Abstract
Premature ovarian insufficiency (POI) is an uncommon cause of infertility in females characterized by hypoestrogenism. Studies have shown that uterine artery embolization (UAE) is associated with POI. Meanwhile, Asherman syndrome (AS) is a rare condition because of intracervical or intrauterine adhesions, which can happen after dilation and curettage. Both these syndromes are causes of amenorrhea and infertility. This case is of a 40-year-old woman who, after cesarean scar pregnancy and subsequent UAE because of uncontrollable vaginal bleeding, developed premature ovarian failure and AS. She underwent hysteroscopic adhesiolysis. She became pregnant with low anti-Müllerian hormone levels. Initial adhesiolysis and intervention in AS can restore uterine endometrium’s ability to host a fetus. Moreover, UAE can cause POI, which might regress to some degree.
INTRODUCTION
Premature ovarian insufficiency (POI) is an uncommon etiology of infertility in females that is characterized by hypoestrogenism, elevated gonadotropin levels and amenorrhea in 1% of women under 40 years of age [1, 2]. Anti-Müllerian hormone (AMH) level is a marker of ovarian reserve and hence an indicator of POI [3]. AMH is produced by the growing follicles and declines with age, becoming undetectable at menopause [2, 3]. Women with POI experience menopause symptoms such as night sweats, hot flush, low sexual desire, hair loss and vaginal dryness [1, 2]. The difference between POI and menopause is that ovarian failure is not permanent in POI, and pregnancy can still occur in 5–10% of cases [4]. Moreover, studies have shown that uterine artery embolization (UAE) severely impacts ovarian reserve and, subsequently, fertility [5–7].
Asherman syndrome (AS) is a rare condition because of intracervical or intrauterine adhesions [8, 9]. The gold standard for the treatment and diagnosis of AS is hysteroscopy [9]. Women with AS experience amenorrhea, hypomenorrhea and recurrent pregnancy loss or infertility [9]. Causes of AS include dilation and curettage, incomplete or missed miscarriage, or treating retained placenta. Both premature ovarian failure and AS are the causes of amenorrhea and infertility [1, 2, 8, 9].
In this case study, a successful pregnancy in a patient who suffered from POI and AS after cesarean scar pregnancy (CSP) is reported.
CASE PRESENTATION
The case is of a 40-year woman who presented to the emergency department in 2015 because of severe vaginal bleeding. At this time, she was Para 1 + 2. She had one living child (born by cesarean section) and two miscarriages. At the time, she was on her third gravidity, one parity and had a living child. According to her last menstrual period, she was 10 weeks into pregnancy and using an ultrasonographic evaluation, the diagnosis of caesarian scar pregnancy was confirmed, although this scar pregnancy was misdiagnosed before being admitted. The severe bleeding indicated suction and curettage; however, a Bakri balloon was placed in as the bleeding could not be stopped. The following morning the balloon was removed, and the patient underwent UAE to stop the bleeding fully. One month after the pregnancy, beta human chorionic gonadotropin concentrations became untraceable. Nine months after the incident, the patient presented to the clinic again with amenorrhea. This peculiar event led to obtaining a hysterosalpingography and laboratory evaluation of the AMH and follicle-stimulating hormone (FSH; 25.2 IU/mL) and luteinizing hormone (LH; 15.2 IU/mL) levels. As the AMH level was low (0.1 ng/mL), we suspected POI and repeated tests within 6 weeks, and the new test results were as follows: FSH (35.4 IU/mL), LH (20.1 IU/mL) and AMH (0.01 ng/mL). Hysterosalpingography was suggestive of total AS (Fig. 1). The first phase of treatment included the hysteroscopic removal of adhesions within the uterine cavity (in 2017). Lower segment adhesions were removed, fundal adhesions were also excised and the left ostium was exposed. It was decided that the patient should undergo another hysteroscopy after hormonal