Abstract
A history of safe use is a backbone of safety assessments for many current probiotic species, however, there is no global harmonization regarding requirements for establishing probiotic safety for use in foods and supplements. As probiotic manufacturers are increasingly seeking to use new strains, novel species, and next-generation probiotics, justification based on a significant history of use may be challenged. There are efforts underway by a variety of stakeholders, including the United States Pharmacopeia (USP), to develop best practices guidelines for assessing the quality and safety of probiotics. A current initiative of the USP seeks to provide expert advice specific to safety considerations for probiotics. Toward this goal, this review provides a helpful summary guide to global regulatory guidelines. We question the suitability of traditional animal toxicology studies designed for testing chemicals for relevance in assessing probiotic safety. This includes discussion of the use of excessive dose levels, the length of repeated dose toxicity studies needed, and the most suitable animal species used in toxicology studies. In addition, the importance of proper manufacturing practices with regard to final product safety are also included. Thus, an outline of essential parameters of a comprehensive safety assessment for a probiotic are provided.
Keywords: Probiotics, Toxicology, United States Pharmacopeia, Safety, Next-generation probiotics, Animal testing, Dietary supplements
1. Introduction
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al., 2014; FAO/WHO 2001). This formal definition established by the Joint FAO/WHO Expert Consultation provided consensual understanding of the term “probiotics”, and has been used by regulatory agencies, scientific institutions, trade associations and consumers in numerous countries for more than two decades. Despite this, a few regulatory agencies instead reference the broader category of “live microbials”, which may include, but does not imply any health benefit. However, for simplicity, the term “probiotics” is used throughout and remains the focus of this manuscript, as the scope is intended to fully align with the FAO/WHO position, as these are intentionally used for the anticipated benefit. Probiotics are used as ingredients in foods, nutritional supplements, and are being developed as live biotherapeutics, amongst other categories (e.g., cosmetics). Specific safety standards, which exist for all these product categories, require a minimum that the probiotic is safe for the intended use and meets standards for purity, identity and potency (Vankerckhoven et al., 2008).
The United States Pharmacopeia (USP) is a non-profit standards-setting organization that publishes quality standards for medicines, foods and dietary supplements (United States Pharmacopeia, 2022a). As such, the USP has initiated development of standards for probiotic ingredients and products.
The USP quality standards (referred to as monographs) are available for a range of ingredients and include the definition of the article, tests for identification, quantification assays (enumeration in the case of probiotics), limits for contaminants, and other quality parameters, as appropriate. In response to an increasing number of probiotics proposed for monograph development, the USP established a probiotic Expert Panel (EP) to advise on quality requirements specific for probiotics. As efforts of the USP EP progressed, it became increasingly clear that in addition to quality, a focus on the safety of probiotics was also warranted. This paper represents efforts by a sub-team of the EP to provide guidance specific to safety considerations for probiotics.
While sometimes under the category of “live microbials”, numerous regulatory guidance documents and organizational initiatives have addressed the topic of probiotic safety, including the FAO/WHO (World Health Organization, 2018), European Food Safety Authority’s (EFSA) Qualified Presumption of Safety (QPS) initiative (EFSA, 2021a), EFSA guidance documents on antimicrobial resistance (McArthur et al., 2013; Jia et al., 2017; Alcock et al., 2020; Hendriksen et al., 2019), International Dairy Federation (IDF) bulletins focused on microbes with a history of safe use in food (IDF, 2002; IDF, 2012; IDF, 2022), the US Food and Drug Administration’s approaches to generally recognized as safe (GRAS) food and the new dietary ingredient notification (NDIN) ingredients (FDA, 2016b; FDA, 2016c; FDA, 2016d), the probiotic monograph from Health Canada (Health Canada, 2021), and the International Probiotics Association (IPA) Guidelines to Qualify a Microorganism to be Termed as ‘Probiotic’, which briefly describes several approaches and requirements from different authorities on establishing the safety of probiotics (IPA, 2017). In addition, some published reviews have also made various recommendations for performing safety assessments of probiotics (Pradhan et al., 2020; Sanders et al., 2010) or reviewing what is known about the safety of probiotic interventions (Hempel et al., 2011). Pariza et al. (2015) provided a comprehensive framework of criteria (in the form of a 13-question decision tree) that is useful when evaluating the safety of new microbial ingredients including those that lack an established history of safe use. Sanders et al. (2016) discussed safety considerations for the use of probiotics in vulnerable populations.
This work aims to move beyond these previous efforts, intended to address safety in a comprehensive manner beyond direct metrics of safety and characterization. In particular, the lack of coordinated guidance regarding the conduct of human safety studies and the use of in vitro and animal models in addition to the appropriateness of traditional animal toxicology studies that are routinely used for chemicals, for use in assessing probiotic safety (Pradhan et al., 2020) is discussed in detail. Specifically, we question the suitability of animal studies as a tool to assess probiotic safety for humans, the use of excessive dose levels, the length of repeated dose toxicity studies, and the most suitable animal species. We also review global regulatory initiatives, discuss essential parameters of a comprehensive safety assessment, and discuss the importance of proper manufacturing practices with regard to final product safety. The scope of this work encompasses probiotics, including next generation probiotic species, administered orally for healthy populations, but does not include genetically modified organisms, or live biotherapeutic products. We do not speak to requirements for use in specific subpopulations, such as immunocompromised, vulnerable and disease populations.
2. Global regulatory initiatives on probiotic safety
Independent of labeling them as “probiotics” these ingredients are widely marketed for use in foods and food supplements around the globe. However, the regulatory requirements for safety vary widely from country to country (Table 1). Regulatory authorities may simply consider the history of safe use of the microbial species or may expand the scope to include a detailed review of strain-level information, including genotypic and phenotypic characterization, animal toxicity studies, and in some limited cases human clinical trials. Although there may be general agreement among global regulatory authorities on the basic safety principals that should be established for probiotics, there is little consensus on the specific types of studies and methods for confirming safety. Thus, it is difficult to provide a comprehensive list of specific techniques and methods for safety requirements sufficient to meet the global regulatory requirements of every country. Such a list would undoubtedly result in implementing a series of tests that would be duplicative and unnecessary – a bureaucratic box-checking exercise rather than an organized, science-based strategy to create data that substantiates safety.
Table 1.
General regulatory structure and reference of microbial strains appropriate for use in foods or food supplements in example countries. Where specifically addressed in guidance or regulation, details of safety requirements including identification (ID), whole genome sequencing (WGS), determination of virulence factors, toxin production, infectivity and toxicity in addition to considerations for assessment of antimicrobial/antibiotic resistance (AbR) are listed.
| General Regulatory Structure | Requirements for Establishing Safety | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | Microbial List and Regulatory Reference | ID | WGS | Virulence Factors | Toxin Production | Infectivity/ Toxicity | AbR Potential | ||
| Australia | Therapeutic Goods Permissible Ingredients https://www.legislation.gov.au/Details/F2021L01449 | Not specified for those species in the substances for use in listed medicines | |||||||
| Brazil | Food Supplements: http://antigo.anvisa.gov.br/documents/10181/5809185/IN_76_2020_COMP.pdf/28f071f8-8079-4671-a1ec-b47c8dd30917 | ID – Genotypic and Phenotypic ID required WGS – required Virulence Factors – Adverse metabolic activity must be investigated Toxins and hemolysins must be investigated Adherence and translocation must be reported Resistance to antimicrobials must be investigated |
|||||||
| Canada | NNHPD: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=probio Food: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/accepted-claims-about-nature-probiotic-microorganisms-food.html |
ID – Genotypic and Phenotypic ID required WGS (complete) and homology to type strains Demonstration of the absence of genetic elements responsible for the production of virulence factors Demonstration of lack of toxigenic activity (i.e. production of toxins) Toxicity not applicable if included in probiotics monograph Full phenotypic and genotypic investigation/explanation of resistance; Demonstration of lack of horizontal antimicrobial resistance transfer ability |
|||||||
| China | For Food Products: http://www.nhc.gov.cn/sps/pztq/201612/712553a5f7554e0e9ec1dfdbcc91e99a.shtml Novel Food Products: Regulations on the safety review of new food ingredients 国家卫生计生委关于印发⟪新食品原料申报与受理规定⟫和⟪新食品原料 安全性审查规程⟫的通知 (nhc.gov.cn) For Health Food: http://www.nhc.gov.cn/sps/s3585/200804/8eafce69b178442eace396b7bc7bf8ef.shtml Technical guidelines for safety inspection and evaluation of strains used as health food raw materials or a health food finished product (2020 edition) |
Not specified for those species in the list of microbial species Taxonomic ID of the strain is required Special properties must be identified If microorganisms do not have history of usual consumption the following is needed: Acute Toxicity/Pathogenicity, three Genotoxicity, 90 Days feeding toxicity, Teratogenicity and Reproduction Toxicity required Antimicrobial resistance should be reported Not specified for those species in the list of microbial species Phenotypic and genotypic strain level and taxonomy defined Complete WGS and homology to type strains required WGS search to demonstrate the absence of genetic elements responsible for the production of virulence factors required WGS search for genetic elements responsible for toxin metabolism required and some microbes may require in-vitro testing Toxicity testing includes intraperitoneal injection and oral gavage |
|||||||
| Denmark | Food Supplements: https://www.foedevarestyrelsen.dk/english/Food/Food-supplements/Pages/default.aspx | Microbial species identification is required Demonstration of absence of potentially pathogenic properties in humans or animals required Demonstration of lack of formation of toxins in harmful quantities during the particular application Toxicity testing may be necessary It must be shown that microbe does not possess transferable antibiotic resistance |
|||||||
| EU | QPS - https://zenodo.org/record/4428353#.YD-upBrsY2w Novel Foods: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2016.4594 |
ID - Genotypic and Phenotypic ID required Detection of genes associated with virulence should be provided via complete strain characterization by fully assembled and validated WGS Toxin potential - Note qualification on QPS list for some species; Toxin potential must be considered for novel foods in regard to potentially adverse metabolic characteristics Animal toxicity testing may be required for novel foods Antimicrobial resistance risk assessment is required for all; determination of intrinsic or acquired resistance and potential transmissible antimicrobial resistance genes is required |
|||||||
| India | Foods with added probiotic ingredients (Schedule VII): https://fssai.gov.in/upload/uploadfiles/files/Compendium_Nutra_29_09_2021.pdf | ID - both Genotypic and Phenotypic identification is required WGS is optional and ID may be carried out using any latest molecular techniques Assessment of the acute, subacute and chronic toxicity of ingestion of extremely large amounts of probiotics must be demonstrated If the strain under evaluation belongs to a species that is a known mammalian toxin producer or of hemolytic potential, it must be tested for each, respectively. Assessment of undesirable side effects must be investigated The risk of transferable antimicrobial resistance must be investigated |
|||||||
| Italy | Guidelines on Prebiotics and Probiotics: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1016_ulterioriallegati_ulterioreallegato_0_alleg.pdf | Taxonomy confirmed as a species with a history of use WGS - Species and strain ID using genetic methods; whole genome sequencing is an option Probiotics shall not be carriers of acquired and/or transmissible antibiotic resistance |
|||||||
| Malaysia | Reg. 26A. Probiotic Culture: https://members.wto.org/crnattachments/2014/sps/MYS/14_4284_00_x.pdf | ID at the strain level is required Demonstration of lack of transmissible antimicrobial resistance genes |
|||||||
| United States | For Food Products: https://www.federalregister.gov/documents/2016/08/17/2016-19164/substances-generally-recognized-as-safe For Dietary Supplements: https://www.federalregister.gov/documents/2016/10/04/2016-23931/dietary-supplements-new-dietary-ingredient-notifications-and-related-issues-revised-draft-guidance |
Taxonomic ID of the strain is required If well-characterized microorganisms within the same species have a history of use, provide percentage homology to demonstrate similarities and differences For strains within species that do not have history of use in foods, 90-day subchronic toxicity testing may be required Antimicrobial resistance should be reported Biogenic amine production should be reported Phenotypic and genotypic strain level and taxonomy defined Identify any human pathogens phylogenetically related to the probiotic Identify any toxins produced by the species or those phylogenetically related to the probiotic Complete WGS and homology to well characterized strains within the species Report resistance to clinically relevant antibiotics and genetic transfer potential WGS search to demonstrate the absence of genetic elements responsible for the production of virulence factors required If no history of use, appropriate human or animal studies that report persistence in body and ability to translocate outside of the GI tract |
|||||||
The Joint WHO/FAO Expert Consultation (FAO/WHO 2001) and Working Group (FAO/WHO, 2002) guidance documents have been used to form a foundation of regulatory structures for probiotics in a number of countries including Indonesia (Badan Pengawas Obat Dan Makanan, 2021), Malaysia (Malaysian Food Act, 1985), Philippines (Republic of the Philippines, 2004), and Thailand (Thailand Ministry of Public Health, 2012). Lists of probiotic species/strains acceptable from a safety perspective were published in the national regulations of Malaysia, Philippines, Korea and Thailand among others. Many parallel efforts have also resulted in a foundational landscapae for probiotics, and since 2002, the IDF and the European Food and Feed Cultures Association (EFFCA) have been collaborating in the determination of safety for microbial food cultures. The FIL-IDF Bulletin 377–2002 was published as a result (Bourdichon et al., 2019). Subsequent updates of the IDF/EFFCA collaboration continue to provide updates on those microorganisms used in foods (Bulletin of the International Dairy Foundation, 2012; 2018, 2022), furthering the evidence of a safe history of use of these species.
