Abstract
BACKGROUND
Splanchnic vein thrombosis (SVT) is a major complication of moderate and severe acute pancreatitis. There is no consensus on whether therapeutic anticoagulation should be started in patients with acute pancreatitis and SVT.
AIM
To gain insight into current opinions and clinical decision making of pancreatologists regarding SVT in acute pancreatitis.
METHODS
A total of 139 pancreatologists of the Dutch Pancreatitis Study Group and Dutch Pancreatic Cancer Group were approached to complete an online survey and case vignette survey. The threshold to assume group agreement was set at 75%.
RESULTS
The response rate was 67% (n = 93). Seventy-one pancreatologists (77%) regularly prescribed therapeutic anticoagulation in case of SVT, and 12 pancreatologists (13%) for narrowing of splanchnic vein lumen. The most common reason to treat SVT was to avoid complications (87%). Acute thrombosis was the most important factor to prescribe therapeutic anticoagulation (90%). Portal vein thrombosis was chosen as the most preferred location to initiate therapeutic anticoagulation (76%) and splenic vein thrombosis as the least preferred location (86%). The preferred initial agent was low molecular weight heparin (LMWH; 87%). In the case vignettes, therapeutic anticoagulation was prescribed for acute portal vein thrombosis, with or without suspected infected necrosis (82% and 90%), and thrombus progression (88%). Agreement was lacking regarding the selection and duration of long-term anticoagulation, the indication for thrombophilia testing and upper endoscopy, and about whether risk of bleeding is a major barrier for therapeutic anticoagulation.
CONCLUSION
In this national survey, the pancreatologists seemed to agree on the use of therapeutic anticoagulation, using LMWH in the acute phase, for acute portal thrombosis and in the case of thrombus progression, irrespective of the presence of infected necrosis.
Keywords: Acute pancreatitis, Splanchnic vein thrombosis, Therapeutic anticoagulation, Bleeding, Recanalization, Outcomes
Core Tip: Splanchnic vein thrombosis is a relatively common complication of moderate and severe acute pancreatitis, but there is still much debate about its treatment with therapeutic anticoagulation. This national survey and case vignette study among 93 pancreatologists demonstrates that the majority prescribe therapeutic anticoagulation for acute portal vein thrombosis and thrombus progression in patients with or without infected necrosis, despite the absence of evidence supporting its use. Whether this collective opinion is accurate needs to be confirmed in future (preferably prospective) studies.
INTRODUCTION
Acute pancreatitis is an inflammatory disorder of the pancreas and is self-limiting in the majority of patients[1,2]. However, approximately 20% of patients develop a moderate or severe disease course, with (peri) pancreatic necrosis and collections[3,4]. Due to the combination of local inflammation and mechanical compression, these complications may cause thrombus formation in the splanchnic circulation, including the portal, splenic and superior mesenteric vein[5,6]. The reported estimates on the incidence of splanchnic vein thrombosis (SVT) in acute pancreatitis range from 17% to 23%, and are even higher in complicated acute pancreatitis[7,8]. The clinical presentation of SVT varies between an asymptomatic thrombus to potential lethal complications, such as portal or left side hypertensive bleeding and small bowel ischemia[9-11]. For this reason, early treatment with therapeutic anticoagulation is recommended in patients with acute SVT[12-14]. However, consistent evidence to drive this decision in acute pancreatitis patients does not exist[15-18]. In fact, a recent meta-analysis from our study group showed that 53% of acute pancreatitis patients do not receive therapeutic anticoagulation[15]. This proportion of untreated patients is substantially higher than previously reported in other SVT populations[19], probably because of the fear of serious bleeding. Variation in clinical practice also became apparent in this meta-analysis[15], as anticoagulation use and the type of agent used were very heterogeneous between studies. Therefore, the aim of this survey was to gain more insight into current opinions of pancreatologists on anticoagulation therapy for SVT following acute pancreatitis.
MATERIALS AND METHODS
We conducted an online national survey and case vignette study among members of the Dutch Pancreatitis Study Group (DPSG) and the Dutch Pancreatic Cancer Group (DPCG). Members were excluded if they were not primary care-takers in the treatment of patients with AP (e.g., radiologists, oncologists, basic scientists). The survey was built in Research Electronic Data Capture, and invitations to participate were sent by e-mail in November 2021, followed by four weekly reminders. Additionally, the survey was promoted through newsletters and during annual study group meetings of the DPSG and DPCG.
Survey design
The survey was developed by a multidisciplinary team of surgeons, gastroenterologists, and radiologists, and included 3 demographical questions, 17 general questions and 3 case vignettes (Supplementary material). Demographic information included the responders’ specialty, type of hospital and working experience. The general questions focused on treatment of SVT and potential factors that may influence the responders’ decision. The case-vignettes addressed the preferred treatment strategy in different clinical cases at different time points. All cases however, concerned a 50-year-old male patient with acute alcoholic necrotising pancreatitis, and can be summarized as follows.
