Abstract
Luteolin (Lut), a polyphenolic compound that belongs to the flavone subclass of flavonoids, possesses anti-inflammatory, cytoprotective, and antioxidant activities. However, little is known regarding its role in mammalian oocyte maturation. This study examined the effect of Lut supplementation during in vitro maturation (IVM) on oocyte maturation and subsequent developmental competence after somatic cell nuclear transfer (SCNT) in pigs. Lut supplementation significantly increased the proportions of complete cumulus cell expansion and metaphase II (MII) oocytes, compared with control oocytes. After parthenogenetic activation or SCNT, the developmental competence of Lut-supplemented MII oocytes was significantly enhanced, as indicated by higher rates of cleavage, blastocyst formation, expanded or hatching blastocysts, and cell survival, as well as increased cell numbers. Lut-supplemented MII oocytes exhibited significantly lower levels of reactive oxygen species and higher levels of glutathione than control MII oocytes. Lut supplementation also activated lipid metabolism, assessed according to the levels of lipid droplets, fatty acids, and ATP. The active mitochondria content and mitochondrial membrane potential were significantly increased, whereas cytochrome c and cleaved caspase-3 levels were significantly decreased, by Lut supplementation. These results suggest that Lut supplementation during IVM improves porcine oocyte maturation through the reduction of oxidative stress and mitochondria-mediated apoptosis.
Keywords: Luteolin, Porcine oocyte maturation, Somatic cell nuclear transfer, Antioxidant, Lipid metabolism, Mitochondrial function, Parthenogenetic activation
Introduction
Because of their physiological and anatomical similarities to humans, transgenic pigs generated by somatic cell nuclear transfer (SCNT) have been used in many areas of biomedical research, including bioreactors, human disease models, and xenotransplantation (Gil et al., 2010; Meurens et al., 2012). In parallel, numerous attempts have been made to increase the developmental competence of SCNT embryos, resulting in the increased production of cloned piglets; however, efficiency remains low compared with in vivo embryo production (Grupen, 2014; Whitworth & Prather, 2010). One reason for this low efficiency is the inadequate in vitro maturation (IVM) environment of oocytes (Herrick, 2019). Oocyte maturation is a complex process that involves both nuclear and cytoplasmic maturation. The low developmental competence of IVM oocytes leads to failed embryonic development, mainly because of improper cytoplasmic maturation despite complete nuclear maturation (Gilchrist & Thompson, 2007). Successful cytoplasmic maturation depends on the IVM environment and thus on the medium composition and culture conditions (Maedomari et al., 2007; Marchal et al., 2001). Optimal IVM conditions are therefore the most important determinant of successful oocyte maturation and a prerequisite for the increased efficiency of transgenic pig production.
Reactive oxygen species (ROS) are commonly generated from byproducts of intracellular energy metabolism, and specifically from the byproducts of mitochondrial respiration in aerobic organisms. Although ROS at moderate levels are essential intracellular signaling molecules involved in normal biological processes, their excessive accumulation (from disrupted redox homeostasis) leads to oxidative stress and thus potentially to cytoskeletal abnormalities, lipid peroxidation, adenosine 5′-triphosphate (ATP) depletion, mitochondrial dysfunction, and apoptosis (Park et al., 2021; Wang et al., 2021). In the in vivo environment, the female reproductive tract (ovary, oviduct, and uterus) protects against ROS accumulation through its antioxidant defense system (Agarwal et al., 2012). However, during IVM, an imbalance between ROS generation and antioxidant activity can induce oxidative stress (Tarin et al., 1996). Compared with other species, the oocytes of pigs are much more sensitive to physiological stress, such as the stress caused by excess light, abnormal oxygen tension, and sudden changes in pH, because of their high cellular lipid and fatty acid contents (Sturmey et al., 2009). Stress-induced excessive ROS generation leads to embryonic arrest or lower embryo viability (Byrne et al., 1999). Attempts to reduce oxidative damage by dietary supplementation with numerous ROS scavengers, such as resveratrol, melatonin, and ascorbic acid, have failed to yield IVM conditions compare to the in vivo environment (Kere et al., 2013; Lee et al., 2018). An understanding of oxidative stress defense mechanisms and the application of more effective antioxidants would protect oocytes against oxidative stress while improving IVM.
Flavonoids are polyphenol secondary metabolites widely distributed in fruits, vegetables, and herbs (de Rijke et al., 2006). Many animals, including humans, consume significant amounts of flavonoids, which are present in a wide variety of edible plants (Mutha, Tatiya & Surana, 2021). The flavonoid luteolin (3, 4, 5, 7-tetrahydroxyflavone; Lut) belongs to the flavone group and is abundant in many vegetables and fruits, especially broccoli, carrots, and rosemary. Lut has been reported to have many biological functions including anti-inflammatory, antibacterial, anti-cancer, antioxidant, and cytoprotective activities (Gupta et al., 2018; Huang, Kim & Cho, 2023). Most previous studies of Lut have focused on therapeutic agents due to its potent anti-cancer and anti-inflammatory activities. Deqiu et al. (2011) reported that Lut can ameliorate insulin resistance-related endothelial dysfunction. Chen et al. (2014) reported that Lut effectively protects mice from lipopolysaccharide-induced lethality by suppressing heat shock protein 90 activity. Kim et al. (2018) reported that Lut suppresses airway inflammation by regulating immune response. In addition, the antioxidant activity of Lut reflects its ability to reduce free radical formation and thus oxidative stress in human umbilical vein endothelial cells and neuroblastoma cells (Chen et al., 2020; Zhang et al., 2013). Although the physiological and biological roles of Lut are well-documented in many cell types, the effect of Lut during meiotic maturation in pigs has not been investigated.
This study examined the effects of Lut supplementation on oocyte maturation and the subsequent developmental competence of SCNT embryos in pigs. Lut was added to the maturation medium; its effects on nuclear maturation, cumulus cell expansion, and subsequent embryonic development after parthenogenetic activation (PA) and SCNT were determined based on intracellular ROS and glutathione (GSH) levels, lipid metabolism, mitochondrial function, and activation of the apoptosis pathway in Lut-treated porcine oocytes.
Materials and Methods
Ethics statement
This study was conducted in strict accordance with the recommendations of the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Institutional Animal Care and Use Committee (approval no. KRIBB-AEC-21107).
Chemicals
All chemicals and reagents used in the present study were acquired from Sigma-Aldrich Chemical Company (St. Louis, MO, USA), unless otherwise indicated.
Oocyte collection and in vitro maturation
Oocyte collection and in vitro maturation were performed as described previously (Park et al., 2021). Porcine ovaries were retrieved from a local slaughterhouse and transported to the laboratory in 0.9% NaCl supplemented with potassium penicillin (75 µg/mL) and streptomycin sulfate (50 µg/mL) at 38.5 °C within 2 h. Cumulus-oocyte complexes (COCs) were recovered from antral follicles (about 3–6 mm in diameter) using an 18-gauge needle fixed to a 10-mL disposable syringe. The COCs were washed three times in 0.9% NaCl supplemented with 0.1% bovine serum albumin (BSA), after which ~50 COCs were matured in 500 µL IVM medium in a four-well multi-dish (Nunc, Roskilde, Denmark) for 44 h at 38.5 °C in 5% CO2 in air. After 22 h, the COCs were cultured in IVM medium without hormones for another 22 h.
Luteolin (Lut) treatment
The concentration of Lut was chosen according to a previous study (Park et al., 2021). Lut was dissolved in dimethyl sulfoxide, then diluted in IVM medium to a final concentration of 5 µM Lut (along with 0.1% dimethyl sulfoxide).
