The review identifies signaling and cell biology processes selectively induced by mechanical compressive forces on normal or pathological tissues such as tumors for the development of novel treatments.
Abstract
In living organisms, cells sense mechanical forces (shearing, tensile, and compressive) and respond to those physical cues through a process called mechanotransduction. This process includes the simultaneous activation of biochemical signaling pathways. Recent studies mostly on human cells revealed that compressive forces selectively modulate a wide range of cell behavior, both in compressed and in neighboring less compressed cells. Besides participating in tissue homeostasis such as bone healing, compression is also involved in pathologies, including intervertebral disc degeneration or solid cancers. In this review, we will summarize the current scattered knowledge of compression-induced cell signaling pathways and their subsequent cellular outputs, both in physiological and pathological conditions, such as solid cancers.
Introduction
Shear, tension, and compression are ubiquitous mechanical forces exerting physiological responses on cells. Shearing force corresponds to the application of a force that is parallel to cell surface; the force applied to cells is perpendicular and directed away from cell surface for tensile force, and perpendicular and directed toward cell surface for compressive force (Fig 1A). Mechanical forces are sensed by mechanosensors that activate biochemical effectors in signaling pathways (see Glossary). This process is called mechanotransduction (Di-Luoffo et al, 2021). Among them, signaling pathways and cellular responses induced by compressive forces are so far the least understood mechanism. We know that shearing and tensile forces lead to different mechanotransduction signaling, especially leading in the activation of different PI3K classes of enzymes and PI3K isoforms (reviewed in Di-Luoffo et al [2021]). Similarly, this difference in term of signal activation patterns is found between stretching and compressive forces applied on cells (Haudenschild et al, 2009; Takemoto et al, 2015; Nordgaard et al, 2022). This differential pathway activation has physiological implications: healthy cells such as osteoblast precursors and periodontal ligament fibroblasts produce and secrete different proteins depending on which mechanical stress they undergo (He et al, 2004; Zhong et al, 2013; Takemoto et al, 2015). To better understand the importance of mechanical forces in biological processes, there is thus a need to discriminate the selective contribution of compressive forces in activating biochemical pathways. Biological processes in vivo are subjected simultaneously to all three types of mechanical forces; disentangling the relative contribution of each physical force in cell processes is thus a complex task. To model in vitro the application of compressive forces to mammalian cells in 2D or in 3D, different methods are available. The use of those methodological approaches is expanding in the cell and tumor biology fields, described in detail in Fig 1B–D. Here, we reviewed the cellular effects of mechanical load (Fig 1B), variation in osmotic and interstitial fluid pressure because of the accumulation of hydrophilic hyaluronic acid in extracellular matrix (Fig 1C) and growth pressure in a confined environment such as rigid matrix (Fig 1D and E), all situations that mimic in vivo settings.
Figure 1. Experimental designs used to mimic in vitro compressive force generation in 2D and 3D cell cultures.
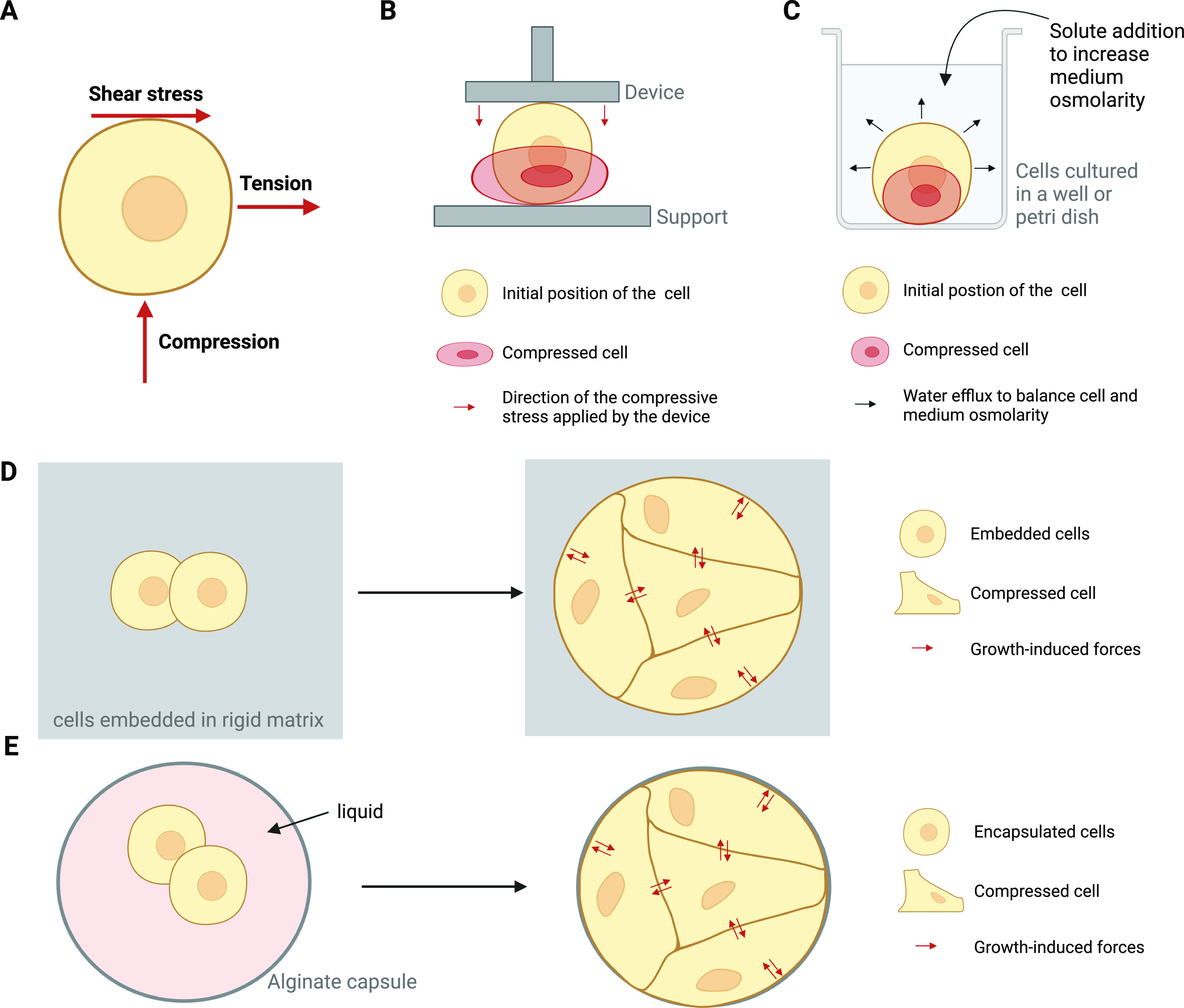
(A) Scheme representing how compression, tension, and shearing forces are applied to the cell membrane. Red arrows represent the direction of the corresponding force applied to the cell. Several methods are used in vitro to reproduce compressive forces that are found in vivo. (B) The compressive force applied can be static (i.e., applied one time during a defined period) or cyclic (i.e., applied in cycles of compression) using the following methods: (B) compression induced by a piston filled with an adjustable weight (static) or by a transmembrane pressure device using compressed gas to press a piston towards the cells (cyclic). Here, only 2D compression is shown; however, compression of 3D multicellular structures can be achieved by those methods. (C) Compression induced by hyperosmotic shock. For example, addition of polyethylene glycol-300 to cell media increases cell media osmolarity resulting in water efflux from cell to media to equilibrate osmolarity and thus causing cell compression. (D) Confined growth of cells or of multicellular structures in rigid matrix (>1 kPa) mimics the buildup of growth-induced compressive forces that are mostly found during tumor development. Refinement of those approaches can be achieved by using gels that can relax and mimic the viscoelastic properties of extracellular matrix. The rigid matrix can also be functionalized to mimic ECM composition and the concomitant biochemical activation through protein/protein interaction of confined cells. (E) Confinement of cells in rigid capsules. They can grow as a mass inside the capsule or line the alginate capsule; the latter mimics the formation of simple epithelium. The described experimental devices were used in the following references (Tse et al, 2012; Guo et al, 2017; Kalli & Stylianopoulos, 2018; Kim et al, 2019; Boyle et al, 2020; Kang et al, 2020; Nia et al, 2020; Li et al, 2021b; Ge et al, 2021).