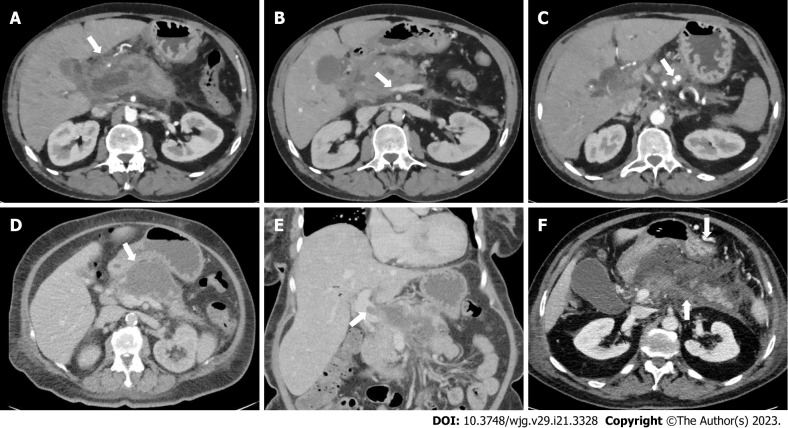
Case vignette 1: A patient visited the emergency department, 5 d after onset of abdominal pain. Contrast-enhanced CT (CECT) showed necrotising pancreatitis with acute necrotic collection in the head of the pancreas (Figure 1A) and: A1: Luminal narrowing of the portal vein without the presence of collateral circulation (Figure 1B); A2: Intraluminal filling defect in the portal vein without the presence of collateral circulation; A3: Intraluminal filling defect in the portal vein without the presence of collateral circulation + a pseudoaneurysm in the proximal splenic artery (Figure 1C).
Figure 1.
Imaging findings of case vignette. A: Acute necrotic collection in the head of the pancreas in case vignette 1; B: Luminal narrowing of the portal vein without the presence of collateral circulation in case vignette 1; C: Pseudoaneurysm in the proximal splenic artery in case vignette 1; D: Almost fully encapsulated pancreatic necrosis without gas configurations in case vignette 2; E: Luminal filling defect in the portal vein without the presence of collateral circulation in case vignette 2; F: Extension of the thrombus to the splenic vein (arrow pointing upwards) and expansion of the collateral pathway in the gastroepiploic veins along the great curvature of the stomach (arrow pointing downwards) in case vignette 3.
Case vignette 2: A patient admitted to the ward, 14 d after onset of abdominal pain. The patient showed signs of clinical detoriation with fever and rising inflammatory parameters. The CECT showed almost fully encapsulated pancreatic necrosis without gas configurations (Figure 1D) and a new intraluminal filling defect in the portal vein without the presence of collateral circulation (Figure 1E). The diagnosis of suspected infected pancreatic necrosis was made.
Case vignette 3: A homeless patient visited the emergency department, 30 d after onset of vague abdominal pain. CECT showed necrotising pancreatitis and: CA: Intraluminal filling defect in the portal vein with the presence of collateral circulation; CB: Thrombus progression and expansion of the collateral circulation (Figure 1F).
The threshold to assume group agreement was set at 75%. If a question ranged from always, usually, sometimes and never, agreement was defined when 75% of the pancreatologists rated it as always or usually (regularly), or sometimes and never (infrequently).
Definitions
SVT was predefined as an actual intraluminal filling defect on imaging of one or more of the splanchnic veins. The chronicity was divided into (sub)acute thrombosis or chronic thrombosis (with concomitant collaterals), anatomical location into portal, splenic and/or superior mesenteric vein, degree into a total or partial occlusion and extent into an isolated thrombus or a thrombus in several venous segments. Thrombus progression was defined as progression into other splanchnic vein(s), into total occlusion, or both.
Statistical analysis
Descriptive data are presented as counts with proportions for categorical data. All analyses were performed using IBM SPSS (20).
RESULTS
A total of 93 of the 139 invited pancreatologists (67%) responded and participated in this survey and case vignette study; 67 gastroenterologists (72%), 25 surgeons (27%) and 1 intensivist (1%). The majority worked in a non-academic centre (70%) and had more than 10 years of experience in treating AP patients (60%). Demographic characteristics are presented in Table 1.
Table 1.
Details of respondents, n (%)
|
Demographics
|
n = 93
|
| Specialty | |
| Surgeon | 25 (27) |
| Gastroenterologist | 67 (72) |
| Intensivist | 1 (1) |
| Type of hospital | |
| Academic | 28 (30) |
| Non-academic, teaching hospital | 60 (65) |
| Non-academic, non-teaching hospital | 5 (5) |
| Experience in treating patients with acute pancreatitis | |
| 0-5 years | 10 (11) |
| 5-10 years | 27 (29) |
| 10-15 years | 17 (18) |
| 15-20 years | 23 (25) |
| > 20 years | 16 (17) |
Indications for and details of treatment with therapeutic anticoagulation
Agreement was reached on whether therapeutic anticoagulation should be prescribed for SVT and luminal narrowing of one or more of the splanchnic veins in acute pancreatitis patients. For SVT, therapeutic anticoagulation was regularly prescribed by 71 (76%) and infrequently by 22 (24%) pancreatologists. In case of luminal narrowing, therapeutic anticoagulation was only regularly prescribed by 12 (13%) pancreatologists. Avoiding complications, such as portal hypertension and bowel ischemia, was the main reason for 81 pancreatologists (87%) to start therapeutic anticoagulation. Screening for an underlying prothrombotic disorder in patients diagnosed with SVT was regularly performed by 14 (15%) pancreatologists, only in patients with a history of one (or more) thrombotic events by 40 (43%), and infrequently by 39 (42%) pancreatologists. There was agreement on the preferred initial type of therapeutic anticoagulation for SVT 81 pancreatologists (87%) preferred subcutaneous low-molecular-weight heparin (LMWH)], but not on the preferred follow-up type. Imaging after the index admission was chosen as follow-up strategy by 79 pancreatologists (85%). Thirteen pancreatologists (13%) indicated that they usually stop anticoagulant therapy in case of achieved radiological recanalization, 35 (38%) after a period of 3 mo, 42 (45%) after 6 mo, and 3 (3%) after 12 mo. All details are provided in Table 2.
