Abstract
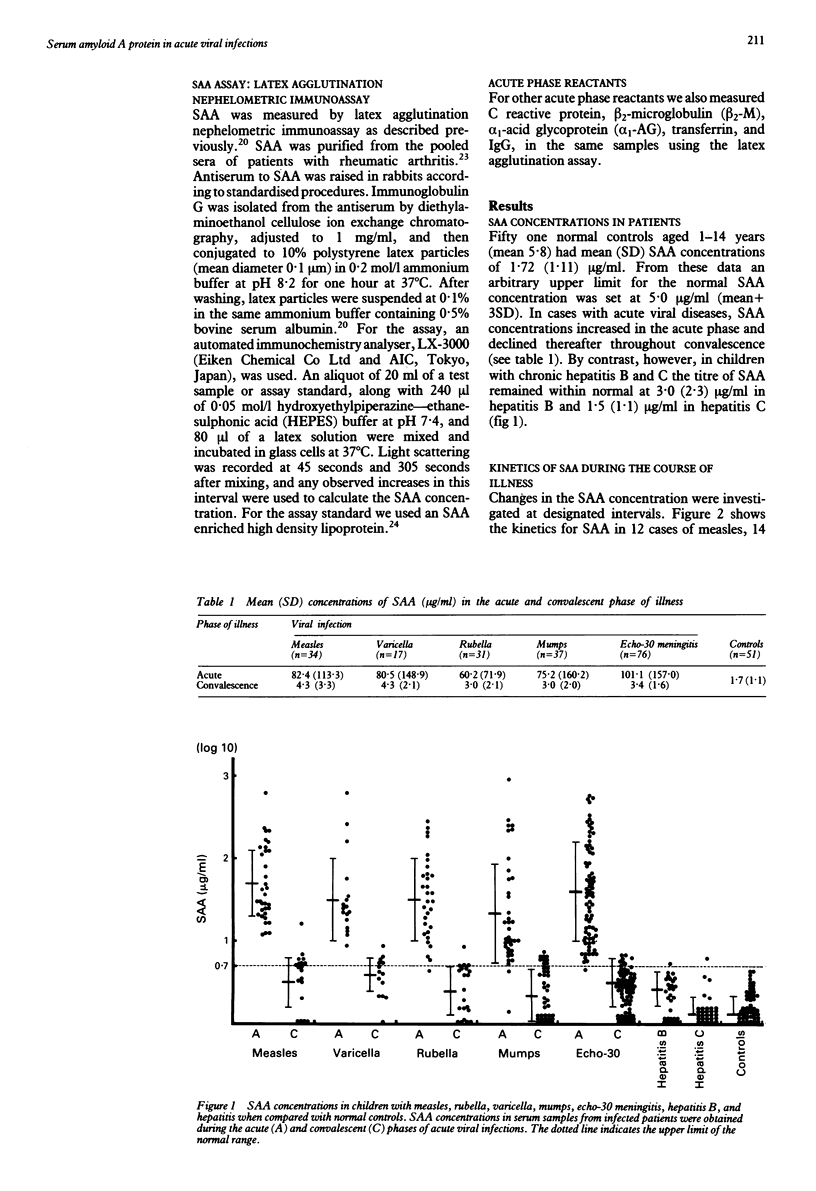
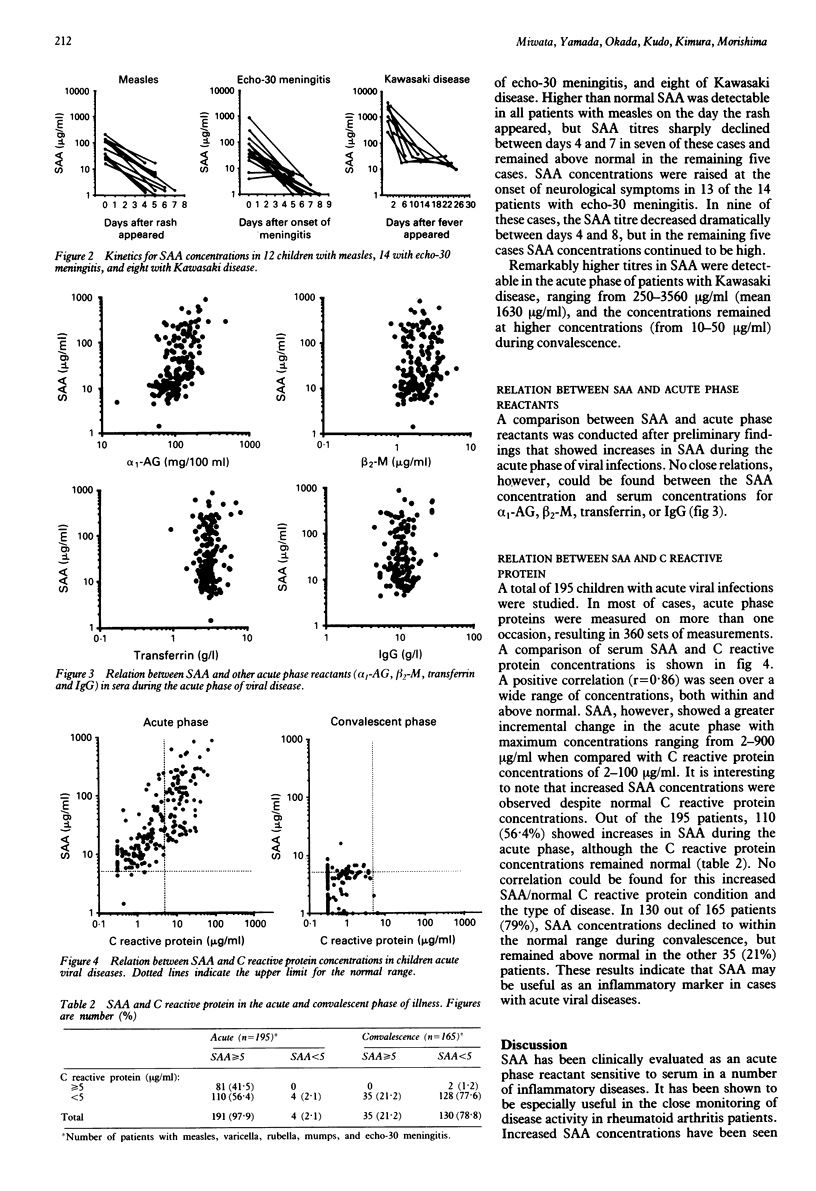
Concentrations of serum amyloid A protein (SAA) were measured in 254 children with viral diseases, including measles, varicella, rubella, mumps, echo-30 meningitis, chronic hepatitis B and C, and in eight with Kawasaki disease. Latex agglutination nephelometric immunoassay was used for assaying SAA. In 191 out of 195 patients (98%), SAA concentrations became markedly raised in the acute phase of the viral disease: measles (97%), varicella (100%), mumps (95%), and echo-30 meningitis (99%) with mean titres of 82.4, 80.5, 60.2, 75.2, and 101.1 micrograms/ml respectively. This increase in SAA was followed by a rapid return to normal concentrations (< 5 micrograms/ml) during convalescence. Remarkably higher concentrations of SAA (mean 1630 micrograms/ml) were detected in the acute phase of patients with Kawasaki disease, but in most of the children with chronic hepatitis B or C, the titres of SAA remained normal. There was no close correlation between SAA and serum concentrations for alpha 1-acid glycoprotein, beta 2-microglobulin, transferrin, and IgG. There was a clear correlation between SAA and C reactive protein concentrations, although SAA showed a greater incremental change than C reactive protein in the acute phase. In the acute phase of these viral diseases, 56% of the patients had raised SAA concentrations (> or = 5 micrograms/ml) with normal C reactive protein concentrations (< 5 micrograms/ml). These results indicate that SAA could be useful as an inflammatory marker in children with acute viral infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- At home and abroad. Lancet. 1982 Jan 2;1(8262):23–24. [PubMed] [Google Scholar]
- Benson M. D., Cohen A. S. Serum amyloid A protein in amyloidosis, rheumatic, and enoplastic diseases. Arthritis Rheum. 1979 Jan;22(1):36–42. doi: 10.1002/art.1780220106. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Cohen A. S. Serum amyloid A protein in amyloidosis, rheumatic, and enoplastic diseases. Arthritis Rheum. 1979 Jan;22(1):36–42. doi: 10.1002/art.1780220106. [DOI] [PubMed] [Google Scholar]