Sensing of compressive forces occurs at various locations in cells (Fig 2A). Mechanotransduction happens in the plasma membrane or in the actin cortical cortex. Nucleus deformation induces biochemical pathways simultaneously or in a sequential manner. Molecular or organelle crowding in cytoplasm participates in the sensing of compressive forces (Guo et al, 2017). Next, compressive forces induce different cellular outputs, ranging from proliferation to cell death (Li et al, 2017; Boyle et al, 2020; Kang et al, 2020; Lin et al, 2021) (Fig 2A–C). The direct application of compressive forces promotes or reduces cell proliferation, survival, and differentiation; it promotes cytoskeleton remodeling, cell motility, and cell migration; it controls cell metabolism. All these cell processes participate to tissue homeostasis. Compressive forces also act indirectly via autocrine or paracrine signaling (Fig 2B and C). Paracrine and autocrine action involves the regulation of secreted cytokines, chemokines (Schreivogel et al, 2019), matrix components (Wright et al, 1997; He et al, 2004; Chowdhury et al, 2006; Fitzgerald et al, 2008; Zhong et al, 2013; Liu & Lee, 2014; Takemoto et al, 2015; Luo et al, 2022), metabolites (McCutchen et al, 2017), and the control of membrane receptor recycling (Baschieri et al, 2020), and cell/cell adhesion (Park & Tschumperlin, 2009; Eisenhoffer et al, 2012; Delarue et al, 2017; Massey et al, 2020; Di Meglio et al, 2022).
Figure 2. General overview of compression mechanotransduction in human cells.
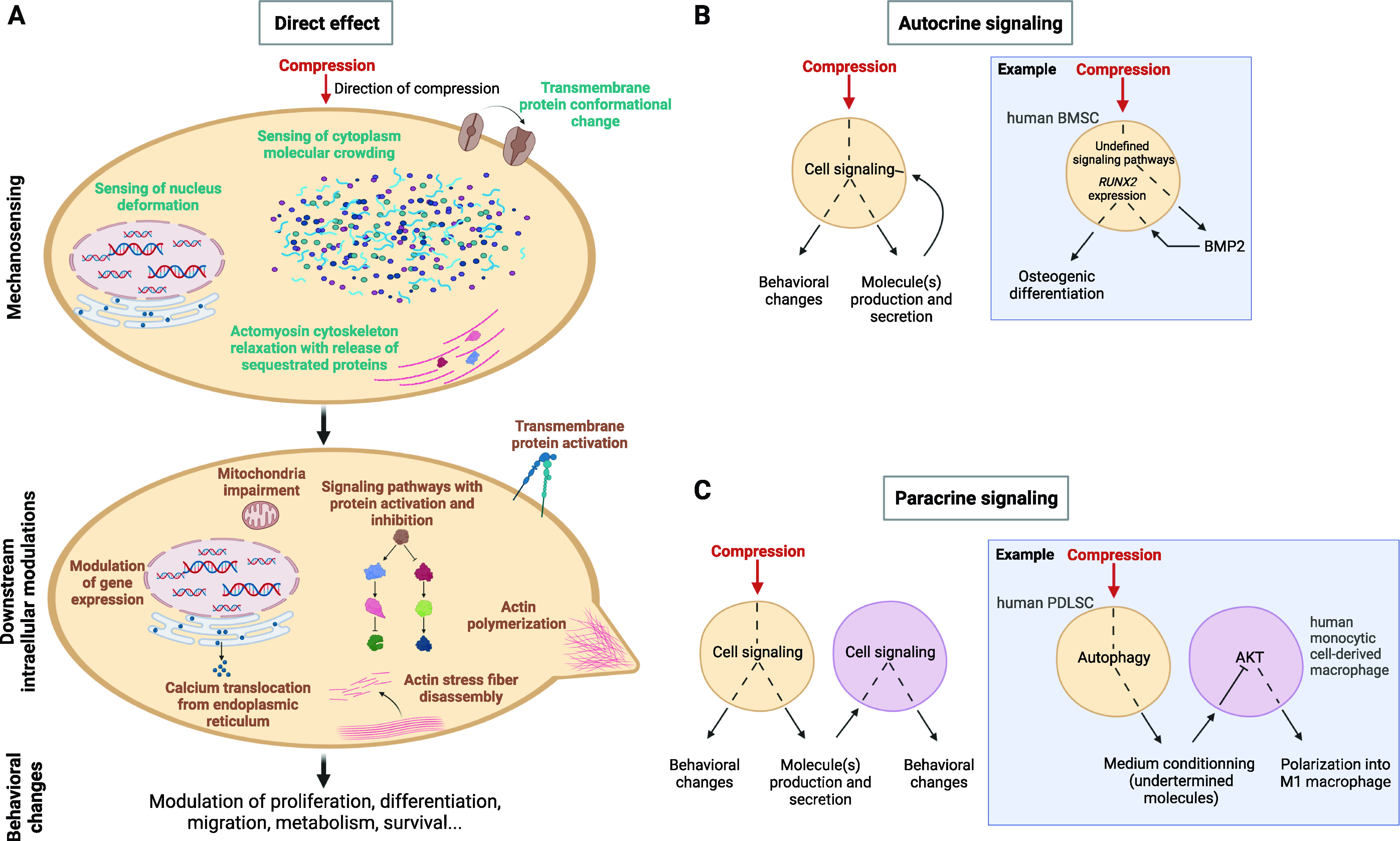
(A) Direct compressive effect on the cell. Supposed mechanosensors and downstream modulations are displayed. This list is not exhaustive (Wright et al, 1997; Park & Tschumperlin, 2009; Eisenhoffer et al, 2012; Liu & Lee, 2014; Chronopoulos et al, 2020; Lomakin et al, 2020; Massey et al, 2020; Park et al, 2020). (B) Compressive stress-induced autocrine signaling. (C) Compressive stress-induced paracrine signaling.