Table 2.
Survey results: Indication for and details of treatment with therapeutic anticoagulation, n (%)
|
Item
|
Total (n = 93)
|
| Do you prescribe therapeutic AC in case of detected thrombosis in one (or more) of the splanchnic veins? | |
| Always | 23 (25) |
| Usually | 48 (52) |
| Sometimes | 21 (23) |
| Never | 1 (1) |
| Do you prescribe therapeutic AC in case of detected luminal narrowing of one (or more) of the splanchnic veins? | |
| Always | 3 (3) |
| Usually | 9 (10) |
| Sometimes | 29 (31) |
| Never | 52 (56) |
| Main reason(s) to start therapeutic AC (multiple answers were possible) | |
| To achieve vessel recanalization | 52 (56) |
| To avoid complications | 81 (87) |
| To prevent formation of altered venous anatomy | 31 (33) |
| To prevent recurrence of SVT | 27 (29) |
| To prevent another venous thromboembolism | 30 (32) |
| Other reason1 | 1 (1) |
| Do you screen for an underlying prothrombotic disorder? | |
| Always | 2 (2) |
| Usually | 12 (13) |
| Sometimes | 25 (27) |
| Only in patients with a history of one (or more) thrombotic events | 40 (43) |
| Never | 14 (15) |
| Which initial type of therapeutic AC do you prefer? | |
| (Low molecular weight) heparin subcutaneous | 81 (87) |
| Unfractionated heparin intravenous | 4 (4) |
| Direct oral anticoagulation | 3 (3) |
| Vitamin K antagonist | 4 (4) |
| Platelet aggregation inhibitor | 1 (1) |
| Urokinase/recombinant tissue plasminogen activator | 0 |
| Which follow-up type of therapeutic AC do you prefer? | |
| (Low molecular weight) heparin subcutaneous | 9 (10) |
| Unfractionated heparin intravenous | 0 |
| Direct oral anticoagulation | 53 (57) |
| Vitamin K antagonist | 29 (31) |
| Platelet aggregation inhibitor | 2 (2) |
| Urokinase/recombinant tissue plasminogen activator | 0 |
| Do you generally follow-up SVT after index admission? | |
| Yes, clinically only | 5 (5) |
| Yes, with imaging | 79 (85) |
| No | 9 (10) |
| After how long do you usually stop the therapeutic AC? | |
| In case of achieved radiological recanalization | 13 (14) |
| 3 mo | 35 (38) |
| 6 mo | 42 (45) |
| 12 mo | 3 (3) |
| Never | 0 |
In free text: expansion of thrombosis.
AC: Anticoagulation; SVT: Splanchnic vein thrombosis.
Determinants of prescribing therapeutic anticoagulation
Seventy-eight pancreatologists (84%) have chosen the time course of thrombosis as the most important factor supporting anticoagulant therapy; 84 pancreatologists (90%) prescribe therapeutic anticoagulation in case of a (sub)acute thrombosis vs 9 (10%) for both (sub)acute and chronic thrombosis. Moreover, 70 pancreatologists (76%) have chosen portal vein thrombosis as the most preferred location to initiate therapeutic anticoagulation, whereas splenic vein thrombosis was chosen as least preferred location by 80 pancreatologists (86%). The majority of pancreatologists (85%) treat both total and partial occlusive thrombosis. There was no agreement whether the risk of different types of bleeding should be considered as a major barrier to prescribe therapeutic anticoagulation. The need for invasive interventions for local complications of acute pancreatitis influenced the decision whether or not to initiate anticoagulation therapy in about half of pancreatologists (52%). All details are outlined in Table 3.
Table 3.
Survey results: Determinants of prescribing therapeutic anticoagulation, n (%)
|
Item
|
Total (n = 93)
|
| Do you consider of ... the thrombosis as an important factor to prescribe therapeutic AC? (multiple answers were possible) | |
| Age (acute or chronic) | 78 (84) |
| Anatomical location (portal, splenic or superior mesenteric vein) | 42 (45) |
| Degree (total or partial) | 45 (48) |
| Extent (isolated thrombosis or thrombosis in several segments) | 49 (53) |
| Progression (over time) | 40 (43) |
| When do you prescribe therapeutic AC? In case of: | |
| (Sub)acute thrombosis | 84 (90) |
| Chronic thrombosis | 0 |
| Both | 9 (10) |
| Rank the anatomical location of the thrombosis from most likely to less likely to start therapeutic AC: | |
| Portal vein-splenic vein-superior mesenteric vein | 9 (10) |
| Portal vein-superior mesenteric vein-splenic vein | 61 (66) |
| Splenic vein-portal vein-superior mesenteric vein | 0 |
| Splenic vein-superior mesenteric vein-portal vein | 1 (1) |
| Superior mesenteric vein-portal vein-splenic vein | 19 (20) |
| Superior mesenteric vein-splenic vein-portal vein | 3 (3) |
| When do you prescribe therapeutic AC? In case of: | |
| Total thrombosis | 9 (10) |
| Partial thrombosis | 5 (5) |
| Both | 79 (85) |
| Do you consider the risk of ... as a major barrier to prescribe therapeutic AC? (multiple answers were possible) | |
| Bleeding in general | 52 (56) |
| Bleeding related to portal hypertension | 17 (18) |
| Bleeding related to pseudoaneurysm | 49 (53) |
| Other risk1 | 1 (1) |
| Does the need for invasive interventions for local complications of acute pancreatitis influence your decision regarding AC therapy? | |
| Yes | 48 (52) |
| No | 45 (48) |
In free text: CVA bleeding history.
