ABSTRACT
Patients with acquired brain injury (ABI) often experience symptoms of anxiety and depression. Until now, evidence-based treatment is scarce. This study aimed to investigate the effectiveness of Acceptance and Commitment Therapy (ACT) for patients with ABI. To evaluate the effect of ACT for people with ABI, a non-concurrent multiple baseline design across four cases was used. Participants were randomly assigned to a baseline period, followed by treatment and then follow-up phases. Anxiety and depressive symptoms were repeatedly measured. During six measurement moments over a year, participants filled in questionnaires measuring anxiety, depression, stress, participation, quality of life, and ACT-related processes. Randomization tests and NAP scores were used to calculate the level of change across phases. Clinically significant change was defined with the Reliable Change Index. Three out of four participants showed medium to large decreases in anxiety and depressive symptoms (NAP = 0.85 till 0.99). Furthermore, participants showed improvements regarding stress, cognitive fusion, and quality of life. There were no improvements regarding psychological flexibility, value-driven behaviour, or social participation. This study shows that ACT is possibly an effective treatment option for people experiencing ABI-related anxiety and depression symptoms. Replication with single case or large scale group studies is needed to confirm these findings.
KEYWORDS: Acceptance and commitment therapy, Anxiety and depressive symptoms, Acquired brain injury, Single-case experimental design
Introduction
Individuals suffering from acquired brain injury (ABI), such as a traumatic brain injury (TBI) or a stroke are at increased risk of developing anxiety or depressive symptoms (Knapp et al., 2020; Kreutzer et al., 2001). These can have a significant impact on patients’ quality of life. Post-ABI depressive and anxiety symptoms are related to higher re-hospitalization rates, less social participation (dependency in daily living and lower return to work rates), and more cognitive and physical impairments (Juengst et al., 2017).
Studies investigating interventions for ABI-related anxiety and depressive complaints are scarce (Gertler et al., 2015; Stalder-Lüthy et al., 2013) and there are therefore limited evidence-based treatment options (Beedham et al., 2020; Knapp et al., 2017). For instance, second wave behavioural therapies such as cognitive behavioural therapy (CBT) have shown to be effective in treating depression and anxiety, but seem less effective for patients with ABI compared to non-ABI samples (Little et al., 2020). Second-wave therapies aim to influence how situations are experienced and dealt with by changing unhelpful or irrational thoughts and cognitive schemas (Beck, 1993). Third-wave therapies such as Acceptance and Commitment Therapy (ACT) utilize a different approach, thoughts and feelings are not changed, but the functions of and one’s relationship to these psychological events are changed. This might be a fitting approach for people with ABI since they often have realistic thoughts which might be hard to challenge. The aim of ACT is to accept one's thoughts and feelings, without judgment, and to commit to pursuing value-driven behaviour, which is named psychological flexibility (Hayes, 2016). ACT might help people to live in a meaningful way with the lasting and impairing consequences of an ABI (Kangas & McDonald, 2011; Robinson et al., 2019). Psychological flexibility is increased using the six core components of ACT (acceptance, cognitive defusion, mindfulness, self-as-context, values, and committed action). For patients to become accustomed to these processes, behavioural change strategies, metaphors, and experiential exercises are used. Although the main treatment aim of ACT is not to reduce symptomatology, a reduction of anxiety and depressive complaints is often observed following ACT interventions (Bai et al., 2020). Furthermore, an increase in psychological flexibility is thought to be related to improved mental health (Gloster et al., 2017).
There is some preliminary evidence that ACT is effective in treating psychological distress following TBI. Whiting et al. (2019) found that patients with severe TBI experienced greater reductions in depression and stress following an ACT treatment (N = 10) compared to a befriending treatment (N = 9) in a pilot randomized controlled trial. However, these results were not maintained in the one-month follow-up. Sander et al. (2020) compared ACT to devised usual care (which consisted of an intake and referral to a psychological service without a follow-up). The study concluded that patients receiving the ACT intervention showed greater improvements regarding psychological distress and psychological flexibility compared to the patients in the usual care group. Moreover, Majumdar and Morris (2019) found that ACT reduced depression and increased self-rated health status and hopefulness in people with a stroke. These studies indicate that ACT can be a suitable treatment for patients with ABI-related anxiety or depressive complaints, however, more research is needed to confirm these findings. Moreover, the long-term effects of ACT in people with ABI remain unknown. Therefore, this study aimed to evaluate the BrainACT intervention, an ACT intervention specifically developed for people with ABI. This was done using single-case experimental design (SCED) methodology. A non-concurrent multiple baseline design across four cases was performed with a follow-up period of one year. It was hypothesized that the BrainACT intervention would reduce anxiety and depressive symptoms. Since the trajectories of change during psychotherapy are diverse and vary per patient (Helmich et al., 2020), both immediate and delayed effects of the interventions were tested. Furthermore, it was expected that the intervention would improve psychological flexibility, cognitive defusion, and value-driven behaviour. Lastly, the study investigated if these outcomes generalized to improvements in levels of stress, social participation, and quality of life.
Methods
Design
In the current study, a non-current multiple baseline design was used, meaning that the study contained four AB designs with a varying baseline length (Coon & Rapp, 2018). Randomization was achieved by randomly assigning patients to a baseline (waiting) period. This was done separately for every patient as you would do in an AB design. The minimum baseline period was 20 days and the maximum baseline period was 42 days. The study was approved by the Ethical Review Committee of Psychology and Neuroscience of Maastricht University (reference number: ERCPN-192_21_04_2018) and the medical ethics committee of Zuyderland Medical Centre (METCZ20180074). All participants gave informed consent.
Participants
Patients were recruited at Zuyderland Medical Centre between August 2018 and January 2019. Patients with ACT treatment indicated as part of their regular care were screened for eligibility in the study. To participate in this study, participants had to meet all of the following criteria: having sustained any type of stroke or traumatic brain injury diagnosed by a neurologist; having a score of seven or higher on the anxiety and/or the depression subscale of the Hospital Anxiety and Depression Scale (HADS); being more than three months post-injury to prevent confounding by spontaneous recovery; being 18 years or older; when using psychopharmacological medication, the dose should be stable four weeks prior to the study and for the duration of the study; having access to the internet and a computer; mastering the Dutch language sufficiently to benefit from treatment; and giving informed consent. Exclusion criteria were: history of brain injury or disease (diagnosed by a neurologist and classified as moderate or severe) or a neurological disorder other than a stroke or traumatic brain injury; pre-morbid disability as assessed with the Barthel Index (score < 19/20); severe co-morbidity that might affect outcome (e.g., cancer or major psychiatric illnesses) for which treatment is given at the moment of inclusion; ongoing mood and/or anxiety disorder based on the DSM 5 (American Psychiatric Association, 2013) for which pharmacological and/or psychological treatment was necessary at the onset of the brain injury; and ACT for comparable problems in the year preceding study entry.
Measures
Repeated measures
During the baseline period, participants were asked daily to rate the following statements “I feel depressed” and “I feel anxious” on a 7-point Likert scale (with 1 being not depressed/anxious and 7 being very depressed/anxious). These questions were based on questions from a study using the experience sampling method, which have shown to be feasible for people with ABI (Lenaert et al., 2019). During the treatment and follow-up phase, patients answered these questions weekly. Participants received a link via email in the morning, which they had the whole day to complete. Since they always received the same link, they occasionally filled in questions more than once per day. If this happened, the first answer was chosen.
Demographic information
Participants completed a demographic questionnaire at the start of the study. Participants filled in information on sex, age, education, employment, and marital status. The patients’ medical files provided clinical information on injury-related factors; type of brain injury, time since brain injury, severity of injury, and lesion location.
Psychological distress
Hospital anxiety and depression scale
The anxiety and depression subscales of the HADS were used to measure anxiety and depressive symptoms (Zigmond & Snaith, 1983). The scores range from 0 to 21 with higher scores indicating higher levels of depression or anxiety. The HADS was found to have good psychometric properties in a TBI sample (Cronbach α depression scale = .88; anxiety scale = .92) (Whelan-Goodinson et al., 2009). Furthermore, a good internal consistency for both subscales (Cronbach α depression scale = .81; anxiety scale = .84) was found in a stroke population (Ayis et al., 2018).
Depression Anxiety Stress Scales-21
The Depression Anxiety Stress Scales-21 (DASS-21) was used to measure the levels of anxiety, depression, and stress of the participants (Antony et al., 1998). It consists of 21 items which are rated on a 4-point Likert scale. The scores range from 0 to 63 with higher scores indicating greater levels of depression, anxiety, or stress. The questionnaire has been validated in a TBI sample. The internal consistency was good for all three scales (Cronbach α depression scale = .90; stress scale = .89; and anxiety scale = .82) (Randall et al., 2017). The DASS-21 was included next to the HADS because it includes a stress scale and includes items on devaluation of life, self-deprecation, and hopelessness which the HADS lacks (Dahm et al., 2013).
Psychological flexibility
Acceptance and Action Questionnaire-Acquired Brain Injury
The Acceptance and Action Questionnaire-Acquired Brain Injury (AAQ-ABI) was used to measure psychological flexibility about the thoughts, feelings, and behaviours that occur as a result of the brain injury (Whiting et al., 2015). The AAQ-ABI is a nine-item self-report measure that is scored on a 5-point Likert scale from 0 (not at all true) to 4 (very true). The total scores range from 0 to 36, with a higher score indicating greater psychological inflexibility. This scale has a good internal consistency in a Dutch sample of patients with ABI (Cronbach’s α = .87) (Rauwenhoff et al., 2021) and in an Australian TBI population (Cronbach α = .90) (Whiting et al., 2015).
