (5.9, 6.8, and 7.7 vs. 9.9%), SBP (114.1, 128.1, and 139.1 vs. 160.4 mmHg), LDL (58.9, 84, and 107 vs. 146 mg/dl), BMI (24.3, 28.6, and 33 vs. 41.4 kg/m2). BMI: body mass index; SBP: systolic blood pressure; LDL: low-density lipoprotein
Figure 3 presents a heat map illustrating the LEs of individuals with T2D by different ages, sex, and biomarker levels. Red denotes high mortality risk (low LE), and white denotes low mortality risk (high LE). Estimated LEs in the lowest (lower left) and highest (upper right) risk groups ranged from 30.1 to 18.2 years in women aged 50 to 60 years, 23.2 to 12.3 in women aged 60 to 70 years, and 14.9 to 6.8 in women aged 70 to 80 years. Among men with T2D, LEs ranged from 15.0 to 25.7 years in those aged 50 to 60, 9.6 to 18.3 in those 60 to 70, and 5.5 to 11.8 in those 70 to 80.
Fig 3.
The estimated remaining life-year in men and women with type 2 diabetes and without cardiovascular diseases.
Notes: The LEs are color-coded for each age-sex group separately.
Discussion
This study quantified the potential gains in LE associated with different levels of biomarkers in patients with diabetes. Differences in A1c and BMI were found to have the largest impact on LE gain from a population perspective. At the individual level, we observed a large variation in the benefits associated with better diabetes care, driven by patients’ individual characteristics. The benefit of biomarker control was most pronounced in younger adults, and diminished as people aged. Better control of biomarkers can potentially increase the LE by three years in an average person with T2D in the US. For individuals with very high levels of A1c, SBP, LDL, and BMI, controlling biomarkers can potentially increase LE by more than ten years.
LE gained through lower BMI was the largest among the four modifiable biomarkers we examined. For people with T2D and a high level of obesity (the 4th quartile, BMI>36 kg/m2), reducing their BMI to below 25 kg/m2 (the 1st quartile) may prolong LE for an estimated 3.9 years. This benefit, however, might not easily achieved in clinical practice because it requires a significant reduction in BMI. Our estimation can be referred to as a “ceiling effect,” which measures the potential LE gains under an optimal scenario. In the real world, patients can achieve halfway and gain a proportion of the estimated LE benefit. Our estimated LE gain from having lower BMI in people with T2D is conservative because weight-loss interventions often lead to reductions in other biomarkers, including A1c, SBP, and LDL, which would add additional life-years. Thus, bodyweight reduction among persons with diabetes and obesity continues to be a clinical and public health priority.
Lifestyle interventions and medical nutrition therapies 24,25 are effective in reducing body weight. In the Look AHEAD study,26 the intensive lifestyle intervention reduced body weight in people with T2D and overweight/obesity by 8.2% in the first year. When administered in a population with diabetes duration < 3 years, intensive lifestyle intervention resulted in a 12% bodyweight reduction. 27 Although the Look AHEAD study did not find a significant reduction in mortality associated with intensive weight control, we believe this was mainly attributable to the fact that Look AHEAD participants were relatively younger and had a shorter duration of diabetes and lower cardiovascular risks than the general population, in which a statistically significant difference requires a much larger sample size and longer follow-up time to detect. Our estimated gain in LE was mainly attributable to potential reductions in cardiovascular diseases associated with weight loss. According to data from ACCORD, a lower BMI level was associated with lower risks of CHF, angina, and revascularization, which in turn was associated with a lower risk of mortality. Bariatric surgery can lead to more than 25% bodyweight reduction.28 It has been reported that after bariatric surgery, a substantial proportion of patients have diabetes remission and stop using glucose-lowering medication.28 Based on our estimates, a 12% weight loss from lifestyle intervention would lead to a LE gain of approximately 1.5 years, and a 25% weight loss from bariatric surgery 3.0 years. This, however, assumes that weight loss can be maintained throughout the lifetime, which has been challenging for both lifestyle intervention and bariatric surgery.29–31 Maintaining weight loss is especially challenging for those under pharmacological therapy, as several commonly used glucose-lowering drugs, such as thiazolidinediones and insulin, are associated with weight gain.32 Newer treatments such as GLP-1 receptor agonists and SGLT2 inhibitors, however, have been shown to reduce body weight. Such features might be especially valuable for patients with BMI at the 4th quartile who can benefit most substantially from weight loss.