In 2005, EFSA’s Scientific Committee began to investigate a qualified presumption of safety “QPS” system to standardize the safety assessments of defined taxonomic groups of microorganisms used in food and feed (EFSA, 2005). Those species determined to be safe, notwithstanding noteworthy qualifications for some species would be included in a QPS list and pending further strain-specific characterization, would be acceptable for use in the European market (EFSA, 2005). The first QPS list was published in 2007 and has been updated routinely with input and refinement from EFSA’s Panel on Biological Hazards (BIOHAZ) (EFSA, 2007; EFSA, 2008). The current version was adopted in December 2021 (EFSA, 2022). The QPS list continues to be the foundation of the safety standards for microorganisms used in food and feed in European countries and provides a regulatory landscape based on a comprehensive and uniform safety review that once attained, negates the need for animal toxicity testing that may be required for non-listed, novel species.
As a result of these initial efforts between 2001 and 2007, regulatory structures for probiotic standards for safety became established across Europe, in a number of countries in Asia, and continue to be developed across the globe. In 2001, China’s Ministry of Health published a list of species for use in health foods, then a comprehensive list of permitted culture species for use in general food in 2010 (China Ministry of Health, 2010) and has since published a list of permitted microbial strains for use in infant and toddler food (China Ministry of Health, 2011). Australia’s Therapeutic Goods Administration (TGA) has published a list of acceptable substances for use in listed medicines that include a number of species of the genus Bifidobacterium and the family Lactobacillaceae, in recognition of safety of these species (TGA Substances that may be used in Listed Medicines in Australian Therapeutic Goods Administration, 2007). In 2009, Health Canada implemented its monograph for probiotics to be used in Natural Health Products prescribing a very clearly defined safety standard and continuous update to reflect latest scientific development and understanding (Health Canada, 2021). Health Canada’s framework specifically developed for probiotics has served as a model for establishment of safety and characterization requirements in a number of countries. In 2016, South Africa published a list of acceptable probiotic species as substances typically considered to be used in a health supplement (MCC, 2016; SAHPRA, 2020) and the Food Safety and Standards Authority of India (FSSAI) in India implemented a list of probiotics species acceptable for use in health supplements, nutraceuticals and foods for special medical purpose (FSSAI, 2002).
Most recently, some regulatory agencies have intentionally focused on building a regulatory structure specifically for probiotics (microorganisms) (ANVISA Resolution RDC 241/2018). There are also newly implemented regional consortia that focus on building a regulatory structure by partnering with industry, academia and health care practitioners within consorting countries to intentionally design an appropriate regulatory landscape for probiotics in Southeast Asia (SEA Probiotics SREN) in addition to efforts at the Codex level (Codex Alimentarius Commission, 2017). Furthermore, a number of countries are also releasing criteria for next generation probiotics and microbiome-derived probiotic strains, outside of the standard Lactobacillaceae and Bifidobacterium species (EFSA, 2016; SAMR, 2020), including the live biotherapeutic product (drug) category (EP 3053, 2019; EP, 20636, 2019; FDA, 2016).
In the United States, probiotics have not been statutorily defined, but have been referred to as “live microbials” in a recent guidance document focused on dietary supplements (United States Food and Drug Administration, 2018). In the US, all regulatory requirements for probiotics as “live microbials” are subsumed into existing regulatory categories (Smith, 2019). Establishing safety, therefore, requires meeting the criteria specifically established for the relevant product category, for example in foods (FDA, 2016a) or for dietary supplements, where extensive criteria for safety requirements have been specified, including the details of toxicity tests required based on intended population and duration of use (GFS. 2016b). The US FDA has not published a list of acceptable probiotic species, however, does publish details on the information submitted for GRAS notifications and for NDI notifications received where safety has been reviewed. Based on the intended use in food products, a number of probiotic strains have been subjected to GRAS self-determination, which can be retained as a confidential self-assessment (although the information supporting safety must still be generally available), but others have been submitted as GRAS Notices to the FDA, voluntarily. The published GRAS Notices reflect many probiotic submissions to which the FDA did not object to the general recognition of safety for use in foods (FDA GRAS Notices, 2021). Specifically for use in dietary supplements, the FDA has also acknowledged certain strains of probiotics as new dietary ingredients through submissions of New Dietary Ingredient (NDI) notifications (US FDA NDIN). It is not unusual for regulatory requirements to be distinctly defined by its intended use in food versus dietary supplements (Table 1) however, FDA takes a unique position of consideration of the history of use of the ingredient. Based on a history of use of the probiotic strain, FDA’s criteria for required submission of evidence of safety will vary, where those strains previously used in foods, and established as GRAS, can be used as ingredients in dietary supplements at equivalent doses with no NDI notification. It should be noted that the information contained within either GRAS or NDI submissions may vary greatly based on a safe history of use and the ability to ‘bridge’ information from previous use within the species for specific probiotics. Because of this variation, in recent years, there has been an industry-wide anticipation of standard requirements and uniformity, which has resulted in probiotics manufacturers routinely providing a standard level of safety information in line with other country regulations and with recent publication (EFSA, 2021a; Pariza et al., 2015; Health Canada, 2021). Most recently, the probiotics industry standards also now focus on addressing many of the criteria in guidance from the FDA (2016b), which can include extensive animal toxicity testing for those species lacking a history of use in foods.
In total, these developments indicate a global progression in probiotic-focused regulatory structures. However, probiotic regulatory structures vary widely, and can range from publishing a list of acceptable genera and species that are recognized as safe, where minimal determination of safety is required at the strain level, to those that require monographs and a fully defined battery of tests at the strain level (Table 1).
Given the range of safety and characterization requirements listed in Table 1, the global probiotics industry has driven industry standards to appropriately and comprehensively assess each probiotic strain used for food and supplements intended for human ingestion. The expansion of requested information from customers and regulators alike, has resulted in the consideration of the below referenced topics, which may be supplemental or exceed what is required for regulatory submission or approval. These topics should be considered for a comprehensive assessment of all probiotic strains intended to be used in foods and human supplements, despite the variation in regulatory requirements in each country. This objective assessment is based on industry experience and the progression in technological advancements, allowing a thorough understanding of these live microbials.
3. In silico and in vitro parameters of a comprehensive assessment of probiotic safety
3.1. Strain identity
The first step in considering marketing of a probiotic includes the unambiguous identification of the probiotic strain(s) of interest. Probiotic identity should be established to the strain level and include determination of inter-strain differences compared to a type strain or well-characterized strain within the same species. Whole genome sequencing (WGS) is used for this purpose, and a description of how to use comparative genomic analysis to demonstrate strain uniqueness was outlined by Jackson et al. (2019). EFSA has further provided information on how to perform WGS analysis and to appropriately summarize and provide the relevant results (EFSA, 2021b). To protect the original stock for which WGS has been completed, the strain should be deposited in a recognized type collection to provide a permanent record of what was evaluated. A master and working cell banks should also be maintained for preserving the genetic integrity of this original stock. The prevention of genetic drift is necessary in maintaining the strain identity and periodic analysis should be conducted.
A high quality, annotated genome sequence is further used to assess genetic elements for antibiotic resistance, virulence factors, hemolytic activity, and toxins. The horizontal transfer capability of antimicrobial resistance genes is determined by identification of these genes either on a plasmid or in near proximity to potential mobile elements (e.g., inverted repeats, transposases). Furthermore, genomic sequence alignment analysis can be used to bridge safety data from one strain to another by identifying homology among strains and identifying differences within each. Even when a safety assessment relies on substantiating a history of safe consumption of the microbe in food, clear identification and characterization at the strain level is needed. Considerations for assessing these critical components of an overall probiotic safety assessment are discussed in detail below.
The determination of a microbial strain within a species acknowledges that traditional aspects of morphology, metabolism and physiology are characteristics common to that species. Currently, due to advances in molecular technology, phylogenetic trees can be developed to demonstrate microbial ancestry and associated physiological functions (vonWright and Axelsson, 2019). This information can be used to identify anticipated functions related to safety, including virulence, toxigenicity, and antibiotic resistance for any strain within well characterized species.
The EFSA system for establishing the safety of a microorganism for food applications is based on characteristics of the microbe’s taxonomic group and a number of conditions (EFSA, 2007). If the taxonomic group does not raise safety concerns, or if safety concerns exist but can be defined and accounted for (i.e., the ‘qualified’ component of QPS), the taxonomic group could be granted QPS status as long as the individual strains meet the established conditions as stipulated in the QPS document. For a microorganism falling into one of the taxonomic groups assigned QPS status by EFSA, further requirements at the strain level include the conditions of safety: antibiotic resistance testing (phenotypic and genotypic), toxigenicity, pathogenicity and any identified qualification gaps. Safety of a taxonomic group is based on identity, body of knowledge, possible pathogenicity, and end use. This taxonomic approach to risk assessment focuses on risks that may be inherent within a genus or species of microbe. The vancomycin-resistance phenotype found in many Lactobacillaceae family species is probably the best characterized example of intrinsic antibiotic resistance and is attributed to properties of the cell wall which prevents the binding of the vancomycin (Gueimonde et al., 2013). Another example includes toxins, such as hemolysins, phospholipases, and other enterotoxins associated with a number of Bacillaceae member species (Jezewska-Frackowiak et al., 2018). This risk is widely known and has led to quality management systems for some Bacillaceae member species including screening for cytoxicity (EFSA, 2021b).
To illustrate the utility of the QPS system, one can turn to the family Lactobacillaceae, many species of which have a history of safe use (Zheng et al., 2020). These species are consumed globally in large quantities on a daily basis through the consumption of fermented foods. EFSA and other authors have concluded that there are no specific safety concerns regarding a number of Lactobacillaceae species that have a long and well-documented history of apparent safe use in foods (Bernardeau et al., 2006; EFSA, 2007). In addition to the history of use, QPS requires strain safety analysis to ensure pre-determined qualifications such as determination of acquired antimicrobial resistance genes (EFSA, 2020a; EFSA, 2020b). Therefore, understanding the level of inherent risk within the species is extremely relevant when bridging safety of the strain to that of the species.
3.2. History of safe use
The general safety of probiotics is presumed for certain species of microbes with a properly documented history of safe use. For example, Lactobacillaceae and Bifidobacterium spp. Historically associated with food have been recognized as safe and have been acknowledged in numerous guidance documents and monographs in addition to publications by thought leaders in peer reviewed journals (Adams and Marteu, 1995; vonWright and Axelsson, 2019). Their established safe use in a diversity of foods and supplement products worldwide coupled with their occurrence in some cases as normal commensals of the mammalian microbiota supports this conclusion.
An adequate description of history of use should include the use across defined geographical areas, with information on intake levels, intake patterns, years of use, and impact on human health, as well as addressing any potential adverse effect issues. Most microorganisms currently marketed as probiotics have been historically used in foods. A safety decision tree was published by Pariza et al. (2015) that defines the significance of such a history of safe use at the species level and defines what additional testing should be completed before the probiotic strain can be considered safe for consumption. For a probiotic strain, it is relevant to include the history of safe use in foods at the species level as pertinent evidence of safety. However, history of safe use alone is not sufficient and certain strain-specific characteristics must accompany a comprehensive safety analysis.
3.3. Virulence and toxigenicity assessment
A safety assessment must consider the potential for a probiotic to express any virulence or toxin genes, which could lead to disease. Typically, the approach to this issue is two-fold. First, determining if the probiotic strain is a member of a species known to be virulent or toxigenic, and second, if there is evidence of virulence or toxin genes in the genome of the microbe.
It is difficult to discreetly define virulence of a microbe at a molecular genetic level, as it can greatly depend on the highly dynamic hostmicrobe relationship (Wiles and Guillemin, 2019). Generally, factors that contribute to the colonization, invasion, and evasion (for host immune-related elements) are considered important genetic underpinnings of virulence. Other factors may enable a microbe to thrive in a particular host and may contribute to virulence by complementing harmful effects of toxins and other direct-acting agents that form the basis of virulence sensu stricto.