Physical compressive forces thus control various biological processes but are also involved in pathologies in humans. In some cases, excessive compression forces lead to pathologies. It is the case of intervertebral disc degeneration (Pauly et al, 2001). During solid cancer development, the tumor growth leads to generation of compressive forces; this phenomenon modifies the tumor and microenvironment cell behavior in return (Kim et al, 2017, 2019; Morikura & Miyata, 2019). It is so far unclear whether normal, cancer, and tumor microenvironment cells respond differently to compressive forces; we will review the recent data that concur to demonstrate that this response is actively promoting cancer development, progression, and resistance to treatment.
In summary, this review synthetizes by which cell signaling pathways’ compression induces cellular phenotypes in mammalian cells. We will also discuss the importance of compression in health and disease with a particular focus on cancer.
Cellular outputs modulated by compression in tissue homeostasis
Homeostatic control of cell numbers in tissues: compression increases cell proliferation of some mesenchymal cells and decreases cell proliferation or extrudes cells in most epithelia.
Cell context determines whether compression promotes or represses cell proliferation. Static (i.e., applied one time during a defined period) or cyclic (i.e., applied in cycles of compression) compressive forces (in 10 of kPa range) increase proliferation of some mesenchymal cells in a direct manner. Compression increases proliferation of rat bone marrow-derived mesenchymal stem cells (rat BMSCs) and rat chondrocytes (Ren et al, 2011; Wang et al, 2013; Boyle et al, 2020). Compression on chondrocytes triggers Ca2+ signaling by activating mechanosensitive ion channels such as Piezo-1 and Piezo-2, two distinct members of stretch-activated channel (SAC) family (Coste et al, 2010; Han et al, 2012; Liu & Lee, 2014). Consequently, transmembrane proteins such as α5β1 integrin are activated (Wright et al, 1997; Chowdhury et al, 2006; Raizman et al, 2010; Liu & Lee, 2014). Proliferative signal is dependent on RHOA and ROCK signaling, and BMP signaling and SRC-induced MAPK/ERK pathway (Ren et al, 2011; Wang et al, 2013; Boyle et al, 2020). In response to compression, an interplay between several pathways is occurring, as an inhibition of a single signal node is not sufficient to fully block the compression-induced proliferation.
In most epithelial cells, it is well established that cell proliferation is triggered by increased mechanical tension (Uroz et al, 2018) and inhibited by compression (Alessandri et al, 2013; Delarue et al, 2014; Dolega et al, 2017). MDCK-II cell epithelial monolayer growing under confinement accumulates pressure that inhibits proliferation (Di Meglio et al, 2022). To maintain tissue homeostasis and control cell number, overcrowding results in live cell extrusion in the lumen. It requires sphingosine 1-phosphate G protein coupled receptor signaling and RHO-kinase-dependent myosin contraction. Compared with other types of cell extrusions, this selective process is distinguished by a signaling through SACs (Piezo-1) (Eisenhoffer et al, 2012). Besides, during epithelium growth, epithelial cells spontaneously buckle (Trushko et al, 2020), and cell proliferation is transiently reactivated within the fold. Whereas compressive force-induced blockage of cell proliferation is dependent on GSK-3β (glycogen synthase kinase 3β) and the β-catenin transcriptional activator signaling pathways (Song et al, 2017; Di Meglio et al, 2022), reactivation of proliferation within folds correlates with the local reactivation of the mechano-sensing YAP/TAZ pathway through curvature sensing (Di Meglio et al, 2022). Other mechanisms that sense epithelium curvature were described and involve nuclear mechanosensing (Luciano et al, 2021). However, activation of YAP/TAZ remains the most well-described mechanosensing signaling process after mechanical and especially tensile stress (Dasgupta & McCollum, 2019; Cobbaut et al, 2020; Cai et al, 2021). YAP and TAZ transcription coactivators are oncoproteins repressed through their phosphorylation by the tumor suppressor LATS1/2 (large tumor suppressor kinases 1 and 2) controlled by the kinases MST1/2 (macrophage stimulating 1 and 2), mammalian homologs of the Hippo kinase (Dasgupta & McCollum, 2019; Cobbaut et al, 2020; Cai et al, 2021).
Control of (stem) cell differentiation
Current research highlights a direct relationship between cellular physical property and (stem) cell fate decision, potentially contributing to organ homeostasis and development. Volumetric compression alone induced by either osmotic, mechanical or matrix rigidity controls stemness and intestinal organoid growth; it activates pro-tumoral pathways such as Wnt-activating β-catenin signaling (Li et al, 2021a).
In bone remodeling, compression forces promote cell differentiation. Prolonged dynamic compression promotes the chondrogenic differentiation of human synovium-derived mesenchymal stem cells in the presence of the transforming growth factor β3 (Ge et al, 2021), describing a physiologically relevant mechanism of stem cell-based cartilage repair and regeneration. Similarly, long-term mechanical load potentiates the osteogenic differentiation of human BMSCs in collagen microtissues (Song et al, 2017; Li et al, 2020). Compression promotes differentiation of mesenchymal cells and their production of collagen matrix, a process that participates in bone healing and remodeling (Wright et al, 1997; He et al, 2004; Chowdhury et al, 2006; Fitzgerald et al, 2008; Zhong et al, 2013; Liu & Lee, 2014; Takemoto et al, 2015).
Mechanistically, different signaling pathways such as the activation of PI3K/AKT or inhibition of MAPK/ERK signaling promote the compression-induced osteogenic differentiation of BMSCs (Pelaez et al, 2012; Song et al, 2017). In response to compression, the PI3K pathway is activated and increases the β-catenin expression level which is involved in osteogenic differentiation of BMSCs (Song et al, 2017). Compression also triggers cell differentiation through paracrine regulations between cell types involved in bone regeneration. Cyclic compressive forces, which mimic compression found in bone healing, enhance production and secretion of BMP2 by human BMSCs that stop their migration. Furthermore, secreted BMP2 is required for the expression of the RUNX2 osteogenic gene (Schreivogel et al, 2019).
Finally, active response to compressive forces is needed for cell differentiation in homeostatic conditions (Nordgaard et al, 2022). In skeletal muscle, the contraction of individual muscle fibers activates p38 MAPK and JNK activation. Osmotic shock and mechanical compression, but not stretching of skeletal muscle cells, selectively activate upstream the ubiquitously expressed but poorly described MAP3K splice form ZAKβ. ZAKβ is necessary for the proper function of skeletal muscle fibers during contraction; its activation is required to prevent muscle pathology (Nordgaard et al, 2022).