The potential gain of LE associated with A1c control diminished as A1c approached normoglycemia. Reducing A1c from 9.9% (the 4th quartile) to 7.7% (the 3rd quartile) was associated with a substantial increase in LE (i.e., 3.4 years on average). However, not much additional LE accumulated from further reducing the A1c. This finding was consistent with the ACCORD trial, in which the intensive glycemic arm (A1c target <6.0%) had escalated mortality rates compared with the control group (A1c target 7%−8%). A similar pattern was also observed from a previous study33 which found that the benefit was reduced when A1c was decreased from 7.0% to normoglycemic level. However, the BRAVO diabetes model utilized ACCORD trial data to formulate its calculation algorithm, so it is not unexpected that these estimations agree with ACCORD findings. Some have suggested that the escalated mortality rate observed in the ACCORD treatment arm was not attributable to the low A1c target, but rather to how the low level of A1c was achieved.34 Since only a small proportion of ACCORD participants utilized GLP-1 receptor agonists, and none used SGLT2 inhibitors to achieve the intensive glycemic goal, whether achieving intensive goals through these newer drugs can produce different results from the ACCORD trial is of great interest to the diabetes community. However, as we await such evidence, our study highlights the importance of controlling A1c levels between 7.0% and 8.0%.
Lowering SBP from the 4th to 1st quartile was associated with a smaller change in LE compared with lowering BMI. However, this does not imply that SBP control is less important than BMI reduction. Our population-level estimates presented in Figure 2 are not designed for clinicians to prioritize one treatment over the other, because treatment effects varied substantially based on patients’ individual characteristics. For example, the LE heatmap presented in Figure 3 shows that a woman aged 50–60 years old with BMI 30 kg/m2, SBP 160 mmHg, and A1c 10% can expect to live an additional 3.0 years by reducing her SBP to 120 mmHg, and can gain 1.2 years through reducing BMI to 25 kg/m2. In addition, findings from economic evaluations35 showed SBP control is cost-saving from a public health perspective. The relatively lower cost of antihypertensive medications and the established strong causal relationship between SBP and macrovascular complications36 make SBP control of high clinical and economic value.
The benefit of treatment goal achievement declined sharply as the patient aged. For example, for a male patient aged 50–60 years with BMI 35 kg/m2, SBP 160 mmHg, A1c 8%, and LDL 130 mg/dl, reducing BMI from 35 to 30 kg/m2 was associated with an additional 1.4 years of LE. However, for a male patient aged 70–80 with the same levels of biomarkers, reducing BMI to 30 kg/m2 was only associated with an additional 0.6 years of LE. This finding emphasizes the importance of biomarker control at an earlier age. It also highlights the potential need for a trade-off between life quality and treatment for elderly patients when the benefit of biomarker control is limited.
Leal and colleagues utilized data from the UKPDS, with mortality rated between 1977–1997, to evaluate the LE gains associated with modifiable biomarker control in the UK population.3,12 Our study, on the other hand, utilized the most updated data (NHANES 2010–2016) to calibrate the BRAVO model to the modern mortality rates. The present estimates are higher than Leal’s estimations for each of the four biomarkers. For a person with T2D and average biomarker level, the estimated LE was 75–80 years based on Leal’s study, and 80–85 years based on the BRAVO simulation. Advancements in medical technology and improvement in public health and healthcare systems in the last three decades could explain the LE gain.
The LE heatmap (fig 3) was designed as a reference tool to support shared decision-making between clinicians and patients. Clinicians can easily locate the cell and the associated remaining life-years corresponding to a patient’s age, sex, and current biomarker values at the point of care. The clinician can then assess the potential gains in LE over a set of treatment goals options, and then show the patient how many additional life-years the patient is likely to achieve by following the treatment plan on the heatmap. This intuitive method provides a tangible platform for the patient to visualize the benefit of the treatment, and thus can enhance the shared decision-making process and potentially improve patients’ motivation for treatment compliance. Although we only included a limited number of biomarker levels in the current heatmap, a more comprehensive LE heat maps that integrate continuous changes of different biomarkers can be generated in the future.