Acceptance and Action Questionnaire II
The Acceptance and Action Questionnaire II (AAQ-II) was administered to measure psychological inflexibility and experiential avoidance (Bond et al., 2011). The answers are scored on a seven-point Likert scale with a range of 0–49. Higher scores indicate less acceptance and psychological flexibility. Whiting et al. (2015) validated the AAQ-II in a sample of patients with ABI (Cronbach α = .90). The AAQ-II was included next to the AAQ-ABI for its added value as a measure with better-studied treatment sensitivity (Ong et al., 2019; Rauwenhoff et al., 2021).
Cognitive fusion
The Cognitive Fusion Questionnaire (CFQ-7) measures cognitive fusion on a 7-point Likert scale (Gillanders et al., 2014). Scores range from 0 to 49. The higher the score, the more fused one is with one’s thoughts. Gillanders et al. (2014) showed that the CFQ-7 has an excellent internal consistency (Cronbach α = .93) in a sample of patients with multiple sclerosis. Furthermore, the CFQ-7 showed excellent internal consistency in a Dutch sample of people with ABI (Cronbach’s α = .97) (Rauwenhoff et al., 2021).
Valued living
The Valued Living Questionnaire (VLQ) is a two-part instrument that measures valued living (Wilson et al., 2010). First, the participant rates the importance of 10 value domains on a 10-point Likert scale. Second, participants rate how consistently they have lived in accordance with their values within these domains. Scores from both parts are used to calculate a valued living component. The internal consistency of the valued living component has been demonstrated as adequate (Cronbach α = .74) (Wilson et al., 2010). The VLQ has been used in earlier research to measure valued living in patients with TBI (Pais et al., 2017).
Quality of life
Quality of life (QoL) was measured with the Short Form Survey (SF-12). The SF-12 was used to measure the health status of the participants, but it can also be described as a broad assessment of the quality of life (Ware Jr et al., 1996). The SF-12 has two subscales, the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The total score of both scales ranges from 0 to 100, with a higher score indicating a better health status. The SF-12 demonstrates good psychometric properties (Cronbach α PCS = .85 and MCS = .81) (Okonkwo et al., 2010).
Participation
The Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P) was used to measure three aspects of participation: frequency of behaviours, participation restrictions experienced due to health condition, and satisfaction with participation (Post et al., 2011). The frequency scale measures the objective level of participation, while the restrictions and satisfaction scales offer an insight into the subjective rating of participation. The questionnaire consists of 31 items. A score ranging from 0 to 100 is calculated for each scale. Higher scores indicate more participation, less restriction, and more satisfaction. It is a valid and reliable measure for patients with ABI with a good internal consistency (Cronbach α = .70–.91) (Post et al., 2011).
Blinding
Blinding of the participants and therapists was not feasible, due to the nature of the intervention. However, research assistants who collected the data were blinded for the phase (baseline, intervention, or follow-up) the participant was in at the moment of assessment.
Intervention
The BrainACT intervention is an ACT treatment adapted for the needs and possible cognitive deficits of people with ABI. The ACT intervention consisted of eight individual sessions of 90 minutes during a period of 3.5 months. The first four sessions were weekly, thereafter the sessions were biweekly, with a 3-week break between the seventh session and last session. Participants did homework exercises lasting approximately 30 minutes for 6 days per week. Homework consisted of reading or listening to summaries of the sessions, practising skills, and doing mindfulness exercises. At the start of the intervention, participants received a workbook with instructions to read at home after each session. We used an eight-session protocol (see Table 1) based on Jansen and Batink (2014), Luoma et al. (2007), and Whiting et al. (2019) in which all six ACT core processes were addressed. The protocol was altered based on the recommendations by Kangas and McDonald (2011) and Broomfield et al. (2011). An expert group of six psychologists experienced in ACT and/or in working with people with ABI gave further advice on the alterations. Alterations consisted of adaptions taking into account the possible cognitive deficits of the participants, and brain-injury-related topics discussed during the treatment. The intervention was provided individually by three certified psychologists who completed an ACT training course of at least five days and are experienced in working with patients with ACT (from four to seven years of experience) and TBI (three to 20 years of experience). Furthermore, the therapists were trained by a researcher (JR) in delivering the protocol.
Table 1.
Overview of the BrainACT intervention.
| Session number | Content | Experiential exercises and metaphors | Homework exercises |
|---|---|---|---|
| 1 | Value exploration and defining core values | –Bus of life metaphor –Values sorting exercise –Describing your gravestone or 80th birthday exercise –Writing down core values |
–Reading or listening to the summary of this session –Explore which values deserve more or less attention –Making pictures of valuable moments |
| 2 | Committed action on the long and short term in relation to values. Education about mindfulness and practising contact with the present moment | –What's the next stop of your bus of life? (defining short-term goals) –Keep driving (defining long-term goals) –Introduction mindfulness: raisin exercise |
–Reading or listening to the summary of this session –Perform a daily activity with full attention –Valuable activity of the week: performing one concrete action that fits within one of the patient's values –Defining obstacles while making these homework exercises |
| 3 | Creative hopelessness; the undeniability of human suffering and the consequences on the long term of trying to control it. Identifying strategies used to cope with negative thoughts and emotions | –Mindfulness exercise: mindful breathing –Identifying how the patient copes with unpleasant experiences –Tug-of war with a monster or bouncing ball metaphor –Mindfulness exercise: body scan |
–Reading or listening to the summary of this session –Keeping track of unpleasant thoughts or feelings and how the patient coped with them –Reread the metaphor of the monster or bouncing ball –Valuable activity of the week |
| 4 | Introducing acceptance as an alternative to control | –Tug-of war with a monster, unwanted guest in your bus of life, or the finger trap metaphor –Explanation of the difference between pain and suffering with the glass of water exercise –Mindfulness exercise: making space, and allowing what is there |
–Reading or listening to the summary of this session –Willingness exercise –Valuable activity of the week |
| 5 | Changing the relationship with thoughts, naming the mind | –Mindfulness exercise: attention for thoughts –The passengers in your bus of life (in combination with post-its) –Defusion exercise: naming the mind –Defusion exercise: singing difficult thoughts –Mindfulness exercise: floating leaves on a river |
–Reading or listening to the summary of this session –Watch the YouTube video of the bus of life –Practice defusion exercises –Valuable activity of the week –Observing the mind during valuable activity |
| 6 | Changing the relationship with thoughts about oneself and introducing the constant self | –Metaphor: the sky and the weather –Exercise: lifeline –Metaphor: Suits (thoughts about oneself) that don’t fit –Exercise: backpack |
–Reading or listening to the summary of this session –Take off a suit that doesn’t fit (anymore) –Practice a mindfulness exercise -Valuable activity of the week |
| 7 | Repetition on defusion and mindfulness | –Mindfulness exercise: the body scan –Defusion exercises: physicalizing the thought –Mindfulness exercise: mindful breathing |
–Reading or listening to the summary of this session –Practice a mindfulness exercise daily –Valuable activity of the week |
| 8 | Review of the different core components, explanation on how these skills together lead to psychological flexibility, and preparation on relapse and setbacks | –Mindfulness exercise: attention for noise –Car metaphor –Bus of life metaphor and exercise –Strategies on how to keep using acquired ACT skills in daily life |
–Reading or listening to the summary of this session –Try to keep practising with the different ACT skills –Start integrating ACT into your daily life –Live your life as is valuable to you! Stop fighting, start living! |
Setting
The sessions were performed at Zuyderland Medical Centre, a general hospital in the Netherlands, at the rehabilitation and medical psychology department. The sessions took place in a therapy room where the psychologist and participant sat across from each other at a table.
Treatment adherence
Treatment adherence was checked using a checklist which the therapists filled out following each session. In this checklist, they indicated which exercises and metaphors they had covered and which ones were skipped or altered. Based on this information, a percentage was calculated from the total number of exercises and metaphors (see Table 1) in the protocol. Likewise, therapists indicated which homework assignments were completed and a percentage was calculated. The treatment adherence rate was 60%, 80%, 82%, and 97% for participants one, two, three, and four respectively. Furthermore, participant one completed 83% of the homework exercises, participant two 86%, participant three 90%, and participant four 97%.
Procedure
Participants with ABI for whom an ACT treatment was indicated were eligible for this study. Psychologists screened patients on the in- and exclusion criteria and when patients met the criteria they were informed about the study. After signing the informed consent, the pre-baseline measurements and baseline randomization took place. Participants were allocated to a baseline varying from 20 to 42 days, after which they started with the ACT intervention (treatment phase) and follow-up phase. During the baseline phase, repeated measures were filled in daily, and during the treatment and follow-up phase, this was filled in weekly. There were five more measuring moments (pre-treatment, during treatment, post-treatment, and at seven and 12 months) during which participants filled in the questionnaires on paper. Research assistants performed these measurements after instructions of a researcher (JR) and following a protocol.
Data analyses
For each participant, levels of anxiety and depressive symptoms were plotted graphically for visual analyses. Visual analyses were conducted following the recommendations of Ledford and Gast (2014). Horizontal lines are depicted to observe changes in the average between phases. The trend was determined by the slope and direction of the best fitting straight line for each phase. This was done using the Split Middle Method as proposed by Ledford and Gast (2014). Trend stability was defined by a stability window ±25% of the trend line. Autocorrelation often occurs in SCED data and is associated with an increase in the occurrence of Type I or II errors (Brossart et al., 2006) and therefore, it is important to assess and report it (Vannest et al., 2018). Autocorrelation was calculated using the formula proposed by Box and Jenkins (1976). A lag-1 autocorrelation coefficient of 0.5 or higher was considered high (Archer et al., 2019). Autocorrelation was not calculated for four phases (both treatment + follow-up phases of participants 1 and 4) because they were constant at floor level. Autocorrelation coefficients were higher than 0.5 for six phases (both baseline phases of participant 1, both treatment + follow-up phases of participant 2, and both baseline phases of participant 3).