Promotion of cytoskeleton rearrangement, cell motility, and migration
Compression induces cell signaling to promote cytoskeleton reorganization, cell motility, and migration. In HEK293 cells, compression promotes the activation of RHOA and ROCK signaling critical for actin cytoskeleton remodeling and cell motility (Boyle et al, 2020). Moreover, bronchial primary epithelial cells transition from a nonmigratory to a migratory phenotype upon compression (De Marzio et al, 2021). Fibrous matrix of collagens helps the cells to migrate along the fibers (Hogrebe et al, 2017), production that is favored by compression (Wright et al, 1997; He et al, 2004; Chowdhury et al, 2006; Fitzgerald et al, 2008; Zhong et al, 2013; Liu & Lee, 2014; Takemoto et al, 2015). Compression promotes key cellular processes involved in migration such as formation of lamellipodia and adhesion to extracellular matrix of human BMSCs cultured in collagen matrices (Li et al, 2020; Lim Lam et al, 2021). This process also plays a key role in the function of immune cells or platelet cells that sense compression when they are colliding in blood vessels or in tissues (Toyjanova et al, 2015). Cytoskeleton rearrangement is also occurring as a protective mechanism to physiological high compressive load. Microtubules can bear compressive loads, which is consistent with models for cellular mechanics in which microtubule compression helps to stabilize cell shape by balancing tensional forces within a prestressed cytoskeleton (Wang et al, 1993); this cytoskeleton rearrangement is particularly relevant for cardiomyocytes subjected to constant contractile compressive stresses (Brangwynne et al, 2006).
Emerging evidence in control of metabolism
Compression modulates the cell metabolism that sustains cell behaviors. Primary human chondrocytes exposed to compression which mimics their natural mechanical load present up-regulation or down-regulation of specific metabolic transcriptional signatures (McCutchen et al, 2017). Nicotinamide metabolism, whose gene signature is down-regulated by compression, is notably essential to produce coenzymes used in glycolysis (McCutchen et al, 2017). The metabolic measurements are, for the moment, too limited to confirm those transcriptomic results. This is an important future field of research as, interestingly, disassembly of actin cytoskeleton network occurs during compression through RHO and ROCK signaling (de Araujo et al, 2014) and could lead to a decrease in glycolysis rate. Indeed, mechanically induced fragmented actin promotes a decreased glycolysis (Park et al, 2020).
Emerging evidence in control of intercellular communication
A new field of research that could expand within the next years is the study of how compressed cells affect their less compressed or uncompressed neighbors through paracrine signaling (Fig 2C). Orthodontic tooth movement generates both tensile and compressive forces. Periodontal ligament stem cells (PDLSCs) are the main mesenchymal stem cells in periodontal tissues (Jiang et al, 2021). Depending on their location in the periodontal tissue, PDLSCs will sense either tension or compression during orthodontic tooth movement (Wise & King, 2008). In vitro compression activates autophagy in PDLCs prompting them to secrete a conditioned medium able to inactivate the AKT signaling of macrophages in a paracrine manner. This causes the polarization of the macrophages into M1 macrophages which next act on bone remodeling and root resorption (Jiang et al, 2021).
Cell compression in pathological conditions
Pathological context involving compression forces
Compressive forces are involved in a large number of pathologies. Asthma is a pathology whose development is associated to sensing of compressive forces. Through a paracrine signaling, the compressed primary human bronchial epithelial cells produce and secrete a vasoconstrictor mediator (Endothelin-1) which acts on the primary human airway smooth muscle cells (HSAM cells) to increase their proliferation and their basal and histamine-induced contractions. Thus, the HSAM cells are more prone to a future bronchoconstriction leading to a deleterious positive feedback loop for which each bronchoconstriction promotes the next one (Lan et al, 2018). Surface glycoproteins such as MUC family members whose level in the plasma membrane is changed in bronchial epithelium upon compression further participate in mechanosensing of compression (Park & Tschumperlin, 2009; Eisenhoffer et al, 2012; Delarue et al, 2017; Massey et al, 2020).
Compression controls both establishment and progression of osteoarthritis, which is a disease characterized by joint inflammation. Thereby, mechanical load induces both human chondrocyte degeneration and cartilage vascular invasion, causing and worsening the pathology, respectively. These effects might be partially mediated by a compression-induced decrease in the activation of TIMP3/TGF-β1 pathway in the compressed chondrocytes (Zhao et al, 2020).
A prolonged high cerebrospinal fluid pressure in the skull causes intracranial hypertension. Compression of rat cortical neurons that mimics this situation reveals that mitochondrial dysfunction and ER stress occur and cause cell death (Chen et al, 2019). Finding ways to target these causes of neuronal cell death would enable to treat patients suffering from intracranial hypertension.