replacement therapy. However, as the patient was frustrated and disappointed about future pregnancies because of likely subfertility, she declined the second hysteroscopy. Within a year of surgery, she started to experience irregular episodes of bleeding again. Her AMH levels were rechecked and had increased to 0.45 ng/mL. She stated that the status of her menstrual periods had become markedly better and that she had experienced irregular episodes of hypomenorrhea. Therefore, the AMH evaluation showed that the levels had increased to 0.45 ng/mL, and the FSH level was 3.0 IU/mL, which was a sign of POI regression. Moreover, ultrasonographic imaging showed that the uterine cavity was free from adhesions (Fig. 2). Three years following her hysteroscopy, she became pregnant again, and an ultrasound scan confirmed that the pregnancy was viable and intrauterine (in 2020). As another episode of pregnancy was suspected, a transvaginal sonographic assessment was carried out, which showed that the gestational sac was not in the cesarean scar site. Albeit with such levels of Asherman involvement, it was assumed that the placenta could become adherent. The placenta showed only some mild levels of placenta accreta. During the pregnancy, the patient had been treated with dydrogesterone as luteal phase support up to the 12th week of pregnancy, after which progesterone suppositories were initiated. Two weeks after the start of the second trimester (15th week of gestation), on the 37th week of pregnancy, the patient underwent an elective cesarean section because of previous cesarean section and the evident placenta accreta (neither on the scar nor previa). The newborn was successfully delivered. However, the placenta was markedly adhesive to the uterine cavity’s fundal site, and the uterus was removed (on the same surgical episode) with an abdominal hysterectomy (Fig. 3).
Figure 1.
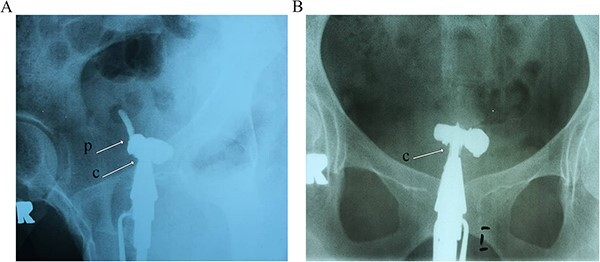
Hysterosalpingography evaluation of the patient before surgical evaluation shows that the radiocontrast agent has covered the cervix and isthmus. However, it has entered a pouch-like space in the middle part, but neither the uterus nor the fallopian tubes are visible. Arrow C: cervix, Arrow P: pouch.
Figure 3.

The uterus during surgery shows marked placental adhesion to the uterine cavity’s fundal site and hypervascularity.
Figure 2.

The follow-up 1 year after surgery transvaginal 3D ultrasonography shows normal uterine myometrium and endometrium with normal echogenicity, whereas no lesions, including polyps or adhesion bands, are seen. Arrow M: myometrium, E: endometrium, C: cervix, U: uterus.
DISCUSSION
Ectopic pregnancy (EP) is defined as the implantation of fertilized egg either outside the uterine cavity. Risk factors include previous EP, intrauterine devices, contraceptives and pelvic inflammatory diseases. While the most common form of EP is tubular, CSP is also a prevalent type [10, 11]. This specific type of EP is usually managed with a combination of methotrexate and surgical approaches such as dilation and curettage [10, 11]. The presented case was of a woman who suffered from misdiagnosed CSP and uncontrollable vaginal bleeding; hence, she underwent a curettage to terminate the pregnancy and stop the bleeding. However, we used a Bakri balloon and UAE to preserve the uterus.
AS is defined as the formation of scar tissue and intrauterine adhesions or intrauterine synechiae formation inside the uterine cavity [8, 9, 12]. The most common causes are endometrial damage during surgeries such as D&C, infections like tuberculosis and congenital anomalies. It usually presents itself with menstrual abnormalities and infertility [8, 9]. The most accurate method of diagnosis is hysteroscopy, but ultrasonographic imaging can also be helpful [9, 12]. Depending on the scale of involvement and the severity of adhesions, this incidence can be managed either surgically with adhesiolysis or expectantly [8, 9, 12]. This case was a complete Asherman (grade IV), and we decided to carry out surgical intervention.