- Chambers R. E., MacFarlane D. G., Whicher J. T., Dieppe P. A. Serum amyloid-A protein concentration in rheumatoid arthritis and its role in monitoring disease activity. Ann Rheum Dis. 1983 Dec;42(6):665–667. doi: 10.1136/ard.42.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenir N. L., Jeenah M. S., Coetzee G. A., Van der Westhuyzen D. R., Strachan A. F., De Beer F. C. Standardisation of the quantitation of serum amyloid A protein (SAA) in human serum. J Immunol Methods. 1985 Nov 7;83(2):217–225. doi: 10.1016/0022-1759(85)90243-1. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Rosenthal C. J., Franklin E. C. Amyloid-related serum component (SAA)--studies in acute infections, medullary thyroid carcinoma, and postsurgery. Clin Immunol Immunopathol. 1976 Jul;6(1):83–93. doi: 10.1016/0090-1229(76)90063-5. [DOI] [PubMed] [Google Scholar]
- Hocke G., Ebel H., Bittner K., Müller T., Kaffarnik H., Steinmetz A. A rapid laser immunonephelometric assay for serum amyloid A (SAA) and its application to the diagnosis of kidney allograft rejection. Klin Wochenschr. 1989 Apr 17;67(8):447–451. doi: 10.1007/BF01725141. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. A serum component related to nonimmunoglobulin amyloid protein AS, a possible precursor of the fibrils. J Clin Invest. 1974 Apr;53(4):1054–1061. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Marhaug G., Husby G. Characterization of human amyloid-related protein SAA as a polymorphic protein: association with albumin and prealbumin in serum. Clin Exp Immunol. 1981 Jul;45(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Marhaug G. Three assays for the characterization and quantitation of human serum amyloid A. Scand J Immunol. 1983 Oct;18(4):329–338. doi: 10.1111/j.1365-3083.1983.tb01804.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Höckerstedt K., Lautenschlager I., Scheinin T. M. Monitoring of high-density lipoprotein-associated amyloid A protein after liver transplantation. Transplant Proc. 1987 Oct;19(5):3825–3826. [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Wegelius O. Relationship between urinary sialylated saccharides, serum amyloid A protein, and C-reactive protein in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1982 Jun;41(3):268–271. doi: 10.1136/ard.41.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Teppo A., Eklund B., Ahonen J. Serum amyloid A protein: a sensitive indicator of renal allograft rejection in humans. Transplantation. 1983 Nov;36(5):501–504. [PubMed] [Google Scholar]