Intervertebral disc degeneration induced by excessive compression is caused by massive cell death
The study of the multistep processes leading to pathologies highlights other cellular outputs induced by excessive compressive forces. Intervertebral disc degeneration (IVDD) is a pathology caused by an inappropriate mechanical load on the intervertebral discs leading especially to low back pain (Kang et al, 2020). Intervertebral discs are formed of the peripheral annulus fibrosus, the cartilage endplate, and the central gelatinous nucleus pulpous (NP), which is the most important part for maintaining the homeostasis of these three structures (Fig 3A). Indeed, NP is made of cells producing NP matrix proteins (aggrecan and collagen II) that enable the mechanical functioning of each disc (Han et al, 2017).
Figure 3. Effects of compression on cell signaling and death-related cellular outputs of nucleus pulposus cells.
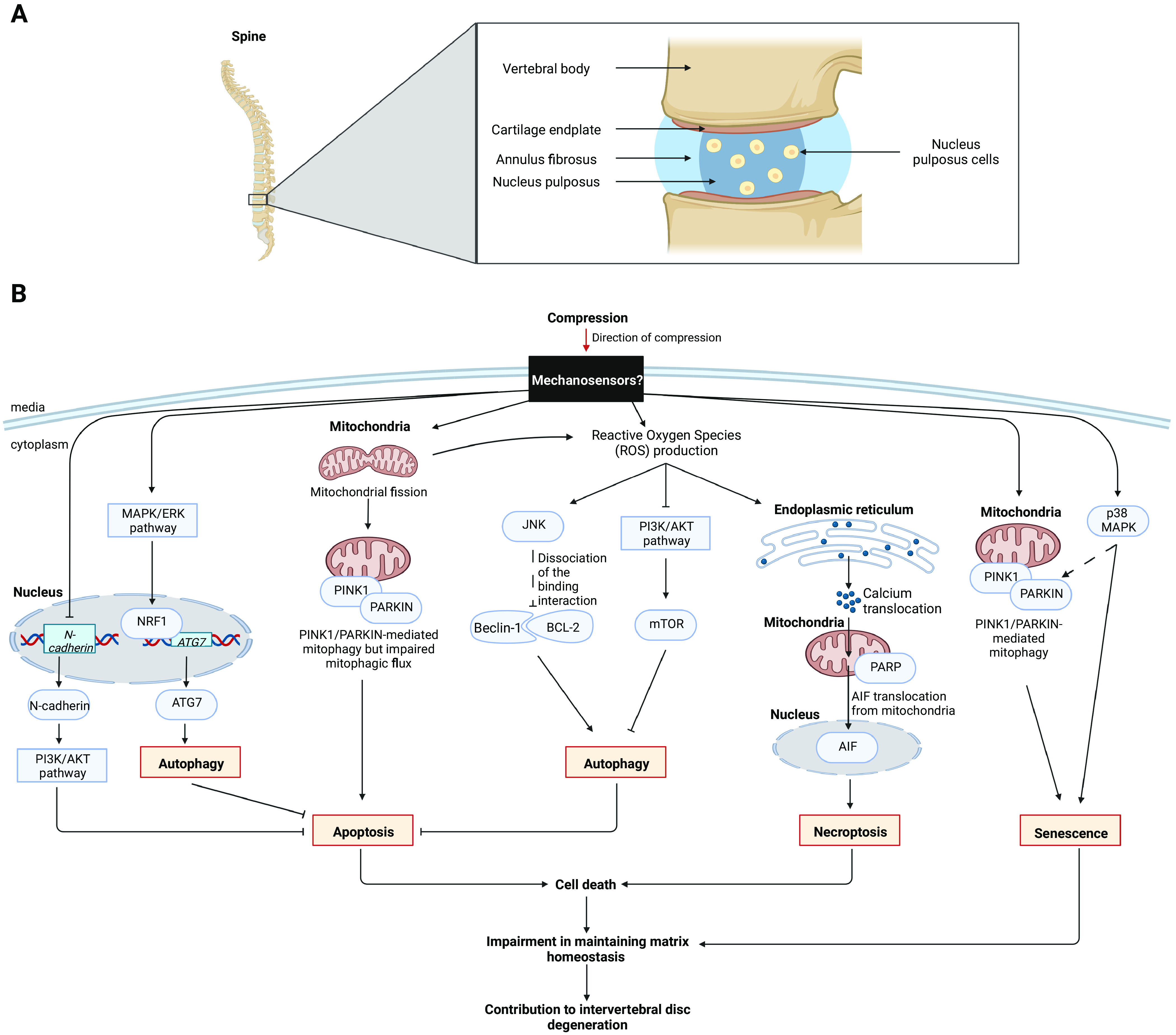
(A) Intervertebral disc structure. (B) Effects of compression in nucleus pulposus cells. Signaling pathways involved after compressive stress in nucleus pulposus cells. The activation of signaling pathways tightly regulate the balance between autophagy, cell death (apoptosis, necroptosis), and senescence in cells. The figure compiles data from Ma et al (2013), Li et al (2017, 2018, 2021b), Huang et al (2020), and Lin et al (2021). Undefined modulations are presented as dotted arrows. AIF, apoptosis-inducing factor; ATG7, autophagy-related protein 7; BCL-2, B-cell lymphoma 2; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; N-cadherin, neural cadherin; NRF1, nuclear respiratory factor 1; p38 MAPK, p38 mitogen-activated protein kinase; PARP, poly(ADP-ribose) polymerase; PI3K, phosphoinositide 3-kinase; PINK1, PTEN (phosphatase and TENsin homolog)-induced kinase 1.
Because of an excessive compression, the production of NP matrix proteins is reduced resulting in an impossibility of maintaining the matrix homeostasis (Li et al, 2017). This impairment was explained by the fact that under a mechanical load, the activation of a wide range of signaling pathways in NP cells converge to the control of cell survival/death balance through induction of either autophagy, apoptosis, necroptosis or senescence (Ma et al, 2013; Li et al, 2017, 2018, 2021b; Pang et al, 2017; Huang et al, 2020; Kang et al, 2020; Lin et al, 2021) (Fig 3B). Autophagy is a cellular process that allows the orderly degradation and recycling of cellular components, hence providing a self-promoted nutrient source benefiting cell homeostasis and survival (Poillet-Perez & White, 2019). The compression-induced signaling pathways and their interplays are a matter of future work. It aims to define how to prevent or attenuate the massive cell death of NP cells in patients suffering from IVDD.
Compression during cancer development—is it promoting or restraining tumor initiation, progression or treatment response?
Unlike IVDD, cancer is not caused by compression, but compressive forces increase during development of solid cancers causing disease progression through various cell modifications (Fig 4). Compressive forces have for origin: (i) the rapid proliferation of cancer cells in a confined environment and, (ii) the accumulation of a non-tumoral environment such as an increase in extracellular matrix content, a remodeling of matrix composition, and matrix swelling with water uptake. Cancer and microenvironment cells sense compressive forces in a solid tumor. In both cell compartments, compression plays a dual role associated with the evolution of tumors (summarized in Fig 4). Epithelial cells respond to compression by arresting cell proliferation, promoting cell death or extruding cells (in lumens for epithelial cancers), which are protective mechanisms in the early steps of cancer development (Fig 4A); however, cancer epithelial cells adapt to this context and later compression leads to increase cancer cell proliferation, migration, and survival (Fig 4B). Similarly, a compressed microenvironment prevents cancer cell proliferation and tumor vascularization in early steps (Fig 4A), but accelerates cell proliferation, migration, survival to harsh environments in late stages (Fig 4C). Finally, compressive force increase in the tumor is sensed by adjacent tissues and favors tumor initiation (Fig 4D).
Figure 4. Impact of compressive forces in cancer initiation, progression, and resistance.
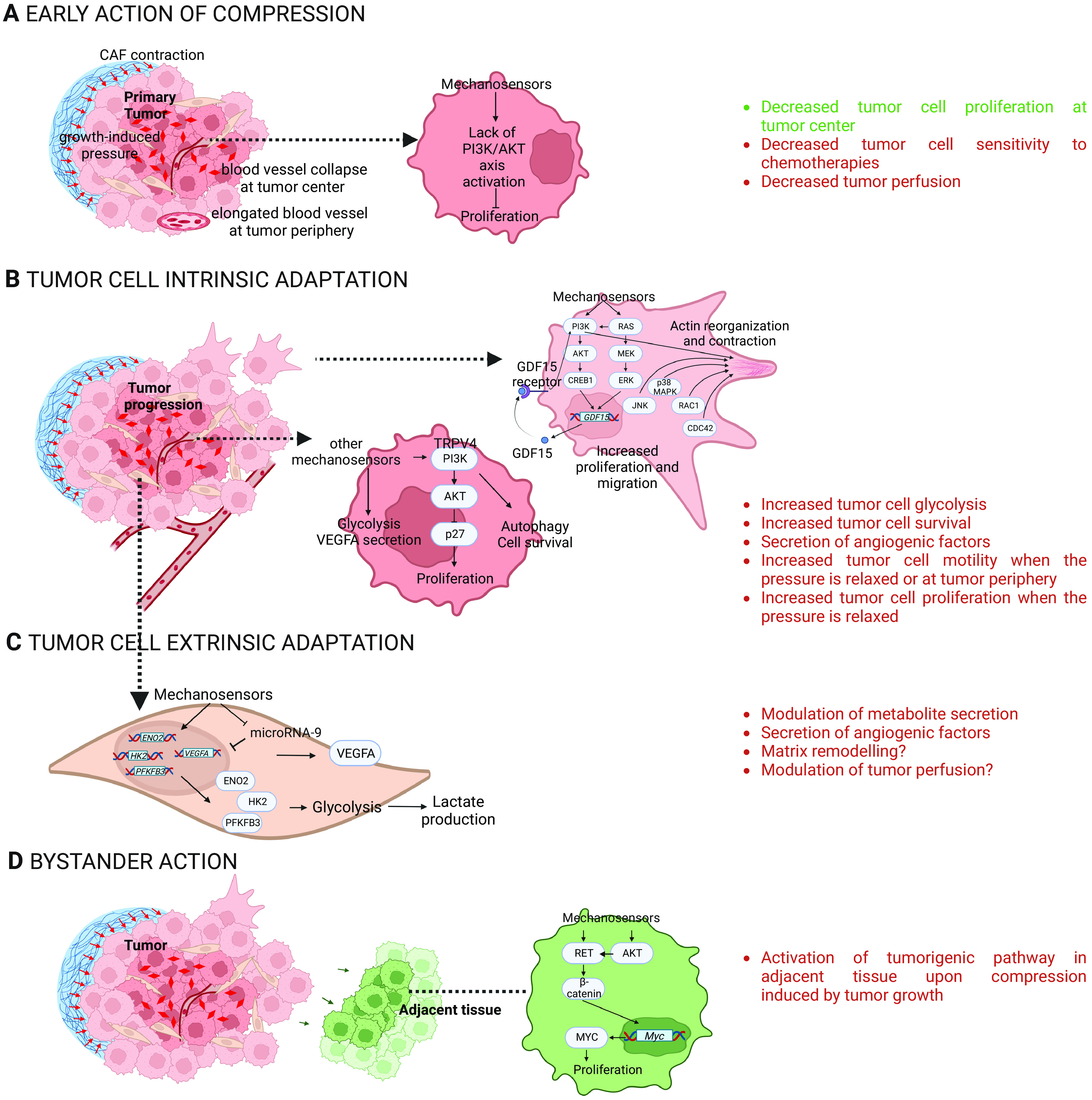
(A, B, C, D) This figure is a summary of studies performed in vitro and in vivo in cancer cells (A, B) from different origins such as the brain, breast, and pancreatic cancer, and in cancer-associated fibroblasts (C), and in cancer adjacent tissues (D). For references, see the main text. Text in green refers to positive action of compression, red to negative actions for patient survival. β-catenin, beta-catenin; CDC42, cell division control protein 42 homolog; CREB1, cAMP responsive element-binding protein 1; ENO2, enolase-2; ERK, extracellular signal-regulated kinase; GDF15, growth/differentiation factor 15; HK2, hexokinase 2; JNK, c-Jun N-terminal kinase; MEK, mitogen-activated protein kinase kinase; MYC, c-Myc; p38 MAPK, p38 mitogen-activated protein kinase; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PI3K, phosphoinositide 3-kinase; RAC1, ras-related C3 botulinum toxin substrate 1; RET, ret proto-oncogene; VEGFA, vascular endothelial growth factor A.
