Abstract
Purpose
COVID-19 pandemic resulted in a significant number of critical care admissions secondary to severe pneumonia and acute respiratory distress syndrome. We evaluated the short-, medium- and long-term outcomes of lung function and quality of life in this prospective cohort study and reported the outcomes at 7 weeks and 3 months from discharge from intensive care unit.
Methods
A prospective cohort study of ICU survivors with COVID-19 was conducted from August 2020 to May 2021 to evaluate baseline demographic and clinical variables as well as determine lung function, exercise capacity, and health-related quality of life (HRQOL) using spirometry and 6-minute walk test (6MWT) conducted in accordance with American Thoracic Society standards, and SF-36 (Rand), respectively. SF-36 is a generic 36 question standardized health survey. Descriptive and inferential statistics (alpha = 0.05) were used to analyse the data.
Results
At baseline, 100 participants were enrolled in the study of whom 76 followed up at 3 months. Majority of the patients were male (83%), Asians (84%) and less than 60 years of age (91%). HRQOL showed significant improvement in all domains of SF-36, except in emotional wellbeing. Spirometry variables also showed significant improvement in all variables over time with greatest improvement in percentage predicted Forced expiratory volume 1 (79% vs 88% p < 0.001). 6MWT showed significant improvement in variables of walk distance, dyspnea, and fatigue with greatest improvement in change in oxygen saturation (3% vs 1.44% p < 0.001). Intubation status did not impact the changes in SF-36, spirometry or 6MWT variables.
Conclusion
Our findings suggest that ICU survivors of COVID-19 have significant improvement in their lung function, exercise capacity and HRQOL within 3 months of ICU discharge regardless of intubation status.
Keywords: COVID-19, lung function, exercise capacity, health related quality of life
Introduction
Pneumonia caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), spread across the globe resulting in coronavirus disease 2019 (COVID-19). COVID-19 has unquestionably been one of the deadliest pandemics of the modern era, resulting in a significant rise in critically ill patients requiring intensive care unit (ICU) admission and treatment across the world.1 Over three distinct waves, the overall number of patients, including those requiring ICU care, has been staggeringly high. In the State of Qatar, with a total population of 2.8 million, up to 7.6% of all COVID-19 hospitalized patients needed ICU care, necessitating an exponential increase in ICU bed capacity of up to 300%.1,2 A prolonged ICU stay due to Acute Respiratory Distress Syndrome (ARDS), the leading cause of ICU admissions in COVID-19, or other critical illnesses, is a well-known risk factor for not only a decline in health-related quality of life (HRQoL) but also physical and psychological sequelae that can last up to 5 years.3
Recent studies have shown that hospitalized patients with COVID-19 (both ICU and non-ICU) can have persistent symptoms such as new-onset fatigue, breathlessness, and psychological distress when followed at 7 weeks.4 Short-term outcomes at 6 months have also reported a decline in physical function and reduced quality of life.5 Even longer-term clinical outcomes at 1 year are predictive of persistence of symptoms in all three areas (physical, mental, and cognitive).6 However, there is a scarcity of data on long-term outcomes that consider pulmonary function, exercise capacity, functional status, physical strength, and radiographic outcomes. Poorer outcomes are to be expected, given the lengthier ICU stay of COVID-19 patients compared to those who do not have COVID-19.7 However, diversity of population (Genetic, racial, gender, age differences) in different countries/regions may lead to different outcomes in different populations. Therefore, it is important for outcome studies to be conducted across varying demographics around the world to better utilize the resources for long-term care of these patients. To explore and address the long-term consequences of this pandemic in our country, it is necessary to establish the incidence of any short- and long-term outcomes, particularly in individuals who required ICU level treatment. As a result, more information would be available to us for proper evaluation, planning, treatment, and rehabilitation of these ICU survivors.8
To address this gap, we designed a prospective cohort study named “Outcomes – Short and Long term in ICU patient with COVID-19: OUTSTRIP COVID” assessing overall mortality, physical and psychiatric co-morbidities, reduction in lung function, and the ability to return to work post-ICU discharge with a follow-up period of 2 years.
In our current interim paper, we describe our findings at 7 weeks and 3 months post-discharge from ICU in relation to health-related quality of life, lung function, and exercise capacity. These were patients during the first two waves of the COVID-19 pandemic caused by the predominant delta variant, and they were vaccine naïve.