Assessment of cumulus cell expansion and the nuclear maturation of oocytes
Cumulus cell expansion and nuclear maturation were assessed as described previously, with modifications (Jeong et al., 2020a). After 44 h of IVM, the degree of cumulus cell expansion in porcine COCs was observed by morphological examination under a microscope (Nikon Corp., Tokyo, Japan) and classified as follows: grade 1: no expansion; grade 2: poor expansion, with spherical and compacted cumulus cells around the oocyte; grade 3: partial expansion, with the expansion of cumulus cell layers except the corona radiata; grade 4: full expansion of all cumulus cell layers. For assessment of nuclear maturation, cumulus cells after 44 h of IVM were removed by gentle pipetting with 0.1% hyaluronidase in Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Grand Island, NY, USA) supplemented with 4 mg/mL BSA. The denuded oocytes were examined under a microscope (Nikon Corp., Tokyo, Japan) and classified as degenerate, immature (without first polar body extrusion), or MII stage.
Parthenogenetic activation
Parthenogenetic activation was performed as described previously (Jeong et al., 2017). MII oocytes in a 1-mm gab wire chamber (CUY5000P1; Nepagene, Chiba, Japan) were parthenogenetically activated with 10 μL of 280 mM mannitol containing 0.1 mM MgSO4∙7H2O, 0.1 mM CaCl2∙2H2O, 0.5 mM HEPES, and 1 mg/mL polyvinyl alcohol (PVA). The oocytes were activated with an electrical pulse (1.1 kV/cm for 50 µs) that was applied using an electro cell fusion generator (LF101; Nepagene, Chiba, Japan). The activated oocytes were then transferred into activation medium, which consisted of in vitro culture (IVC) medium (porcine zygote medium-3 containing 4 mg/mL BSA) supplemented with 5 μg/mL cytochalasin B and 2 mM 6-dimethylaminopurine, at 38.5 °C under 5% CO2. After 4 h, the oocytes were transferred to IVC medium at 38.5 °C under 5% CO2. Cleavage and blastocyst formation were evaluated at 48 and 144 h, respectively.
Somatic cell nuclear transfer
SCNT was performed as described previously, with modifications (Jeong et al., 2020b, 2021, 2017). MII oocytes in DPBS supplemented with 4 mg/mL BSA, 75 µg/mL penicillin G, 50 µg/mL streptomycin sulfate, and 7.5 µg/mL cytochalasin B were cut using a sharp pipette and then the first polar body and cytoplasm-containing chromosomes were removed using the squeezing method under an inverted microscope (DMI 3000B; Leica Microsystems, Wetzlar, Germany) equipped with a micromanipulator (NT-88-V3; Nikon Narishige, Tokyo, Japan). We used KRIBB small miniature pig kidney cells established in a previous study as donor cells (Song et al., 2018). Donor cell injection and fusion were conducted using the Sendai virus (SV)-mediated method. Briefly, the freeze-dried inactivated SV envelope was dissolved in 260 mL of suspending buffer and diluted 1:4 (v/v) with cell fusion buffer, in accordance with the manufacturer’s instructions (Cosmo Bio, Osaka, Japan). Donor cells were immersed in the inactivated SV solution for 1 min, then introduced into the perivitelline space of cytoplasts. After 2 h, oocyte-cell couplets that had completely fused, as confirmed using an inverted microscope, were selected and activated for 4 h in activation medium containing 1 µM oxamflatin at 38.5 °C in 5% CO2 in air. The activated embryos were transferred to IVC medium supplemented with 1 μM oxamflatin, cultured for 20 h, washed in fresh IVC medium and then cultured in IVC medium at 38.5 °C in 5% CO2 in air. Cleavage and blastocyst rates were determined at 48 and 144 h, respectively.
Indirect immunofluorescence assay
Indirect immunofluorescence assay was performed as described previously (Jeong et al., 2021). Oocytes or blastocysts were fixed in 4% paraformaldehyde overnight at 4 °C and washed three times for 10 min each in DPBS supplemented with 1 mg/mL PVA (DPBS-PVA). The fixed oocytes/blastocysts were permeabilized by incubation for 1 h at RT in PBS containing 0.5% Triton X-100. After three washes in DPBS-PVA, the oocytes/blastocysts were stored overnight at 4 °C in DPBS-PVA supplemented with 1 mg/mL BSA (DPBS-PVA-BSA). For CDX2 staining, blastocysts were blocked with 10% normal goat serum for an additional 45 min, then incubated at 4 °C overnight with cytochrome c (Abcam, Cambridge, UK), cleaved caspase 3 (Cell Signaling Technology, Danvers, MA, USA) or mouse monoclonal anti-CDX2 (Biogenex Laboratories Inc., San Ramon, CA, USA) as the primary antibody. After three washes in DPBS-PVA-BSA, the oocytes/blastocysts were incubated for 1 h at room temperature (RT) with conjugated secondary antibody, either Alexa-Fluor-488-labeled goat anti-mouse or anti-rabbit IgG (1:200 in DPBS-PVA-BSA), washed three times for 10 min each in DPBS-PVA-BSA, stained for DNA using 2 µg/mL DAPI, and observed using a fluorescence microscope (Olympus, Tokyo, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP-digoxygenin nick end-labeling (TUNEL) assay
TUNEL assay was conducted using an in situ cell death detection kit (Roche, Basel, Switzerland), as described previously (Jeong et al., 2021). Fixed Blastocysts were washed three times for 10 min each in DPBS-PVA and were incubated in DPBS containing 0.5% Triton X-100 for 1h at RT. After three washes for 10 min each in DPBS-PVA, they were incubated in DPBS containing 10 mg/mL BSA for 1 h at RT. Blastocysts were incubated with TUNEL reaction medium for 1 h at 38.5 °C, washed three for 10 min each in DPBS-PVA, and mounted on clean glass slides with DAPI. Whole-mount blastocysts were observed under a fluorescence microscope (Olympus, Tokyo, Japan) by using detection for DAPI labeled (total cell numbers) or TUNEL positive (apoptotic cell numbers) nuclei. Five to 10 blastocysts per treatment group were used in the TUNEL assay in each independent experiment.
Measurement of intracellular ROS and GSH levels
The oocytes were washed three times with DPBS-PVA and incubated in DPBS-PVA containing 5 μM CM-H2DCFDA (Invtrogene, Carlsbad, CA, USA) for 30 min or 10 μM CMF2HC (Invitrogen, Waltham, MA, USA) for 10 min. After incubation, the oocytes were washed three times with DPBS-PVA. Fluorescence images were captured with a fluorescence microscope (DMi8; Leica Microsystems) equipped with an ultraviolet filter (460 nm for ROS or 370 nm for GSH). Fluorescence intensity was analyzed using ImageJ software (version 1.47; National Institutes of Health, Bethesda, MD, USA).
Analysis of ATP, lipid droplet, and fatty acid contents
ATP, lipid droplet, and fatty acid analysis were performed as described previously (Joo et al., 2023). The oocytes were washed three times in DPBS-PVA and fixed in 4% paraformaldehyde overnight at 4 °C. For ATP staining, the fixed oocytes were washed three times in DPBS-PVA and incubated for 1 h at RT in the dark in DPBS containing 500 nM BODIPY FL ATP (BODIPY-ATP; A12410; Molecular Probes, Eugene, OR, USA). Subsequently, the oocytes were washed three times with DPBS-PVA, mounted on clean glass slides, and stained with DAPI. For lipid droplet and fatty acid staining, the fixed oocytes were washed three times in DPBS-PVA and incubated for 1 h at RT in the dark in DPBS containing 10 μg/mL BODIPY 493/503 (BODIPY-LD; D3922; Molecular Probes, Eugene, OR, USA) or 6 μM BODIPY 558/568 C12 (BODIPY-FA; D3835; Molecular Probes, Eugene, OR, USA). After three washes in DPBS-PVA, the oocytes were mounted on glass slides and imaged using a fluorescence microscope (DMi8; Leica Microsystems, Wetzlar, Germany). Fluorescence intensity was analyzed using ImageJ software after data normalization via subtraction of background intensity from each oocyte size.