Early action of compression in primary tumors
The thickening of the non-tumoral environment around tumors because of inflammation lead to a compression of the whole tumor (Stylianopoulos et al, 2012; Jain et al, 2014; Northcott et al, 2018; Morikura & Miyata, 2019). In addition, in colon polyps, cancer-associated fibroblasts encapsulate and actively compress epithelial cells. Spontaneous cancer-associated fibroblasts actomyosin contractility is sensed by cancer cells leading to the cytoplasmic relocalization of their YAP proteins, preventing YAP/TAZ transcriptional effect, and reducing epithelial cell growth (Barbazan et al, 2021 Preprint). Hippo pathway containing transcriptional regulators YAP/TAZ can reprogram cancer cells into cancer stem cells and incite tumor initiation, progression, and metastasis. Furthermore, the Hippo pathway crosstalks with morphogenetic signals, such as Wnt growth factors, and is also regulated by RHO and G protein-coupled receptor, cAMP (cyclic adenosine monophosphate), and PKA (protein kinase A) pathways (Dasgupta & McCollum, 2019; Cobbaut et al, 2020; Cai et al, 2021). Class I PI3Ks are known to be upstream activators of YAP/TAZ transcriptional pathway under tensile stress, positioning class I PI3Ks proteins as potential regulators of an essential mechanotransduction signaling (Chronopoulos et al, 2020; Di-Luoffo et al, 2021).
Compressive forces reduce cancer cell proliferation, especially in the solid tumor center (Stylianopoulos et al, 2013; Nam et al, 2019; Rizzuti et al, 2020). With this knowledge, we previously tested and validated in vitro the hypothesis that the compression-induced decreased proliferation of cancer cells reduces the efficiency of chemotherapeutics known to target cycling cells (Rizzuti et al, 2020). It is the case with the use of gemcitabine, reference treatment in pancreatic cancer. Compressive forces modify the blood vessel shape until collapsing its structure, and as a consequence, decreasing the tumor perfusion (Stylianopoulos et al, 2013). By decreasing the tumor perfusion, this would prevent molecular agents to reach the cancer cells, further reducing the drug efficiency (Stylianopoulos et al, 2012) (Fig 4A).
Tumor cell intrinsic adaptation to compression
Whole transcriptomics analysis reveals that signaling outputs induced by compression converge to control the expression of genes involved in glycolysis in glioblastoma cells (Calhoun et al, 2020). The fact that glycolysis seems to be up-regulated in cancer upon compression is opposite to what is described on chondrocytes (McCutchen et al, 2017). This process might enable cancer cell survival in compressed environments with low access to nutrients and oxygen (Kim et al, 2019), because of vessel collapse.
Studies converge to show that compression promotes cancer cell motility and their migratory behavior (Pathak & Kumar, 2012). Both the MAPK/ERK pathway, in brain cancer cell lines, and the PI3K/AKT pathway, in pancreatic cancer cell lines, increase the expression of the migration-related GDF15 (growth/differentiation factor 15) gene which mediates cancer cell migration after their compression (Kalli et al, 2019a, 2019b). In compressed pancreatic cancer cell lines, up-regulation of p38 MAPK and JNK signaling pathways and cytoskeleton remodelers (RAC1 and CDC42) were also shown to promote migration (Kalli et al, 2022). Besides, the compression-induced motility of tumoral cells also depends on the cancer cell type studied. Brain neuroglioma cell lines migrate more under compression than the more aggressive brain glioblastoma cell lines even though both cell lines display an increased motility in response to compression (Kalli et al, 2019b; Calhoun et al, 2020). Molecular mechanism that explains this process possibly involves the regulation of PI3K pathway, as PI3Kα is critical to control metastatic behavior (Ippen et al, 2019; Thibault et al, 2021; Tehranian et al, 2022). Compression also enhances the invasive phenotype of cancer cells by specifically increasing the motility of peripheral tumoral cells (Tse et al, 2012). The increased motility of peripheral tumoral cells upon compressive forces was also found in a 3D experiment for which mouse colon carcinoma cells were encapsulated and grown in alginate capsules (Alessandri et al, 2013).
Compression of cancer cells could further enhance their proliferation when the forces are relaxed. When various epithelial tumor spheroids are grown in softer hydrogels enabling a reduced mechanical confinement in time compared with stiffer hydrogels, increased cell growth occurs and activates SACs such as transient receptor potential vanilloide 4 (TRPV4) that themselves activate the PI3K/AKT axis. This would enable the cytoplasmic relocalization and therefore inhibition of p27 (an inhibitor of cell cycle) resulting in a cell cycle acceleration through a G1 to S phase transition (Nam et al, 2019). This adaptive behavior in response to compression is only unleashed when tumor cells are relieved from their confinement. Indeed, compression without confinement increases the proliferation of colon and glioblastoma cancer cells (Mary et al, 2022).