Materials and Methods
Study Design, Setting and Population
This is a national prospective cohort study conducted across medical ICUs under the tertiary health care facility in the state of Qatar (Hamad Medical Corporation (HMC), Doha, Qatar). The study was approved and funded by the Medical Research Centre, HMC under MRC-01-20-860. The study was conducted in compliance with the Declaration of Helsinki.
One hundred patients with COVID-19 who required ICU care were enrolled in the study within seven weeks of discharge from ICU (from August 10th-2020-May 5th, 2021). Patients over the age of 18 who were SARS CoV-2 positive based on Nucleic Acid Amplification Testing by reverse transcriptase PCR test detecting viral RNA in any respiratory secretion, admitted to ICU because of severe/critical COVID-19 illness, and able to provide valid informed consent before discharge or at first appointment in COVID chest clinics within 7 weeks of ICU discharge were eligible. Anyone with a suspected acute brain lesion that could cause global impairment of consciousness or cognition, such as traumatic brain injury, stroke, intracranial hemorrhage, or hypoxic brain injury, a preexisting neuro-psychological condition, moderate-to-severe COPD, asthma, cystic fibrosis, or parenchymal lung disease, ie, interstitial lung disease, was excluded. Severe or Critical disease was defined as a positive COVID-19 test along with any of the following: dyspnea (respiratory rate ≥30 breath/min), hypoxia (SpO2 ≤93% on room air), radiological changes affecting ≥50% of the lung, or severe disease complications such as respiratory failure, the need for mechanical ventilation, septic shock, or non-respiratory organ failure.8
Study Variables
Baseline Data and Demographics
Several baseline variables at ICU admission were collected using a case record form, including patient demographics (age, nationality), body mass index, data on blood investigations, radiology, comorbidities, Acute Physiology and Chronic Health Evaluation (APACHE) score at 24 hours, disease-related potential predictors such as oxygen saturation index and arterial oxygen tension/fraction of inspired oxygen ratio (PaO2/FiO2 ratio). Length of stay (LOS) in the hospital and the ICU, as well as the overall number of ventilator days, were also recorded.
Six-Minute Walk Test
A six-minute standardized walk test was performed to determine exercise capacity, in accordance with American Thoracic Society (ATS) standards.9
Spirometry
Spirometry to determine lung function was performed as spirometry in accordance with the ATS guidelines.10 Results were expressed in absolute values, and percentage of the predicted value, based on the Global Lung Function Initiative (GLI) reference values.11
Health-Related Quality of Life
Quality of life score was assessed using Health-Related Quality of Life Questionnaire (HRQoL) SF-36 (Rand). SF-36 is a generic standardized (non-preference-based) health survey questionnaire used in clinical practice and research, health policy and assessments, and general population surveys. The 36 questions on the SF-36 are meant to evaluate eight health concepts (scores range from 0 to 100, with 0 indicating maximum disability and 100 indicating no disability), including physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health.12
Two physicians and a research assistant checked all the data. The translated and validated SF-36 questionnaires in English, Arabic, Hindi, Bengali only were used for the study.13
Statistical Analysis
Data was managed and analyzed using Microsoft Excel and SPSS version 28 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp). Descriptive statistics using mean (SD) and frequency (%) were used to describe the data. Data normality was checked using Kolmogorov–Smirnov test. Comparison of two groups was analyzed using independent t-test or Mann–Whitney U-test, while Chi-Square test (or Fisher’s exact test) was used for categorical data. Paired t-test or Wilcoxon test and general linear model (GLM) were applied for repeated measure with follow-up and controlling for covariates. We performed correlation analyses using Pearson correlation and Point Biserial correlation tests. Alpha level of 0.05 was used for all inferential statistical tests.