Analysis of mitochondria content and mitochondrial membrane potential
MitoTracker and JC-1 staining were performed as described previously (Jeong et al., 2019). The oocytes were incubated for 1 h in DPBS-PVA containing MitoTracker Red CMXRos (Invitrogen, Waltham, MA, USA) or JC-1 (1:100) (Cayman Chemical, Ann Arbor, MI, USA), washed three times for 10 min each in DPBS-PVA, and fixed for 2 h at RT in 4% paraformaldehyde. After three more 10 min washes in DPBS-PVA, the oocytes were either immediately observed under a fluorescence microscope (DMi8; Leica Microsystems, Wetzlar, Germany) for JC-1 staining or mounted on glass slides in Vectashield containing DAPI (Vector Laboratories, Newark, CA, USA) and then observed under a fluorescence microscope (DMi8; Leica Microsystems, Wetzlar, Germany) for MitoTracker staining. Fluorescence intensity was analyzed using ImageJ software after data normalization via subtraction of background intensity from each oocyte size.
Statistical analyses
All experiments were repeated at least three times. The results are expressed as means ± standard errors of the mean. Data were analyzed by analysis of variance, followed by Student’s t-test, using SigmaStat software (SPSS Inc., Chicago, IL, USA). P-values < 0.05 were considered indicative of statistical significance.
Results
Luteolin enhances oocyte maturation and cumulus cell expansion in porcine COCs
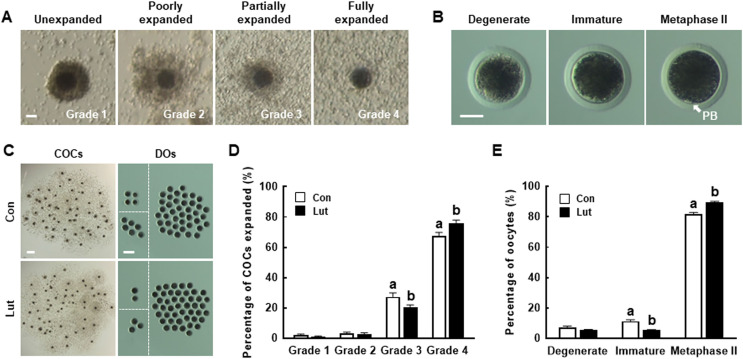
To investigate the effects of Lut on meiotic progression in porcine oocytes, COCs were cultured in IVM medium with or without 5 µM Lut during IVM; cumulus cell expansion and oocyte nuclear maturation were examined after 44 h. A comparison of the control and Lut-supplemented groups revealed no significant differences in the proportions of grade 1 (Con, 2.0 ± 0.7% vs. Lut, 1.0 ± 0.4%) and grade 2 (Con, 3.3 ± 0.7% vs. Lut, 2.7 ± 0.8%) cumulus cell expansion (Figs. 1A, 1C and 1D). However, Lut supplementation significantly decreased the proportion of cumulus cells with partial expansion (grade 3; Con, 27.3 ± 2.6% vs. Lut, 20.3 ± 1.7%) and increased the proportion of cumulus cells with full expansion (grade 4; Con, 67.3 ± 2.5% vs. Lut, 76.0 ± 2.0%). Lut supplementation also significantly increased the proportion of MII oocytes (Con, 81.7 ± 1.1% vs. Lut, 89.3 ± 0.8%) and decreased the proportion of immature oocytes (Con, 11.3 ± 1.0% vs. Lut, 5.3 ± 0.4%); compared with the control group, there was no significant difference in the proportion of degenerated oocytes (Con, 7.0 ± 1.1% vs. Lut, 5.3 ± 0.7%) (Figs. 1B, 1C and 1E). These results suggest that Lut supplementation during IVM improves the meiotic maturation of porcine oocytes.
Figure 1. Effects of luteolin (Lut) supplementation on porcine oocyte maturation.
Morphological classes of (A) cumulus cell expansion and (B) denuded oocytes (DOs) in cumulus-oocyte complexes (COCs). Bar = 50 µm. (C) Representative images of COCs (left, bar = 400 µm) and DOs (right, bar = 200 µm) in the control and Lut-treated groups after 44 h of in vitro maturation (IVM). (D) The percentage of cumulus cell expansion and (E) the proportions of different stages of nuclear maturation in the control and Lut-treated groups (n = 300 per group). Data are from six independent experiments and different superscript letters indicate significant differences (P < 0.05).
Luteolin supplementation during IVM improves the developmental competence of porcine PA embryos
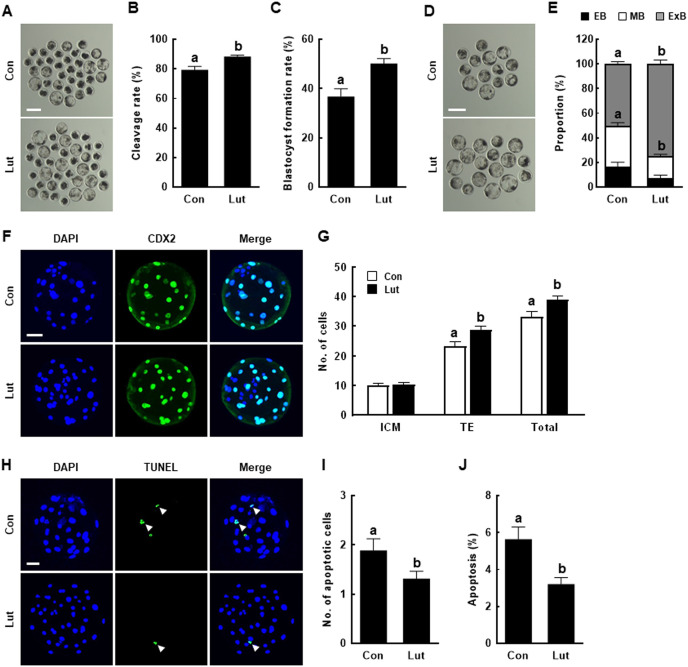
PA embryos derived from Lut-supplemented MII oocytes exhibited significantly higher rates of cleavage (Con, 79.3 ± 2.4% vs. Lut, 88.3 ± 1.0%) and blastocyst formation (Con, 36.8 ± 3.1% vs. Lut, 50.2 ± 2.0%), compared with control embryos (Figs. 2A–2C). Given the beneficial effects of Lut supplementation during IVM on PA embryonic development, post-blastulation development, inner cell mass (ICM)/trophectoderm (TE) cell number, and apoptosis rate were also evaluated as indicators of blastocyst quality. Within the Lut-supplemented group, the proportion of middle stage blastocysts was considerably lower (Con, 33.4 ± 2.4% vs. Lut, 17.9 ± 1.5%), whereas the proportion of expanded stage blastocysts was higher (Con, 50.2 ± 2.0% vs. Lut, 74.9 ± 3.2%), than in the control group (Figs. 2D and 2E). CDX2 staining revealed no difference in ICM cell number (Con, 10.0 ± 0.7 vs. Lut, 10.3 ± 0.7); in the Lut supplementation group, both TE (Con, 23.2 ± 1.6 vs. Lut, 28.7 ± 1.3) and the total cell number (Con, 33.2 ± 2.0 vs. Lut, 39.0 ± 1.4) were significantly increased compared with the control group (Figs. 2F and 2G). The TUNEL assay results showed that Lut supplementation significantly reduced the apoptotic cell number (Con, 1.9 ± 0.2 vs. Lut, 1.3 ± 0.2) and the apoptosis rate (Con, 5.6 ± 0.7% vs. Lut, 3.2 ± 0.4%) (Figs. 2H–2J).
Figure 2. Effects of Lut supplementation during IVM on the developmental competence after parthenogenetic activation (PA).