AC: Anticoagulation; SVT: Splanchnic vein thrombosis.
Statements on prognosis
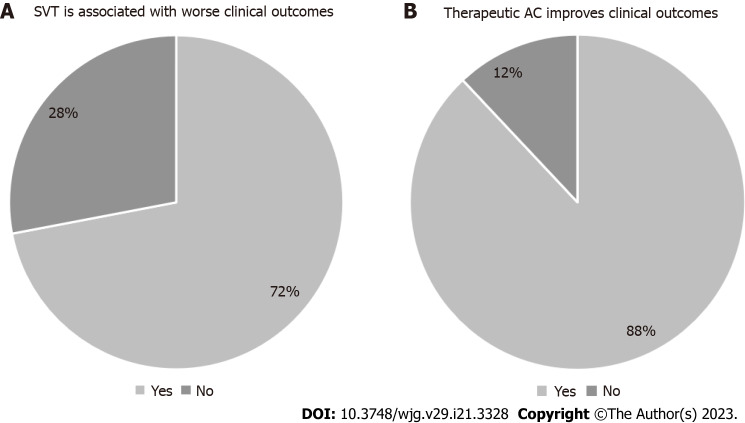
An association between the presence of SVT and worse clinical outcomes in patients with acute pancreatitis was assumed by 67 pancreatologists (72%) (Figure 2). Moreover, the vast majority (88%) agreed that therapeutic anticoagulation for splanchnic vein thrombosis improves clinical outcomes in these patients. Insufficient evidence was the most frequently quoted reason among pancreatologists who disagreed with this second statement.
Figure 2.
Statements on prognosis. A: Splanchnic vein thrombosis is associated with worse clinical outcomes; B: Therapeutic anticoagulation improves clinical outcomes. AC: Anticoagulation; SVT: Splanchnic vein thrombosis.
Case-vignettes
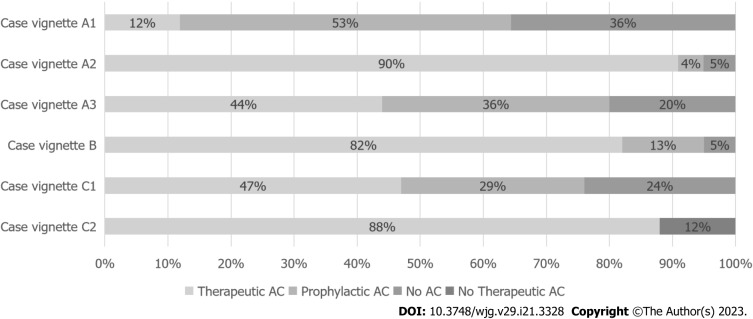
The results of the case vignettes are summarized in Figure 3. In the first case vignette (patient 1, day 5 of acute necrotising pancreatitis), 11 pancreatologists (12%) would prescribe a therapeutic dose anticoagulation if luminal narrowing without collateral circulation was detected in the portal vein. Of the 82 pancreatologists (88%) who opted for no therapeutic dose anticoagulation, 73 (89%) would change treatment strategy in case an actual filling defect in the portal vein was detected. In total, 84 pancreatologists (90%) would prescribe therapeutic dose anticoagulation to this patient with an actual portal vein thrombosis without collateral circulation. If a pseudoaneurysm was concomitantly present, 43 of those 84 pancreatologists (51%) who favoured a therapeutic dose anticoagulation would switch to a prophylactic dose anticoagulation (n = 28, 65%) or no anticoagulation at all (n = 15, 35%), leaving 41 pancreatologists (44%) in the therapeutic anticoagulation group.
Figure 3.
Case vignettes results: Choice of treatment. AC: Anticoagulation.
In the second case vignette (patient 2, day 14 of suspected infected necrotising pancreatitis), 77 pancreatologists (82%) would prescribe therapeutic dose anticoagulation if a portal vein thrombosis without collateral circulation was detected. The presence of (suspected) infected pancreatic necrosis influenced the choice of anticoagulation agent in 49 pancreatologists (52%). Almost all of these pancreatologists pointed out that once infected pancreatic necrosis is suspected, they would choose an agent with a short half-life because of the potentially need of invasive intervention.
In the third case vignette (patient 3, day 30 of acute necrotising pancreatitis), 44 pancreatologists (47%) would prescribe a therapeutic dose anticoagulation if a portal vein thrombosis with collateral circulation was detected. Of these 44 pancreatologists, 19 (43%) would perform upper endoscopy to screen for and-if present-treat oesophageal varices before starting anticoagulation therapy. In case of thrombus progression (extension of the thrombus to the splenic vein and expansion of the collateral pathway), 11 pancreatologists (12%) would stay conservative (i.e., no therapeutic dose of anticoagulation), 82 (88%) would start or continue a therapeutic dose anticoagulation and none would proceed to an intervention.
DISCUSSION
This first nationwide survey and case vignette study gives insight into the clinical scenarios in which therapeutic anticoagulation is currently used, and not used to treat or prevent splanchnic vein thrombosis in acute pancreatitis patients. In an earlier study[15], we found 7 retrospective cohort studies evaluating therapeutic anticoagulation in this patient category with conflicting results in clinical outcome[20-27]. These studies were of moderate quality and therefore the pancreatologist’ preference and belief predominate in current decision making rather than scientific evidence.