These “virulence” factors are not causative agents in isolation, but importantly, it is the combined actions of such genetic underpinnings and their coordinated expression that contribute to disease potential in a comprehensive manner. A characteristic example is the species Escherichia coli, which contains many strains that are common commensals, while others act as pathotypes and cause disease. The E. coli example provides a window into the complexity of host-microbe interactions and the role and designation of “virulence factors” to pathogenicity. It is important to mention that determination of pathogenicity requires a systems biology approach and a deeper understanding of the mechanisms of probiotic beneficial relationships with the host (Sanders et al., 2010). As such, proper taxonomy becomes an initial first step in categorization of a respective microbe into pathogen or beneficial microbe and what may be respective “characteristic” genetic baggage.
Such high-resolution information brings clarity to core genome relationships along with accessory genome elements that may further develop and inform our understanding of genetic content of concern. These recent developments led the European Food Safety Authority (EFSA) to update its Qualified Presumption of Safety (QPS) list of microorganisms to address such changes (EFSA BIOHAZ Panel, 2020). Certainly, such first pass identification has been facilitated by highly curated databases and genomic-scale analytical resources such as the Pathosystems Resource Integration Center (PATRIC) shown in Table 2 (Binda et al., 2020). Analytical tools that target horizontally acquired loci, mobilization structures, and associated content within respective genomes have also been included as potential resources. A few examples of potential virulence and toxin factors that should be considered as part of an overall safety assessment are described in further detail below.
Table 2.
Summary of phylogenomic resources for virulence and antibiotic resistance factor identification.a.
| Name | Link | Profiling Category | Key Description and References | |
|---|---|---|---|---|
| Virulence | ||||
| Virulence Factors Database (VFDB) with VFanalyzer | http://www.mgc.ac.cn/VFs/ | Global virulence |
|
|
| Pathogen-host Interactions (PHI- base) with PHIB-BLAST | http://www.phi-base.org/ | Global virulence interactions |
|
|
| Pathosystems Resource Integration Center (PATRIC) VF module | https://www.patricbrc.org/ | Curated priority pathogens |
|
|
| Mobilization Targets | ||||
| VRprofile | https://bioinfo-mml.sjtu.edu.cn/VRprofile/ | Contextual virulence |
|
|
| Pathogenicity Island Database (PAIDB) version 2.0 | http://www.paidb.re.kr | Horizontal gene transfer regions |
|
|
| ICEberg 2.0 | https://db-mml.sjtu.edu.cn/ICEberg2/ | Integrative and conjugative elements |
|
|
| PHAge Search Tool Enhanced Release (PHASTER) | https://phaster.ca/ | Prophages |
|
|
| INTEGRALL | http://integrall.bio.ua.pt/ | Integrons |
|
|
| IS finder | https://isfinder.biotoul.fr/ | Insertion sequences |
|
|
| Antibiotic Resistance | ||||
| Antibiotic Resistance Genes Database (ARDB) | http://ardb.cbcb.umd.edu/ b | Global resistance |
|
|
| Comprehensive Antibiotic Resistance Database (CARD) | https://card.mcmaster.ca/ | Global resistance |
|
|
| ResFinder and Pointfinder | https://cge.food.dtu.dk/services/ResFinder/ | Resistance genotype-to-phenotype |
|
|
| Antibiotic Resistance Gene Annotation (ARG-ANNOT) | https://www.mediterranee-infection.com/acces-ressources/base-de-donnees/arg-annot-2/ | Global resistance bioinformatic tool |
|
|
| Resfams | http://www.dantaslab.org/resfams | Resistance prediction and annotation |
|
|
Current publicly available resources reflect rapidly changing landscape for genome annotation and hypothetical gene identification; list should not be considered exhaustive, but representative of categorical, commonly used platforms.
Last website of record.
Most recently, an increase in the biofilm-forming capacity of probiotic strains may lead to pathogenicity as determined by variable genetic traits (Rossi et al., 2022). These authors re-assessed the pathogenic potential of various strains of Lactobacilli using infection case reports published between 2019 and 2021. As a result of their findings and to protect particularly vulnerable populations, it is recommended that genetic stability should be periodically re-evaluated using WGS.
3.4. Antibiotic resistance
Only probiotic strains that do not contribute to the spread of functional antibiotic resistance should be selected for use in food and supplements (Ouwehand et al., 2016). Antibiotic resistance in probiotic strains is a concern if the resistance can be transferred directly or through mediator microbes to potentially pathogenic members of the microbiota, leading to antibiotic resistant pathogens refractory to treatment. The phenotypic assessment of antibiotic resistance involves testing the susceptibility of the probiotic strain of interest to a set of clinically relevant antibiotics, and determination of the minimal inhibitory concentration (MIC) for each. The EFSA has a protocol for assessing antibiotic resistance that is most often used and includes determination of phenotypic resistance, based on an MIC above the pre-established cut-off for a particular antibiotic and mining the genome for the presence of antibiotic resistance genes (EFSA, 2012; EFSA, 2018). According to the guidance given by EFSA in 2012, any functional antibiotic resistance above a pre-determined threshold should be further characterized as intrinsic or transmissible and the genetic basis of resistance should be determined and explained (Fig. 1).
Fig. 1.
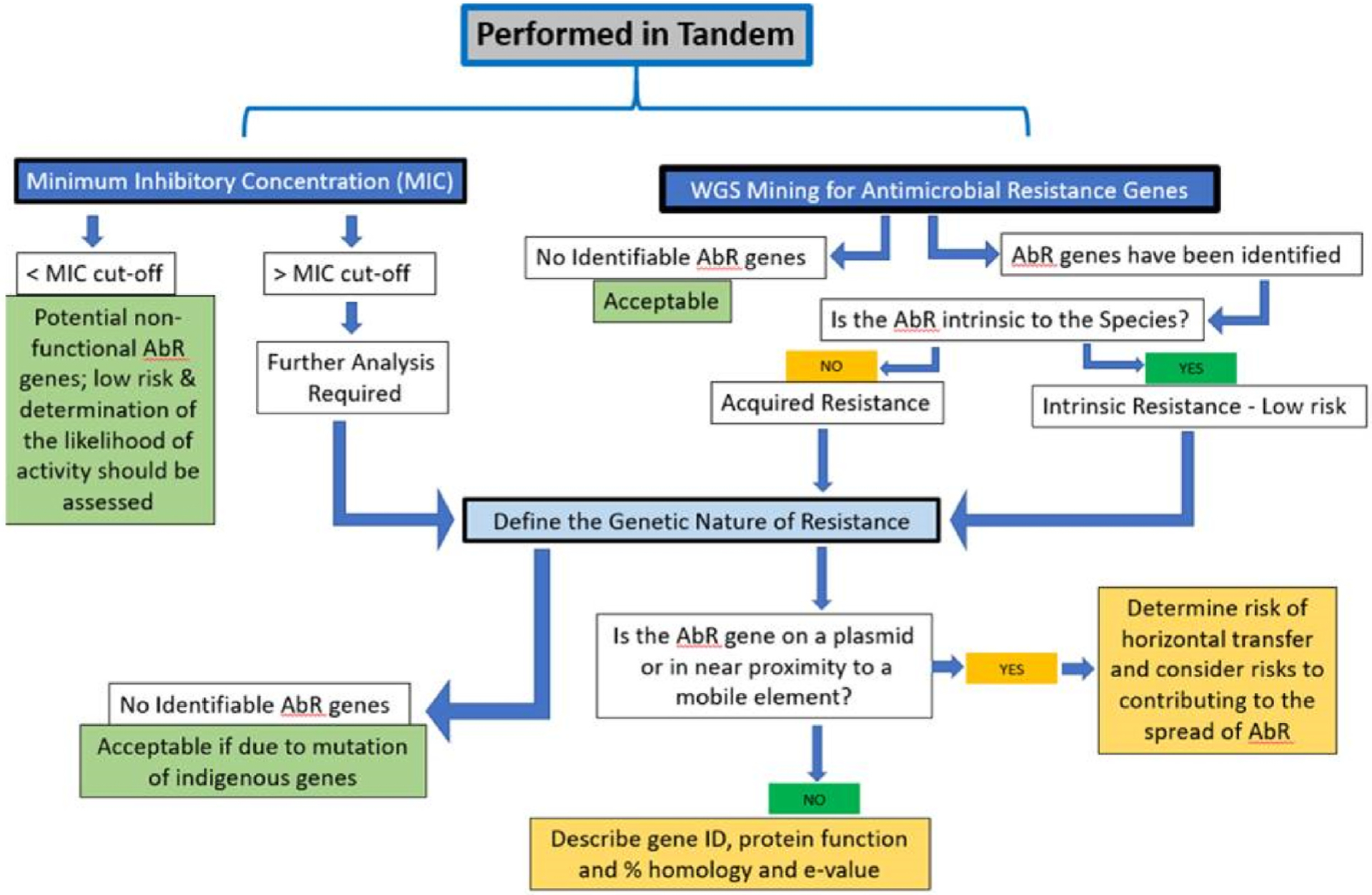
Phenotypic and Genotypic analysis of antimicrobial resistance genes for consideration of strain safety.
The updated EFSA guidance in 2018, indicates that both a phenotypic (MIC) and genotypic analysis of antimicrobial resistance must be determined and does not limit the analysis to functional antimicrobial resistance above a pre-determined threshold or cut-off (EFSA, 2018). This is in line with the safety requirements for a number of countries globally.
Given this guidance and the expanded inclusion of regulatory requirements in regard to antibiotic resistance, an attempt to provide a comprehensive approach to the determination of antibiotic resistance as part of a safety determination for all probiotic strains is described in Fig. 1.
Understanding the genetic nature of the antibiotic resistance is key to determining the risk of potential spread, which is a major concern given the promiscuous nature of bacteria. It is well known that live microbes harbor antibiotic resistance genes and the possibility of horizontal gene transfer in the human gut has been established (McInnes et al., 2020). Intrinsic resistance common to a species presents a very low potential for horizontal spread and can be utilized safely as a result (EFSA, 2018). Any antimicrobial resistance not normally harbored in a species is considered to be acquired resistance. Chromosomal mutation leading to acquired resistance has been determined to be low risk, as low potential for horizontal spread exists (EFSA, 2012). Essentially, EFSA guidance and global regulatory requirements, dictate that the genetic nature of clinically relevant antibiotic resistance genes must be explained. Strains carrying transmissible resistance flanked by genetic elements known to mediate horizontal spread, must be addressed and explained per EFSA guidance, whereas more global requirements are to determine the risk of transfer potential (see Table 1). Most importantly, strains where the nature of the resistance cannot be explained should be avoided.
The search for antibiotic resistance genes within the WGS of a probiotic strain can be facilitated by available antimicrobial resistance databases (Table 2). Of note, because the microbial coverage of these databases is constantly expanding, it is important that gene annotation be conducted with the most current releases. Antifungal resistance genes are also included in at least one database, Mycology Antifungal Resistance Database (MARDγ) (Nash et al., 2018). Clinically relevant antibiotics that should be considered are those listed as critically important antimicrobials (CIAs) and can be found as published by the World Health Organization (WHO) (2018).
3.5. Biogenic amines
Biogenic amines are basic organic compounds that contain an amine group. Although most concern for toxicity of biogenic amines is associated with microbial production in foods such as some cheeses, they can be produced in situ as well. The primary source of biogenic amines is metabolic activity of the resident gut microbiota through amino acid decarboxylases. Although biogenic amines are crucial for maintaining many biological functions, in excess they may lead to many toxic effects (Wojciech et al., 2020). Common biogenic amines of interest include monoamines (e.g., histamine, tyramine), diamines (e.g., cadaverine, putrescine), and polyamines (e.g., spermidine, spermine). Histamine and tyramine are of greatest concern due to potential toxicities. The potential for additional biogenic amine production by administered probiotic strains can be addressed by determining the presence of genes encoding biogenic amine production using WGS, or via targeted PCR amplification. Phenotypic confirmation of biogenic amine production by bacterial species can be analyzed using a decarboxylase screening medium and high-performance liquid chromatography (Bover-Cid and Holzapfel, 1999).
3.6. Mucin degradation
Mucin is a protein that is the core structural element of mucus, which comprises a crucial component of mucosal surfaces throughout the digestive tract. Among other roles, mucins provide a barrier that protects the host epithelium from microbial and chemical invasion (Grondin et al., 2020). There are over 40 bacterial genes identified that are related to the metabolism of mucin (Tailford et al., 2015). WGS can be used to identify bacterial genes related to mucin degradation and its activity can be analyzed in vitro by evaluating the ability of bacterial strains to grow in liquid or on agar plates containing mucin as the sole carbon source.