Interestingly, we and others identify that in the adaptive response to compression of cancer cells, PI3K/AKT activation leads either to cell proliferation, survival or to migration (Kalli et al, 2019a; Nam et al, 2019; Di-Luoffo et al, 2023 Preprint), that is either pro-tumoral or pro-migratory. Identifying ways to understand how to block this adaptive response to compression could be a way to limit cancer progression. As a proof of concept, we use a panel of breast and pancreatic cancer cells where the PI3K pathway actively controls cell survival and proliferation. We demonstrate that we can further push the cell fate of cancer cells to trigger cell death under compression if PI3Kα is inactivated by increasing autophagic flux (Di-Luoffo et al, 2023 Preprint) data that are reminiscent to the molecular processes described in IVDD (Li et al, 2021b). Increased levels of autophagy in tumor cells promote growth of established tumors and treatment resistance at the progressive stage (Bryant et al, 2019). Combined control of PI3K and autophagy needs to be tested as a novel way to control deleterious adaptive response of cancer cells to compression (Fig 4B).
Tumor cell extrinsic adaptation to compression
The microenvironment also adapts to compressive forces and is likely to contribute to cancer progression. Compression controls gene expression involved in glycolysis in breast cancer-associated fibroblasts (Kim et al, 2019; Calhoun et al, 2020), that might provide a different metabolite supply to cancer cells; the impact on tumor matrix remodeling is poorly investigated so far (Luo et al, 2022). A recent study revealed that compression increases VEGFA gene expression and protein level in breast cancer cells and associated fibroblasts (Kim et al, 2017). Because VEGFA contributes to the formation of new blood vessels (Claesson-Welsh & Welsh, 2013), angiogenesis might be increased upon compression. Nowadays, the impact of compressive forces on tumor vascularization and, consequently, on drug delivery is not fully understood (Fig 4C).
Bystander action of compression induced by tumors in neighboring normal cells
Growth-induced compressive forces result in an increased compression of the tumor interior that spreads to the boundaries of the tumor (called radial compression) where conjunctive stroma also sense compressive forces. Studies on colon cancers revealed that a growing solid tumor exerts a mechanical pressure on adjacent non-tumoral cells that induces a signaling into cancer-initiating cells causing the formation of adjacent tumoral foci through a pathway involving RET and the β-catenin protein (Fernández-Sánchez et al, 2015; Nguyen Ho-Bouldoires et al, 2022) (Fig 4D).
Discussion and Future Directions
In homeostasis, compression modifies the signaling pathways and, ultimately, the behaviors of the compressed cells and even indirectly, the adjacent uncompressed cells. Depending on the context, compression either induces or prevents cell proliferation, survival, differentiation, and migration and changes the cell metabolism. These behaviors occur during developmental processes (Xiong et al, 2020).
The impact of compressive forces during development is a vast topic, beyond the field covered in this review and likely to expand. Indeed, some studies show that the fate of stem cells depends on the intensity of the mechanical load: in the presence of an adipogenic medium, rat BMSCs subjected to a small-magnitude stress undergo an osteogenic differentiation, whereas rat BMSCs subjected to a large-magnitude stress undergo an adipogenic differentiation (Song et al, 2017). Stemness is controlled by compression in normal intestinal organoids (Li et al, 2021a). As stemness property is relevant for cancer studies and is notably important in tumor and metastatic dormancy, it is crucial to pursue this line of research in both model organisms and cancer context.
Organ size is thought to be regulated in part by mechanical forces. One hypothesis is that compressive forces increase as the organ grows to reach a threshold inhibiting further organ growth. Inhibition of cell proliferation by compression likely participates in this termination of organ growth (Buchmann et al, 2014). The compressive forces found in adult organs might also regulate cell proliferation to maintain a constant organ size. Indeed, one study suggests that compression might decrease proliferation of skeletal myoblast cells (Takemoto et al, 2015). How tumor overcomes this physical limitation is a matter of importance.
Compression can drive pathologies (such as IVDD) or buildup during pathology development (such as in cancer). Knowing the selective biochemical signals that are triggered by compression and that contribute to diseases could lead to the discovery of efficient signal-targeted therapies. For example, the studies of IVDD give insight on how to push cancer cells undergoing compression towards cell death. Both the mitochondria-targeted antioxidant MitoQ and the resveratrol molecule could positively impact IVDD in vitro by stabilizing mitochondrial functions and increasing NP matrix synthesis, respectively (Han et al, 2017; Kang et al, 2020). The benefit or detriment of those strategies could be investigated in cancer, where mitochondrial function plays a key role despite the Warburg effect.
To reach the aim of developing therapies that consider the influence of compression in cancer, several key outstanding questions remain.
First, different mechanosensors of compressive forces were identified: compression could change the conformation of ion channels like Piezo-1 and Piezo-2 and activate as a consequence transmembrane proteins like α5β1 integrin, MUC family. Recent studies emphasized that intracellular, molecular, and organelle crowding and nucleus deformation occur and could participate in the starting point of the compression mechanotransduction (Guo et al, 2017; Lomakin et al, 2020; Venturini et al, 2020). However, we still need to identify the selective mechanosensors or the selective mechanism of their activation by compression.
Furthermore, studies should now focus on confirming the implicated pathways in vivo or in refined biomimetic devices. For 3D studies, attention should be paid to the geometry of the produced tissue as it can impact the cell response to compression (Berg et al, 2021). Besides, few in vivo compression methods have been developed. By using magnets subcutaneously inserted close to the mouse colon, Fernández-Sánchez et al deliver an in vivo mechanical pressure, mimicking the one undergone by non-tumoral tissues adjacent to a growing early colon tumor. This study revealed that this compressive force could cause the formation of new tumoral foci in the non-tumoral adjacent tissues (Fernández-Sánchez et al, 2015). Another group, Nia et al used modified cranial windows on mice to recapitulate compressive forces caused by brain tumors (primary and metastatic tumors of the cerebellar cortex and tumors of the cerebellum) (Nia et al, 2020). Combining this device with intravital imaging or other assays could represent a powerful way to study the effect of in vivo compression in cancer.
The signaling pathways induced in compressed cells lead to both cancer and microenvironment cell adaptation by feedback loops (Kalli et al, 2019a, 2019b; Kim et al, 2019; Calhoun et al, 2020). This should be analyzed more comprehensively in various cancer cell types to identify a selective factor critical for the adaptive response to compressive forces. In this topic, we are interested in investigating how PI3K signaling is a generic adaptive response of cancer and microenvironment cells to compression (Di-Luoffo et al, 2021), and in particular, on how autophagy response is a determinant of cancer cell death upon compression and PI3K inhibition (Di-Luoffo et al, 2023 Preprint).
In regenerative medicine, some studies attempt to use compressive forces, or even to combine them with other constraints exerting naturally (e.g., shearing forces), to obtain a model mimicking the human cartilage (Guo et al, 2016). One should be careful to use modulation of compression to treat cancer. If therapies by modifying extracellular matrix could alleviate the pressure within the tumor, they could improve drug efficiency of chemotherapeutic agents (Stylianopoulos et al, 2012; Rizzuti et al, 2020). However, once unconfined, compressed cancer cells might be more aggressive and resist therapies. Fig 4 shows that compressed cancer cells evolve and adapt. They ultimately increase their proliferative and invasive phenotype and their capacity to survive and proliferate in harsh environments (Stylianopoulos et al, 2013; Nam et al, 2019; Rizzuti et al, 2020). This process might trigger selection of selective genetic and epigenetic traits that need to be characterized. Those traits might lead to both cancer progression and to cancer resistance to targeted therapies.