Results
Study Population
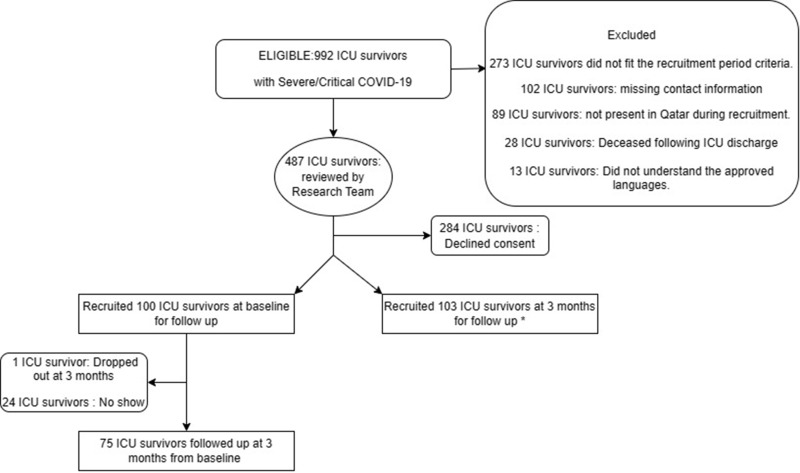
A total of 992 patients were screened with laboratory confirmed COVID-19 disease, who were discharged from our Intensive Care Unit (ICU) from June 1, 2020, to May 31, 2021. One hundred participants who fit the inclusion criteria were enrolled into the study (Figure 1). One patient dropped out of the study in view of relocation back to their home country. Only 75 patients attended the follow-up at 3 months with the rest not attending despite reminders over the phone.
Figure 1.
Flow chart of patient selection and follow up at 3 months.
Demographics and Baseline Characteristics
Table 1 describes the demographic characteristics of the patients. Most of the patients were male (n = 83, 83.0%), with a mean (SD) age of 47.9 (8.4). Most were below 60 years of age (n = 91, 91.0%), Asian (n = 84, 84.0%) and non-Qatari (n = 99, 99.0%). The baseline parameters were categorized into two groups based on intubation. There were significant differences between patients who were intubated versus those who were not for the following baseline parameters: APACHE at 24 hours (p = 0.024), APACHE II death risk % (p = 0.005), number of extrapulmonary organ failure (p < 0.001), prone positioning (p < 0.001), length of stay in the ICU (p < 0.001) and total length of hospital stay (p < 0.001).
Table 1.
Baseline Characteristics of Study Participants
| Baseline Variables | Mean ± SD or n (%) | Intubated (n=20) | Non-Intubated (n=80) | p value** |
|---|---|---|---|---|
| Age | 47.8 ± 8.4 | 47.8 ± 8.1 | 47.9 ± 8.6 | 0.944 |
| Age category | ||||
| Below 60 | 91 (91) | 18 (90.0) | 73 (91.3) | 0.573 |
| 60 and above | 9 (9) | 2 (10.0) | 7 (8.8) | |
| Gender | ||||
| Male | 82 (82) | 17 (85) | 65 (81.3) | 1.000 |
| Female | 18 (18) | 3 (15) | 15 (18.8) | |
| Nationality | ||||
| Qatari | 1 (1.0) | Not applicable | ||
| Non-Qatari * | 99 (99.0) | |||
| BMI | 30.5 ± 5.9 | 28.0 (6.9) | 30.2 (8.7) | 0.613 |
| Smoking | 6(6) | 2 (10) | 4 (5) | 0.597 |
| DM | 56 (56) | 12 (60) | 44 (55) | 0.687 |
| HTN | 44 (44) | 12 (60) | 32 (40) | 0.107 |
| APACHE at 24 hours | 8.5 ± 3.27 | 9.5 (6.8) | 8.0 (4) | 0.024 |
| APACHE II Death risk % | 10.71 ± 5.39 | 12.0 (15) | 8.0 (7) | 0.005 |
| Corticosteroids | 99 (99) | 20 (100) | 79 (98.8) | 1.000 |
| Days Received Corticosteroids | 11.38 ± 4.78 | 10.0 (7.0) | 10.0 (4.0) | 0.256 |
| Muscle relaxants | 98 (98) | 20 (100) | 78 (97.5) | 1.000 |
| Prone Positioning | 27 (27) | 18 (90) | 9 (11.3) | < 0.001 |
| Length of stay in ICU | 10.7 ± 11.4 | 14.0 (8.0) | 7.0 (5.0) | < 0.001 |
| Total Hospital Stay | 19.72 ± 17.54 | 21.0 (19.0) | 14.0 (11.0) | < 0.001 |
Notes: **Independent t-test; others by Mann–Whitney U-test and Chi-Square or Fisher’s exact test. *Arabs:10 (10%) Asian:84 (84%) Other 6 (6%). All significant p values in bold text.