(A) Representative images of day 6 PA embryos from control and Lut-treated oocytes. Bar = 200 µm. (B) Cleavage rates and (C) blastocyst formation rates of embryos from control and Lut-treated oocytes after PA (Con; n = 208, Lut; n = 221). (D) Representative images of blastocysts and (E) quantification of the proportion of each blastocyst stage from control and Lut-treated oocytes after PA (Con; n = 77, Lut; n = 111). Bar = 200 µm. (F) Representative images of CDX2 staining. Bar = 50 µm. (G) Quantification of the inner cell mass (ICM) and trophectoderm (TE) as well as total cell numbers (n = 30 per group). (H) Representative images of the TUNEL assay results. Green and blue fluorescence indicate TUNEL-labeled embryos (white arrow) and nuclei, respectively. Bar = 50 µm. Quantification of the (I) apoptotic cell number and (J) apoptosis rate (n = 35 per group). Data are from five independent experiments and different superscript letters indicate significant differences (P < 0.05).
Luteolin supplementation during IVM improves the developmental competence of porcine SCNT embryos
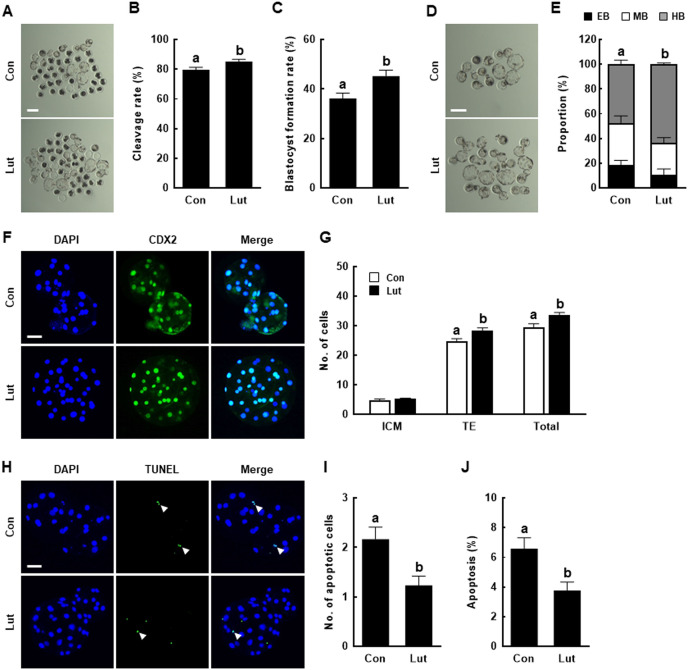
The effect of Lut supplementation during IVM on developmental competence after SCNT was also investigated. Consistent with the result for PA, SCNT embryos from Lut-supplemented MII oocytes exhibited significantly increased rates of cleavage (Con, 79.6 ± 1.7% vs. Lut, 85.2 ± 1.4%) and blastocyst formation (Con, 36.2 ± 2.2% vs. Lut, 45.2 ± 2.4%), compared with the controls (Figs. 3A–3C). Although the proportions of early (Con, 18.7 ± 3.7% vs. Lut, 10.9 ± 4.5%) and middle (Con, 33.7 ± 5.8% vs. Lut, 25.3 ± 4.5%) blastocyst stages did not significantly differ between the control and Lut-supplemented groups, a large increase in the proportion of the hatched (hatching or already hatched; Con, 47.6 ± 3.4% vs. Lut, 63.8 ± 1.1%) blastocyst stage was observed in the Lut-supplemented group (Figs. 3D and 3E). According to the CDX2 staining results, there was no difference in ICM cell number (Con, 4.8 ± 0.4 vs. Lut, 5.2 ± 0.3), but both TE (Con, 24.6 ± 1.0 vs. Lut, 28.4 ± 0.9) and total cell number (Con, 29.4 ± 1.2 vs. Lut, 33.5 ± 0.9) were significantly increased in the Lut supplementation group compared with the control group (Figs. 3F and 3G). In the TUNEL assay, the number of apoptotic cells (Con, 2.2 ± 0.2 vs. Lut, 1.2 ± 0.2) and the apoptosis rate (Con, 6.6 ± 0.7% vs. Lut, 3.8 ± 0.6%) were significantly reduced in the Lut supplementation group (Figs. 3H–3J). These results provided further evidence of the beneficial effects of Lut supplementation during IVM on porcine embryonic development after PA and SCNT.
Figure 3. Effects of Lut supplementation during IVM on developmental competence after somatic cell nuclear transfer (SCNT).
(A) Representative images of day 6 SCNT embryos from control and Lut-treated oocytes. Bar = 200 µm. (B) The cleavage rate and (C) blastocyst formation rates of embryos from control and Lut-treated oocytes after SCNT (Con; n = 183, Lut; n = 190). (D) Representative images of blastocysts and (E) quantification of the proportion of each blastocyst stage from control and Lut-treated oocytes after SCNT (Con; n = 67, Lut; n = 86). Bar = 200 µm. (F) Representative images of CDX2 staining. Bar = 50 µm. (G) Quantification of ICM and TE, as well as total cell numbers (n = 34 per group). (H) Representative images of the TUNEL assay results. Green and blue fluorescence indicate TUNEL-labeled embryos (white arrow) and nuclei, respectively. Bar = 50 µm. Quantification of (I) apoptotic cell number and (J) the apoptosis rate (n = 30 per group). Data are from five independent experiments and different superscript letters indicate significant differences (P < 0.05).
Luteolin prevents intracellular ROS accumulation in porcine oocytes
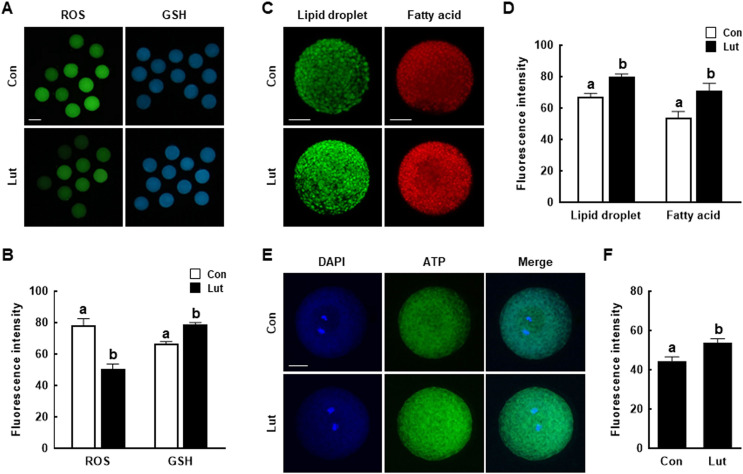
Analysis of the antioxidant properties of Lut during IVM revealed lower ROS levels (Con, 78.2 ± 4.4 vs. Lut, 50.7 ± 3.0) and higher GSH levels (Con, 66.4 ± 1.5 vs. Lut, 78.6 ± 1.5) in Lut-supplemented oocytes than in control MII oocytes (Figs. 4A and 4B).
Figure 4. Effects of Lut supplementation during IVM on oxidative stress and lipid metabolism.
(A) Fluorescence images of oocytes treated with CM-H2DCFDA to measure the intracellular levels of reactive oxygen species (left) and with CMF2HC to measure the intracellular levels of glutathione (right) in the indicated groups. Bar = 100 um. (B) Quantification of fluorescence intensity (n = 50 per group). (C) Fluorescence images of oocytes stained with BODIPY-LD (left) and BODIPY-FA (right). Bar = 50 um. (D) Quantification of fluorescence intensity (LD: lipid droplets; n = 30 per group, FA, fatty acids; n = 25 per group). (E) Fluorescence images of BODIPY-ATP-stained oocytes. Bar = 50 um. (F) Quantification of fluorescence intensity (n = 35 per group). Data are from three independent experiments and different superscript letters indicate significant differences (P < 0.05).