Randomization tests were used to determine if the levels of anxiety and depression significantly differed between phases (baseline versus treatment + follow-up phase). This was done as described by Bulte and Onghena (2008). These tests are permutation tests relying on random assignment (baseline length in our case) to test a null hypothesis (Heyvaert & Onghena, 2014; Perdices & Tate, 2009), the null hypothesis for the current study being no difference between phases (no treatment effect). In order to compare the phases, a t-statistic was calculated for each possible permutation. This t-statistic is the absolute difference between the mean of the baseline phase and the mean of the treatment + follow-up phase (mean of baseline phase – mean of treatment + follow-up phase). The t-statistic is calculated for every possible starting point of the intervention. The t-statistics that are as large as or larger than the observed data point (starting point of treatment) are divided by the total number of possible permutations, which is the probability association (Perdices & Tate, 2009). Therefore, there is a minimum number of possible starting points in order to obtain a p-value of <.05. In the current study 23 possible randomization assignments permit a p-value of <.05 (1/23 = 0.043). Since it was unclear beforehand when participants would start to respond to the intervention (i.e., decrease in anxiety or depressive symptoms), randomization tests were performed assuming a delayed effect. After the main randomization test, to test for immediate effect, the randomization tests were repeated until the smallest p-value was reached (ter Kuile et al., 2009; Winkens et al., 2020). The smallest p-value is equivalent to the moment in which the difference between means (and thus the t-statistic) of both phases is the largest. We hypothesized that the delayed effect should take place during the first thirteen measurements of the treatment + follow-up phase since we did expect the effect to take place during the treatment. Additionally, the p-values of the immediate and delayed effects were combined following Edgington (1972) additive method. Randomization tests and combining of the p-values were performed using the SCDA plug-in package in R (Bulté & Onghena, 2013).
Non-overlap of all pairs (NAP) was used as a measure of effect (Parker & Vannest, 2009). Since the participants were all more than three months post-ABI, we did not expect spontaneous improvements in the baseline phase. Therefore, NAP is a more appropriate measure than baseline-corrected TAU (Manolov et al., 2021). NAP is a non-overlap index that can be derived from Mann–Whitney U (Parker et al., 2011). NAP summarizes the data overlap between each baseline data point and each treatment + follow-up phase data point in turn. NAP is calculated by dividing the number of comparisons with no overlap by the total number of comparisons (Parker & Vannest, 2009). Parker and Vannest (2009) proposed the following ranges in order to interpret NAP; scores ranging from 0 to 0.65 are weak effects, from 0.66 to 0.92 are medium effects, and from 0.93 to 1.0 are large or strong effects. An online calculator was used to calculate NAP (Pustejovsky et al., 2021). High levels of autocorrelation can lead to overestimation of the effect measure and therefore NAP scores should be interpreted with some caution, however, confidence intervals computed for NAP do assume independence (Barnard-Brak et al., 2021).
In order to define clinical significant change, the reliable change index (RCI) was calculated between the pre-treatment measurements and the other measurements. This was done using the formula of Jacobson and Truax (1991). Since the outcomes are z-scores they are significant if z < −1.96 or z > 1.96.
Results
Four participants were included in the study. The demographic and injury-related characteristics can be found in Table 2. There were some deviations from protocol. First, during the follow-up phase of participant 1, he did not want to continue to answer the weekly questions. He had been filling out the same answers for weeks and expected to continue to do so. It was therefore agreed that the researcher (JR) would fill in the answers for him and if the answer was different, he would let the researcher know. Participant 1 did fill in the questionnaires during the follow-up measurements, the scores of which corresponded with the answers that he had filled in. Second, participant 2 filled in 1 (the lowest score) for the anxiety score for the first 16 days. However, during the pre-treatment measuring moment, he explained that he misunderstood the question and that the score should be higher in these first 16 days. It was agreed upon to impute these 16 days with the average score of the remaining 11 days (score of 3.18).
Table 2.
Participant characteristics.
| Participant | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Age | 59 | 63 | 35 | 47 |
| Educational level | Higher professional education | Secondary vocational education | Primary vocational education | Higher professional education |
| Occupation | Incapacitated | Employed | Incapacitated | Employed |
| Marital status | Married | Married | Married | Married |
| Time since injury (months) | 22 | 64 | 10 | 36 |
| Type of injury | Traumatic brain injury | Brainstem stroke | Traumatic brain injury | Right hemispheric stroke |
Case formulation
Participant 1
Participant 1 is a 59-year old man, who sustained a TBI after falling down a set of stairs in 2016. After the accident, he lost consciousness (unknown for how long) and experienced post-traumatic amnesia for one hour. He had no psychiatric history. After the accident, he completed an outpatient rehabilitation programme, during which he started an e-health module for panic disorder, which he did not finish. Several months after the rehabilitation programme, he experienced a severe increase in fatigue, cognitive, and emotional complaints. His physiatrist (rehabilitation physician) then referred him to the department of medical psychology. He worried a lot about his future. He avoided busy or stressful situations. He for instance stopped seeing his friends from a cycling group. He did this to control his complaints, however, he felt frustrated that this did not reduce his complaints. The participant is married and has two adult children. He works in finance and was on sick leave.
During the intervention, he started to share his complaints and negative feelings with his wife and his friends. Instead of hiding his feelings, pretending he was okay, or avoiding others. This helped him to reconnect to others and to restart undertaking activities together. When introduced to the theme of fusion, he initially found it hard to recognize that this could apply to him. However, looking back at the intervention he felt that fusion, defusion, and self as context had been the most helpful and valuable parts of the intervention. Moreover, it is good to mention that during the treatment a financial dispute, which had caused a lot of stress, was resolved.
Participant 2
Participant 2 is a 63-year-old man, who suffered from an ischemic brainstem stroke in 2013. He had no psychiatric history. He was referred by his general practitioner to the mental health care department because of a depressive disorder based on the DSM criteria. He reported mood problems, excessive worrying, fatigue, and poor concentration. He was regarded as a very active and sporty man, who sets high standards for himself. In his free time, he was active as a swimming trainer, four evenings a week, for more than 30 years. In the year before the intervention, the emotional problems and fatigue slowly increased. He stopped running and slept a lot, also during the day. He was working as a lawyer, 32 hours a week. The patient is married and has two children and three grandchildren.
During the intervention, he decided to stop with his activities as a swimming coach, because he realized that this role cost him a lot of energy and was no longer satisfactory. He told the therapist: “my mind told me that I have to do this, but I realized that it was no longer satisfactory for me.” He also restarted running and going to the cinema with his wife. After the intervention, he needed less sleep during the day and the depressive disorder was in remission (based on the DSM criteria). His partner joined the treatment sessions and was actively involved in the homework assignments.
Participant 3
Participant 3 is a 35-year-old man, who sustained a TBI after a bike accident in 2018. Symptoms he experienced following the accident were nausea, emesis, headache, tinnitus, and diplopia. He had no psychiatric history. He was referred by the neurologist to the mental health care department. He reported mood problems, including apathy, irritability, and restlessness. In addition, he suffered from fatigue, memory and attention problems, and tinnitus. Because of his symptoms, he often cancelled social appointments and was worrying about the future. He could not carry out his work as a house painter because of severe shoulder dysfunction and was receiving a disability payment. The patient is married.
During the intervention, he discovered he did many activities because it was expected by others. He has become more assertive and makes more value-oriented choices regarding activities. After the intervention, he indicated that he mainly lived more in the here and now and he experienced fewer worries, also regarding his tinnitus. His wife was actively involved in the process at home, partly because of his severe dyslexia. The patient was satisfied with the treatment and experienced fewer emotional complaints.
Participant 4
Participant 4 is a 47-year old woman who had a lacunar stroke in 2016. She had no psychiatric history. Following the stroke, the patient followed a rehabilitation programme, which included psychological treatment consisting of 10 sessions of CBT. After this first rehabilitation period, she had a 100% return to work. The patient tried to bring everything back to normal, leaving this difficult period in her life behind her and putting a lot of effort into her return to work. Soon, however, she noticed that things were everything but normal. She suffered from fatigue and could not return to her normal activities like leisure time with her family and sports. The patient reported an agitated mood, fatigue, and a general loss of pleasure. Moreover, she reported feelings of guilt because of her lack of energy to spend time with her children. Next to that, she feared a recurrence of a stroke when experiencing sudden dizziness or nausea. Finally, she experienced her relapse of these symptoms as a failure. She worked as a logistic manager in a peripheral hospital for 20 hours a week. She is married and has two children of which the eldest has an intellectual disability.
During the intervention, the patient was confronted with her experiential avoidance. Strategies she used to avoid difficult emotions were to endure in work and to trivialize or rationalize problems. Once confronted with her behaviour she started to open up, especially to her husband. Particularly, she discussed with him her negative feelings when her oldest child showed difficult behaviour. She learned to allow her negative feelings instead of suppressing them. The theme of cognitive defusion was difficult for her at first. In the end, however, it gave her new insights on how to accept her thoughts and feelings as they were. Additionally, she recognized her tendency to take control of everything herself. She began to share and delegate, both at home and work. This resulted in more time to spend with her family. The patient reported feeling happier at the end of the intervention.