Our study has several limitations. First, SGLT2 inhibitors were not included in the ACCORD trial. The benefits of A1c reduction through SGLT2 inhibitors may be larger than we estimated as evidence shows that SGLT2 inhibitors may have additional cardio-protective effects in addition to A1c control.37 However, most of this evidence is from people with established cardiovascular disease, which is not the target population of this study. We excluded patients with a history of cardiovascular disease, because for secondary prevention in patients with established cardiovascular disease, detailed drug-specific recommendations (e.g., SGLT2 inhibitors for CHF prevention in people with T2D) are often recommended rather than simple biomarker control.38 Secondly, our estimations are limited by the prediction accuracy of the BRAVO simulation model. In this study, the BRAVO model had an RB as low as 2% when predicting 30-year mortality against a nationally representative sample in the US even before the calibration process. Such high accuracy is due to the previous two rounds of extensive model validation and calibration, utilizing data from 18 international clinical trials.14,15 We therefore believe that the estimations generated using the BRAVO simulation model have good scientific validity. Third, we were unable to distinguish type 1 diabetes from T2D because self-reported diabetes status in NHANES does not differentiate diabetes type. However, as more than 90% of US adults with diabetes have T2D,39 this bias will have limited impact on our estimations.
Conclusion
We quantified the potential gains in LE associated with improvement in biomarkers, finding that improving each of the four biomarkers toward the recommended levels was associated with gains in LE, although the pattern and magnitude differed between them and according to patients charactheristics (e.g age). Our findings can be used by clinicians and patients in selecting optimal treatment goals, to motivate patients in achieving them, and to measure potential health benefits for interventions and programs to improve diabetes care in the US.
Supplementary Material
Acknowledgments:
Dr. Kianmehr is the guarantor of this work and has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
H.S. and H.K. analyzed data and prepared the results. H.S., H.K., and P.Z. wrote the manuscript. J.L., J.G., N.S.O., and V.F. provided clinical expertise, L.S, M.P., E.G., and K.B. provided public health expertise. All authors contributed critically to the discussion and participated in the manuscript development.
Financial Support:
N.S.O. was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA248972.
Footnotes
Disclosure: Authors have disclosed no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Reference
- 1.Baena-Díez JM, Peñafiel J, Subirana I, et al. Risk of Cause-Specific Death in Individuals With Diabetes: A Competing Risks Analysis. Diabetes Care. 2016;39(11):1987–1995. doi: 10.2337/dc16-0614 [DOI] [PubMed] [Google Scholar]
- 2.Mellitus Diabetes, Glucose Fasting, and Risk of Cause-Specific Death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30(7):834–839. doi: 10.1093/eurheartj/ehn567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Experiment Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- 5.Stiefel MC, Perla RJ, Zell BL. A Healthy Bottom Line: Healthy Life Expectancy as an Outcome Measure for Health Improvement Efforts. Milbank Q. 2010;88(1):30–53. doi: 10.1111/j.1468-0009.2010.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care. 2007;30(6):1562–1566. doi: 10.2337/dc06-2544 [DOI] [PubMed] [Google Scholar]
- 7.Astrup A, Finer N. Redefining Type 2 diabetes: ‘Diabesity’ or ‘Obesity Dependent Diabetes Mellitus’? Obes Rev. 2000;1(2):57–59. doi: 10.1046/j.1467-789x.2000.00013.x [DOI] [PubMed] [Google Scholar]
- 8.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Mamun AA, Bonneux L. Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008 [DOI] [PubMed] [Google Scholar]
- 9.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of Different Blood Pressure–Lowering Regimens on Major Cardiovascular Events in Individuals With and Without Diabetes Mellitus: Results of Prospectively Designed Overviews of Randomized Trials. Arch Intern Med. 2005;165(12):1410–1419. doi: 10.1001/archinte.165.12.1410 [DOI] [PubMed] [Google Scholar]
- 10.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet Lond Engl. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6 [DOI] [PubMed] [Google Scholar]
- 12.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–1759. doi: 10.1007/s00125-004-1527-z [DOI] [PubMed] [Google Scholar]
- 13.Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: An Appraisal of Its Benefits and Limitations. Am Heart Hosp J. 2007;5(2):91–96. doi: 10.1111/j.1541-9215.2007.06350.x [DOI] [PubMed] [Google Scholar]