Luteolin enhances lipid metabolism of porcine oocytes
Because lipid metabolism contributes to the energy needed during oocyte maturation and embryonic development, the lipid droplet, fatty acid, and ATP contents of porcine oocytes supplemented with Lut during IVM were measured. Lut-supplemented MII oocytes showed significantly higher the intensity of lipid droplets (Con, 67.3 ± 2.1 vs. Lut, 80.0 ± 1.8) and fatty acids (Con, 54.1 ± 3.8 vs. Lut, 70.9 ± 4.8) compared to control (Figs. 4C and 4D). Moreover, ATP content was higher in Lut-supplemented MII oocytes than control (Con, 44.5 ± 2.1 vs. Lut, 53.8 ± 2.0) (Figs. 4E and 4F).
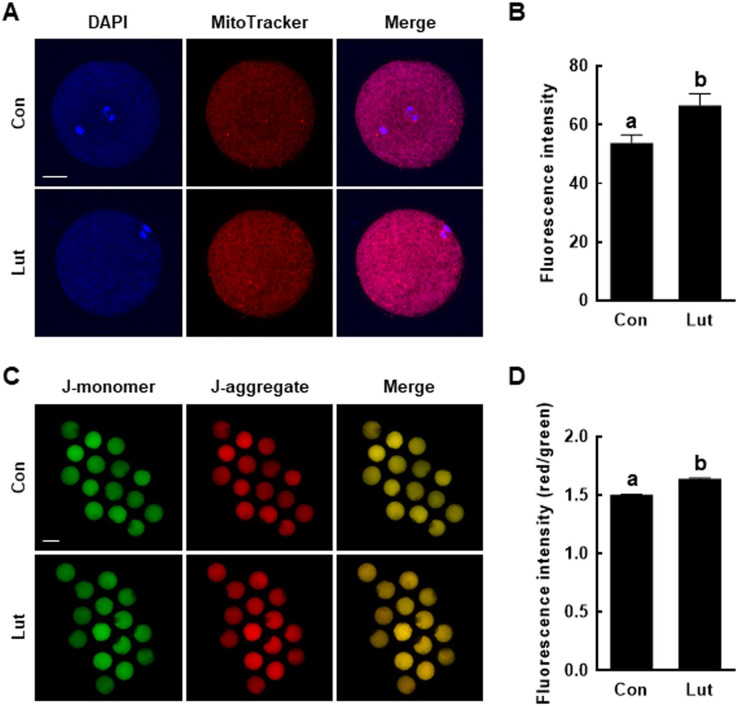
Luteolin enhances the mitochondrial functions of porcine oocytes
As sites of oxidative phosphorylation, mitochondria are essential to cellular energy production during oocyte maturation and subsequent embryonic development. Accordingly, the effects of Lut on the developmental competence of porcine oocytes during IVM were examined by measuring both the mitochondrial content and mitochondrial membrane potential. Compared with the control group, the mitochondrial content of Lut-supplemented oocytes was higher (Con, 53.8 ± 2.8 vs. Lut, 66.6 ± 3.9) (Figs. 5A and 5B) and the J-aggregate (high membrane potential)/J-monomer (low membrane potential) ratio was significantly increased (Con, 1.50 ± 0.01 vs. Lut, 1.64 ± 0.01) (Figs. 5C and 5D).
Figure 5. Effects of Lut supplementation during IVM on mitochondrial function.
(A) Fluorescence images of oocytes stained with MitoTracker. Bar = 100 um. (B) Quantification of fluorescence intensity (n = 44 per group). (C) Fluorescence images of oocytes stained with JC-1. Bar = 100 um. (D) Quantification of the (red/green) fluorescence intensity (n = 45 per group). Data are from three independent experiments and different superscript letters indicate significant differences (P < 0.05).
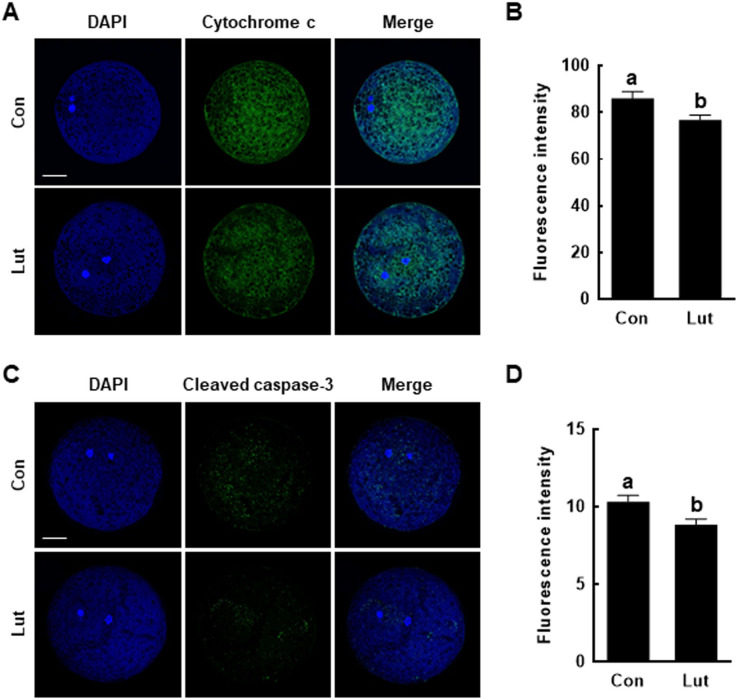
Luteolin protects mitochondrial-mediated apoptosis of porcine oocytes
The effects of Lut on the apoptosis pathway were assessed by measuring the levels of cytochrome c and cleaved caspase-3 in porcine MII oocytes. Cytochrome c levels were lower in Lut-supplemented oocytes than in control MII oocytes (Con, 85.9 ± 3.1 vs. Lut, 76.7 ± 2.1) (Figs. 6A and 6B); cleaved caspase-3 levels were also lower in Lut-supplemented oocytes (Con, 10.3 ± 0.4 vs. Lut, 8.8 ± 0.4) (Figs. 6C and 6D).
Figure 6. Effects of Lut supplementation during IVM on mitochondria-mediated apoptosis.
(A) Fluorescence images of oocytes stained with cytochrome c. Bar = 100 um. (B) Quantification of fluorescence intensity (n = 26 per group). (C) Fluorescence images of oocytes stained with cleaved caspase-3. Bar = 100 um. (D) Quantification of fluorescence intensity (n = 28 per group). Data are from three independent experiments and different superscript letters indicate significant differences (P < 0.05).
Discussion
The production of transgenic pigs by SCNT has attracted interest from biomedical researchers; however, the production efficiency resulting from this approach is suboptimal because of factors related to oocyte quality and culture conditions. Poor IVM conditions are mainly the result of the oxidative stress induced by the overproduction of ROS, which disrupts normal cell structures and processes, including cell membranes, RNA transcription, DNA/protein synthesis, and lipid metabolism, leading to decreased oocyte quality (Guerin, El Mouatassim & Menezo, 2001). Previous studies showed that antioxidant supplementation during IVM allows the successful cytoplasmic maturation of porcine oocytes and enhances the developmental capacity of SCNT embryos by reducing ROS levels (Kere et al., 2013; Qi et al., 2020; Taweechaipaisankul et al., 2016). Lut is a powerful antioxidant with excellent radical-scavenging and cytoprotective abilities (Heim, Tagliaferro & Bobilya, 2002). Luteolin has a double bond between C2 and C3 that provides hydrogen/electrons, and can stabilize the radical group by blocking the Fenton reaction using the oxo group of C4 that binds to transition metal ions such as iron and copper (Gendrisch et al., 2021). These structural features of Lut prevent oxidative damage by inhibiting pro-oxidant enzymes and inducing antioxidant enzymes. A previous study reported that Lut upregulates the transcription of ROS scavenging enzymes by activating the SIRT and FOXO families in human monocytes (Kim, Lee & Yun, 2017). Moreover, the antioxidant abilities of Lut are synergistically reinforced by its interactions with other antioxidants, including vitamins and cellular redox systems (Wolfle, Haarhaus & Schempp, 2013). The results of this study demonstrated that Lut supplementation during IVM improves porcine oocyte maturation and subsequent embryonic development after PA and SCNT. These effects can be at least partly attributed to the benefits conferred by the reduction of oxidative stress on lipid metabolism and mitochondrial function.