A certain level of mucus degradation by gut microbiota occurs as many bacteria use it as a carbon source. For example, Akkermansia muciniphila feeds on mucins, converting them in to short-chain fatty acids (SCFAs). These SCFAs are a critical energy source for cells lining the gastrointestinal tract. A change in the physiological balance caused by a shift in mucin degrading bacteria is thought by some to lead to disease and infection in humans (EFSA, 2020a; EFSA, 2020b), however this trait is reported to be very tightly regulated within gut microbes and influenced by the availability of dietary polysaccharides (Flint et al., 2012). Mucin degradation capability is a part of a comprehensive safety analysis for probiotics, as the determination of risk associated with this trait requires a systems biology approach and a deeper understanding of the mechanisms of probiotic beneficial relationships with the host. Thus, an understanding of the number of mucin-degrading genes contained within a probiotic species genome is part of a holistic review of the safety of a probiotic strain, as mucin degradation potential in isolation should not be seen as a virulence characteristic.
D(−)-Lactate production.
D(−)-lactate production by probiotics is suggested to be a risk factor for the development of acidosis. D(−)-lactic acid is produced by some probiotic species, particularly Lactobacillaceae species. Common species that are known to produce D-lactic acid include L. acidophilus, L. gasseri, L. delbrueckii subsp. bulgaricus, L. fermentum, L. lactis, L. brevis, L. helveticus, L. plantarum and L. reuteri (Quigley et al., 2018). Probiotic strains should be tested for their ability to produce D(−)-lactic acid. Although acidosis can result from D(−)-lactic acid production in the gut, there have been no reported cases of healthy individuals with elevated D(−)-lactic acid levels, and no link of acidosis to the ingestion of D(−)-lactic acid producing bacteria as probiotics (Connolly et al., 2005). In most individuals, production of D-lactate is limited under normal conditions (Pohanka, 2020). Furthermore, it should be noted that the documented cases of D(−)-lactic acidosis have been limited to infants whose intestinal tract has been significantly surgically altered, most often linked with short bowel syndrome and the direct feeding of D-lactate, not from administration of D-lactic acid producing bacteria (Satoh et al., 1982; Munakata et al., 2009). Impairment of metabolism and excretion factors associated with liver disease and chronic renal failure, respectively, are also believed to be risk factors for developing acidosis (Fabian et al., 2017).
4. Animal toxicity and human clinical studies
4.1. Animal toxicity studies
When a history of use cannot be established for a particular probiotic strain, then most authoritative guidance documents suggest the need to do additional safety studies, including in animals. However, there is little specific guidance on the design and conduct of animal toxicity studies. Unlike chemicals, a prescriptive non-clinical toxicology testing paradigm would not be relevant to probiotics due to their unique nature and may provide limited information regardless (Sanders et al., 2010; Ishibashi and Yamazaki, 2001). Thus, specific requirements for testing of probiotics should be carefully considered on a case-by-case basis.
If animal toxicology studies are deemed necessary to establish safety for a particular probiotic strain the following questions must be addressed:
What animal model is best suited for extrapolating adverse findings to human clinical relevance?
What duration of dosing in the animal model is necessary to support use of the probiotics in humans?
What study endpoints are relevant to the safety assessment of a probiotic?
It seems reasonable that some degree of animal testing may be necessary for certain probiotic strains. For example, when a sufficient history of use cannot be established for a particular probiotic strain/species, or certainly in the case of “novel” or next-generation probiotic species, then some animal toxicity assessment may be necessary prior to human clinical studies. Even when candidate probiotic strains are human commensal microbes, they are “not-self” and cannot be considered harmless.
Recently, Rousseau et al. (2020) provided an overview of in vitro, ex vivo and in vivo non-clinical models that they believed were relevant for conducting microbiome research on various products, including probiotics. Although these models may hold promise for microbiome research purposes, the majority are not ready for use in assessing standard toxicology endpoints.
The pig (and minipig) model has received particular interest due to many anatomic, physiological, and metabolic similarities with humans (Ziegler et al., 2016). The microbiota within the pig small and large intestine are close to the gastrointestinal microbiota of humans (Crespo-Piazuelo et al., 2018). However, rodents are the most widely used animal models for the conduct of toxicity studies, including studies with probiotics. The intestines of mice have anatomical, histological, and physiological similarities with human intestines, and they share 90% or more genetic homology with humans (Monaco et al., 2015). The rat gastrointestinal tract also shares anatomical and physiological similarities with humans and has an advantage of size over the mouse which facilitates evaluation of various study parameters (e.g., allows greater volume of blood collection). Between the two rodent models, rats have a more similar microbiota to humans than mice (Nagpal et al., 2018).
There are notable differences between rodents and humans that may be particularly relevant to studies with probiotics. The mucus growth rate is higher in the human colon than in rodent colons, and the diet is clearly different between rodents (herbivores) and humans (omnivores). In the end, no animal model will fully represent humans; however, when toxicity studies are deemed necessary for a probiotic, it seems prudent to rely on standard rodent models (i.e., rats or mice). Overall, rodents provide the advantage of tremendous historical data on toxicity findings that are known to be rodent-specific with no human correlate (i.e., lack clinical relevance).
Once an appropriate rodent model has been chosen, the next logical question is related to the duration of dosing that is adequate to support further testing of safety in human clinical trials. Interestingly, a review of traditional oral repeated dose animal toxicity studies with probiotics, and published in the scientific literature, reveals no findings of any adverse effects regardless of duration (Pradhan et al., 2020). This includes acute (single dose), repeated dose (e.g., 14–28 days), sub-chronic (e.g., 28–90 days), and in at least one case, chronic (12+ months) studies conducted at very high doses of the probiotic under investigation (Isa et al., 2016 and references therein). For example, Endres et al. (2009) dosed a proprietary preparation of B. coagulans to rats for 90 days with no observed toxicity up to 1.36 × 1011 CFU/kg. The resulting safety factor based on anticipated human use level of this probiotic preparation was as much as 95,200 times. Isa et al. (2016) report on a 12-month study conducted in rats with Clostridium butyricum MIYAIR588® (CBM588®) at 5000 mg/kg in diet with no observed toxicity. The same is true for a sub-chronic rodent study of a probiotic product, HOWARU® Restore, equivalent to 2.64 × 1012 CFU/kg, 1.72 × 1012 CFU/kg for L. acidophilus NCFM, 3.35 × 1012 CFU/kg for L. paracasei Lpc-37, 4.05 × 1012 CFU/kg for B. lactis BI-04 and 3.07 × 1012 CFU/kg for B. lactic BI-07 strains studied at 5000 mg/kg with no toxicity demonstrated (Morovic et al., 2017).
Based on the studies mentioned above, when the same probiotic strain was tested across study durations, from acute/repeated dose (e.g., from 1 to 28 days) to sub-chronic or chronic (3 months–1 year) there were no findings of toxicity with increased chronicity of exposure. The apparent low order of toxicity observed with probiotics when tested in traditional animal toxicity studies raises the question of whether shorter-term animal studies, such as 14 or 28-days, might not provide enough assurance of safety to proceed to human safety and tolerability studies. Such an approach is used for the testing of industrial chemicals to meet the European Union REACH legislation (Registration, Evaluation, Authorisation and Restriction of Chemicals), where it has been proposed that when a chemical has a low toxicity profile in repeated dose studies there is little added value to conducting 90-day repeated dose studies (Taylor et al., 2014).
The question on duration of dosing in toxicity studies is also connected to the consideration of what toxicity endpoints are relevant to assess probiotic safety. Safety endpoints unique to in vivo administration of live microorganisms should include translocation and infectivity potential in healthy animals. Translocation involves the passage of live microbes from the gastrointestinal tract to other sites within the body, and is assessed in lymph nodes, spleen, liver, bloodstream, or other tissues where the aforementioned organs are collected at necropsy, homogenized and plated for enumeration (Liong, 2008). Confirmation of bacterial colonies may be assessed via genetic methods. Translocation of microbes to other organs of the body is of concern as it could cause infection in the host (e.g., bacteremia or septicemia). Although the exact mechanism(s) for microbes to translocate is unknown, it is related to a defective intestinal barrier, immunosuppression, or gut prematurity. Translocation as a result of a compromised gut barrier has been studied in diseased rodent models (e.g., induced colitis); however, these models may be of little value in understanding translocation potential as opportunistic pathogenicity in healthy humans.
Similar to the conduct of other in vivo studies as part of the safety program, healthy animals are more relevant for studying translocation potential of probiotics (Lahtinen et al., 2009). A number of in vivo studies to assess the translocation potential of various probiotic strains have been conducted in health mice or rats; all of which have been 4 weeks in duration (Zhou et al., 2000; Kabeir et al., 2008; Tompkins et al., 2008). Thus, it seems reasonable to assume that translocation and infectivity could be observed within the timeframe of a repeated dose toxicity study.
Another important safety consideration with probiotic strains is whether they have the ability to produce primary or secondary metabolites within the gastrointestinal tract that could pose toxicity to the host. Information about the production of known toxic metabolites associated with a particular microbial strain may be gathered from WGS analysis and/or through a literature search of the species or related species (EFSA, 2018). To date, there is no way of knowing the full spectrum of potential metabolites produced by a microbial strain; however, the potential toxic effects of any microbial metabolic products upon the host would also be assessed in an in vivo animal study.
Other toxicity endpoints that have, on occasion, been explored using both in vitro and in vivo models are genotoxicity or developmental and reproductive toxic effects of probiotic strains. From a genotoxicity standpoint, concern may be more relevant related to ingredients used in the processing and manufacturing of the probiotic, and any impurities subsequently generated. This concern may be alleviated by a careful assessment of processing ingredients and impurities. Some assurance of a lack of reproductive effects could be obtained from repeated-dose toxicity studies which includes an assessment of reproductive organs. However, similar to findings from repeated dose oral toxicity studies at high dose levels, no probiotic strain tested in standard (OECD-compliant) genotoxicity or developmental and reproductive studies have been reported to have any toxic effects (Chiu et al., 2013; Isa et al., 2016). Thus, the routine assessment of these endpoints seems unnecessary.
In summary, some level of animal toxicity assessment may be necessary in certain cases prior to human clinical studies with a particular probiotic strain, primarily those with little history of safe use in foods. However, based on what has become a substantial dataset of repeated dose toxicity studies including sub-chronic and chronic studies with high doses of probiotics resulting in no negative findings, it seems reasonable to consider alternative testing paradigms. Shorter-term studies, such as repeated dose studies could provide sufficient assurance of safety to move to clinical studies in healthy humans. These first-in-human studies should be appropriately designed to collect safety endpoint data. A summary of the basic safety parameters that should be covered for any probiotic under consideration, along with suggestions for additional safety data that may be warranted on a case-by-case basis is provided in Box 1.
Box 1.
Essential Parameters of a Comprehensive Safety Assessment for a Probiotic.
Basic Safety Information - all probiotics
|
Additional Safety Information - case-by-case basis
|
4.2. Human clinical studies
Given the underlying attribute within the definition of a probiotic strain of conferring a health benefit, clinical trials are routinely generated on probiotics to appropriately build substantiation, as this is required for products that bear any claim of health benefit. Within these clinical trials, the safety of probiotics is inconsistently reported in published clinical studies, which may be due in part to the lack of consensus on what safety endpoints should be assessed in clinical evaluations. One published review has noted that the majority of published clinical studies have not adequately assessed the safety of probiotics (Hempel et al., 2011) An exhaustive review of 622 probiotic clinical studies conducted in 2011 by the Agency for Healthcare Research and Quality (AHRQ) has found no evidence of increased risk for probiotics, but the report concluded that the available literature was “not well equipped to answer questions on the safety of probiotics in intervention studies with confidence.” The majority of human clinical probiotic studies available in the literature were not designed as safety and tolerability studies and many fail to adequately report adverse events. Safety parameters are usually not reported in efficacy studies and conclusions on safety are not well defined.
Here we discuss the role and design of human studies to assess probiotic safety (Shane et al., 2010). Human studies should be conducted in a manner to minimize bias and increase confidence in outcomes as well as ensure the rights, safety and well-being of study subjects. Therefore, including randomization, blinding, a placebo or other appropriate control and other good clinical practices are important considerations (US FDA, 2016a).
If sufficient history of safe use is known for oral consumption of a specific probiotic species, and the strain of interest has been properly identified to the strain level, its genome properly sequenced, annotated and shown to not contain genes of concern, and intended use of the probiotic falls within an exposure considered to be safe, phase 1 safety studies are likely not needed for use by generally healthy humans. The EFSA Qualified Presumption of Safety approach follows this same basic premise (EFSA, 2020a; EFSA, 2020b). A phase 1 clinical study should not be considered essential to establish safety for those strains with a history of safe use. But in the event that a clinical study for safety is conducted, it should be properly designed to assess safety where the dose and duration are specified; and adverse events (AEs) are appropriately monitored. Rather than a phase 1 safety study, harms may reasonably be monitored in the conduct of a well-designed efficacy trial.