Alt Text for Graphical Figures 1 and 2:
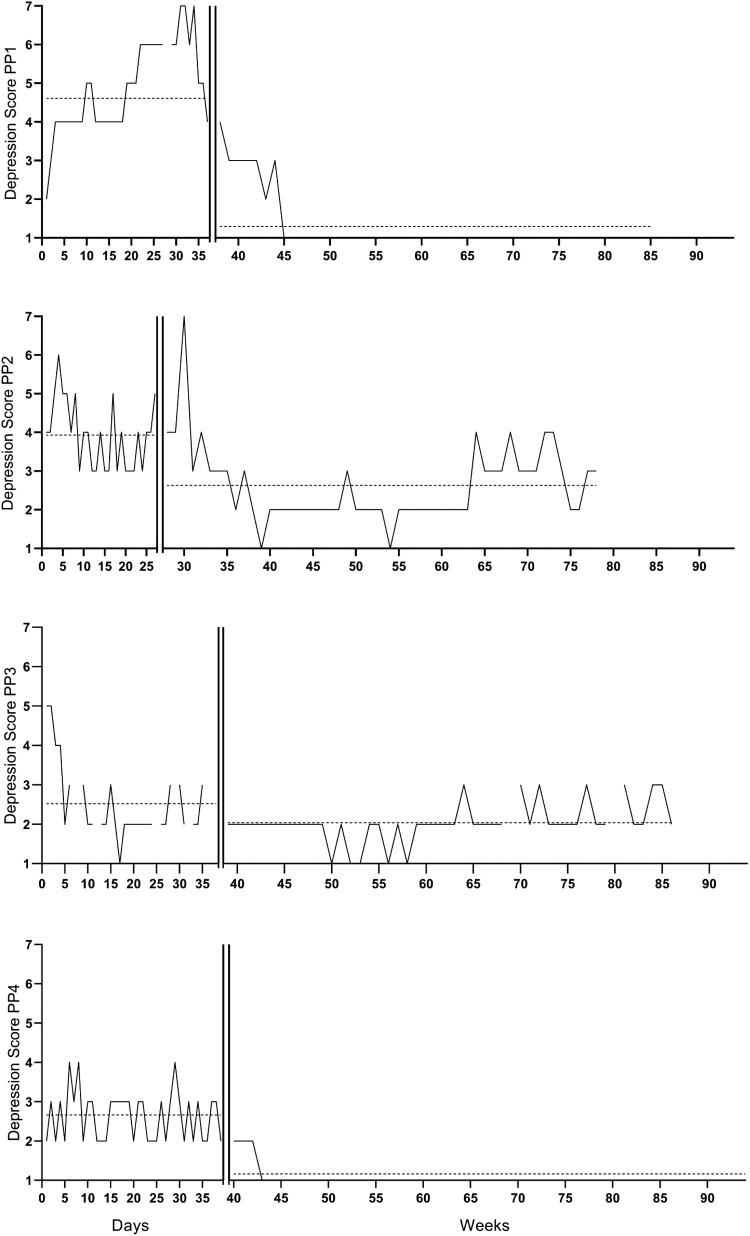
Figure 1.
Repeated measures of depressive symptoms per participants 1, 2, 3, and 4 over time. The vertical lines are the start of the intervention. The horizontal line is the average score per phase. The participants started with the baseline phase at different dates (participant one started on 6 August 2018, participants two on 9 August 2018, participant 3 on 31 October 2018, and participant 4 on 22 January 2019).
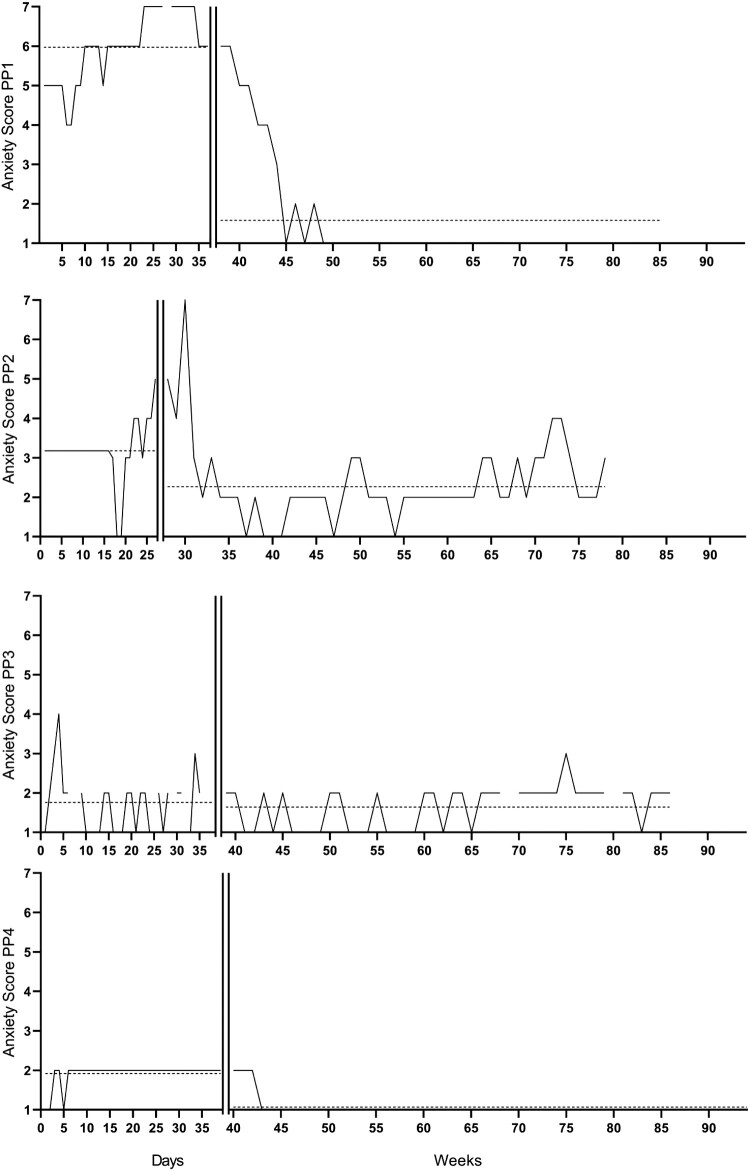
Figure 2.
Repeated measures of anxiety symptoms per participants 1, 2, 3, and 4 over time. The vertical line is the start of the intervention. The horizontal line is the average score per phase. The participants started with the baseline phase at different dates (participant one started on 6 August 2018, participants two on 9 August 2018, participant 3 on 31 October 2018, and participant 4 on 22 January 2019).
Visual analyses, randomization tests, and TAU-U
The repeated anxiety and depression scores are represented visually in Figures 1 and 2. Characteristics of phase lengths and measurements are represented in Tables 3 and 4.
Table 3.
Phase characteristics of the repeated depression scores.
| Participant | Number of measurements baseline phase | Baseline Mean (SD) | Number of measurements intervention + FU phase | Intervention + FU Mean (SD) |
|---|---|---|---|---|
| 1 | 36 | 4.86 (1.17) | 48 | 1.29 (0.74) |
| 2 | 27 | 3.93 (0.87) | 51 | 2.63 (1.00) |
| 3 | 30 | 2.52 (0.95) | 46 | 2.04 (0.51) |
| 4 | 39 | 2.66 (0.63) | 55 | 1.16 (0.46) |
Table 4.
Phase characteristics of the repeated anxiety scores.
| Participant | Number of measurements baseline phase | Baseline Mean (SD) | Number of measurements intervention + FU phase | Intervention + FU Mean (SD) |
|---|---|---|---|---|
| 1 | 36 | 5.97 (0.88) | 48 | 1.58 (1.38) |
| 2 | 27 | 3.18 (2.37) | 51 | 2.37 (1.06) |
| 3 | 30 | 1.76 (0.74) | 46 | 1.64 (0.53) |
| 4 | 39 | 1.92 (0.27) | 55 | 1.07 (0.26) |
Participant 1
Participant one was assigned to a baseline length of 37 days.
Depression scores
Visual analyses
There was a decrease in the average depression scores in the baseline compared to the treatment + follow-up phases. The trend in the baseline phase was accelerating, this levelled to zero-celerating in the treatment phase. Both phases had high trend stability, which was 88.89% for the baseline phase and 85.42% for the treatment + follow-up phase.
Response to the intervention
Randomization tests (Table 5) showed a statistically significant delayed effect (t = 3.63, p = .043), which reached significance after two weeks. Furthermore, there was a large difference between the baseline and treatment + follow-up phase (NAP = 0.99, 90% CI = 0.95–1).
Table 5.
Immediate and delayed randomization tests of the depression scores with combined p-values.
| Participant | Immediate effect p-values | Delayed effect after two week | Delayed effect after three weeks | Delayed effect after four weeks | Delayed effect after five weeks | Delayed effect after six weeks | Effect was significant at week |
|---|---|---|---|---|---|---|---|
| 1 | .261 | .043* | .087 | .174 | .217 | .130 | 2 |
| 2 | .522 | .478 | .435 | .087 | .217 | .043* | 6 |
| 3 | .217 | .391 | .478 | .956 | .607 | .652 | – |
| 4 | .217 | .174 | .130 | .087 | .043* | (not within randomization possibilities) | 5 |
| Combined p-values | .091 | .058 | .072 | .119 | .057 | .935 |
*Significant immediate or delayed effect.
Anxiety scores
Visual analyses
There was a decrease in the average anxiety scores in the baseline compared to the treatment + follow-up phases. The trend in the baseline phase was accelerating, this levelled to zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 100% for the baseline phase and 81.25% for the treatment + follow-up phase.
Response to the intervention
Randomization tests (Table 6) showed a statistically significant delayed effect (t = 4.72, p = .043), which reached significance after six weeks. Furthermore, there was a largedifference between the baseline and treatment + follow-up phase (NAP = 0.97, 90% CI = 0.92–0.99).
Table 6.
Immediate and delayed randomization tests of the anxiety scores with combined p-values.
| Participant | Immediate effect p-values | Delayed effect after two week | Delayed effect after three weeks | Delayed effect after four weeks | Delayed effect after five weeks | Delayed effect after six weeks | Effect was significant at week |
|---|---|---|---|---|---|---|---|
| 1 | .261 | .217 | .174 | .130 | .087 | .043* | 6 |
| 2 | .565 | .478 | .348 | .087 | .043* | .174 | 5 |
| 3 | .043* | .217 | .130 | .083 | .261 | .565 | 1 |
| 4 | .217 | .174 | .130 | .087 | .043 | (not within randomization possibilities) | 5 |
| Combined p-values | .058 | .058 | .016* | .016* | <.01* | .079 |
*Significant immediate or delayed effect.