The cumulus cells surrounding the oocyte provide nutrients, hormones, and an optimal microenvironment for oocyte growth and maturation (Assidi, Dieleman & Sirard, 2010). A previous study showed that the removal of cumulus cells before IVM decreases the oocyte maturation rate and subsequent embryonic development after fertilization, indicating that cumulus cells are crucial for oocyte maturation and quality (Wongsrikeao et al., 2005). Cumulus cell expansion has been used as a morphological indicator of oocyte quality, based on the direct correlation of the optimal expansion of cumulus cells with proper oocyte maturation and the acquisition of developmental competence (Brown et al., 2017). Cumulus cells also play an important role in protecting oocytes from environmental stress, including oxidative stress (Tatemoto, Sakurai & Muto, 2000). However, excessive oxidative stress in cumulus cells induced cellular apoptosis and reduced CX43 levels (gap junction related factors), resulting in reduced oocyte quality (Qian et al., 2022). Moreover, oxidative stress caused by hydrogen peroxide exposure reduced not only expression of cumulus cell expansion- and maternal factor-related genes but also blastocyst formation and total cell number; interestingly, isorhamnetin treatment, another flavonoid compound, rescued by these defects (Oh et al., 2023). In this study, Lut supplementation prevents oxidative stress in porcine COCs by reducing ROS levels and increasing GSH levels. Moreover, Lut supplementation during IVM significantly increased the rate of COCs with fully expanded cumulus cells and MII oocytes, which then improved porcine IVM efficiency by reducing oxidative stress.
Oocyte quality is gradually acquired during ovarian follicular development, which includes oocyte growth and maturation (Eppig et al., 1994). Both preimplantation embryonic development and pregnancy maintenance to full term can be traced to oocyte quality. Although many transgenic pigs obtained by SCNT have been produced from IVM-derived oocytes, only a small percentage of those oocytes develop to the blastocyst stage, suggesting that current IVM systems do not adequately support the acquisition of oocyte developmental competence (Trounson, Anderiesz & Jones, 2001). Improving oocyte quality would thus increase the rate of successful embryonic development after SCNT. This study showed that, in PA and SCNT embryos, Lut supplementation during IVM significantly increases the cleavage rate, blastocyst formation, proportions of expanded or hatching blastocysts, cell number, and cellular survival rate; these results demonstrate the beneficial effects of Lut on the developmental competence of PA and SCNT embryos through enhancement of oocyte quality.
Lipid droplets are crucial organelles that store oocyte intracellular lipids to regulate cellular metabolism and energy homeostasis. During oocyte maturation, oocytes synthesize lipid droplets using fatty acids, liquid-liquid phase separation and lipid accumulation in the membrane of the endoplasmic reticulum. Simultaneously, fatty acids are cleaved from triglycerides by lipase activation and transported into the mitochondria, where they undergo β-oxidation for the ATP production that is necessary to support successful oocyte growth and maturation (Ferguson & Leese, 2006). Abnormal lipid metabolism, including elevated levels of saturated fatty acids, impairs oocyte maturation and quality, thus interrupting subsequent embryonic development (Bradley & Swann, 2019). Immature porcine oocytes are particularly sensitive to the damage caused by oxidative stress because their lipid contents are much higher than the contents in other mammalian species, including cows, mice, and sheep, suggesting an important role for lipid metabolism in porcine oocyte maturation (McEvoy et al., 2000; Somfai et al., 2011). The significantly higher contents of lipid droplets, fatty acids, and ATP in Lut-supplemented oocytes, compared with control MII oocytes, suggest that the improved IVM efficiency achieved by Lut supplementation is mediated by enhancement of lipid metabolism. However, further detailed experiments are needed to provide direct evidence of the effects of Lut supplementation on lipid metabolism during porcine oocyte maturation.
Mitochondria are cytoplasmic organelles that regulate ATP synthesis, ROS generation, calcium signaling, fatty acid oxidation, apoptosis, and other cellular metabolic processes (McBride, Neuspiel & Wasiak, 2006). They also support important aspects of mammalian reproduction, including oocyte maturation, fertilization, and embryonic development (Van Blerkom, 2011). However, oxidative stress by excessive ROS accumulation triggers a decrease in cellular mitochondrial content through the suppression of mitochondrial biogenesis involving mtDNA replication, membrane formation, and mitochondrial division (Bouchez & Devin, 2019; Nisoli et al., 2004). Oxidative stress also disrupts the mitochondrial membrane potential, which reduces the oxidative phosphorylation reaction and ATP synthesis (Aiken, Cindrova-Davies & Johnson, 2008). Numerous studies have reported that antioxidant supplementation during IVM is commonly able to overcome mitochondria injury by reducing ROS levels (Chen et al., 2022; Li et al., 2022; Liu et al., 2023). A previous study reported that Lut increased PGC-1α and nitric oxide levels, which would correct cellular impairment and may reduce mitochondrial ROS production, suggesting that Lut may promote mitochondrial biogenesis (Suh, Chon & Choi, 2016). Our results showed that Lut-supplemented oocytes not only reduced ROS levels but also increased mitochondrial number and membrane potential compared to the control, indicating that Lut supplementation increased mitochondrial number and function due to its antioxidant activity. In addition, oxidative stress disrupts the mitochondrial membrane potential, resulting in the release of cytochrome c from the mitochondria into the cytoplasm (Liu et al., 1996). Cytochrome c is a member of the mitochondria-dependent apoptotic pathway; its activation triggers the caspase cascade, including caspase-9 and caspase-3, to induce apoptosis (Sun et al., 2018). A previous study reported that Lut suppressed lysophosphatidylcholine-induced apoptosis by blocking calcium/mitochondria/caspase-dependent pathway in human umbilical vein endothelial cells (Song et al., 2010). In this study, Lut supplementation significantly reduced the levels of cytochrome c and cleaved caspase-3 compared to the control, thereby preventing apoptosis. Taken together, these results indicate that Lut supplementation during IVM improves oocyte quality by enhancing mitochondrial function through the reduction of intracellular ROS levels.
Conclusions
Our results demonstrate that Lut supplementation during IVM improves porcine oocyte quality and subsequent embryonic development after PA and SCNT. The positive effects of Lut can be attributed to the reduction of oxidative stress and enhancements of both lipid metabolism and mitochondrial function. These findings strongly suggest that Lut supplementation can improve the production of high-quality porcine oocytes, thus be used to improve the production of transgenic pigs for biomedical research.
Supplemental Information
Funding Statement
This research was supported by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4252331) and the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (2021M3H9A1096895), Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Pil-Soo Jeong conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Hae-Jun Yang performed the experiments, prepared figures and/or tables, and approved the final draft.
Se-Been Jeon performed the experiments, prepared figures and/or tables, and approved the final draft.
Min-Ah Gwon performed the experiments, prepared figures and/or tables, and approved the final draft.
Min Ju Kim performed the experiments, prepared figures and/or tables, and approved the final draft.
Hyo-Gu Kang performed the experiments, prepared figures and/or tables, and approved the final draft.
Sanghoon Lee performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Young-Ho Park analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Bong-Seok Song analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Sun-Uk Kim conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Deog-Bon Koo conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Bo-Woong Sim conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.