An important finding of the current study was that more than 75% of pancreatologists regularly prescribe therapeutic anticoagulation for SVT, particularly for a thrombus that acutely developed. This is in line with recommendations from general guidelines for SVT management[12-14]. In the absence of a visualized thrombus, most pancreatologists indicated not to treat compressed veins with anticoagulation. Although wall shear stress in a compressed vessel may promote platelet activation, and subsequently thrombus formation[28], there is no data yet to question the opinion of the pancreatologists.
In this study, the most important reason to administer therapeutic anticoagulation was to avoid complications including bowel ischemia and portal hypertension. Bowel ischemia has been reported in up to 33% of acute pancreatitis patients treated with therapeutic anticoagulation vs 16% of untreated patients[22,24,25]. A potential explanation for this discrepancy could be that bowel ischemia was already present prior to the start of therapy, therefore being an indication for therapeutic anticoagulation rather than a consequence. In addition, the presence of varices and other collaterals have been equally reported[15], and only one of the aforementioned studies described one case of bleeding from oesophageal varices in an anticoagulated patient[24]. Again, it is likely that a perceived bleeding risk influenced the decision whether or not to prescribe therapeutic anticoagulation. This confounding by indication clearly limits the interpretation of these retrospective studies.
Achieving vessel recanalization was chosen as the second goal. A recent meta-analysis showed that the pooled rate of recanalization of SVT was similar between treated (36%) and untreated patients (31%)[15]. However, there is reason to believe that the benefit of anticoagulation therapy may alter when considering the anatomical location of the thrombosis[21,29]. Patients with portal vein or superior mesenteric vein thrombosis may have an increased risk of complications, while having lower spontaneous recanalization rates. In particular, mortality rates of patients with superior mesenteric vein thrombosis are reported up to 50%[30,31]. On the other hand, splenic vein thrombosis, which is by far the most common site of thrombosis in acute pancreatitis patients, forms a less serious concern for gastrointestinal bleeding and insufficient recanalization[8,26,32]. A selective anticoagulation policy, in which therapeutic anticoagulation was reserved for portal- and superior mesenteric vein thrombosis, was recently assessed in a retrospective study[33]. This study showed a recanalization rate of 67% in portal- and superior mesenteric vein thrombosis, which is substantially higher than previously reported[15]. In addition, a recent practice guideline from the Pancreas study group, Chinese Society of Gastroenterology, recommends a selective anticoagulation policy[34]. In this survey, portal vein thrombosis, followed by superior mesenteric vein thrombosis, was also the pancreatologists’ preferred location for prescribing therapeutic anticoagulation, while splenic vein was the least preferred location.
With respect to chronic SVT, the current guidelines do not recommend therapeutic anticoagulation[10]. This is in line with the reported use in case vignette 3, with the exception of the case of the patient with thrombus progression and expansion of the collateral circulation. In this scenario, 88% of pancreatologists would treat such patient with therapeutic anticoagulation. A recent multicentre randomised controlled trial comparing daily rivaroxaban 15 mg/d to no anticoagulation in patients with noncirrhotic chronic portal vein thrombosis[35], formally challenged the guideline recommendations. This study showed that rivaroxaban, even in prophylactic dose, reduced the incidence of venous thromboembolism; therefore, this study may initiate a shift towards a more frequent use of anticoagulation in chronic SVT. On that note, primary prophylaxis of portal hypertensive bleeding should be performed, as laid out by the BAVENO IV guideline[13]. In this survey, however, the minority of pancreatologists followed this recommendation. Improvements should also be made to distinguish acute from chronic SVT. Currently, no clear definition for chronic SVT exists other than a presumed time course of more than 6 mo or the presence of multiple small collaterals around the obstructed veins[10,36], which is not useful to diagnose a nonocclusive chronic thrombosis (i.e., absence of collateral pathways). A promising invention to overcome this problem is magnetic resonance noncontrast thrombus imaging, though validation is still needed[37].
According to our survey, subcutaneous LMWH was the favoured initial type of therapeutic anticoagulation, while no agreement regarding the choice of long-term anticoagulation and its duration was found. In current guidelines, switching LMWH to a vitamin K antagonist once reaching the target range is the reported strategy for patients with SVT[12-14,38]. The use of direct oral anticoagulation (i.e., apixaban) in acute pancreatitis patients with SVT is reported in two studies and showed comparable results[21,33]. However, in the case of (suspected) infected pancreatic necrosis, LMWH seems to be preferred by more than half of the pancreatologists, due to its short half-life and reversibility. Besides, many acute pancreatitis patients have reduced caloric intake limiting the absorption of DOACS. Therefore, it seems fair to advise LWMH, especially in the acute phase. Looking at the duration of anticoagulation therapy for provoked SVT in patients with a transient risk factor, such as acute pancreatitis, the suggested duration is 3 mo to 6 mo[12-14,38]. Consistently, 38% and 45% of the pancreatologists in our survey preferred 3 mo and 6 mo treatment duration, respectively.
Based on the available literature, it remains unclear whether therapeutic anticoagulation is associated with higher rates of bleeding. An increased bleeding risk with therapeutic anticoagulation has been reported up to 33% of patients[21,25,26], but there are also studies showing lower rates of bleeding[24]. The theory for this latter finding is that therapeutic anticoagulation prevents thrombus progression, therefore reducing the portal pressure and consequently the risk of bleeding[19]. In this study, the risk of bleeding was not identified as a significant discouraging factor, as only about half of the pancreatologists considered bleeding in general and bleeding related to pseudoaneurysm as a major barrier to prescribe therapeutic anticoagulation. Also, the possible need for invasive intervention, due to suspected infected necrosis, did not significantly influence the treatment strategy. Another critical question is whether SVT influences the disease course of acute pancreatitis patients, but again this remains unanswered[15]. In this survey, the majority of pancreatologists assumed that the occurrence of SVT is associated with worse clinical outcomes, and interestingly, even more pancreatologists were convinced that the use of therapeutic anticoagulation leads to improved patient outcomes.