Health-Related Quality of Life SF 36 at Baseline and 3 Months
The SF36 scores were completed by 90 patients at baseline and 68 patients at 3 months follow-up. Due to a language barrier hindering completion of the SF36, 10 individuals were excluded and 22 were lost to follow up. Table 2 shows the general health status SF-36 scores overtime, ie, at baseline and 3-month period. Seven domains (except the emotional wellbeing, p = 0.052) significantly improved during the follow-up period (p < 0.001). The greatest change/improvement was in the domain on role functioning/physical (84.4%). Further analysis using GLM indicated that intubation did not affect any changes in SF-36.
Table 2.
Mean Scores of SF-36 Domains at Baseline, 3 Months Period and Changes Overtime
| Items | At Baseline (Mean ± SD) | At 3 Months | SF 36 Over Time | Intubation Status (Covariate)** |
|---|---|---|---|---|
| (n=90) | (n=68) | p value* | p value | |
| Physical functioning | 59.11 ± 22.08 | 73.97 ± 22.34 | < 0.001 | 0.220 |
| Role functioning/physical | 41.67 ± 41.39 | 76.84 ± 33.55 | < 0.001 | 0.245 |
| Role functioning/emotional | 60.72 ± 42.57 | 79.40 ± 34.09 | < 0.001 | 0.586 |
| Energy/fatigue | 55.48 ± 20.45 | 67.06 ± 18.81 | < 0.001 | 0.539 |
| Emotional well-being | 72.92 ± 19.11 | 77.34 ± 16.83 | 0.052 | 0.586 |
| Social functioning | 64.06 ± 24.51 | 77.93 ± 22.35 | < 0.001 | 0.402 |
| Pain | 68.00 ± 23.69 | 80.69 ± 19.90 | < 0.001 | 0.056 |
| General health | 63.23 ± 18.07 | 73.20 ± 18.33 | < 0.001 | 0.626 |
Notes: *Paired t-test was used at an alpha level of 0.05. **General Linear Model (GLM) was used. All significant p values in bold text.
Spirometry at Baseline and 3 Months
A total of 99 participants underwent spirometry at baseline and 75 at 3 months. One patient at baseline was unable to perform the spirometry despite multiple attempts because of persistent cough. The spirometry measures overtime, and the effect of intubation are depicted in Table 3. The FVC, FEV1% and PEFR were significantly improved after 3 months into the study (p < 0.001). The greatest change/improvement was in FEV1% (158.8%). Further analysis showed that intubation did not affect the spirometry measures over time.
Table 3.
Lung Function: Average Measures and Percentage Predicted of FEV1, FVC, PEFR
| Baseline (Mean ± SD) | 3 Months (Mean ± SD) | PFT Overtime | Intubation Status (Covariate)** | ||
|---|---|---|---|---|---|
| N= 99 | N = 75 | p value | p value | ||
| FVC | L | 2.92 ± 0.97 | 3.12 ± 0.89 | < 0.001 | 0.481 |
| % | 78.27 ± 22.57 | 85.29 ± 22.56 | < 0.001 | 0.410 | |
| FEV1% | L | 2.43 ± 0.74 | 6.29 ± 31.45 | < 0.001* | 0.074 |
| % | 79.12 ± 20.23 | 87.99 ± 18.26 | < 0.001 | 0.415 | |
| FEV1/FVC | L | 84.86 ± 8.35 | 86.36 ± 5.47 | 0.249 | 0.483 |
| % | 107.44 ± 11.11 | 109.00 ± 6.13 | 0.266 | 0.344 | |
| PEFR | 96.72 ± 24.72 | 106.93 ± 21.76 | < 0.001 | 0.245 | |
Notes: *Wilcoxon test was used due to skewed distribution. **General Linear Model (GLM) was used. All significant p values in bold text.
Six-Minute Walk Test at Baseline and 3 Months
The measures of 6MWT at baseline and 3 months of follow-up are illustrated in Table 4. Only the final heart rate and the heart rate 1 min after 6MWT were not significantly changed; other measures, however, showed significant improvement. The biggest change was in the O2 desaturation during the 6 MWT from baseline to 3 months (−52.2%). Intubation as a covariate did not impact any changes in 6MWT outcomes.
Table 4.