Meanwhile, premature ovarian failure (POI) is an uncommon etiology of infertility in females characterized by hypoestrogenism, elevated gonadotropin levels and amenorrhea in 1% of women under 40 years of age [1–3]. Although pregnancy is a rare incidence among these patients, it is not unheard of [4, 13]. UAE, in many instances, has been reported to have decreased ovarian reserve; it is presumed that the decrease in ovarian perfusion as a consequence of embolization in the uterine artery, which provides a portion of the overall blood supply to ovaries might cause this [5, 7, 14]. Interestingly, a transient form of ovarian failure could happen in cases of UAE, as did our patient [5–7, 14]. In our case, we only resorted to this method only after uncontrollable uterine bleeding [5–7].
CONCLUSION
This case was of a woman who developed AS after the treatment for CSP. Despite extremely low initial AMH levels, the patient became pregnant. We assume that the initial hysteroscopic adhesiolysis made the endometrium suitable for implantation. This case highlights that appropriate surgical and medical intervention can improve the chances of future fertility. We assume that AS was developed because of extensive endometrial manipulation and embolization. POI was developed in this case because of UAE, and this risk has been well-reported in previous studies.
ACKNOWLEDGEMENTS
None.
Contributor Information
Azadeh Tarafdari, Department of Obstetrics and Gynecology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
Fahimeh G Vahdani, Department of Obstetrics and Gynecology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
Alireza Hadizadeh, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran; Research Centre for Advanced Technologies in Cardiovascular Medicine, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
Sahar Khoshravesh, School of Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran.
Shiva Hadizadeh, Women Reproductive Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
DECLARATIONS
Ethics approval and consent to participate
This study was approved by the research and ethics committee of the Tehran University of Medical Sciences. The patient’s family has given their informed consent to publish this case.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
DATA AVAILABILITY
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
AUTHOR CONTRIBUTIONS
A.H. and A.T. contributed to developing the research idea and composing and revising the manuscript. F.G.V. contributed to composing and revising the manuscript. S.K. and S.H. contributed to developing the research idea and revising the manuscript.
REFERENCES
- 1. Rebar RW. Premature ovarian failure. Obstet Gynecol 2009;113:1355–63. [DOI] [PubMed] [Google Scholar]
- 2. Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005;11:391–410. [DOI] [PubMed] [Google Scholar]
- 3. Anderson RA, Nelson SM. Anti-Müllerian hormone in the diagnosis and prediction of premature ovarian insufficiency. Semin Reprod Med 2020;2020:263–9. [DOI] [PubMed] [Google Scholar]
- 4. Asbagh FA, Ebrahimi M. A case report of spontaneous pregnancy during hormonal replacement therapy for premature ovarian failure. Iran J Reprod Med 2011;9:47. [PMC free article] [PubMed] [Google Scholar]
- 5. Czuczwar P, Stępniak A, Wrona W, Woźniak S, Milart P, Paszkowski T. The influence of uterine artery embolisation on ovarian reserve, fertility, and pregnancy outcomes–a review of literature. Prz Menopauzalny 2016;15:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod 2007;22:1996–2005. [DOI] [PubMed] [Google Scholar]
- 7. Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. J Vasc Interv Radiol 2013;24:459–67. [DOI] [PubMed] [Google Scholar]
- 8. Baramki TA, Alexander CJ. Asherman's Syndrome, 2004.
- 9. Smikle C, Yarrarapu SNS, Khetarpal S. Asherman Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448088/ (27 Jun 2022, date last accessed). [PubMed]
- 10. Morlando M, Buca D, Timor-Tritsch I, Cali G, Palacios-Jaraquemada J, Monteagudo A et al. Reproductive outcome after cesarean scar pregnancy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2020;99:1278–89. [DOI] [PubMed] [Google Scholar]
- 11. Petersen KB, Hoffmann E, Larsen CR, Nielsen HS. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958–67. [DOI] [PubMed] [Google Scholar]
- 12. Guo EJ, Chung JPW, Poon LCY, Li TC. Reproductive outcomes after surgical treatment of Asherman syndrome: a systematic review. Best Pract Res Clin Obstet Gynaecol 2019;59:98–114. [DOI] [PubMed] [Google Scholar]
- 13. Alper MM, Jolly EE, Garner PR. Pregnancies after premature ovarian failure. Obstet Gynecol 1986;67:59S–62. [DOI] [PubMed] [Google Scholar]
- 14. Amato P, Roberts AC. Transient ovarian failure: a complication of uterine artery embolization. Fertil Steril 2001;75:438–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