All human studies should be designed, conducted and reported in a manner consistent with well-developed principles such as Good Clinical Practices (GCPs), which are applicable broadly, not just to probiotics. Many useful guidelines have been published, including a checklist of items that should be considered during the planning of a human trial (SPIRIT) (Chan et al., 2013), Good Clinical Practices (United States Food and Drug Administration, 2022a), accurate and standardized approaches to reporting harms (CONSORT) (Ioannidis et al., 2004), and a list of components for a properly reported clinical trial (CONSORT). Recently, a checklist designed specifically for human studies focused on microbiome endpoints was proposed and may be a more relevant checklist for probiotic studies. Too often, human probiotic trials are inadequately conducted or reported, and they include deficiencies in trial registration, outcomes (primary and secondary) statements, compliance measure, treatment allocation and randomization methods, blinding, defining and reporting adverse events, sample size calculations, data analysis plans, and disclosure of roles of sponsors and investigators in study design or data access. These errors make it difficult to draw conclusions regarding safety from published studies.
Measurement of participant compliance is an important factor, which in the case of orally administered probiotics may be assessed through fecal recovery of the probiotic strain. This will also provide insight into persistence of the probiotic being administered, a useful component of a probiotic safety assessment.
Adverse event (AE) reporting is a critical component of human clinical trials. The first step to proper reporting of AEs is clarity of how, in the context of the study, they will be defined and collected. For studies in healthy populations, in addition to vital signs, investigators should track symptoms such as loose stools, diarrhea (≥3 loose stools per day for 2 consecutive days), constipation, fever, flatulence, lack of appetite, pain, rash, vomiting, cough, earache, nasal congestion, runny nose, and sore throat. These should be graded as mild, moderate, severe and life threatening. An all-too-frequent mistake made in reporting of AEs is failure to fully report all AEs. Recording an AE and making a determination on its causal relationship to the intervention are two separate actions. Any AE must be recorded; causally attributing the event to the intervention is an important, but subsequent step.
A comprehensive guide for reporting harms was developed under the aegis of CONSORT (Ioannidis et al., 2004). This review nicely summarized common poor practices in reporting harms. Further, they advise that in general, it is preferable to report harms and benefits in the same manuscript; this enables comparison of risks to harms. Although the balance of risks and harms is often not invoked from a regulatory perspective for foods or supplements, there is value for clinicians and scientists active in this area of research to understand how they compare.
Errors and incompleteness in reporting AEs are unfortunately quite common. In today’s publishing environment, biases against using journal space for AE reports cannot be defended. Authors are strongly encouraged to submit detailed AE reports with all human trials and journals should make this information available as supplementary information, if not in the primary paper. Further, AEs should not be reported in the aggregate, but as comparisons between control and placebo group. To the extent that AEs occur infrequently, formal statistical analysis may not be possible or appropriate. Serious AEs, in general defined as adverse events that may threaten life or function of the study subject or may require medical or surgical intervention, must be tracked and reported to regulatory authorities.
5. Safety considerations for processing and manufacture
To ensure that dietary supplements such as probiotics are safe for human consumption, it is mandatory for them to be produced in facilities that comply with appropriate Good Manufacturing Practices (GMP), and this is recognized globally (FDA, 2010; FDA, 2022b; Health Canada, 2015; Regulation EC, 2004). Even though a probiotic may pass release testing, other risks may be present in addition to release criteria. Complying with appropriate GMP regulations provides assurance that operations and systems are traceable, that facilities and equipment are of proper design, materials are appropriate for the manufactured product, and that monitoring, and controls of manufacturing processes in place are commensurate to risk. Ultimately, systems should be in place to assure safety, quality, identity, purity and potency of the products produced and released by the manufacturer (FDA, 2010; FDA, 2022b).
A risk management system should be in place to monitor chemical, allergenic, physical, biological/microbiological contaminants during the process. By integrating a hazard analysis to GMPs, the facility is better equipped to identify operational pre-requisites as well as critical control points, defined as the stages of the process where the hazards must be controlled to ensure safety and quality of the products. As such, critical control points are identified, limits established and monitored accordingly, and corrective/preventive actions are developed when needed. An effective system will integrate these complementary requirements through good recordkeeping (Accessed June 2021Standard Global Services,).
Purity considerations are also an important aspect of probiotic process and manufacturing. Chemical contaminants such as pesticides, residual solvents, heavy metals, polycyclic aromatic hydrocarbons, allergens, and microbial contaminants could find their way in the supply chain via the raw materials used in the dietary supplements production. An efficient surveillance plan according to the source and risk of the raw materials, in-process and end products must be in place to monitor, control and address the presence of such contaminants to provide safe products to the consumers.
Qualifying raw materials and establishing their specifications are required by GMPs (FDA, 2010; FDA 2022b; Health Canada, 2015). Any new raw material requires an adequate evaluation. Analysis, such as identification, physical properties, assays and contaminants should be part of the evaluation. These elements, therefore, are integrated to a vendor qualification program to ensure consistency and safety of raw materials.
Many ingredients are involved in the production process of probiotic products. These include media culture ingredients, composed of nitrogen sources, proteins, sugars, vitamins and minerals, to obtain optimal probiotic growth, the cryoprotectant agents to protect the strains during the freeze-drying process, and additives to standardize the blends to the required concentration. All raw materials must go through the evaluation process and quality control analysis prior to use to ensure that the final product is safe for human consumption and meets safety, quality, identity, purity and potency (SQIPP) as mentioned in the previous section. Furthermore, additional steps and in-process checks are conducted throughout the process to ensure product safety, for example, sterilization or pasteurization of the media culture prior to inoculation to reduce any possible bioburden and testing for microbial contaminants and hygiene criteria. As most probiotic manufacturers have optimized the growth media components to ensure complete utilization during fermentation, it is rare that media components would be carried over following centrifugation of the probiotic cell mass. Removal of the spent fermentation media via centrifugation allows a physical removal of the fermentation media, however, if the manufacturing process does not involve centrifugation to remove the media, these components must be accounted for in the safety considerations and labeling of the probiotic product.
As per GMPs, the required overage to assure the guaranteed concentration at the end of the shelf life of the product is determined by the stability of the product, its recommended storage temperature and final packaging (FDA, 2010; FDA, 2022; Health Canada, 2015). Real-time stability studies must be conducted on the finished product in its final packaging and storage requirements to confirm if the overages are sufficient for the intended storage period (EMA, 1996; EMA, 2003; USP, Accessed June 2021). When assessing the safety of probiotics, it is important to take into consideration not just the labeled daily serving of probiotic, but one should account for the overage as well. Although probiotics at high concentrations have not revealed safety concerns actual exposure to the probiotic should be recorded (Alisi et al., 2014; Guandalini et al., 2010; Mack, 2011; EMA, 1996).
To ensure consumer safety, the presence of major food allergens, must be declared on the product label (Health Canada, 2020; FDA, 2006). If the supplier fails to declare the presence of a priority food allergen, the supplier may be subjected to a product recall and sanctions (Health Canada,2020; United States Food and Drug Administration, 2006). The most frequent allergens found in probiotics, due to their popularity as ingredients used in the production process, are milk, soy and gluten. In order to safely claim an allergen free product, the supplier must have an efficient allergen management program in place and demonstrate that the product does not contain any of the priority allergens as determined by the local regulations. Only then, a product can be labeled allergen-free; the same principle applies for dairy, soy and gluten-free products.
6. Discussion and conclusions
By 2026, the global probiotics market is projected to reach 91.1 billion USD (MarketsandMarkets, 2021). Probiotics are the focus of a great deal of scientific research in both healthy individuals, where a number of beneficial effects have been demonstrated, as well as in subjects with underlying health conditions. However, it is unreasonable to argue that probiotics have substantial biological activity impacting human physiology to result in health benefits without acknowledging that those same mechanisms have the potential to impact human physiology in a negative manner. Therefore, safety of probiotics should not be concluded without convincing evidence.
Establishing probiotic safety is a challenge due in part to a lack of harmonized requirements among regulatory bodies, making it unclear what requirements need to be fulfilled and a lack of scientific consensus about the need for certain studies, such as animal testing in the safety assessment process. The existing patchwork of safety requirements from different regions globally requires manufacturers to develop a clear understanding of where their product will be sold so they can address the safety requirements specific to that country. Compilation of country-specific lists of genera, species and/or strains approved by the appropriate country’s regulatory authority and any qualifications necessary to establish safety may be useful to companies. Proactively including all globally known regulatory requirements in a manufacturer’s safety testing regime is an assured way to establish safety globally. For strategic regulatory planning, it is important to identify target countries and highlight a few of the requirements in countries with known or anticipated regulatory landscape for future strategic planning in establishing safety.
Countries with modern regulatory structures often include requirements in line with advanced technology, relying more on whole genome sequencing mining and relying less on animal testing (Health Canada, 2019; EFSA, 2018; Agencia Nacional de Vigilancia Sanitaria, 2018). Yet, many countries do not provide any regulatory landscape specific to probiotics, and lump this ingredient type in with all others to be used in foods and food supplements. This approach is lacking, however, as the expectation is often that probiotic safety be established using the same means as other ingredients, which are not always appropriate or sufficient for probiotics (Sanders et al., 2010).
From a toxicology perspective, live microorganisms represent unique challenges that are quite different from chemicals. A safety consideration of a probiotic strain must begin with taxonomic and strain identification, the latter which is dependent on WGS. This genomic information is crucial for genotypic characterization, which when combined with phenotypic activity can be very powerful to understand relevancy of various pathogenicity genes associated with adhesion, colonization and invasion.
Once confirmation of the probiotic is complete, an initial tier assessment considers whether there is a significant history of safe use of the strain in question. If a history of safe use of the strain can be established at the same or lower anticipated exposure level, then no additional safety testing of the bacterial strain itself is likely needed. If there are limitations on the history of safe use of the strain and/or the species exists on the EFSA QPS list for example, then some limited testing may be necessary. This should include a search of phylogenomic databases using the WGS to determine presence of various antibiotic resistance, virulence and toxin genes and phenotypic testing for antibiotic resistance should be conducted according to standard antibiotic screens.
When a history of safe use does not exist and the microbial species is not on any established list (e.g., QPS), then more extensive testing may be required including animal and/or human clinical studies. A summary of the safety testing paradigm described previously, and principles presented in this manuscript is presented in Fig. 2.
Fig. 2.
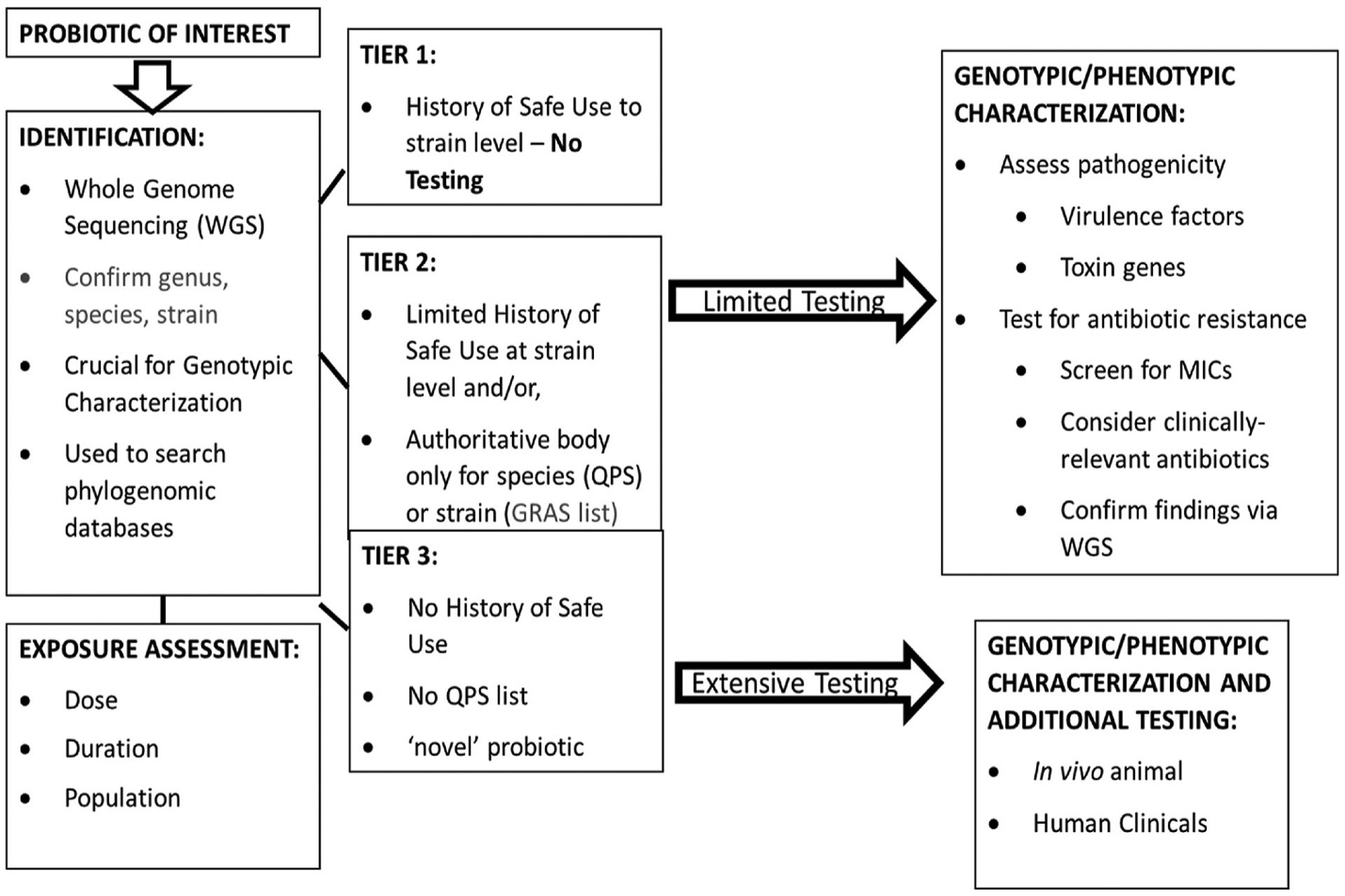
A proposed testing tier to guide data needs and studies for assessing probiotic safety.