6-Minute Walk Test: Mean Values at Baseline and 3 Months Follow Up
| Variables | Baseline (Mean ± SD) | 3 Months (Mean ± SD) | 6MWT Overtime | Intubation Status (Covariate)** |
|---|---|---|---|---|
| N = 100 | N= 76 | p value | p value | |
| Initial heart rate, b/m | 84.28 ± 12.41 | 78.93 ± 12.2 | < 0.001 | 0.417 |
| Final heart rate, b/m | 107.28 ± 17.00 | 104.85 ± 15.34 | 0.160 | 0.316 |
| Δ Heart rate reduced by at 1 min | 17.26 ± 11.07 | 19.16 ± 10.57 | 0.161 | 0.871 |
| Heart rate 1 min post 6MWT, b/m | 89.95 ± 13.34 | 84.99 ± 13.04 | < 0.001 | 0.416 |
| Initial Borg Dyspnea score | 1.35 ± 0.48 | 1.09 ± 0.30 | < 0.001 | 0.429 |
| Final Borg Dyspnea score | 2.47 ± 0.90 | 2.11 ± 0.59 | < 0.001 | 0.427 |
| Initial Borg Fatigue score | 1.23 ± 0.46 | 1.07 ± 0.30 | 0.013 | 0.598 |
| Final Borg Fatigue score | 1.77 ± 0.75 | 1.41 ± 0.62 | < 0.001 | 0.468 |
| Initial SpO2, % | 96.76 ± 1.86 | 97.84 ± 1.07 | < 0.001 | 0.747 |
| Lowest SpO2, % | 93.7 ± 5.0 | 96.3 ± 2.0 | < 0.001 | 0.772 |
| ΔSpO2 | 3.01 ± 3.69 | 1.44 ± 1.85 | < 0.001* | 0.820 |
| Δ SpO2 1 min post 6MWT, % | 96.28 ± 4.58 | 97.92 ± 0.17 | 0.003 | 0.660 |
| 6MWD, m | 386.50 ± 78.45 | 437.22 ± 58.91 | < 0.001 | 0.631 |
Notes: *Wilcoxon test was used due to skewed distribution. **General Linear Model (GLM) was used. All significant p values in bold text.
Correlation Analyses
We performed further correlation analyses using Pearson correlation and Point Biserial correlation tests at alpha level = 0.05. The domains of physical functioning, role functioning – physical, and general health of the SF36 were correlated with all baseline patient variables, FEV1 and FVC, as well as the 6MWT and its domains of oxygen saturation and heart rate. In our analysis, we found no significant correlations. Similarly, a correlation between FEV1 and FVC, as well as six-minute walk distance, oxygen saturation, and heart rate reduction, was performed against all baseline patient characteristics, with no significant correlation found again.
Discussion
In Qatar, around 3.7 per 10,000 COVID-19 cases had severe or critical disease, requiring ICU admission.14 In this paper, we report the short-term outcome data related to health-related quality of life, lung function, and exercise capacity from our prospective cohort study of ICU survivors with COVID-19 (OUTSTRIP COVID-19). Majority of the patients were males in our study representative of the demographics of the country that comprises 76% males.15
Health-Related Quality of Life
When compared to healthy controls in a similar representative sample of Qatar, HRQOL following 7 weeks of ICU discharge was substantially lower in our group as expected in all domains except emotional wellbeing, which was numerically higher.16 When compared to patients with mild COVID-19 from the same study or similar other studies, it was significantly lower in all domains.16–18 The surprising disparity in emotional wellbeing between ICU survivors and the general population could be attributed to a variety of factors, including an increased prevalence of depression, anxiety, stress, psychological distress and post-traumatic stress among the general population during the COVID-19 pandemic, which resulted in lower scores.19 In contrast, the holistic multidisciplinary team care, including psychological support provided to ICU survivors,20 better level of nursing care (1:1 or 1:2 nurse: patient ratio) and different expectations of ICU survivors versus the general population (positive thoughts of surviving a severe illness from a disease with high mortality) might explain the numerically higher scores in survivors.21
At 3 months, the HRQOL improved considerably from baseline in all components except emotional wellbeing and was equivalent to patients with severe COVID-19 reported by Arnold et al.22 However, when compared to ICU survivors from the Dutch23 or the French cohort,24,25 our patient group had fewer role limitations owing to physical health, which might be attributed to our cohort’s lower mean age and comorbidities. Similarly, compared to our sample at 3 months, ICU survivors with non-COVID-19 illness appear to have a lower score in role limitation related to physical, mental, and general health,17,26 which can again be due to our cohort’s younger mean age.