References
- Agarwal et al. (2012).Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology. 2012;10(1):49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken, Cindrova-Davies & Johnson (2008).Aiken CE, Cindrova-Davies T, Johnson MH. Variations in mouse mitochondrial DNA copy number from fertilization to birth are associated with oxidative stress. Reproductive BioMedicine Online. 2008;17(6):806–813. doi: 10.1016/S1472-6483(10)60409-9. [DOI] [PubMed] [Google Scholar]
- Assidi, Dieleman & Sirard (2010).Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction. 2010;140(6):835–852. doi: 10.1530/REP-10-0248. [DOI] [PubMed] [Google Scholar]
- Bouchez & Devin (2019).Bouchez C, Devin A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cells. 2019;8(4):287. doi: 10.3390/cells8040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley & Swann (2019).Bradley J, Swann K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. The International Journal of Developmental Biology. 2019;63:93–103. doi: 10.1387/ijdb.180355ks. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2017).Brown HM, Dunning KR, Sutton-McDowall M, Gilchrist RB, Thompson JG, Russell DL. Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153(3):R109–R120. doi: 10.1530/REP-16-0426. [DOI] [PubMed] [Google Scholar]
- Byrne et al. (1999).Byrne AT, Southgate J, Brison DR, Leese HJ. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. Journal of Reproduction and Fertility. 1999;117(1):97–105. doi: 10.1530/jrf.0.1170097. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2014).Chen D, Bi A, Dong X, Jiang Y, Rui B, Liu J, Yin Z, Luo L. Luteolin exhibits anti-inflammatory effects by blocking the activity of heat shock protein 90 in macrophages. Biochemical and Biophysical Research Communications. 2014;443(1):326–332. doi: 10.1016/j.bbrc.2013.11.122. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2022).Chen Q, Gao L, Li J, Yuan Y, Wang R, Tian Y, Lei A. Alpha-ketoglutarate improves meiotic maturation of porcine oocytes and promotes the development of PA embryos, potentially by reducing oxidative stress through the Nrf2 pathway. Oxidative Medicine and Cellular Longevity. 2022;2022:7113793. doi: 10.1155/2022/7113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen HI, Hu WS, Hung MY, Ou HC, Huang SH, Hsu PT, Day CH, Lin KH, Viswanadha VP, Kuo WW, Huang CY. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutrition, Metabolism and Cardiovascular Diseases. 2020;30(6):1032–1043. doi: 10.1016/j.numecd.2020.02.014. [DOI] [PubMed] [Google Scholar]
- de Rijke et al. (2006).de Rijke E, Out P, Niessen WM, Ariese F, Gooijer C, Brinkman UA. Analytical separation and detection methods for flavonoids. Journal of Chromatography A. 2006;1112:31–63. doi: 10.1016/j.chroma.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Deqiu et al. (2011).Deqiu Z, Kang L, Jiali Y, Baolin L, Gaolin L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie. 2011;93(3):506–512. doi: 10.1016/j.biochi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Eppig et al. (1994).Eppig JJ, Schultz RM, O’Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Developmental Biology. 1994;164(1):1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- Ferguson & Leese (2006).Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Molecular Reproduction and Development. 2006;73:1195–1201. doi: 10.1002/mrd.20494. [DOI] [PubMed] [Google Scholar]
- Gendrisch et al. (2021).Gendrisch F, Esser PR, Schempp CM, Wolfle U. Luteolin as a modulator of skin aging and inflammation. BioFactors. 2021;47(2):170–180. doi: 10.1002/biof.1699. [DOI] [PubMed] [Google Scholar]
- Gil et al. (2010).Gil MA, Cuello C, Parrilla I, Vazquez JM, Roca J, Martinez EA. Advances in swine in vitro embryo production technologies. Reproduction in Domestic Animals. 2010;45(Suppl 2):40–48. doi: 10.1111/j.1439-0531.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist & Thompson (2007).Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67(1):6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Grupen (2014).Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology. 2014;81(1):24–37. doi: 10.1016/j.theriogenology.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Guerin, El Mouatassim & Menezo (2001).Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Human Reproduction Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Gupta et al. (2018).Gupta G, Tiwari J, Dahiya R, Kumar Sharma R, Mishra A, Dua K. Recent updates on neuropharmacological effects of luteolin. EXCLI Journal. 2018;17:211–214. doi: 10.17179/excli2018-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, Tagliaferro & Bobilya (2002).Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Herrick (2019).Herrick JR. Assisted reproductive technologies for endangered species conservation: developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biology of Reproduction. 2019;100(5):1158–1170. doi: 10.1093/biolre/ioz025. [DOI] [PubMed] [Google Scholar]
- Huang, Kim & Cho (2023).Huang L, Kim MY, Cho JY. Immunopharmacological activities of luteolin in chronic diseases. International Journal of Molecular Sciences. 2023;24(3):2136. doi: 10.3390/ijms24032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2020a).Jeong PS, Lee S, Park SH, Kim MJ, Kang HG, Nanjidsuren T, Son HC, Song BS, Koo DB, Sim BW, Kim SU. Butylparaben is toxic to porcine oocyte maturation and subsequent embryonic development following in vitro fertilization. International Journal of Molecular Sciences. 2020a;21(10):3692. doi: 10.3390/ijms21103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2020b).Jeong PS, Sim BW, Park SH, Kim MJ, Kang HG, Nanjidsuren T, Lee S, Song BS, Koo DB, Kim SU. Chaetocin improves pig cloning efficiency by enhancing epigenetic reprogramming and autophagic activity. International Journal of Molecular Sciences. 2020b;21(14):4836. doi: 10.3390/ijms21144836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2021).Jeong PS, Yang HJ, Park SH, Gwon MA, Joo YE, Kim MJ, Kang HG, Lee S, Park YH, Song BS, Kim SU, Koo DB, Sim BW. Combined chaetocin/trichostatin a treatment improves the epigenetic modification and developmental competence of porcine somatic cell nuclear transfer embryos. Frontiers in Cell and Developmental Biology. 2021;9:709574. doi: 10.3389/fcell.2021.709574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2017).Jeong PS, Yoon SB, Choi SA, Song BS, Kim JS, Sim BW, Park YH, Yang HJ, Mun SE, Kim YH, Kang P, Jeong KJ, Lee Y, Jin YB, Huh JW, Lee SR, Koo DB, Park YI, Kim SU, Chang KT. Iloprost supports early development of in vitro-produced porcine embryos through activation of the phosphatidylinositol 3-kinase/AKT signalling pathway. Reproduction, Fertility and Development. 2017;29(7):1306–1318. doi: 10.1071/RD15391. [DOI] [PubMed] [Google Scholar]
- Jeong et al. (2019).Jeong PS, Yoon SB, Lee MH, Son HC, Lee HY, Lee S, Koo BS, Jeong KJ, Lee JH, Jin YB, Song BS, Kim JS, Kim SU, Koo DB, Sim BW. Embryo aggregation regulates in vitro stress conditions to promote developmental competence in pigs. PeerJ. 2019;7(1):e8143. doi: 10.7717/peerj.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo et al. (2023).Joo YE, Jeong PS, Lee S, Jeon SB, Gwon MA, Kim MJ, Kang HG, Song BS, Kim SU, Cho SK, Sim BW. Anethole improves the developmental competence of porcine embryos by reducing oxidative stress via the sonic hedgehog signaling pathway. Journal of Animal Science and Biotechnology. 2023;14(1):32. doi: 10.1186/s40104-022-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere et al. (2013).Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. Journal of Reproduction and Development. 2013;59(1):78–84. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Lee & Yun (2017).Kim A, Lee W, Yun JM. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutrition Research and Practice. 2017;11(5):430–434. doi: 10.4162/nrp.2017.11.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2018).Kim SH, Saba E, Kim BK, Yang WK, Park YC, Shin HJ, Han CK, Lee YC, Rhee MH. Luteolin attenuates airway inflammation by inducing the transition of CD4(+)CD25(−) to CD4(+)CD25(+) regulatory T cells. European Journal of Pharmacology. 2018;820:53–64. doi: 10.1016/j.ejphar.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2018).Lee S, Jin JX, Taweechaipaisankul A, Kim GA, Lee BC. Synergistic effects of resveratrol and melatonin on in vitro maturation of porcine oocytes and subsequent embryo development. Theriogenology. 2018;114:191–198. doi: 10.1016/j.theriogenology.2018.03.040. [DOI] [PubMed] [Google Scholar]