A strength of this study is the response rate of 67%, which is relatively high compared to previous surveys among pancreatologists[39-41]. Furthermore, the ratio of 30:70 between academic and non-academic pancreatologists attributed to a valuable insight into the pancreatologists’ opinions on the use of therapeutic anticoagulation. This study also has several limitations. First, the results may not directly reflect the actual practice in other countries as only members of two Dutch associations of pancreatology were invited. This decision was made to avoid selection based on publication record, and consequently include pancreatologists who are not actively involved in the treatment of acute pancreatitis[40]. Another advantage of our method is that it allowed us to calculate the survey’s response rate by bypassing the confidentiality of membership lists of international pancreatic associations. Second, the clinical presentation of SVT is very heterogeneous, as well as the patient characteristics and clinical disease course among acute pancreatitis patients, which influences current decision making. For this reason, it might have been difficult for pancreatologists to answer some of the general questions. Therefore, case vignettes were used to explore what considerations underpin their decisions. As the descriptions throughout the case vignettes were consistently formulated and only one clinical detail was changed at a time, treatment of patients with superior mesenteric vein and splenic vein thrombosis was not assessed in the case vignettes. Consequently, the pancreatologists’ preference on this manifestation of SVT in acute pancreatitis remained unknown. Finally, the rationale behind the “nonprescribing trend” was not assessed adequately, which could be a focus for future research.
CONCLUSION
In conclusion, this national survey demonstrates the tendency of pancreatologists to prescribe therapeutic anticoagulation for acute thrombosis, in particular for acute portal vein thrombosis and in case of thrombus progression, irrespective of the presence of infected necrosis. With therapeutic anticoagulation, the majority of pancreatologists believed that the clinical outcomes of acute pancreatitis patients with splanchnic vein thrombosis will improve. Furthermore, this study reflects on several knowledge gaps in literature, and sets out clear points for future research. Specifically, a deeper understanding of the pathophysiology and natural course of splanchnic vein thrombosis secondary to acute pancreatitis would allow us to clarify the therapeutic role of anticoagulation.
ARTICLE HIGHLIGHTS
Research background
Splanchnic vein thrombosis (SVT) is a severe complication of acute pancreatitis that may cause portal hypertensive complications and bowel ischemia. To prevent such complications, therapeutic anticoagulation is recommended in the general population of patients with an acute SVT.
Research motivation
Evidence to support this recommendation in acute pancreatitis patients does however not exist and as a result, clinical decision-making is mostly based on the preferences and beliefs of the pancreatologists.
Research objectives
To gain insight into current opinions on the use of therapeutic anticoagulation for SVT in acute pancreatitis.
Research methods
An online survey was sent to 139 Dutch pancreatologists. The threshold to assume agreement was set at 75%.
Research results
The response rate was 67% (n = 93). Seventy-one pancreatologists (77%) regularly prescribed therapeutic anticoagulation for SVT, using LMWH in the acute phase (87%). The majority favored therapeutic anticoagulation for acute thrombosis (90%), portal vein thrombosis in patients with or without infected necrosis (82% and 90%) and in case of thrombus progression (88%). There was no agreement whether the risk of bleeding is a barrier for initiation of therapeutic anticoagulation.
Research conclusions
The pancreatologists reached agreement regarding the use of therapeutic anticoagulation for SVT, particularly in cases of acute thrombosis, portal vein thrombosis and thrombus progression.
Research perspectives
To get a better understanding of the therapeutic role of anticoagulation, it is crucial to conduct prospective studies targeting the pathophysiology and natural course of SVT in acute pancreatitis patients.
ACKNOWLEDGEMENTS
We are very grateful to all 93 pancreatologists who participated in this study, 54 of whom agreed to be included in the acknowledgement: M Anten; A Bhalla; M Boermeester; K Bosscha; S Bouwense; H Braat; M Bruno; F van Delft; W Derksen; L van Driel; P van Duijvendijk; P Dura; R Eichhorn; P Fockens; E van Geenen; M Hadithi; M van der Have; J Haveman; M Hemmink; I de Hingh; H Hofker; L Holl; A Inderson; J Jansen; M Liem; D Lips; L Kager; V de Meijer; M Meijssen; H Moeniralam; V Nieuwenhuijs; M van de Poll; R Quispel; T Romkens; J Schreinemakers; M Schwartz; M Spanier; J Sprakel; M Stommel; J Straathof; J Tenthof van Noorden; W Thijs; M Tielemans; N Venneman; R Verbeek; F Vleggaar; R Voermans; J Vrolijk; L van der Waaij; R van Wanrooij; M Wielenga; R de Wilde; R Zoutendijk.
Footnotes
Institutional review board statement: This survey research is not subject to the Dutch Medical Research involving Human Subjects Acts (WMO) as participants are not subject to procedures or are required to follow rules of behavior. Consequently, this study does not need a full review by an accredited MREC or the CCMO.
Conflict-of-interest statement: All authors declare no conflict of interest.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 3, 2023
First decision: March 24, 2023
Article in press: April 25, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Liu C, China; Pécsi D, Hungary S-Editor: Chen YL L-Editor: A P-Editor: Fan JR
Contributor Information
Noor J Sissingh, Department of Gastroenterology and Hepatology, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands; Department of Research and Development, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands.