Participant 2
Participant two was assigned to a baseline length of 27 days.
Depression scores
Visual analyses
There was a decrease in the average depression scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was decelerating and zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 96.3% for the baseline phase and 50.98% for the treatment phase.
Response to the intervention
Randomization tests showed a statistically significant delayed effect (t = 1.56, p = .043), which reached significance after six weeks. Furthermore, there was a medium difference between the baseline and treatment + follow-up phase (NAP = 0.85, 90% CI = 0.75–0.91).
Anxiety scores
Visual analyses
There was a decrease in the average anxiety scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was zero-celerating, which stayed zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 85.19% for the baseline phase and 49.02% for the treatment phase.
Response to the intervention
Randomization tests showed a statistically significant delayed effect (t = 1.21, p = .043), which reached significance after five weeks. Furthermore, there was a medium difference between the baseline and treatment + follow-up phase (NAP = 0.85, 90% CI = 0.77–0.91).
Participant 3
Participant three was assigned to a baseline length of 38 days.
Depression scores
Visual analyses
There was a decrease in the average depression scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was decelerating and zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 62.07% for the baseline phase and 74.47% for the treatment + follow-up phase.
Response to the intervention
Randomization tests showed no statistically significant immediate or delayed effect (t = 3,63, p = .27). Furthermore, there was a weak difference between the baseline and treatment + follow-up phase (NAP = 0.63, 90% CI = 0.52–0.73).
Anxiety scores
Visual analyses
There was a small decrease in the average anxiety scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was zero-celerating and accelerating in the treatment + follow-up phase. Both phases had high trend stability, which was 51.72% for the baseline phase and 74.47% for the treatment + follow-up phase.
Response to the intervention
Randomization tests showed a statistically significant immediate effect (t = 0.120, p = .043). However, there was a small difference between the baseline and treatment + follow-up phase (NAP = 0.53, 90% CI = 0.42–0.64).
Participant 4
Participant four was assigned to a baseline length of 39 days.
Depression scores
Visual analyses
There was a decrease in the average depression scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was zero-celerating, which stayed zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 50% for the baseline phase and 87.27% for the treatment + follow-up phase.
Response to the intervention
Randomization tests showed a statistically significant delayed effect (t = 1.58, p = .043), which reached significance after five weeks. Furthermore, there was a large difference between the baseline and treatment + follow-up phase (NAP = 0.96, 0.90–0.98).
Anxiety scores
Visual analyses
There was a decrease in the average anxiety scores in the baseline compared to treatment + follow-up phase. The trend in the baseline phase was zero-celerating, which stayed zero-celerating in the treatment + follow-up phase. Both phases had high trend stability, which was 92.11% for the baseline phase and 92.73% for the treatment phase.
Response to the intervention
Randomization tests showed a statistically significant delayed effect (t = 0,91, p = .043), which reached significance after five weeks. Furthermore, there was a large difference between the baseline and treatment phase (NAP = 0.93, 90% CI = 0.86–0.96).
Reliable change index
In Table 7 the reliable change index can be found between the different measurement moments of the HADS, DASS-21 stress scale, AAQ-II, AAQ-ABI, CFQ-7, and VLQ. The complete table including the DASS-21 anxiety and depression scale, Sf-12, and USER-P can be found in the supplemental materials.
Table 7.
Reliable change index.
| PP1 | PP2 | PP3 | PP4 | |
|---|---|---|---|---|
| HADS-D | ||||
| Pre-baseline | 7 | 9 | 9 | 5 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 6 (0.40) | 11 (−0.80) | 9 (0) | 9 (−1.61) |
| During-treatment (RCI pre-treatment/During-treatment) | 5 (0.40) | 3 (3.21*) | 8 (0.40) | 6 (1.21) |
| Post-treatment (RCI pre-treatment/post-treatment) | 2 (1.61) | 7 (1.61) | 5 (1.61) | 3 (2.41*) |
| 7 month FU (RCI pre-treatment/7FU) | 1 (2.01*) | 3 (3.22*) | 11 (−0.80) | 3 (2.41*) |
| 12 months FU (RCI pre-treatment/12FU) | 0 (2.41*) | 3 (3.22*) | 10 (−0.40) | 0 (3.62*) |
| HADS-A | ||||
| Pre-baseline | 10 | 3 | 6 | 7 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 10 (0) | 16 (−5.66) | 8 (−0.87) | 8 (−0.43) |
| During-treatment (RCI pre-treatment/During-treatment) | 10 (0) | 6 (4.35*) | 7 (0.44) | 6 (0.87) |
| Post-treatment (RCI pre-treatment/post-treatment) | 4 (2.61*) | 8 (3.48*) | 3 (2.17*) | 5 (1.31) |
| 7 month FU (RCI pre-treatment/7FU) | 2 (3.48*) | 4 (5.22*) | 7 (0.43) | 5 (1.31) |
| 12 months FU (RCI pre-treatment/12FU) | 0 (4.35*) | 6 (4.35*) | 10 (−0.87) | 5 (1.31) |
| DASS-21 stress | ||||
| Pre-baseline | 15 | 9 | 12 | 5 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 11 (1.65) | 18 (−3.72) | 11 (0.41) | 6 (−0.41) |
| During-treatment (RCI pre-treatment/During-treatment) | 12 (−0.41) | 5 (5.37*) | 9 (0.83) | 6 (0) |
| Post-treatment (RCI pre-treatment/post-treatment) | 5 (2.48*) | 6 (4.96*) | 6 (2.07*) | 5 (0.41) |
| 7 month FU (RCI pre-treatment/7FU) | 3 (3.31*) | 5 (5.37*) | 8 (1.24) | 4 (0.83) |
| 12 months FU (RCI pre-treatment/12FU) | 0 (4.54*) | 4 (5.78*) | 10 (0.41) | 4 (0.83) |
| AAQ-II | ||||
| Pre-baseline | 15 | 15 | 14 | 9 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 12 (0.66) | 34 (−4.17) | 11 (0.66) | 11 (−0.44) |
| During-treatment (RCI pre-treatment/During-treatment) | 15 (−0.66) | 17 (3.73*) | 12 (−0.22) | 10 (0.22) |
| Post-treatment (RCI pre-treatment/post-treatment) | 9 (0.66) | 7 (5.93*) | 9 (0.44) | 9 (0.44) |
| 7 month FU (RCI pre-treatment/7FU) | 8 (0.88) | 14 (4.39*) | 16 (−1.09) | 8 (0.66) |
| 12 months FU (RCI pre-treatment/12FU) | 8 (0.88) | 11 (5.05*) | 13 (−0.44) | 7 (0.88) |
| AAQ-ABI | ||||
| Pre-baseline | 12 | 7 | 8 | 9 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 7 (1.20) | 27 (−4.80) | 10 (−0.48) | 9 (0) |
| During-treatment (RCI pre-treatment/During-treatment) | 12 (−1.20) | 10 (4.08*) | 9 (0.24) | 7 (0.48) |
| Post-treatment (RCI pre-treatment/post-treatment) | 1 (1.44) | 11 (3.84*) | 7 (0.72) | 5 (0.96) |
| 7 month FU (RCI pre-treatment/7FU) | 2 (1.20) | 6 (5,04*) | – | 3 (1.44) |
| 12 months FU (RCI pre-treatment/12FU) | 1 (1.44) | 8 (4,56*) | 12 (−0.48) | 1 (1.92) |
| CFQ-7 | ||||
| Pre-baseline | 33 | 35 | 24 | 17 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 19 (3.61*) | 44 (−2.32) | 36 (−3.10) | 12 (1.28) |
| During-treatment (RCI pre-treatment/During-treatment) | 23 (−1.03) | 32 (3.10*) | 33 (0.77) | 11 (0.26) |
| Post-treatment (RCI pre-treatment/post-treatment) | 10 (2.32*) | 17 (6.97*) | 14 (5.68*) | 12 (0) |
| 7 month FU (RCI pre-treatment/7FU) | 7 (3.10*) | 11 (8.51*) | 26 (2.58*) | 10 (0.52) |
| 12 months FU (RCI pre-treatment/12FU) | 10 (2.32*) | 20 (6.19*) | 24 (3.10*) | 9 (0.77) |
| VLQ composite score | ||||
| Pre-baseline | – | 53.50 | – | 56.80 |
| Pre-treatment (RCI pre-baseline/pre-treatment | 45.20 | 52.00 (0.14) | 47.40 | 61.90 (−0.49) |
| During-treatment (RCI pre-treatment/During-treatment) | 55.90 (−1.02) | 48.20 (0.36) | 33.90 (1.29) | 53.20 (0.83) |
| Post-treatment (RCI pre-treatment/post-treatment) | – | 57.60 (−0.54) | 35.80 (1.11) | 58.90 (0.29) |
| 7 month FU (RCI pre-treatment/7FU) | 53.60 (−0.80) | 59.20 (−0.69) | 34.40 (1.25) | 66.70 (−0.46) |
| 12 months FU (RCI pre-treatment/12FU) | – | 53.50 | – | 56.80 |
Abbreviations: HADS: Hospital Anxiety and Depression Scale; DASS-21: Depression, anxiety and stress scale-21; AAQ-II: Acceptance and Action Questionnaire II; AAQ-ABI: Acceptance and Action Questionnaire after brain injury; VLQ: Valued Living Questionnaire; CFQ-7: Cognitive Fusion Questionnaire.
Note: Table includes raw scores with the reliable change index between brackets.
*Clinical significant change.
Discussion
The main goal of this study was to examine the effectiveness of the BrainACT intervention for people experiencing ABI-related anxiety and depressive symptoms. This was done using a non-concurrent multiple baseline design across four cases.