- 14.Shao H, Fonseca V, Stoecker C, Liu S, Shi L. Novel Risk Engine for Diabetes Progression and Mortality in USA: Building, Relating, Assessing, and Validating Outcomes (BRAVO). PharmacoEconomics. 2018;36(9):1125–1134. doi: 10.1007/s40273-018-0662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao H, Shi L, Fonseca VA. Using the BRAVO Risk Engine to Predict Cardiovascular Outcomes in Clinical Trials With Sodium–Glucose Transporter 2 Inhibitors. Diabetes Care. 2020;43(7):1530–1536. doi: 10.2337/dc20-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao H, Yang S, Stoecker C, Fonseca V, Hong D, Shi L. Addressing Regional Differences in Diabetes Progression: Global Calibration for Diabetes Simulation Model. Value Health. 2019;22(12):1402–1409. doi: 10.1016/j.jval.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Si L, Willis MS, Asseburg C, et al. Evaluating the Ability of Economic Models of Diabetes to Simulate New Cardiovascular Outcomes Trials: A Report on the Ninth Mount Hood Diabetes Challenge. Value Health. Published online August 6, 2020. doi: 10.1016/j.jval.2020.04.1832 [DOI] [PubMed] [Google Scholar]
- 18.Shao H, Fonseca V, Furman R, Meneghini L, Shi L. Impact of Quality Improvement (QI) Program on 5-Year Risk of Diabetes-Related Complications: A Simulation Study. Diabetes Care. 2020;43(11):2847–2852. doi: 10.2337/dc20-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kianmehr H, Yang S, Fonseca V, et al. 1401-P: Life Expectancy of Individuals with Type 2 Diabetes Mellitus (T2DM) in the United States. Diabetes. 2020;69(Supplement 1). doi: 10.2337/db20-1401-P [DOI] [Google Scholar]
- 20.National Death Index Dataset. Published February 8, 2021. Accessed March 18, 2021. https://www.cdc.gov/nchs/ndi/index.htm [Google Scholar]
- 21.NHANES 2015–2016 Questionnaire Data. Accessed June 30, 2021. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2015 [Google Scholar]
- 22.Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with Type 2 diabetes. J Diabetes Complications. 2010;24(2):84–89. doi: 10.1016/j.jdiacomp.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 23.Association AD. Professional Practice Committee: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S3-S3. doi: 10.2337/dc21-Sppc [DOI] [PubMed] [Google Scholar]
- 24.Wc K EB-C, Se F, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/nejmoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raynor HA, Davidson PG, Burns H, et al. Medical Nutrition Therapy and Weight Loss Questions for the Evidence Analysis Library Prevention of Type 2 Diabetes Project: Systematic Reviews. J Acad Nutr Diet. 2017;117(10):1578–1611. doi: 10.1016/j.jand.2017.06.361 [DOI] [PubMed] [Google Scholar]
- 26.Wadden TA, West DS, Neiberg RH, et al. One-year Weight Losses in the Look AHEAD Study: Factors Associated With Success. Obesity. 2009;17(4):713–722. doi: 10.1038/oby.2008.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taheri S, Zaghloul H, Chagoury O, et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8(6):477–489. doi: 10.1016/S2213-8587(20)30117-0 [DOI] [PubMed] [Google Scholar]
- 28.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015;150(10):931–940. doi: 10.1001/jamasurg.2015.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah M, Simha V, Garg A. Long-Term Impact of Bariatric Surgery on Body Weight, Comorbidities, and Nutritional Status. J Clin Endocrinol Metab. 2006;91(11):4223–4231. doi: 10.1210/jc.2006-0557 [DOI] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. The Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity. 2014;22(1):5–13. doi: 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provilus A, Abdallah M, McFarlane SI. Weight gain associated with antidiabetic medications. Clin Pract. 2011;8(2):113. [Google Scholar]
- 33.Valentine WJ, Palmer AJ, Nicklasson L, Cobden D, Roze S. Improving life expectancy and decreasing the incidence of complications associated with type 2 diabetes: a modelling study of HbA1c targets. Int J Clin Pract. 2006;60(9):1138–1145. doi: 10.1111/j.1742-1241.2006.01102.x [DOI] [PubMed] [Google Scholar]
- 34.Seaquist ER, Miller ME, Bonds DE, et al. The Impact of Frequent and Unrecognized Hypoglycemia on Mortality in the ACCORD Study. Diabetes Care. 2012;35(2):409–414. doi: 10.2337/dc11-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao H, Laxy M, Rolka DB, Gregg EW, Zhang P. New 2017 ACC/AHA Hypertension Guidelines—Cost-Effectiveness among U.S. Adults with Type 2 Diabetes. Diabetes. 2018;67(Supplement 1). doi: 10.2337/db18-173-OR [DOI] [Google Scholar]
- 36.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117. doi: 10.1007/s00125-018-4670-7 [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S125–S150. [DOI] [PubMed] [Google Scholar]
- 39.Control C for D, Prevention. National diabetes statistics report, 2020. Atlanta GA Cent Dis Control Prev US Dep Health Hum Serv. Published online; 2020:12–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.