Jesse V Groen, Department of Surgery, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands.
Hester C Timmerhuis, Department of Research and Development, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands; Department of Surgery, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands.
Marc G Besselink, Department of Surgery, Amsterdam UMC, Location University of Amsterdam, Amsterdam 1081 HZ, Netherlands; Amsterdam Gastroenterology Endocrinology Metabolism, Amsterdam 1105 AZ, Netherlands.
Bas Boekestijn, Department of Radiology, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands.
Thomas L Bollen, Department of Radiology, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands.
Bert A Bonsing, Department of Surgery, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands.
Frederikus A Klok, Department of Medicine-Thrombosis and Hemostasis, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands.
Hjalmar C van Santvoort, Department of Surgery, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands; Department of Surgery, University Medical Centre Utrecht, Utrecht, Utrecht 3584 CX, Netherlands.
Robert C Verdonk, Department of Gastroenterology and Hepatology, St. Antonius Hospital, Nieuwegein 3420 EM, Netherlands.
Casper H J van Eijck, Department of Surgery, Erasmus Medical Centre, Rotterdam 3015 GD, Netherlands.
Jeanin E van Hooft, Department of Gastroenterology and Hepatology, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands.
Jan Sven D Mieog, Department of Surgery, Leiden University Medical Centre, Leiden 2333 ZA, Netherlands. j.s.d.mieog@lumc.nl.
Data sharing statement
Requests for data can be made to the corresponding author and will be discussed during a meeting of the Dutch Pancreatitis Study Group. After approval by the Dutch Pancreatitis Study Group, data that underlie the results reported in this study, will be shared.
References
- 1.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 2.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85–96. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- 3.Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC, Bruno MJ Dutch Pancreatitis Study Group. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 4.van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263. doi: 10.1053/j.gastro.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni NA, Khanna S, Vege SS. Splanchnic venous thrombosis and pancreatitis. Pancreas. 2013;42:924–931. doi: 10.1097/MPA.0b013e318287cd3d. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073–1079. doi: 10.1111/j.1445-2197.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 7.Anis FS, Adiamah A, Lobo DN, Sanyal S. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37:446–454. doi: 10.1111/jgh.15711. [DOI] [PubMed] [Google Scholar]
- 8.Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford) 2011;13:839–845. doi: 10.1111/j.1477-2574.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaander MC, Hoekstra J, Hansen BE, Van Buuren HR, Leebeek FW, Janssen HL. Anticoagulant therapy in patients with non-cirrhotic portal vein thrombosis: effect on new thrombotic events and gastrointestinal bleeding. J Thromb Haemost. 2013;11:452–459. doi: 10.1111/jth.12121. [DOI] [PubMed] [Google Scholar]
- 10.Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic Vein Thrombosis: Current Perspectives. Vasc Health Risk Manag. 2019;15:449–461. doi: 10.2147/VHRM.S197732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawacki Ł, Matykiewicz J, Stochmal E, Głuszek S. Splanchnic Vein Thrombosis in Acute Pancreatitis and Its Consequences. Clin Appl Thromb Hemost. 2021;27:10760296211010260. doi: 10.1177/10760296211010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 13.de Franchis R Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, van Santvoort HC, Verdonk RC, Bonsing BA, van Eijck CHJ, van Hooft JE, Mieog JSD Dutch Pancreatitis Study Group. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022;22:235–243. doi: 10.1016/j.pan.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Hajibandeh S, Hajibandeh S, Agrawal S, Irwin C, Obeidallah R, Subar D. Anticoagulation Versus No Anticoagulation for Splanchnic Venous Thrombosis Secondary to Acute Pancreatitis: Do We Really Need to Treat the Incidental Findings? Pancreas. 2020;49:e84–e85. doi: 10.1097/MPA.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 17.Norton W, Lazaraviciute G, Ramsay G, Kreis I, Ahmed I, Bekheit M. Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2020;19:116–121. doi: 10.1016/j.hbpd.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Chandan S, Buddam A, Khan SR, Mohan BP, Ramai D, Bilal M, Dhindsa B, Bhogal N, Kassab LL, Goyal H, Perisetti A, Facciorusso A, Adler DG. Use of therapeutic anticoagulation in splanchnic vein thrombosis associated with acute pancreatitis: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:862–871. doi: 10.20524/aog.2021.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valeriani E, Di Nisio M, Riva N, Cohen O, Garcia-Pagan JC, Magaz M, Porreca E, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis. Blood. 2021;137:1233–1240. doi: 10.1182/blood.2020006827. [DOI] [PubMed] [Google Scholar]
- 20.IBM Corp. IBM SPSS Statistics for Windows, version 26.0. Armonk, NY: IBM Corp. 2019. [cited 20 March 2023]. Available from: https://www.ibm.com/products/spss-statistics .