Main outcome measures
Participants 1, 2, and 4 showed medium to large improvements in anxiety and depressive complaints. Furthermore, these three participants responded to the treatment within two to six weeks after starting the BrainACT intervention. The delayed effect of participant 1 reached significance after two weeks. A study by Keinonen et al. (2018) found that 25% of patients can experience a sudden gain (an abrupt reduction in symptom severity after which they stabilize) after the first two sessions of an ACT intervention. It seems likely, also looking at his graph, that participant 1 experienced a sudden gain in depression scores after session two. Interestingly, all other delayed effects reached significance at week five or six. Furthermore, these three participants (except for the anxiety and stress symptoms of participant four, which however were low at the start of the intervention) showed clinically significant improvements in anxiety, depressive, and stress symptoms. These lasted up to a year after the start of the intervention. Based on these results it can be concluded that the BrainACT intervention was successful in decreasing anxiety and depressive complaints.
For participant 3 the results were less evident. NAP scores indicated that he experienced a weak decrease in depression and anxiety scores, while the randomization tests showed a significant immediate effect for anxiety and no significant effects for depression. There was a clinically significant change on the HADS-anxiety post-treatment of participant 3. However, this did not last and the HADS scores on the follow-up moments are even higher than before the treatment. The scores of the repeated measures on both graphs show a similar pattern. This could be an indication that the treatment was not long enough or that extra booster sessions after a few months would have been beneficial. While the patient did state that he was satisfied with the treatment and experienced fewer emotional complaints, these results do not indicate that the BrainACT intervention decreased his anxiety and depressive complaints.
For all four participants, the clinically significant changes in anxiety and depression are mostly seen on the subscales of the HADS and not on DASS-21. Although the DASS-21 was primarily included to measure stress and not anxiety or depression, it would have been more convincing if anxiety and depression had reached clinically significant change on the DASS-21 as well. Patients scored lower pre-treatment anxiety and depression scores on the DASS-21 compared to the HADS scores. As a result, there was less room for improvement on the DASS-21.
ACT-process measures
Participants 1, 3, and 4 showed no clinically significant improvement regarding psychological flexibility, experiential avoidance, or psychological flexibility related to thoughts and feelings about ABI, although the scores did improve for these patients. Participant 2 did show clinically significant improvements in these domains. However, this seems to be a result of a very high score for both measures on the pre-treatment measurement moment, making these results quite unreliable. The non-clinical significant improvement in psychological flexibility corresponds with the results of Whiting et al. (2019), where an improvement in stress and depression was also found, but no improvement in psychological flexibility in severe TBI participants, who had received an ACT intervention. However, Sander et al. (2020) did find a significant improvement regarding psychological flexibility in TBI participants who had received an ACT intervention. Clearly, more research is needed into the role of psychological flexibility during an ACT intervention for ABI-related anxiety and depression symptoms. Although the psychometric properties of the AAQ-II and AAQ-ABI have been studied in patients with ABI (Rauwenhoff et al., 2021; Whiting et al., 2015), there has been critique on the validity of the AAQ-II. Studies found that rather than measuring psychological flexibility it measures psychological distress or neuroticism (Cherry et al., 2021; Doorley et al., 2020). This should be taken into account when using the AAQ-II. However, if the AAQ-II is a measure of psychological distress, one would expect greater reductions in scores, similar to the other measures of psychological distress in this study.
Furthermore, no improvements were found in value-driven behaviour for all four participants. This is somewhat surprising since the treatment protocol places a strong emphasis on the identification of values and afterwards, valued behaviours were followed up and encouraged each session (valuable activity of the week). Nonetheless, it might be possible that this was still not sufficient to elicit behavioural change, which has proven to be complex (Bouton, 2014). Another explanation could be found in the instrument used for the measurement of valued behaviour, the VLQ. This questionnaire uses ten broad value domains, which leaves no room for personal values (that might have been targeted during treatment) outside these domains and the second part of the questionnaire is quite difficult to fill in. Moreover, changes in only one or two domains, may not be noticed in the total score of the VLQ. On the other hand, in other studies, the VLQ has shown improvements following ACT interventions (Reilly et al., 2019). Currently, an adapted version for patients with ABI of the VLQ is being developed specifically to tackle these problems (Miller et al., 2021). It would be interesting to know if this adapted scale would be able to measure change regarding valued behaviour.
There was a reduction in cognitive fusion for all four participants, which was clinically significant for participants 1, 2, and 3. This could be the result of the emphasis placed on cognitive defusion in the treatment protocol. For instance, in session seven, information on cognitive defusion was rehearsed and practised to improve this skill. In other medical populations, cognitive fusion (and not illness-related symptoms) was related to levels of depression (Carvalho et al., 2019; Trindade et al., 2018). Furthermore, previous research has found that cognitive defusion significantly mediates changes in quality of life, behavioural avoidance, and depression in both ACT and CBT interventions (Arch et al., 2012; Zettle et al., 2011). Although this was not studied, the change in mood complaints and quality of life could have been mediated by the improvements in cognitive fusion for the participants in this study.
Secondary outcome measures
There were no clear improvements in social participation or physical health-related quality of life. However, mental health-related quality of life did improve for all participants, which was a clinically significant improvement for two participants and very close to significance for the other two participants. These findings are in line with results from previous studies which also found improvements in mental health-related quality of life but not in physical health-related quality of life following ACT interventions (Eilenberg et al., 2016; Wicksell et al., 2013). It can be concluded that all four participants, to some extent, benefited from the treatment regarding this outcome.
Strengths and limitations
This study has several strengths. The follow-up time of a year is long compared to other single case studies. Therefore, there were a considerable number of data points in each phase (ranging from 27 to 54), especially considering the minimum of five data points per phase (Tate et al., 2013) and Michiels and Onghena (2019) advise at least 30 measurements in total to achieve adequate power for randomization tests. Furthermore, the study succeeded in replicating the treatment effect in three different participants, thus strengthening the hypotheses that ACT is an effective treatment for psychological distress following ABI. Lastly, no adverse events occurred for any of the participants.
However, this study is not without its limitations. First, increased levels of autocorrelation were found for six phases. As mentioned earlier, NAP does not control for autocorrelation. High levels of autocorrelation can lead to overestimation of the effect measure and therefore NAP scores should be interpreted with some caution (Barnard-Brak et al., 2021). This should be taken into account when the results in the phases with high autocorrelation. Second, the two participants with a stroke were relatively young and three participants had high education levels. As a result, these findings might not translate to older stroke patients or patients with a lower education level. Third, the treatment adherence rating was not done completely independently, which is recommended (Tate et al., 2013). Although the treatment adherence rate was calculated by the researcher, the checklists were filled in by the psychologists. Furthermore, the treatment adherence rate of the treatment delivered to participant 1, is quite low with 60%, while the minimum is an 80% compliance with the rating protocol (Tate et al., 2013). The patient experienced the treatment as cognitively challenging and in agreement with the researcher, it was decided to decrease the number of exercises and metaphors discussed in the sessions. Fourth, from these results, no conclusions can be made as to whether ACT is more effective for patients with stroke or TBI, which was also not within the scope of this study. However, it seems that levels and aetiology of depression generally do not differ between stroke or TBI patients (Blicher & Nielsen, 2008), nor does their coping style (Herrmann et al., 2000). Levels of anxiety can be higher for patients with a stroke, since they can experience fear of stroke recurrence (Chun et al., 2018; Verberne et al., 2021). Further research should focus on possible differences in the effectiveness of ACT for patients with different types of ABI.
Conclusion
In conclusion, this study adds to the existing knowledge that ACT is likely helpful in treating psychological distress following ABI and that the BrainACT intervention can decrease anxiety and depressive complaints in individuals with ABI. Larger randomized controlled trials or replication with single case studies are needed to obtain additional evidence on the efficacy of ACT for individuals with ABI.
Supplementary Material
Acknowledgements
The authors would like to thank the participants for participating in this study and the participating site, Zuyderland Medical Centre.