Similar testing paradigms to what is summarized in Fig. 2 have been proposed and published by others (Pariza et al., 2015; Brodmann et al., 2017; EFSA, 2017; Saarela, 2019; Pradhan et al., 2020; Rouanet et al., 2020). However, what has been less defined and where additional guidance is needed is to what extent traditional animal toxicity studies are relevant for assessing the safety of probiotics. Repeated dose toxicity studies with probiotic strains have typically been conducted in rodent species. As mentioned previously, a review of traditional oral repeated dose animal toxicity studies with probiotics available in the scientific literature, reveals no findings of any adverse effects regardless of dose and duration (Pradhan et al., 2020). The extensive list of studies provided in Pradhan et al. includes examples of acute, repeated dose, sub-chronic, and in at least one case, a chronic study of 12 months in duration. All of these toxicity studies were conducted at very high doses of the probiotic under investigation. When the same probiotic strain was tested across study durations there were no findings of toxicity with increased chronicity of exposure. The apparent low order of toxicity observed with probiotics tested to date in traditional animal toxicity studies leads us to suggest that shorter-term animal studies provide enough assurance of safety to proceed to human safety and tolerability studies.
The question of duration of dosing in toxicity studies is also connected to the consideration of what toxicity endpoints are relevant to assess for probiotic safety. An important safety endpoint that is unique to in vivo administration of live microorganisms is that of translocation. Translocation involves the passage of live microbes from the gastrointestinal tract to other sites within the body, and is typically assessed in lymph nodes, spleen, liver, bloodstream, or other tissues (Liong, 2008). A number of in vivo studies to assess translocation potential of various probiotic strains have been conducted in either mice or rats; all of which have been 4 weeks in duration (Zhou et al., 2000; Kabeir et al., 2008; Tompkins et al., 2008). When healthy animals are used, there have not been findings of translocation or infectivity in strains tested to date. Thus, it seems reasonable to assume that translocation and infectivity could be observed within the timeframe of a repeated dose toxicity study.
Furthermore, the long history of safe consumption of live microbes throughout human history argues against the lurking dangers from genetic transfer of dangerous genes from probiotics into commensal microbiota, perilous immune system activation, or threats of sepsis. Negative effects from currently consumed ‘traditional’ probiotics of course have been reported, but these are rare, especially in the context of wide exposure. No intervention is 100% safe, and taken in context, these reports are not alarming.
In addition to the potential toxicity considerations for the microorganism itself, a number of other factors related to final product must be taken under consideration. These include the ingredients used in the production and processing of probiotics and any carryover impurities (e. g., allergens associated with soy and milk) as well as delivery forms for finished products, cryoprotectants, and other inactive ingredients. Although dietary supplements are meant for healthy populations, many manufacturers use cautionary labeling to address potential exposure in certain sensitive populations. Lastly, soft chew product forms are extremely popular and because of their potential attractiveness to children should be considered for child-resistant packaging to prevent accidental exposure.
In this paper we highlight the basic safety information needed for any probiotic strain prior to release into the marketplace. We also include additional safety information that may be needed on a case-by-case basis depending on the probiotic under consideration (Box 1 and Fig. 2). The combination of advanced technologies such as WGS which includes genomic-scale analytical resources and tools that target horizontally acquired loci, mobilization structures, and associated content within respective genomes with highly curated databases (Table 2) may provide more relevant safety information resulting in less reliance on traditional animal testing. This approach appears to be gaining support from some regulatory authoritative bodies (Health Canada, EFSA, ANVISA); however, global harmonization of these modern technologies and approaches is still needed.
Acknowledgements
The authors would like to thank the many internal reviewers from the institutions for which the authors are associated.
Funding body information
There was no funding source involved in development of this manuscript, and the authors did not receive any funding in its preparation.
Footnotes
Disclaimer & declaration of competing interest
Amy L. Roe, Amy Smith, Marie-Eve Boyte, James Heimbach, Mary Ellen Sanders, Jay Sirois: These authors are involved with probiotic dietary supplement manufacturers through employment, trade associations, or as consultants.
Chris A. Elkins: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention. C.A. Elkins contributions as government co-author are attributed to respective in silico AR and virulence profiling sections.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Amy L. Roe: Conceptualization, Literature search and review, Writing (major) - reviewing, editing, formatting. Marie-Eve Boyte: Conceptualization, Writing (minor) – reviewing. Chris A. Elkins: Conceptualization, Writing (minor) – reviewing. Virginia S. Goldman: Conceptualization, Writing (minor) – reviewing, editing. James Heimbach: Conceptualization, Writing (minor), reviewing. Emily Madden: Writing (minor) – reviewing. Hellen Oketch-Rabah: Conceptualization, Writing (minor) – reviewing. Mary Ellen Sanders: Conceptualization, Writing (minor) – reviewing, editing. Jay Sirois: Writing (minor) – reviewing. Amy Smith: Conceptualization, Writing (major) – reviewing, editing.
References
- Adams MR, Marteu P, 1995. On the safety of lactic acid bacteria from food. Int. J. Food Microbiol 27 (2–3), 263–264. 10.1016/0168-1605(95)00067-t. [DOI] [PubMed] [Google Scholar]
- Agencia Nacional de Vigilancia Sanitaria (ANVISA), 2018. Resolution of the Collegiate Board RDC No. 241. RESOLUTION of the COLLEGIATE BOARD RDC No. 241. OF JULY 26, 2018 - National Press (in.gov.br). (Accessed 20 May 2022). [Google Scholar]
- Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen ALV, Cheng AA, Liu S, Min SY, Miroshnichenko A, Tran HK, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B, Faltyn M, Hernandez-Koutoucheva A, Sharma AN, Bordeleau E, Pawlowski AC, Zubyk H, Dooley D, Griffiths E, Maguire F, Winsor GL, Beiko RG, Brinkman FSL, Hsiao WWL, Domselaar GV, McArthur AG, 2020. Card 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48 (D1), D517–d525. 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V, 2014. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther 39, 1276–1285. 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango-Argoty G, Garner E, Pruden A, Health LS, Vikesland P, Zhang L, 2018. DeepARG: a Deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 6, 23. 10.1186/s40168-018-0401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt D, Marcu A, Liang Y, Wishart DS, 2019. PHAST, PHASTER and PHASTEST: tools for finding prophage in bacterial genomes. Briefings Bioinf. 20 (4), 1560–1567. 10.1093/bib/bbx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Therapeutic Goods Administration (Tga), 2007. Substances that May Be Used in Listed Medicines in Australia. Australian Government, Dept. of Health and Ageing Therapeutic Goods Administration https://www.legislation.gov.au/Details/F2021L01449. (Accessed 20 May 2022). [Google Scholar]
- Badan Pengawas Obat Dan Makanan, 2021. Health Supplement Containing Probiotic Product Assessment Guidelines. REPUBLIK INDONESIA NADFC, No. 1. Badan Pengawas Obat & Makanan - Republic of Indonesia (pom.go.Id) (Accessed 20 May 2022). [Google Scholar]
- Bernardeau M, Guguen M, Vernoux JP, 2006. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. Rev 30 (4), 487–513. 10.1111/j.1574-6976.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME, Tremblay A, Ouwehand AC, 2020. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol 11, 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwartz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, Fagelhauer L, Chakraborty T, Neumann B, Werner G, Bender JK, Stingl K, Nguyen M, Coppes J, Xavier BB, Malhotra-Kumar S, Wieczorek K, Amaro A, Clemente L, Mossong J, Losch S, Ragimbeau C, Lund O, 2020. ResFinder 4.0 for predictions of phenotypes from genotypes. Antimicrob. Chemother 75 (12), 3491–3500. 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdichon F, Laulund S, Tenning P, 2019. Inventory of microbial species with a rationale: a comparison of the IDF/EFFCA inventory of microbial food cultures with the EFSA Biohazard Panel qualified presumption of safety. FEMS Microbiol. Lett 366 (5), fnz048. 10.1093/femsle/fnz048. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Holzapfel WH, 1999. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol 53, 33–41. 10.1016/s0168-1605(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Brodmann T, Endo A, Gueimonde M, Vinderola G, Kneifel W, de Vos WM, Salminen S, Gomez-Gallego C, 2017. Safety of novel microbes for human consumption: practical examples of assessment in the European Union. Front. Microbiol 8, 1–15. 10.3389/fmicb.2017.01725. Article 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletin of the International Dairy Federation (IDF), 2012. Safety Demonstration of Microbial Food Cultures (MFC) in Fermented Food Products. 455/2012. Bulletin of the IDF N◦ 455/2012: Safety Demonstration of Microbial Food Cultures (MFC) in Fermented Food Products – FIL-IDF (Accessed 20 May 2022). [Google Scholar]
- Bulletin of the International Dairy Federation (IDF), 2018. Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products. N◦ 495/2018. Bulletin of the IDF N◦ 495/2018: Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products – FIL-IDF. (Accessed 20 May 2022). [Google Scholar]
- Bulletin of the International Dairy Federation (IDF), 2022. Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products: Update of the Bulletins of the IDF N◦377–2002, N◦455–2012 and N◦495–2018. N◦514/2022. Bulletin of the IDF N◦ 514/2022: Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products – FIL-IDF. (Accessed 20 May 2022). [Google Scholar]
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA, Dore CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D, 2013. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med 158 (3), 200–207. 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Ministry of Health (MOH), 2010. List of Microorganisms that Can Be Used in Food (Notification No 65 [2010]). http://www.gov.cn/gzdt/2010-04/28/content_1594897.htm. (Accessed 20 May 2022).
- China Ministry of Health (MOH), 2011. The List of Culture Strains Permitted for Use in Infant and Toddler Food by NHC, No.25. http://www.nhc.gov.cn/sps/s7891/201111/a10fe4a0b1dd477c9884649220368cc2.shtml. (Accessed 20 May 2022).
- Chiu YJ, Nam MK, Tsai YT, Huang CC, Tsai CC, 2013. Genotoxicity assessment of multispecies probiotics using reverse mutation, mammalian chromosomal aberration, and rodent micronucleus tests. Sci. World J 254239 10.1155/2013/254239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codex Alimentarius Commission, 2017. Proposal for New Work on Harmonized Probiotic Guidelines for Use in Foods and Dietary Supplements. Joint FAO/WHO Food Standards Programme, Agenda Item 11, NFSDU/39 CRD/3. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-720-39%252FCRDs%252FCRD03.pdf. (Accessed 23 January 2022). [Google Scholar]
- Connolly E, Abrahamsson T, Bjorksten B, 2005. Safety of D(−)-lactic acid producing bacteria in the human infant. J. Pediatr. Gastroenterol. Nutr 41 (4), 489–492. 10.1097/01.mpg.0000176179.81638.45. [DOI] [PubMed] [Google Scholar]
- Crespo-Piazuelo D, Estelle J, Revilla M, Criado-Mesas L, Ramayo-Calda Y, Ovilo C, Fernandez AI, Ballester M, Folch JM, 2018. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci. Rep 8, 12727 10.1038/s41598-018-30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2005. Opinion of the scientific committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 226, 1–12. https://www.efsa.europa.eu/en/efsajournal/pub/226#:~:text=DOI%3A,j.efsa.2005.226. (Accessed 20 May 2022). [Google Scholar]
- EFSA, 2007. Introduction of a Qualified Presumption of Safety (QPS) Approach for Assessment of Selected Microorganisms Referred to EFSA. https://www.efsa.europa.eu/en/efsajournal/pub/587#:~:text=DOI%3A,j.efsa.2007.587. (Accessed 20 May 2022).