- 21.Pagliari D, Cianci R, Brizi MG, Mancarella FA, Musso M, Cintoni M, Franza L, Flore RA, Gasbarrini A, Tondi P. Anticoagulant therapy in the treatment of splanchnic vein thrombosis associated to acute pancreatitis: a 3-year single-centre experience. Intern Emerg Med. 2020;15:1021–1029. doi: 10.1007/s11739-019-02271-5. [DOI] [PubMed] [Google Scholar]
- 22.Junare PR, Udgirkar S, Nair S, Debnath P, Jain S, Modi A, Rathi P, Rane S, Contractor Q. Splanchnic Venous Thrombosis in Acute Pancreatitis: Does Anticoagulation Affect Outcome? Gastroenterology Res. 2020;13:25–31. doi: 10.14740/gr1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toqué L, Hamy A, Hamel JF, Cesbron E, Hulo P, Robert S, Aube C, Lermite E, Venara A. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: A 6-year single-center experience. J Dig Dis. 2015;16:734–740. doi: 10.1111/1751-2980.12298. [DOI] [PubMed] [Google Scholar]
- 24.Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas. 2013;42:1251–1254. doi: 10.1097/MPA.0b013e3182968ff5. [DOI] [PubMed] [Google Scholar]
- 25.Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854–862. doi: 10.1016/j.cgh.2013.09.068. [DOI] [PubMed] [Google Scholar]
- 26.Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford) 2011;13:860–864. doi: 10.1111/j.1477-2574.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garret C, Péron M, Reignier J, Le Thuaut A, Lascarrou JB, Douane F, Lerhun M, Archambeaud I, Brulé N, Bretonnière C, Zambon O, Nicolet L, Regenet N, Guitton C, Coron E. Risk factors and outcomes of infected pancreatic necrosis: Retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United European Gastroenterol J. 2018;6:910–918. doi: 10.1177/2050640618764049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg. 2015;61:1068–1080. doi: 10.1016/j.jvs.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 29.Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J, Morard I, Lasser L, Langlet P, Denninger MH, Vidaud D, Condat B, Hadengue A, Primignani M, Garcia-Pagan JC, Janssen HL, Valla D European Network for Vascular Disorders of the Liver (EN-Vie) Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210–218. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–1688. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Kamath PS. Acute superior mesenteric venous thrombosis: one disease or two? Am J Gastroenterol. 2003;98:1299–1304. doi: 10.1111/j.1572-0241.2003.07338.x. [DOI] [PubMed] [Google Scholar]
- 32.Besselink MG. Splanchnic vein thrombosis complicating severe acute pancreatitis. HPB (Oxford) 2011;13:831–832. doi: 10.1111/j.1477-2574.2011.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.K T, Chan SJ, Varghese C, Lim WB, Cheemungtoo GM, Akter N, Nayar M, Pandanaboyana S. A selective anticoagulation policy for splanchnic vein thrombosis in acute pancreatitis is associated with favourable outcomes: experience from a UK tertiary referral centre. HPB (Oxford) 2022;24:1937–1943. doi: 10.1016/j.hpb.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Pancreas Study Group; Chinese Society of Gastroenterology Chinese Medical Association. Practice guidance for diagnosis and treatment of pancreatitis-related splanchnic vein thrombosis (Shenyang, 2020) J Dig Dis. 2021;22:2–8. doi: 10.1111/1751-2980.12962. [DOI] [PubMed] [Google Scholar]
- 35.Plessier A, Goria O, Cervoni JP, Ollivier I, Bureau C, Poujol-Robert A, Minello A, Houssel-Debry P, Rautou PE, Payancé A, Scoazec G, Bruno O, Corbic M, Durand F, Vilgrain V, Paradis V, Boudaoud L, de Raucourt E, Roy C, Gault N, Valla D. Rivaroxaban Prophylaxis in Noncirrhotic Portal Vein Thrombosis. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2200104. [DOI] [PubMed] [Google Scholar]
- 36.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dam LF, Dronkers CEA, Gautam G, Eckerbom Å, Ghanima W, Gleditsch J, von Heijne A, Hofstee HMA, Hovens MMC, Huisman MV, Kolman S, Mairuhu ATA, Nijkeuter M, van de Ree MA, van Rooden CJ, Westerbeek RE, Westerink J, Westerlund E, Kroft LJM, Klok FA Theia Study Group. Magnetic resonance imaging for diagnosis of recurrent ipsilateral deep vein thrombosis. Blood. 2020;135:1377–1385. doi: 10.1182/blood.2019004114. [DOI] [PubMed] [Google Scholar]
- 38.DeLeve LD, Valla DC, Garcia-Tsao G American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boxhoorn L, Timmerhuis HC, Verdonk RC, Besselink MG, Bollen TL, Bruno MJ, Elmunzer BJ, Fockens P, Horvath KD, van Santvoort HC, Voermans RP Dutch Pancreatitis Study Group. Diagnosis and treatment of pancreatic duct disruption or disconnection: an international expert survey and case vignette study. HPB (Oxford) 2021;23:1201–1208. doi: 10.1016/j.hpb.2020.11.1148. [DOI] [PubMed] [Google Scholar]
- 40.de Rijk FEM, van Veldhuisen CL, Besselink MG, van Hooft JE, van Santvoort HC, van Geenen EJM, Hegyi P, Löhr JM, Dominguez-Munoz JE, de Jonge PJF, Bruno MJ, Verdonk RC Dutch Pancreatitis Study Group. Diagnosis and treatment of exocrine pancreatic insufficiency in chronic pancreatitis: An international expert survey and case vignette study. Pancreatology. 2022;22:457–465. doi: 10.1016/j.pan.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Sperna Weiland CJ, Engels MML, Poen AC, Bhalla A, Venneman NG, van Hooft JE, Bruno MJ, Verdonk RC, Fockens P, Drenth JPH, van Geenen EJM Dutch Pancreatitis Study Group. Increased Use of Prophylactic Measures in Preventing Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Dig Dis Sci. 2021;66:4457–4466. doi: 10.1007/s10620-020-06796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for data can be made to the corresponding author and will be discussed during a meeting of the Dutch Pancreatitis Study Group. After approval by the Dutch Pancreatitis Study Group, data that underlie the results reported in this study, will be shared.