6-Minute Walk Test
As measured by exercise capacity in a 6-minute walk test, physical health improved significantly from baseline to 3 months, with a substantial increase in distance walked, change in oxygen saturation, and Borg dyspnea and fatigue scores for our cohort. We also showed significant improvement in end exercise oxygen saturation during follow-up. This can be explained by previously well-reported improvement of radiological findings of ground glass opacities and parenchymal bands in patients with Severe COVID-19 at 3 months17 to complete radiological resolution in 65% of patients at 6 months in a cohort study of 114 patients with severe COVID-19.27 The distance travelled was comparable to other ICU survivors, however among those who were intubated due to ARDS, the 6-minute walk test was much lower and comparable to SARS and MERS survivors or those with non-COVID-19 ARDS.28,29 If we define the groups as ARDS versus non-ARDS, then the distance walked by those who were intubated versus those who were not is similar to that of non-COVID-19 patients.30 In patients with severe COVID-19, the 6-minute walk distance reported in other studies ranges from 425m ± 94m to 517m ± 44m, with a pooled estimate of 461m,29,31–35 which is numerically higher by more than 30 m compared to our cohort. We were, however, not able to identify any ICU parameter that predicted this exercise impairment.
Pulmonary Function Tests
Lung function improved significantly from baseline to three months in our cohort. In previous trials at 3 months in patients with severe or critical COVID-19, FEV1 ranged from 75% to 110% with a mean estimate of 89.4% (percentage predicted) and FVC from 93% to 64.8 with mean estimates of 83.6%17,32,36,37 comparable to our study.
Impact of Intubation on Lung Function Recovery
Intubation had no significant impact on pulmonary function improvement. This might be explained by the fact that in COVID −19 like intubation, prolonged noninvasive respiratory support is also a risk factor for fibrotic changes on follow-up CT scans.27 The vigorous effort of breathing required by spontaneously breathing patients with acute hypoxic respiratory failure from COVID-19 results in the patients developing - self-inflicted lung injury secondary to the mechanical forces similar to ventilator-induced lung injury.38,39
Study Limitations
Our real-life prospective study has some limitations, including the loss of more than 20% of patients to follow up, which was most likely attributable to our study’s patient mix. Because the majority of patients are not Qataris, there is a significant proportion of expats returning to their home countries. A considerable percentage of patients work in low skilled labor jobs, which makes appropriate follow-up difficult owing to employment obligations and financial constraints.
The absence of extensive pulmonary function evaluations, including lung volumes and diffusion capacity, restricts the evaluation of these measures in this study. Our study also included relatively young people and therefore may not be a true representation for a region with an older cohort of COVID-19 patients. Our study’s strengths include its prospective nature, which represents a real-life cohort of COVID-19 ICU survivors in Qatar and a genuine representation of the population in most regional countries with a large expatriate young male population.
Conclusion
Our study showed that ICU survivors with severe COVID-19 have limitations in quality of life, exercise capacity and lung function following discharge. However, these parameters improve significantly within 3 months of discharge from ICU. We recommend that ICU survivors be followed up on a regular basis in order to identify individuals with poor quality of life and lung function and to address their needs in a timely and efficient way. Long-term follow-up of these individuals may aid in understanding the disease’s long-term course and the needs of our patients.
Funding Statement
Medical Research Center (MRC), Hamad medical Corporation, Doha, Qatar.
Data Sharing Statement
With primary author upon request can be shared.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Omrani AS, Almaslamani MA, Daghfal J, et al. The first consecutive 5000 patients with Coronavirus disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis. 2020;20(1):777. doi: 10.1186/s12879-020-05511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dashboard of COVID-19 in Qatar. Available from: https://www.data.gov.qa/explore/dataset/covid-19-cases-in-qatar/table/?sort=date. Accessed June 15, 2023.