- Li et al. (2022).Li J, Wang R, Chen Q, Tian Y, Gao L, Lei A. Salidroside improves porcine oocyte maturation and subsequent embryonic development by promoting lipid metabolism. Theriogenology. 2022;192:89–96. doi: 10.1016/j.theriogenology.2022.08.028. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2023).Liu H, An ZY, Li ZY, Yang LH, Zhang XL, Lv YT, Yin XJ, Quan LH, Kang JD. The ginsenoside Rh2 protects porcine oocytes against aging and oxidative stress by regulating SIRT1 expression and mitochondrial activity. Theriogenology. 2023;200:125–135. doi: 10.1016/j.theriogenology.2023.02.006. [DOI] [PubMed] [Google Scholar]
- Liu et al. (1996).Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Maedomari et al. (2007).Maedomari N, Kikuchi K, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T, Kashiwazaki N. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilization and embryonic development in vitro. Theriogenology. 2007;67(5):983–993. doi: 10.1016/j.theriogenology.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Marchal et al. (2001).Marchal R, Tomanek M, Terqui M, Mermillod P. Effects of cell cycle dependent kinases inhibitor on nuclear and cytoplasmic maturation of porcine oocytes. Molecular Reproduction and Development. 2001;60:65–73. doi: 10.1002/mrd.1062. [DOI] [PubMed] [Google Scholar]
- McBride, Neuspiel & Wasiak (2006).McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Current Biology. 2006;16(14):R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- McEvoy et al. (2000).McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. Journal of Reproduction and Fertility. 2000;118(1):163–170. doi: 10.1530/reprod/118.1.163. [DOI] [PubMed] [Google Scholar]
- Meurens et al. (2012).Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends in Microbiology. 2012;20(1):50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha, Tatiya & Surana (2021).Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Future Journal of Pharmaceutical Sciences. 2021;7(1):25. doi: 10.1186/s43094-020-00161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli et al. (2004).Nisoli E, Clementi E, Moncada S, Carruba MO. Mitochondrial biogenesis as a cellular signaling framework. Biochemical Pharmacology. 2004;67(1):1–15. doi: 10.1016/j.bcp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Oh et al. (2023).Oh SH, Lee SE, Yoon JW, Park CO, Park HJ, Kim SH, Lee DG, Pyeon DB, Kim EY, Park SP. Isorhamnetin improves in vitro maturation of oxidative stress-exposed porcine oocytes and subsequent embryo development. Zygote. 2023;31(1):14–24. doi: 10.1017/S0967199422000399. [DOI] [PubMed] [Google Scholar]
- Park et al. (2021).Park SH, Jeong PS, Joo YE, Kang HG, Kim MJ, Lee S, Song BS, Kim SU, Cho SK, Sim BW. Luteolin orchestrates porcine oocyte meiotic progression by maintaining organelle dynamics under oxidative stress. Frontiers in Cell and Developmental Biology. 2021;9:689826. doi: 10.3389/fcell.2021.689826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi et al. (2020).Qi JJ, Li XX, Diao YF, Liu PL, Wang DL, Bai CY, Yuan B, Liang S, Sun BX. Asiatic acid supplementation during the in vitro culture period improves early embryonic development of porcine embryos produced by parthenogenetic activation, somatic cell nuclear transfer and in vitro fertilization. Theriogenology. 2020;142:26–33. doi: 10.1016/j.theriogenology.2019.09.027. [DOI] [PubMed] [Google Scholar]
- Qian et al. (2022).Qian Y, Zou X, Liang X, Lu N, Cui Y, Liu J, Meng Y. Peroxiredoxin 4, a new favorable regulator, can protect oocytes against oxidative stress damage during in vitro maturation. Biochemical and Biophysical Research Communications. 2022;601:52–58. doi: 10.1016/j.bbrc.2022.02.049. [DOI] [PubMed] [Google Scholar]
- Somfai et al. (2011).Somfai T, Kaneda M, Akagi S, Watanabe S, Haraguchi S, Mizutani E, Dang-Nguyen TQ, Geshi M, Kikuchi K, Nagai T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reproduction, Fertility and Development. 2011;23(7):912–920. doi: 10.1071/RD10339. [DOI] [PubMed] [Google Scholar]
- Song et al. (2018).Song BS, Jeong PS, Lee JH, Lee MH, Yang HJ, Choi SA, Lee HY, Yoon SB, Park YH, Jeong KJ, Kim YH, Jin YB, Kim JS, Sim BW, Huh JW, Lee SR, Koo DB, Chang KT, Kim SU. The effects of kinase modulation on in vitro maturation according to different cumulus-oocyte complex morphologies. PLOS ONE. 2018;13(10):e0205495. doi: 10.1371/journal.pone.0205495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2010).Song J, Liu K, Yi J, Zhu D, Liu G, Liu B. Luteolin inhibits lysophosphatidylcholine-induced apoptosis in endothelial cells by a calcium/mitocondrion/caspases-dependent pathway. Planta Medica. 2010;76(5):433–438. doi: 10.1055/s-0029-1186197. [DOI] [PubMed] [Google Scholar]
- Sturmey et al. (2009).Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reproduction in Domestic Animals. 2009;44(Suppl 3):50–58. doi: 10.1111/j.1439-0531.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Suh, Chon & Choi (2016).Suh KS, Chon S, Choi EM. Luteolin alleviates methylglyoxal-induced cytotoxicity in osteoblastic MC3T3-E1 cells. Cytotechnology. 2016;68(6):2539–2552. doi: 10.1007/s10616-016-9977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2018).Sun X, Zhang H, Zhang Y, Yang Q, Zhao S. Caspase-dependent mitochondrial apoptotic pathway is involved in astilbin-mediated cytotoxicity in breast carcinoma cells. Oncology Reports. 2018;40:2278–2286. doi: 10.3892/or.2018.6602. [DOI] [PubMed] [Google Scholar]
- Tarin et al. (1996).Tarin JJ, Vendrell FJ, Ten J, Blanes R, van Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Molecular Human Reproduction. 1996;2(12):895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- Tatemoto, Sakurai & Muto (2000).Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biology of Reproduction. 2000;63(3):805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- Taweechaipaisankul et al. (2016).Taweechaipaisankul A, Jin JX, Lee S, Kim GA, Lee BC. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reproduction in Domestic Animals. 2016;51(6):870–876. doi: 10.1111/rda.12748. [DOI] [PubMed] [Google Scholar]
- Trounson, Anderiesz & Jones (2001).Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- Van Blerkom (2011).Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2021).Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, Li F. Oxidative stress in oocyte aging and female reproduction. Journal of Cellular Physiology. 2021;236(12):7966–7983. doi: 10.1002/jcp.30468. [DOI] [PubMed] [Google Scholar]
- Whitworth & Prather (2010).Whitworth KM, Prather RS. Somatic cell nuclear transfer efficiency: how can it be improved through nuclear remodeling and reprogramming? Molecular Reproduction and Development. 2010;77(12):1001–1015. doi: 10.1002/mrd.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle, Haarhaus & Schempp (2013).Wolfle U, Haarhaus B, Schempp CM. The photoprotective and antioxidative properties of luteolin are synergistically augmented by tocopherol and ubiquinone. Planta Medica. 2013;79:963–965. doi: 10.1055/s-00000058. [DOI] [PubMed] [Google Scholar]
- Wongsrikeao et al. (2005).Wongsrikeao P, Kaneshige Y, Ooki R, Taniguchi M, Agung B, Nii M, Otoi T. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reproduction in Domestic Animals. 2005;40(2):166–170. doi: 10.1111/j.1439-0531.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang YC, Gan FF, Shelar SB, Ng KY, Chew EH. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food and Chemical Toxicology. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.