- McAdam K. P., Elin R. J., Sipe J. D., Wolff S. M. Changes in human serum amyloid A and C-reactive protein after etiocholanolone-induced inflammation. J Clin Invest. 1978 Feb;61(2):390–394. doi: 10.1172/JCI108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy P. L., Frank A. L., Ablow R. C., Masters S. J., Dolan T. F., Jr Value of the C-reactive protein test in the differentiation of bacterial and viral pneumonia. J Pediatr. 1978 Mar;92(3):454–456. doi: 10.1016/s0022-3476(78)80448-x. [DOI] [PubMed] [Google Scholar]
- Raynes J. G., Cooper E. H. Comparison of serum amyloid A protein and C-reactive protein concentrations in cancer and non-malignant disease. J Clin Pathol. 1983 Jul;36(7):798–803. doi: 10.1136/jcp.36.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Sullivan L. M. Serum amyloid A to monitor cancer dissemination. Ann Intern Med. 1979 Sep;91(3):383–390. doi: 10.7326/0003-4819-91-3-383. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. C-reactive protein in acute viral infections. J Med Virol. 1981;8(3):161–167. doi: 10.1002/jmv.1890080302. [DOI] [PubMed] [Google Scholar]
- Saxstad J., Nilsson L. A., Hanson L. A. C-reactive protein in serum from infants as determined with immunodiffusion techniques. II. Infants with various infections. Acta Paediatr Scand. 1970 Nov;59(6):676–680. doi: 10.1111/j.1651-2227.1970.tb17704.x. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Zimlichman S., Winikoff Y., Pras M., Chaimovitz C., Sarov I. Serum amyloid A (SAA) in viral infection: rubella, measles and subacute sclerosing panencephalitis (SSPE). Clin Exp Immunol. 1982 Dec;50(3):503–506. [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Morishima T., Kudo T., Maki T., Maki S., Nagai Y. Serum hepatitis C virus sequences in posttransfusion non-A, non-B hepatitis. Blood. 1991 Mar 15;77(6):1157–1160. [PubMed] [Google Scholar]
- Whicher J. T., Chambers R. E., Higginson J., Nashef L., Higgins P. G. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J Clin Pathol. 1985 Mar;38(3):312–316. doi: 10.1136/jcp.38.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Uchiyama K., Yakata M., Gejyo F. Sandwich enzyme immunoassay for serum amyloid A protein (SAA). Clin Chim Acta. 1989 Feb 15;179(2):169–175. doi: 10.1016/0009-8981(89)90163-0. [DOI] [PubMed] [Google Scholar]
- Yap S. H., Moshage H. J., Hazenberg B. P., Roelofs H. M., Bijzet J., Limburg P. C., Aarden L. A., van Rijswijk M. H. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991 Feb 19;1091(3):405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]