Funding Statement
This work was supported by ZonMW under Grant (project number 636310003). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Antony, M. M., Bieling, P. J., Cox, B. J., Enns, M. W., & Swinson, R. P. (1998). Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment, 10(2), 176–181. 10.1037/1040-3590.10.2.176 [DOI] [Google Scholar]
- Arch, J. J., Wolitzky-Taylor, K. B., Eifert, G. H., & Craske, M. G. (2012). Longitudinal treatment mediation of traditional cognitive behavioral therapy and acceptance and commitment therapy for anxiety disorders. Behaviour Research and Therapy, 50(7-8), 469–478. 10.1016/j.brat.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Archer, B., Azios, J. H., Muller, N., & Macatangay, L. (2019). Effect sizes in single-case aphasia studies: A comparative, autocorrelation-oriented analysis. Journal of Speech, Language, and Hearing Research, 62(7), 2473–2482. 10.1044/2019_JSLHR-L-18-0186 [DOI] [PubMed] [Google Scholar]
- Ayis, S. A., Ayerbe, L., Ashworth, M., & DA Wolfe, C. (2018). Evaluation of the Hospital Anxiety and Depression Scale (HADS) in screening stroke patients for symptoms: Item response theory (IRT) analysis. Journal of Affective Disorders, 228, 33–40. 10.1016/j.jad.2017.11.037 [DOI] [PubMed] [Google Scholar]
- Bai, Z., Luo, S., Zhang, L., Wu, S., & Chi, I. (2020). Acceptance and commitment therapy (ACT) to reduce depression: A systematic review and meta-analysis. Journal of Affective Disorders, 260, 728–737. 10.1016/j.jad.2019.09.040 [DOI] [PubMed] [Google Scholar]
- Barnard-Brak, L., Watkins, L., & Richman, D. M. (2021). Autocorrelation and estimates of treatment effect size for single-case experimental design data. Behavioral Interventions, 36(3), 595–605. 10.1002/bin.1783 [DOI] [Google Scholar]
- Beck, A. T. (1993). Cognitive therapy: Past, present, and future. Journal of Consulting and Clinical Psychology, 61(2), 194–198. 10.1037//0022-006x.61.2.194 [DOI] [PubMed] [Google Scholar]
- Beedham, W., Belli, A., Ingaralingam, S., Haque, S., & Upthegrove, R. (2020). The management of depression following traumatic brain injury: A systematic review with meta-analysis. Brain Injury, 34(10), 1287–1304. 10.1080/02699052.2020.1797169 [DOI] [PubMed] [Google Scholar]
- Blicher, J. U., & Nielsen, J. F. (2008). Does long-term outcome after intensive inpatient rehabilitation of acquired brain injury depend on etiology? NeuroRehabilitation, 23(2), 175–183. 10.3233/NRE-2008-23207 [DOI] [PubMed] [Google Scholar]
- Bond, F. W., Hayes, S. C., Baer, R. A., Carpenter, K. M., Guenole, N., Orcutt, H. K., Waltz, T., & Zettle, R. D. (2011). Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A revised measure of psychological inflexibility and experiential avoidance. Behaviour Therapy, 42(4), 676–688. 10.1016/j.beth.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Bouton, M. E. (2014). Why behavior change is difficult to sustain. Preventive Medicine, 68, 29–36. 10.1016/j.ypmed.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box, G. E., & Jenkins, G. M. (1976). Time series analysis: Forecasting and control. Holden-Day. [Google Scholar]
- Broomfield, N. M., Laidlaw, K., Hickabottom, E., Murray, M. F., Pendrey, R., Whittick, J. E., & Gillespie, D. C. (2011). Post-stroke depression: The case for augmented, individually tailored cognitive behavioural therapy. Clinical Psychology & Psychotherapy, 18(3), 202–217. 10.1002/cpp.711 [DOI] [PubMed] [Google Scholar]
- Brossart, D. F., Parker, R. I., Olson, E. A., & Mahadevan, L. (2006). The relationship between visual analysis and five statistical analyses in a simple AB single-case research design. Behavior Modification, 30(5), 531–563. 10.1177/0145445503261167 [DOI] [PubMed] [Google Scholar]
- Bulte, I., & Onghena, P. (2008). An R package for single-case randomization tests. Behavior Research Methods, 40(2), 467–478. 10.3758/brm.40.2.467 [DOI] [PubMed] [Google Scholar]
- Bulté, I., & Onghena, P. (2013). The single-case data analysis package: Analysing single-case experiments with R software. Journal of Modern Applied Statistical Methods, 12(2), 450–478. 10.22237/jmasm/1383280020 [DOI] [Google Scholar]
- Carvalho, S. A., Trindade, I. A., Gillanders, D., Pinto-Gouveia, J., & Castilho, P. (2019). Cognitive fusion and depressive symptoms in women with chronic pain: A longitudinal growth curve modelling study over 12 months. Clinical Psychology & Psychotherapy, 26(5), 616–625. 10.1002/cpp.2386 [DOI] [PubMed] [Google Scholar]
- Cherry, K. M., Vander Hoeven, E., Patterson, T. S., & Lumley, M. N. (2021). Defining and measuring “psychological flexibility”: A narrative scoping review of diverse flexibility and rigidity constructs and perspectives. Clinical Psychology Review, 84. Article 101973. 10.1016/j.cpr.2021.101973 [DOI] [PubMed] [Google Scholar]
- Chun, H. Y., Whiteley, W. N., Dennis, M. S., Mead, G. E., & Carson, A. J. (2018). Anxiety after stroke: The importance of subtyping. Stroke, 49(3), 556–564. 10.1161/STROKEAHA.117.020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon, J. C., & Rapp, J. T. (2018). Application of multiple baseline designs in behavior analytic research: Evidence for the influence of new guidelines. Behavioral Interventions, 33(2), 160–172. 10.1002/bin.1510 [DOI] [Google Scholar]
- Dahm, J., Wong, D., & Ponsford, J. (2013). Validity of the Depression Anxiety Stress Scales in assessing depression and anxiety following traumatic brain injury. Journal of Affective Disorders, 151(1), 392–396. 10.1016/j.jad.2013.06.011 [DOI] [PubMed] [Google Scholar]
- Doorley, J. D., Goodman, F. R., Kelso, K. C., & Kashdan, T. B. (2020). Psychological flexibility: What we know, what we do not know, and what we think we know. Social and Personality Psychology Compass, 14(12), 1–11. 10.1111/spc3.12566 [DOI] [Google Scholar]
- Edgington, E. S. (1972). An additive method for combining probability values from independent experiments. The Journal of Psychology, 80(2), 351–363. 10.1080/00223980.1972.9924813 [DOI] [Google Scholar]
- Eilenberg, T., Fink, P., Jensen, J., Rief, W., & Frostholm, L. (2016). Acceptance and commitment group therapy (ACT-G) for health anxiety: A randomized controlled trial. Psychological Medicine, 46(1), 103–115. 10.1017/S0033291715001579 [DOI] [PubMed] [Google Scholar]
- Gertler, P., Tate, R. L., & Cameron, I. D. (2015). Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database of Systematic Reviews, 2015(12). 10.1002/14651858.CD009871.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillanders, D. T., Bolderston, H., Bond, F. W., Dempster, M., Flaxman, P. E., Campbell, L., Kerr, S., Tansey, L., Noel, P., Ferenbach, C., Masley, S., Roach, L., Lloyd, J., May, L., Clarke, S., & Remington, B. (2014). The development and initial validation of the cognitive fusion questionnaire. Behavioral Therapy, 45(1), 83–101. 10.1016/j.beth.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Gloster, A. T., Meyer, A. H., & Lieb, R. (2017). Psychological flexibility as a malleable public health target: Evidence from a representative sample. Journal of Contextual Behavioral Science, 6(2), 166–171. 10.1016/j.jcbs.2017.02.003 [DOI] [Google Scholar]
- Hayes, S. C. (2016). Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies – Republished article. Behavior Therapy, 47(6), 869–885. 10.1016/j.beth.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Helmich, M. A., Wichers, M., Olthof, M., Strunk, G., Aas, B., Aichhorn, W., Schiepek, G., & Snippe, E. (2020). Sudden gains in day-to-day change: Revealing nonlinear patterns of individual improvement in depression. Journal of Consulting and Clinical Psychology, 88(2), 119–127. 10.1037/ccp0000469 [DOI] [PubMed] [Google Scholar]
- Herrmann, M., Curio, N., Petz, T., Synowitz, H., Wagner, S., Bartels, C., & Wallesch, C.-W. (2000). Coping with illness after brain diseases a comparison between patients with malignant brain tumors, stroke, Parkinson's disease and traumatic brain injury. Disability and Rehabilitation, 22(12), 539–546. 10.1080/096382800416788 [DOI] [PubMed] [Google Scholar]
- Heyvaert, M., & Onghena, P. (2014). Analysis of single-case data: Randomisation tests for measures of effect size. Neuropsychological Rehabilitation, 24(3-4), 507–527. 10.1080/09602011.2013.818564 [DOI] [PubMed] [Google Scholar]
- Jacobson, N., & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. 10.1037//0022-006x.59.1.12 [DOI] [PubMed] [Google Scholar]
- Jansen, G., & Batink, T. (2014). Time to ACT! Het basisboek voor professionals. Thema. [Google Scholar]
- Juengst, S. B., Kumar, R. G., & Wagner, A. K. (2017). A narrative literature review of depression following traumatic brain injury: Prevalence, impact, and management challenges. Psychology Research and Behavior Management, 10, 175–186. 10.2147/PRBM.S113264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas, M., & McDonald, S. (2011). Is it time to act? The potential of acceptance and commitment therapy for psychological problems following acquired brain injury. Neuropsychological Rehabilitation, 21(2), 250–276. 10.1080/09602011.2010.540920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinonen, K., Kyllönen, H., Astikainen, P., & Lappalainen, R. (2018). Early sudden gains in an acceptance and values-based intervention: Effects on treatment outcome for depression and psychological flexibility. Journal of Contextual Behavioral Science, 10, 24–30. 10.1016/j.jcbs.2018.07.010 [DOI] [Google Scholar]
- Knapp, P., Campbell Burton, C. A., Holmes, J., Murray, J., Gillespie, D., Lightbody, C. E., Watkins, C. L., Chun, H. Y. Y., & Lewis, S. R. (2017). Interventions for treating anxiety after stroke. Cochrane Database of Systematic Reviews, 5(5). 10.1002/14651858.CD008860.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp, P., Dunn-Roberts, A., Sahib, N., Cook, L., Astin, F., Kontou, E., & Thomas, S. A. (2020). Frequency of anxiety after stroke: An updated systematic review and meta-analysis of observational studies. International Journal of Stroke, 15(3), 244–255. 10.1177/1747493019896958 [DOI] [PubMed] [Google Scholar]