- EFSA, 2008. The maintenance of the list of QPS microorganisms intentionally added to food or feed-scientific opinion of the panel on biological hazards. EFSA J. 923, 1–48. https://www.efsa.europa.eu/en/efsajournal/pub/923#:~:text=DOI%3A,j.efsa.2008.923. (Accessed 20 May 2022). [Google Scholar]
- EFSA, 2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10 (6), 2740. 10.2903/j.efsa.2012.2740. [DOI] [Google Scholar]
- EFSA, 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 14 (11), 4594. 10.2903/j.efsa.2016.4594. [DOI] [Google Scholar]
- EFSA, 2017. Guidance on the assessment of the safety of feed additives for the consumer. EFSA J. 10.2903/j.efsa.2017.5022. Guidance adopted: 27 September 2017. [DOI] [Google Scholar]
- EFSA, 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 16 (3), 5206. 10.2903/j.efsa.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel, 2020. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 18 (7), 6174. 10.2903/j.efsa.2021.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2020a. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 18 (2), 5966. 10.2903/j.efsa.2020.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2020b. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 13: suitability of taxonomic units notified to EFSA until September 2020. EFSA J. 19 (1), 6377. 10.2903/j.efsa.2021.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2021a. Qualified presumption of safety (QPS). European food safety authority (EFSA). Available at: https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps. (Accessed 19 January 2021). [Google Scholar]
- EFSA, 2021b. Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain | European Food Safety Authority (europa.eu). Available at: (Accessed 2 June 2021). [Google Scholar]
- EFSA, 2022. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 15: Suitability of Taxonomic Units Notified to EFSA until September 2021 10.2903/j.efsa.2022.7045. (Accessed 8 May 2022). [DOI] [PMC free article] [PubMed]
- Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG, 2009. Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol 47 (6), 1231–1238. 10.1016/j.fct.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian E, Kramer L, Siebert F, Hogenauer C, Raggam RB, Wenzl H, Krejs GJ, 2017. D-lactic acidosis – case report and review of the literature. Z. Gastroenterol 55, 75–82. 10.1055/s-0042-117647, 01. [DOI] [PubMed] [Google Scholar]
- FAO/WHO, 2001. Report of a Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. https://www.fao.org/documents/pub_dett.asp?lang=en&pub_id=61756. (Accessed 26 January 2022).
- FAO/WHO, 2002. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food 2002. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E, 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microb. 3 (4), 289–306. 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Safety and Standards Authority of India (FSSAI), 2002. https://fssai.gov.in/upload/uploadfiles/files/Nutraceuticals_Regulations.pdf. (Accessed 7 July 2022).
- Gibson MK, Forsberg KJ, Dantas G, 2015. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 9 (1), 207–216. 10.1038/ismej.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin JA, Kwon YH, Far PM, Haq S, Khan WI, 2020. Mucins in intestinal mucosal defense and inflammation: learning from clinical and experimental studies. Front. Immunol 11, 2054. 10.3389/fimmu.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guandalini S, Magazzu G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M, 2010. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter randomized, placebo-controlled, double-blind, crossover study. J. Pediatr. Gastroenterol. Nutr 51 (1), 24–30. 10.1097/mpg.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- Gueimonde M, Sanchez B, de los Reyes-Gavilan CG, Margolles A, 2013. Antibiotic resistance in probiotic bacteria. Front. Microbiol 4 (202), 1–6. 10.3389/fmicb.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain J-M, 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother 58 (1), 212–220. 10.1128/aac.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada, 2021. Monograph on Probiotics. http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=probioAccessed. (Accessed 8 May 2022).
- Health Canada, 2015. Good Manufacturing Practices Guidance Document., Version 3.0. Available at: Good Manufacturing Practices Guidance Document - Canada.ca. (Accessed 20 May 2022). [Google Scholar]
- Health Canada, 2020. Allergens and gluten sources labelling. Available at: https://www.canada.ca/en/health-canada/services/food-allergies-intolerances/avoiding-allergens-food/allergen-labelling.html. (Accessed 20 May 2022).
- Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JNV, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, Smith A, Roth B, Polak J, Motala A, Perry T, Shekelle PG, 2011. Safety of probiotics used to reduce risk and prevent or treat disease. Evid. Rep. Technol. Assess 200, 1–645. Apr. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4780970/. (Accessed 15 January 2021). [PMC free article] [PubMed] [Google Scholar]
- Hendriksen RS, Bortolaia V, Tate H, Tyson GH, Aarestrup FM, McDermott PF, 2019. Using genomics to track global antimicrobial resistance. Front. Public Health 7. 10.3389/fpubh.2019.00242. Article 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME, 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol 11 (8), 506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- IDF, 2002. Bulletin No. 377: Inventory of Microorganisms with a Documented History of Use in Food. https://store.fil-idf.org/product/health-benefits-and-safety-evaluation-of-certain-food-components-inventory-of-microorganisms-with-a-documented-history-of-use-in-food-trans-fatty-acids-milk-lipids-in-diet-and-health-med/. (Accessed 19 April 2021).
- IDF, 2012. Bulletin No. 455, Safety demonstration of microbial food cultures (MFC) in fermented food products. https://store.fil-idf.org/product/safety-demonstration-of-microbial-food-cultures-mfc-in-fermented-food-products-2/. (Accessed 19 January 2021).
- IDF, 2022. Bulletin No. 514, Inventory of microbial food cultures with safety demonstration in fermented food products. https://store.fil-idf.org/product/bulletin-idf-n-495-2018-inventory-microbial-food-cultures-safety-demonstration-fermented-food-products/. (Accessed 19 January 2021).
- Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, Moher D, Group C., 2004. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann. Intern. Med 141 (10), 781–788. 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- IPA, 2017. International Probiotics Association (IPA) Guidelines to Qualify a Microorganism to Be Termed as ‘probiotic’. https://internationalprobiotics.org/wp-content/uploads/20170602-IPA-guidelines-to-qualify-a-microorganism-as-probiotic.pdf. (Accessed 19 January 2021).
- Isa K, Oka K, Beauchamp N, Sato M, Wada K, Ohtani K, Nakanishi S, McCartney E, Tanaka M, Shimizu T, Kamiya S, Kruger C, Takahashi M, 2016. Safety assessment of the Clostridium butyricum MIYAIRI 588® probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Hum. Exp. Toxicol 35 (8), 818–832. 10.1177/0960327115607372. [DOI] [PubMed] [Google Scholar]
- Ishibashi N, Yamazaki S, 2001. Probiotics and safety. Am. J. Clin. Nutr 73 (2 Suppl. l), 465S–470S. 10.1093/ajcn/73.2.465s. [DOI] [PubMed] [Google Scholar]
- Jackson SA, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M, Goldman VS, Burguiere P, Atwater JB, Sanders ME, 2019. Improving end-user trust in the quality of commercial probiotic products. Front. Microbiol 10, 739. 10.3389/fmicb.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezewska-Frackowiak J, Serocyznska K, Banaszczyk J, Jedrzejczak G, Zylicz-Stachula A, Skowron PM, 2018. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol 65 (4), 509–519. 10.18388/abp.2018_2652. [DOI] [PubMed] [Google Scholar]
- Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FSL, Wright GD, McArthur AG, 2017. Card 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 45 (D1), D566–d573. 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeir BM, Abd Manap Y, Wong S, Hakim M, Othaman M, Shuhaimi M, 2008. Safety evaluation of Bifidobacterium pseudocatenulatum G4 as assessed in BALB/c mice. Lett. Appl. Microbiol 46, 32–37. 10.1111/j.1472-765X.2007.02255.x. [DOI] [PubMed] [Google Scholar]
- Lahtinen SJ, Boyle RJ, Margolles A, Frias R, Gueimonde M, 2009. Safety assessment of probiotics, chapter 31. In: Charalampopoulos D, Rastall RA (Eds.), Prebiotics and Probiotics Science and Technology, 1page 1193. Springer; Reference. 10.1016/B978-0-12-823733-5.00021-0. [DOI] [Google Scholar]
- Li J, Tai C, Deng Z, Zhong W, He Y, Ou H-Y, 2018. VRprofile: gene-cluster-detection-based profiling of virulence and antibiotic resistance traits encoded within genome sequences of pathogenic bacteria. Briefings Bioinf. 19 (4), 566–574. 10.1093/bib/bbw141. [DOI] [PubMed] [Google Scholar]
- Liong M-T, 2008. Safety of probiotics: translocation and infection. Nutr. Rev 66 (4), 192–202. 10.1111/j.1753-4887.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Pop M, 2009. ARDB-Antibiotic resistance genes database. Nucleic Acids Res. 37 (Issue Suppl. 1), D443–D447. 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zheng D, Jin Q, Chen L, Yang J, 2019a. Vfdb 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. v. 47 (Database Issue), D687–D692. 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li X, Xie Y, Bi D, Sun J, Li J, Tai C, Deng Z, Ou H-Y, 2019b. ICEberg 2.0: an updated database of bacterial integrative and conjugative elements. Nucleic Acids Res. 47 (D1), D660–D665. 10.1093/nar/gky1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack DR, 2011. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients 3 (2), 245–264. 10.3390/nu3020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaysian Food Act, 1985. P.U.(A) 437/85 Twelfth A Schedule, Regulations 26A. Available online: http://fsq.moh.gov.my/v6/xs/page.php?id=72.
- Mao C, Abraham D, Wattam AR, Wilson JJC, Shukla M, Yoo HS, Sobral BW, 2015. Curation, integration and visualization of bacterial virulence factors in PATRIC. Bioinformatics 31 (2), 252–258. 10.1093/bioinformatics/btu631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MarketsandMarkets, 2021. Probiotics Market by Applications (Functional Food & Beverages (Dairy Products, Non-dairy Beverages, Infant Formula, Cereals), Dietary Supplements, Feed), Ingredient (Bacteria, Yeast), Form (Dry, Liquid), End User, and Region – Global Forecast to 2026. Probiotics Market Growth Analysis, Trends, and Forecasts to 2026 | MarketsandMarkets. (Accessed 21 January 2022). [Google Scholar]
- McArthur AG, Waglechner N, Nizam F, Austin Y, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O’Brien JS, Pawlowski AC, Piddock LJV, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wrigth GD, 2013. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother 57 (7), 3348–3357. 10.1128/aac.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes RS, McCallum GE, Lamberte LE, van Schaik W, 2020. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol 53, 35–43. 10.1016/j.mib.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Monaco G, van Dam S, Ribeiro JLCN, Larbi A, de Magalhaes JP, 2015. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels. BMC Evol. Biol 15, 259. 10.1186/s12862-015-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morovic W, Roper J, Smith AB, Mukerji P, Stahl B, Rae JC, Ouwehand AC, 2017. Safety evaluation of HOWARU®Restore (Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Bifidobacterium animalis subsp. Lactis B1-04 and B. lactis Bi-07) for antibiotic resistance, genomic risk factors, and acute toxicity. Food Chem. Toxicol 110, 316–324. 10.1016/j.fct.2017.10.037. [DOI] [PubMed] [Google Scholar]
- Moura A, Soares M, Pereira C, Leitao N, Henriques I, Correia A, 2009. INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics 25 (8), 1096–1098. 10.1093/bioinformatics/btp105. [DOI] [PubMed] [Google Scholar]
- Munakata S, Arakawa C, Kohira R, Fujita Y, Fuchigami T, Mugishima H, 2009. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev. 32 (8), 691–694. 10.1016/j.braindev.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Wang S, Solberg Woods LC, Seshie O, Chung ST, Shively CA, Register TC, Craft S, McClain DA, Yadav H, 2018. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol 9 (2897), 1–13. 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A, Sewell T, Farrer RA, Abdolrasouli A, Shelton JMG, Fisher MC, Rhodes J, 2018. MARD mycology antifungal resistance database. Bioinformatics 34, 3233–3234. 10.1093/bioinformatics/bty321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B, 2016. Probiotic approach to prevent antibiotic resistance. Ann. Med 48 (4), 246–255. 10.3109/07853890.2016.1161232. [DOI] [PubMed] [Google Scholar]
- Pariza MW, Gillies KO, Kraak-Ripple SF, Leyer G, Smith AB, 2015. Determining the safety of microbial cultures for consumption by humans and animals. Regul. Toxicol. Pharmacol 73 (1), 164–171. 10.1016/j.yrtph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Pradhan D, Mallappa RH, Grover S, 2020. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 108, 106872. 10.1016/j.foodcont.2019.106872. [DOI] [Google Scholar]
- Pohanka M, 2020. D-lactic acid as a metabolite: toxicology, diagnosis, and detection. Biomed. Res. 2020 10.1155/2020/3419034. ID 3419034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EMM, Pot B, Sanders ME, 2018. ‘Brain fogginess’ and D-lactic acidosis: probiotics are not the cause. Clin. Transl. Gastroenterol 9 (9), 187. 10.1038/s41424-018-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation (EC), 2004. No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32004R0852&from=EN. (Accessed 26 May 2022).