- 3.Herridge MS, Tansey CM, Matté A, et al.; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 4.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 5.Carenzo L, Protti A, Dalla Corte F, et al.; the Humanitas COVID-19 Task Force. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann Intensive Care. 2021;11(1):91. doi: 10.1186/s13613-021-00881-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559. doi: 10.1001/jama.2022.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan E, Song J, Deane AM, Plummer MP. Global impact of coronavirus disease 2019 infection requiring admission to the ICU. Chest. 2021;159(2):524–536. doi: 10.1016/j.chest.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlgren C, Divanoglou A, Larsson M, et al. Rehabilitation needs following COVID-19: five-month post-discharge clinical follow-up of individuals with concerning self-reported symptoms. eClinicalMedicine. 2022;43:101219. doi: 10.1016/j.eclinm.2021.101219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 10.Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society Technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. doi: 10.1164/rccm.201710-1981ST [DOI] [PubMed] [Google Scholar]
- 11.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 13.The surveys from RAND Health Care are public documents. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed June 15, 2023.
- 14.Seedat S, Chemaitelly H, Ayoub HH, et al. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates in Qatar. Sci Rep. 2021;11(1):18182. doi: 10.1038/s41598-021-97606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022; 2022. Available from: https://population.un.org/wpp/. Accessed June 15, 2023.
- 16.Ouanes S, Al-Amin H, Hussein NB, et al. Physical and psychosocial well-being of hospitalized and non-hospitalized patients with COVID-19 compared to the general population in Qatar. Front Psychiatry. 2021;12:792058. doi: 10.3389/fpsyt.2021.792058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines. 2021;9(8):900. doi: 10.3390/biomedicines9080900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nochaiwong S, Ruengorn C, Thavorn K, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep. 2021;11(1):10173. doi: 10.1038/s41598-021-89700-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadoo O, Latoo J, Reagu SM, Amro RAA, Masoodi NA, Alabdulla M. Mental health during COVID-19 in Qatar. Gen Psychiatr. 2020;33(6):e100313. doi: 10.1136/gpsych-2020-100313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apitzsch S, Larsson L, Larsson AK, Linder A. The physical and mental impact of surviving sepsis - a qualitative study of experiences and perceptions among a Swedish sample. Arch Public Health. 2021;79(1):66. doi: 10.1186/s13690-021-00585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment 3 months after recovery from acute Coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;73(5):e1089–e1098. doi: 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerth AMJ, Hatch RA, Young JD, Watkinson PJ. Changes in health-related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia. 2019;74(1):100–108. doi: 10.1111/anae.14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valent A, Dudoignon E, Ressaire Q, Dépret F, Plaud B. Three-month quality of life in survivors of ARDS due to COVID-19: a preliminary report from a French academic centre. Anaesth Crit Care Pain Med. 2020;39(6):740–741. doi: 10.1016/j.accpm.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Wu J, Hao S, Yang M, Lu X, Chen X. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci Rep. 2017;7(1):17275. doi: 10.1038/s41598-017-17497-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry SM, Nalamalapu SR, Nunna K, et al. Six-minute walk distance after critical illness: a systematic review and meta-analysis. J Intensive Care Med. 2021;36(3):343–351. doi: 10.1177/0885066619885838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed H, Patel K, Greenwood DC. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. doi: 10.2340/16501977-2694 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed H, Patel K, Greenwood D, et al. Long-term clinical outcomes in survivors of coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis of follow-up studies. MedRxiv. 2020;9:99. [DOI] [PubMed] [Google Scholar]
- 31.Wong AW, López-Romero S, Figueroa-Hurtado E, et al. Predictors of reduced 6-minute walk distance after COVID-19: a cohort study in Mexico. Pulmonology. 2021;27(6):563–565. doi: 10.1016/j.pulmoe.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González J, Benítez ID, Carmona P, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eksombatchai D, Wongsinin T, Phongnarudech T, et al. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: a prospective cohort study. PLoS One. 2021;16(9):e0257040. doi: 10.1371/journal.pone.0257040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truffaut L, Demey L, Bruyneel AV, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22(29). doi: 10.1186/s12931-021-01625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Gassel RJJ, Bels JLM, Raafs A, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203(3):371–374. doi: 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battaglini D, Robba C, Ball L, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth. 2021;127(3):353–364. doi: 10.1016/j.bja.2021.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver L, Das A, Saffaran S, et al. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: a computational modelling study. Ann Intensive Care. 2021;11(1):109. doi: 10.1186/s13613-021-00904-7 [DOI] [PMC free article] [PubMed] [Google Scholar]