- Kreutzer, J. S., Seel, R. T., & Gourley, E. (2001). The prevalence and symptom rates of depression after traumatic brain injury: A comprehensive examination. Brain Injury, 15(7), 563–576. 10.1080/02699050010009108 [DOI] [PubMed] [Google Scholar]
- Ledford, J. R., & Gast, D. L. (2014). Single case research methodology: Applications in special education and behavioral sciences. Routledge. [Google Scholar]
- Lenaert, B., Colombi, M., van Heugten, C., Rasquin, S., Kasanova, Z., & Ponds, R. (2019). Exploring the feasibility and usability of the experience sampling method to examine the daily lives of patients with acquired brain injury. Neuropsychological Rehabilitation, 29(5), 754–766. 10.1080/09602011.2017.1330214 [DOI] [PubMed] [Google Scholar]
- Little, A., Byrne, C., & Coetzer, R. (2020). The effectiveness of cognitive behaviour therapy for reducing anxiety symptoms following traumatic brain injury: A meta-analysis and systematic review. NeuroRehabilitation, 48(1), 67–82. 10.3233/NRE-201544 [DOI] [PubMed] [Google Scholar]
- Luoma, J. B., Hayes, S. C., & Walser, R. D. (2007). Learning ACT: An acceptance & commitment therapy skills-training manual for therapists. New Harbinger Publications. [Google Scholar]
- Majumdar, S., & Morris, R. (2019). Brief group-based acceptance and commitment therapy for stroke survivors. Journal of Clinical Psychology, 58(1), 70–90. 10.1111/bjc.12198 [DOI] [PubMed] [Google Scholar]
- Manolov, R., Moeyaert, M., & Fingerhut, J. E. (2021). A priori justification for effect measures in single-case experimental designs. Perspectives on Behavior Science, 45, 1–34. 10.1007/s40614-021-00282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels, B., & Onghena, P. (2019). Randomized single-case AB phase designs: Prospects and pitfalls. Behavior Research Methods, 51(6), 2454–2476. 10.3758/s13428-018-1084-x [DOI] [PubMed] [Google Scholar]
- Miller, H., Lawson, D., Power, E., Borschmann, K., Sathananthan, N., Kamberis, N., Armstrong, A., & Wong, D. (2021). Development and validation of the Valued Living Questionnaire – Comprehension Support Version (VLQ-CS) 6th Pacific Rim Conference. Online Virtual Event. [DOI] [PubMed]
- Okonkwo, O. C., Roth, D. L., Pulley, L., & Howard, G. (2010). Confirmatory factor analysis of the validity of the SF-12 for persons with and without a history of stroke. Quality of Life Research, 19(9), 1323–1331. 10.1007/s11136-010-9691-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, C. W., Lee, E. B., Levin, M. E., & Twohig, M. P. (2019). A review of AAQ variants and other context-specific measures of psychological flexibility. Journal of Contextual Behavioral Science, 12, 329–346. 10.1016/j.jcbs.2019.02.007 [DOI] [Google Scholar]
- Pais, C., Ponsford, J. L., Gould, K. R., & Wong, D. (2017). Role of valued living and associations with functional outcome following traumatic brain injury. Neuropsychological Rehabilitation, 29(4), 625–637. 10.1080/09602011.2017.1313745 [DOI] [PubMed] [Google Scholar]
- Parker, R. I., & Vannest, K. (2009). An improved effect size for single-case research: Nonoverlap of all pairs. Behavior Therapy, 40(4), 357–367. 10.1016/j.beth.2008.10.006 [DOI] [PubMed] [Google Scholar]
- Parker, R. I., Vannest, K. J., & Davis, J. L. (2011). Effect size in single-case research: A review of nine nonoverlap techniques. Behavior Modification, 35(4), 303–322. 10.1177/0145445511399147 [DOI] [PubMed] [Google Scholar]
- Perdices, M., & Tate, R. L. (2009). Single-subject designs as a tool for evidence-based clinical practice: Are they unrecognised and undervalued? Neuropsychological Rehabilitation, 19(6), 904–927. 10.1080/09602010903040691 [DOI] [PubMed] [Google Scholar]
- Post, M. W. M., van der Zee, C. H., Hennink, J., Schafrat, C. G., Visser-Meily, J. M. A., & van Berlekom, S. B. (2011). Validity of the Utrecht Scale for evaluation of rehabilitation-participation. Disability and Rehabilitation, 34(6), 478–485. 10.3109/09638288.2011.608148 [DOI] [PubMed] [Google Scholar]
- Pustejovsky, J. E., Chen, M., & Swan, D. M. (2021). Single-case effect size calculator (Version 0.5.2) [Web application]. https://jepusto.shinyapps.io/SCD-effect-sizes/
- Randall, D., Thomas, M., Whiting, D., & McGrath, A. (2017). Depression Anxiety Stress Scales (DASS-21): Factor structure in traumatic brain injury rehabilitation. Journal of Head Trauma Rehabilitation, 32(2), 134–144. 10.1097/HTR.0000000000000250 [DOI] [PubMed] [Google Scholar]
- Rauwenhoff, J., Peeters, F., Bol, Y., & Van Heugten, C. (2021). Measuring psychological flexibility and cognitive defusion in individuals with acquired brain injury. Brain Injury, 35(10), 1–17. 10.1080/02699052.2021.1972155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, E. D., Ritzert, T. R., Scoglio, A. A. J., Mote, J., Fukuda, S. D., Ahern, M. E., & Kelly, M. M. (2019). A systematic review of values measures in acceptance and commitment therapy research. Journal of Contextual Behavioral Science, 12, 290–304. 10.1016/j.jcbs.2018.10.004 [DOI] [Google Scholar]
- Robinson, P. L., Russell, A., & Dysch, L. (2019). Third-wave therapies for long-term neurological conditions: A systematic review to evaluate the status and quality of evidence. Brain Impairment, 20(1), 58–80. 10.1017/BrImp.2019.2 [DOI] [Google Scholar]
- Sander, A. M., Clark, A. N., Arciniegas, D. B., Tran, K., Leon-Novelo, L., Ngan, E., Bogaards, J., Sherer, M., & Walser, R. (2020). A randomized controlled trial of acceptance and commitment therapy for psychological distress among persons with traumatic brain injury. Neuropsychological Rehabilitation, 31(7), 1105–1129. 10.1080/09602011.2020.1762670 [DOI] [PubMed] [Google Scholar]
- Stalder-Lüthy, F., Messerli-Bürgy, N., Hofer, H., Frischknecht, E., Znoj, H., & Barth, J. (2013). Effect of psychological interventions on depressive symptoms in long-term rehabilitation after an acquired brain injury: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 94(7), 1386–1397. 10.1016/j.apmr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Tate, R. L., Perdices, M., Rosenkoetter, U., Wakim, D., Godbee, K., Togher, L., & McDonald, S. (2013). Revision of a method quality rating scale for single-case experimental designs and n-of-1 trials: The 15-item Risk of Bias in N-of-1 Trials (RoBiNT) Scale. Neuropsychological Rehabilitation, 23(5), 619–638. 10.1080/09602011.2013.824383 [DOI] [PubMed] [Google Scholar]
- ter Kuile, M. M., Bulte, I., Weijenborg, P. T. M., Beekman, A., Melles, R., & Onghena, P. (2009). Therapist-aided exposure for women with lifelong vaginismus: A replicated single-case design. Journal of Consulting and Clinical Psychology, 77(1), 149–159. 10.1037/a0014273 [DOI] [PubMed] [Google Scholar]
- Trindade, I. A., Marta-Simoes, J., Ferreira, C., & Pinto-Gouveia, J. (2018). Chronic illness-related cognitive fusion explains the impact of body dissatisfaction and shame on depression symptoms in breast cancer patients. Journal of Consulting and Clinical Psychology, 25(6), 886–893. 10.1002/cpp.2323 [DOI] [PubMed] [Google Scholar]
- Vannest, K. J., Peltier, C., & Haas, A. (2018). Results reporting in single case experiments and single case meta-analysis. Research in Developmental Disabilities, 79, 10–18. 10.1016/j.ridd.2018.04.029 [DOI] [PubMed] [Google Scholar]
- Verberne, D. P. J., Ponds, R., Kroese, M., Wijenberg, M. L. M., Barten, D. G., Pasmans, R., Staals, J., & van Heugten, C. M. (2021). Long-term psychosocial outcome following mild traumatic brain injury and minor stroke: A direct longitudinal comparison. Journal of Neurology, 268(6), 2132–2140. 10.1007/s00415-020-10385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware Jr, J. E., Kosinski, M., & Keller, S. D. (1996). A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- Whelan-Goodinson, R., Ponsford, J., & Schönberger, M. (2009). Validity of the Hospital Anxiety and Depression Scale to assess depression and anxiety following traumatic brain injury as compared with the structured clinical interview for DSM-IV. Journal of Affective Disorders, 114(1), 94–102. 10.1016/j.jad.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Whiting, D., Deane, F., Ciarrochi, J., McLeod, H. J., & Simpson, G. K. (2015). Validating measures of psychological flexibility in a population with acquired brain injury. Psychological Assessment, 27(2), 415–423. 10.1037/pas0000050 [DOI] [PubMed] [Google Scholar]
- Whiting, D., Deane, F., McLeod, H., Ciarrochi, J., & Simpson, G. (2019). Can acceptance and commitment therapy facilitate psychological adjustment after a severe traumatic brain injury? A pilot randomized controlled trial. Neuropsychological Rehabilitation, 30(7), 1348–1371. 10.1080/09602011.2019.1583582 [DOI] [PubMed] [Google Scholar]
- Wicksell, R., Kemani, M., Jensen, K., Kosek, E., Kadetoff, D., Sorjonen, K., Ingvar, M., & Olsson, G. (2013). Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. European Journal of Pain, 17(4), 599–611. 10.1002/j.1532-2149.2012.00224.x [DOI] [PubMed] [Google Scholar]
- Wilson, K. G., Sandoz, E. K., Kitchens, J., & Roberts, M. (2010). The Valued Living Questionnaire: defining and measuring valued action within a behavioral framework. The Psychological Record, 60(2), 249. 10.1007/BF03395706 [DOI] [Google Scholar]
- Winkens, I., Heugten, C., Oyens, I., Geschwind, N., & Bol, Y. (2020). Acceptance and commitment therapy for people with multiple sclerosis: A nonconcurrent multiple baselines design. Neurology and Neurobiology, 1–10. 10.31487/j.NNB.2020.02.13 [DOI] [Google Scholar]
- Zettle, R. D., Rains, J. C., & Hayes, S. C. (2011). Processes of change in acceptance and commitment therapy and cognitive therapy for depression: A mediation reanalysis of Zettle and Rains. Behavior Modification, 35(3), 265–283. 10.1177/0145445511398344 [DOI] [PubMed] [Google Scholar]
- Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.