- Republic of the Philippines, 2004. Bureau Circular No. 16. https://www.fda.gov.ph/wp-content/uploads/2021/04/Bureau-Circular-No.−16-s.−2004.pdf. (Accessed 29 January 2022).
- Rossi F, Amadoro C, Gasperi M, Colavita G, 2022. Lactobacilli infection case reports in the last three years and safety implications. Nutrients 14, 1178. 10.3390/nu14061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouanet A, Bolca S, Bru A, Claes I, Cvejic H, Girgis H, Harper A, Lavergne SN, Mathys S, Pane M, Pot B, Shortt C, Alkema W, Bezulowsky C, Blanquet-Diot S, Chassard C, Claus SP, Hadida B, Hemmingsen C, Jeune C, Lindman B, Midzi G, Mogna L, Movitz C, Nasir N, Oberreither M, Seegers JFM, Sterkman L, Valo A, Vieville F, Cordaillat-Simmons M, 2020. Live biotherapeutic products, a road map for safety assessment. Front. Med published June 19, 2020. 10.3389/fmed.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau CF, Desvignes C, Kling F, Voisin EM, Ruthsatz M, 2020. Microbiome product toxicology: regulatory view on translational challenges. Regul. Toxicol. 1–29 10.1007/978-3-642-36206-4_140-1. [DOI] [Google Scholar]
- Saarela MH, 2019. Safety aspects of next generation probiotics. Curr. Opin. Food Sci 30, 8–13. 10.1016/j.cofs.2018.09.001. [DOI] [Google Scholar]
- SAHPRA, 2020. Complementary Medicines – Health Supplements Safety and Efficacy. 7.04_CM_SE_Health_Supplements_Mar22_v4_3.Pdf (sahpra.org.Za). (Accessed 20 May 2022). [Google Scholar]
- Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hormannsperger G, Huys G, Levy DD, Lutgendorff F, Mack D, Phothirath P, Solano-Aguilar G, Vaughan E, 2010. Safety assessment of probiotics for human use. Gut Microb. 1 (3), 164–185. 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, Leyer G, 2016. Probiotic use in at-risk populations. J. Am. Pharm. Assoc. JAPhA 56, 680–686. 10.1016/j.japh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Satoh T, Narisawa K, Konno T, Katoh T, Fujiyama J, Tomoe A, Metoki K, Hayasaka K, Tada K, Ishibashi M, Yamane N, Mitsuoka T, Benno Y, 1982. D-lactic acidosis in two patients with short bowel syndrome: bacteriological analyses of the fecal flora. Eur. J. Pediatr 138 (4), 324–326. 10.1007/bf00442509. [DOI] [PubMed] [Google Scholar]
- Shane AL, Cabana MD, Vidry S, Merenstein D, Hummelen R, Ellis CL, Heimbach JT, Hempel S, Lynch SV, Sanders ME, Tancredi DJ, 2010. Guide to designing, conducting, publishing and communicating results of clinical studies involving probiotic applications in human participants. Gut Microb. 1 (4), 243–253. 10.4161/gmic.1.4.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P, Gourbeyre E, Chandler M, 2014. Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol. Rev 38 (5), 865–891. 10.1111/1574-6976.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, 2019. Chapter 40: the regulation of probiotics in the United States. In: Lactic Acid Bacteria, Microbiological and Functional Aspects, fifth ed. Gabriel Vinderola, Arthur Ouwehand, Seppo Salminen, Atte von Wright. CRC Press. Published. (Accessed 5 April 2019). [Google Scholar]
- SEA Probiotics SREN. Available at:: SEA PROBIOTICS SREN - PERGIZI PANGAN Indonesia Accessed on May 20, 2022.
- Standard Global Services (SGS). Agriculture and Food Hazard Analysis and Critical Control Points (HACCP) Certification. Available at: HACCP Certification | SGS Canada; Accessed on May 20, 2022. [Google Scholar]
- State Administration for Market Regulation (SAMR), 2020. Technical Guidelines for Safety Inspection and Evaluation of Strains Used for Health Food Raw Materials. https://www.cirs-group.com/en/food/analysis-on-guideline-for-safety-inspection-and-evaluation-of-strains-used-as-health-food-raw-materials-2020-version. (Accessed 20 May 2022).
- Tailford LE, Crost EH, Kavanaugh D, Juge N, 2015. Mucin glycan foraging in the human gut microbiome. Front. Genet 6 10.3389/fgene.2015.00081. Article 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Andrew DJ, Rego L, 2014. The added value of the 90-day repeated dose oral toxicity test for industrial chemicals with a low (sub)acute toxicity profile in a high-quality dataset. Regul. Toxicol. Pharmacol 69, 320–332. 10.1016/j.yrtph.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Thailand Ministry of Public Health, 2012. Notification of the Ministry of public health: use of probiotic microorganisms in food. Government gazette. Special Part 189 Ngor. http://food.fda.moph.go.th/law/data/announ_moph/V.English/No.%20339%20Use%20of%20Probiotic%20Microorganisms%20in%20Foods.pdf. (Accessed 20 May 2022), 129. [Google Scholar]
- Tompkins TA, Hagen KE, Wallace TD, Fillion-Forte V, 2008. Safety evaluation of two bacterial strains used in Asian probiotic products. Can. J. Microbiol 54, 391–400. 10.1139/w08-022. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration (US FDA), 2006. Food allergen labeling and consumer protection act of 2004 questions and answers. Available at: https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-questions-and-answers#q17. (Accessed 20 May 2022).
- United States Food and Drug Administration (FDA), 2016a. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information; Guidance for Industry (fda.Gov). (Accessed 20 May 2022). [Google Scholar]
- United States Food and Drug Administration (FDA), 2018. Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials: Guidance for Industry. Draft Guidance. Draft Guidance for Industry: Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials (fda.Gov). (Accessed 13 August 2022). [Google Scholar]
- United States Food and Drug Administration (US FDA), 2022a. Good clinical practices. Available at: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/good-clinical-practice. (Accessed 20 May 2022).
- United States Pharmacopeia (USP), 2022a. About USP. Available at: : About U.S. Pharmacopeia (usp.org). (Accessed 20 May 2022). [Google Scholar]
- Urban M, Cuzick A, Seager J, Wood V, Rutherford K, Venkatesh SY, De Silva N, Martinez MC, Pedro H, Yates AD, Hassani-Pak K, Hammond-Kosack KE, 2020. PHI-base: the pathogen-host interactions database. Nucleic Acids Res. 48 (D1), D613–D620. 10.1093/nar/gkz904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankerckhoven V, Huys G, Vancanneyt M, Vael C, Klare I, Romond MB, Entenza JM, Moreillon P, Wind RD, Knol J, Wiertz E, Pot B, Vaughan EE, Kahlmeter G, Goossens H, 2008. Biosafety assessment of probiotics used for human consumption: recommendations from the EU-PROSAFE project. Trends Food Sci. Technol 19 (2), 102–114. 10.1016/j.tifs.2007.07.013. [DOI] [Google Scholar]
- vonWright A, Axelsson L, 2019. Chapter 1: lactic acid bacteria, microbiological and functional aspects. In: Lactic Acid Bacteria, Microbiological and Functional Aspects, fifth ed. Gabriel Vinderola, Arthur Ouwehand, Seppo Salminen, Atte von Wright, CRC Press. Published. (Accessed 5 April 2019). [Google Scholar]
- Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW, 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 42 (Database Issue), D581–D591. 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles TJ, Guillemin K, 2019. The other side of the coin: what beneficial microbes can teach us about pathogenic potential. J. Mol. Biol 431 (16), 2946–2956. 10.1016/j.jmb.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Wojciech W, Lukasiewicz M, Puppel K, 2020. Biogenic amines: formation, action and toxicity – a review. J. Sci. Food Agric 10.1002/jsfa.10928. Published online. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2018. Critically Important Antimicrobials for Human Medicine, 6th Revision. Critically Important Antimicrobials for Human Medicine : 6th Revision (who.Int). (Accessed 20 May 2022). [Google Scholar]
- Yoon SH, Park Y-K, Kim JF, 2015. PAIDB v2.0: exploration and analysis of pathogenicity and resistance islands. Nucleic Acids Res. 43 (D1), D624–D630. 10.1093/nar/gku985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Ganzle MG, Lebeer S, 2020. A taxanomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol 70 (4), 2782–2858. 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- Zhou JS, Shu Q, Rutherford KJ, Prasad J, Gopal PK, Gill HS, 2000. Acute oral toxicity and bacterial translocation studies on potentially probiotic strains of lactic acid bacteria. Food Chem. Toxicol 38 (2), 153–161. 10.1016/s0278-6915(99)00154-4. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Gonzalez L, Blikslager A, 2016. Large animal models: the key to translational discovery in digestive disease research. Cell Mol. Gastroenterol. Hepatol 2, 716–724. 10.1016/j.jcmgh.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- European Medicines Agency (Ema), 1996. ICH topic Q5C quality of biotechnological products: stability testing of biotechnological/biological products. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-topic-q-5-c-quality-biotechnological-products-stability-testing-biotechnological/biological-products_en.pdf. (Accessed 6 June 2021).
- European Medicines Agency (Ema), 2003. ICH topic Q1E evaluation of stability data. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-e-evaluation-stability-data-step-5_en.pdf. (Accessed 6 June 2021).
- European Pharmacopeia, 2019a. Live Biotherapeutic Products for Human Use, 04/2019/3053.
- European Pharmacopeia, 2019b. Microbiological Examination of Live Biotherapeutic Products: Tests for Specified Micro-organisms, 04/2019/20638.
- Medical Control Counsel (MCC), 2016. Complementary Medicines – Health Supplements Safety and Efficacy, 7.04 SE Health Supplements June 16, v2.doc. 7.04_CM_SE_Health_Supplements_Jun20_v4.pdf (sahpra.org.za).
- Regulation (EC), 2004. No 853/2004 of the European Parliament and of the Council of 29 April 2004: laying down specific hygiene rules for the hygiene of foodstuffs. J. Eur. Union [Google Scholar]
- United States Food and Drug Administration (US FDA), 2010. Small entity compliance guide: current good manufacturing practice in manufacturing, packaging, labeling, or holding operations for dietary supplements. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-current-good-manufacturing-practice-manufacturing-packaging-labeling#:~:text=The%20Dietary%20Supplement%20(DS)%20CGMP,the%20dietary%20supplement%20is%20packaged. (Accessed 20 May 2022). [PubMed]
- United States Food and Drug Administration (FDA), 2016b. Substances Generally Recognized as Safe. Generally Recognized as Safe (GRAS) | FDA (Accessed 20 May 2022).
- United States Food and Drug Administration (FDA), 2016c. Draft Guidance for Industry: New Dietary Ingredient Notifications and Related Issues. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-new-dietary-ingredient-notifications-and-related-issues. (Accessed 20 May 2022).
- United States Food and Drug Administration (FDA), 2016d. Dietary Supplements: New Dietary Ingredient Notifications and Related Issues: Guidance for Industry. https://www.fda.gov/media/99538/download. (Accessed 20 May 2022).
- United States Food and Drug Administration (FDA), 2021. GRAS Substances (SCOGS) Database. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices. (Accessed 20 May 2022).
- United States Food and Drug Administration (US FDA), 2022b. Facts about the current good manufacturing practices (CGMPs). Available at: https://www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practices-cgmps. (Accessed 20 May 2022).
- United States Pharmacopeia (USP), 2022b. Chapter 64 probiotic tests. Available at: https://online.uspnf.com/uspnf/document/1_GUID-804A3FF9-39F3-4C5D-A4B0-2A387EF95F96_2_en-US. (Accessed 20 May 2022).
- United States Pharmacopeia (USP), 2022c. USP <659> packaging and storage requirements. Available at: https://online.uspnf.com/uspnf/document/1_GUID-48C0134E-8117-4B3B-8DC4-EA70C93EEE8F_5_en-US?source=TOC. (Accessed 6 June 2021).
- Zhou CE, Smith J, Lam M, Zemla A, Dyer MD, Siezak T, 2007. MvirDB – a microbial database of protein toxins, virulence factors and antibiotic resistance genes for bio-defence applications. Nucleic Acids Res. Jan 35, D391–D394. Database issue). 10.1093/nar/gkl791. [DOI] [PMC free article] [PubMed] [Google Scholar]