One aspect of the compression-induced cell response that is currently understudied is the relationship between genetic alterations and cancer response to a compressive environment. Emerging evidence on the studied panel of glioblastoma cells suggests that compression-induced cell signaling and its cellular output might depend on the cell aggressivity and their genetic background (Kalli et al, 2019b; Calhoun et al, 2020). We are thus currently developing novel approaches to study this association in an unbiased way.
In conclusion, understanding selective compression-induced effects in cancer is needed for the success of cancer mechanotherapeutics, such as cancer treatment targeting matrix sensing or morphogenetic programs (Sheridan, 2019). Estimating compressive forces in patients is necessary to know more about mechanobiology in cancers and tailor mechanotherapy to each patient. Alleviating or increasing the tumor pressure for patient therapy is still a matter of debate (Leite & Barbosa, 2019). However, as the compressive forces activate selective oncogenic pathways (Di-Luoffo et al, 2023 Preprint), compression could induce novel tumor vulnerabilities that can be targeted by novel emerging mechanotherapies.
Glossary
Mechanosensor: Protein-sensing mechanical stresses and transmitting this mechanical signal in a biochemical signal such as signaling pathways.
Mechanotransduction: Transformation of a mechanical stress into chemical–biological signals.
BMP (bone morphogenetic protein): Group of growth factors involved in development of tissues including bones. BMP2 is a member of this family.
β-catenin: Member of the catenin protein family which is a subunit of the cadherin protein complex, and which acts as an intracellular signal transducer. It plays amongst others an important role in the canonical Wnt pathway.
CDC42 (cell division control protein 42 homolog): Member of the Rho family (Ras homolog family) which is a member of the Ras superfamily of small GTPases.
GPCR (G-protein-coupled receptors): Also called seven-transmembrane receptors or heptahelical receptors, they are proteins located in the cell membrane that bind extracellular substances and transmit signals from these substances to intracellular tripartite molecules called a heterotrimeric G protein (guanine nucleotide-binding proteins).
GSK-3 (glycogen synthase kinase 3): Originally identified as a regulator of glycogen metabolism, GSK-3 acts as a downstream regulatory switch for numerous signaling pathways, including cellular responses to WNT, growth factors, insulin, receptor tyrosine kinases (RTK), Hedgehog pathways, and G-protein-coupled receptors (GPCR). Two isoenzymes encoded by two different genes exist (GSK-3α and GSK-3β).
Hippo/YAP/TAZ pathway: Cell signaling pathway which negatively regulates YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif). YAP and TAZ are both transcription coactivators which bind to the TEAD transcription factors (TEAD1–4) and notably promote cell proliferation and survival. When active, the Hippo pathway causes YAP and TAZ nuclear export or degradation. YAP and TAZ activities is also modulated by Hippo pathway-independent mechanisms.
JNK (c-Jun N-terminal kinase): Group of kinases which are members of the mitogen-activated protein kinase (MAPK) family.
MAPK/ERK pathway: Cell signaling pathway activated by growth factors or cytokines. It involves the cascade activation of RAS (GTPase), RAF [serine/threonine kinase, member of mitogen-activated protein (MAP) kinase kinase kinase or MAP3K family], MEK1/2, also called mitogen-activated protein kinase kinases 1 and 2 (kinases with serine/threonine kinase and tyrosine kinase activities) and ERK1/2, also called extracellular signal-regulated kinases 1 and 2 (serine/threonine kinases). ERK1/2 are members of the mitogen-activated protein kinase (MAPK) family of protein.
p38 MAPK: Group of kinases which are members of the mitogen-activated protein kinase (MAPK) family.
PI3K/AKT pathway: Cell signaling pathway in which PI3Ks (phosphoinositide 3-kinases) are activated by several signals (e.g., hormones, growth factors, extracellular matrix) and phosphorylate phosphatidylinositol on 3- hydroxyl group positions of the inositol ring to produce PIP3 [Phosphatidylinositol (3,4,5)-trisphosphate]. Through recruitment of various proteins at the PIP3, the AKT protein is phosphorylated and thus activated to phosphorylate other proteins at serine and threonine sites. This pathway also closely controls actin cytoskeleton rearrangements through RHO and ROCK activation.
RET (rearranged during transfection): Tyrosine kinase receptor which is a subunit of a complex binding to growth factors of the glial-derived neurotropic factor (GDNF) family.
RAC1 (Ras-related C3 botulinum toxin substrate 1): Member of the Rho family (Ras homolog family), which is a member of the Ras superfamily of small GTPases.
RHO (Ras homolog family member): Members of the Rho family (Ras homolog family), which are part of the Ras superfamily of small GTPases. RHOE and RHOA have antagonistics action in actin cytoskeleton regulation.
ROCK (RHO-associated protein kinase): Member of the AGC (PKA/PKG/PKC) family of serine–threonine protein kinases.
RUNX2 (runt-related transcription factor 2): Transcription factor associated with osteoblast differentiation.
SRC (proto-oncogene tyrosine-protein kinase Src): Family of non-receptor tyrosine kinases able to activate, in particular, MAPK/ERK signaling.
Stretched-activated channels (SACs): SACs are described to respond to mechanical forces along the plane of the cell membrane (membrane tension), but not to hydrostatic pressure perpendicular to it.
TIMP3/TGF-β1 pathway: Cell signaling pathway in which TGF-β1 (transforming growth factor 1) binds to its cell membrane receptor, leading to the phosphorylation, and thus activation of SMAD2 and SMAD3, which in turn can induce the expression of TIMP3 (tissue inhibitor of matrix metalloproteinase).
WNT: Wnt proteins belong to an evolutionarily conserved family of secreted cysteine-rich glycoproteins. Wnts can activate β-catenin-dependent canonical Wnt pathway and β-catenin-independent noncanonical Wnt pathway. A key feature of the canonical Wnt pathway is the regulated degradation of transcription coactivator β-catenin by the β-catenin destruction complex which includes glycogen synthase kinase 3α and 3β (GSK-3α and GSK-3β).
Supplementary Material
Acknowledgements
A particular thanks to Christophe Trehin, Ecole Normale Supérieure review tutor for advice, and Morgan Delarue and the “CRCT-SigDYN/LAAS-DelarueLab Mechagroup” for their constant discussions on the topic. Our work is funded by Labex Toucan (ANR), Fondation Toulouse Cancer Santé (Mecharesist), Inserm Plan Cancer (PressDiagTherapy followed by MechaEvo), INCA-PLBIO2021, Fondation ARC (ARCPJA2021060003932, ARCPGA2022120005630_6362-3). J Guillermet-Guibert obtained a Fondation Fonroga prize for C Schmitter to perform a master degree on this project. All figures were created using Biorender software.
Author Contributions
C Schmitter: conceptualization, data curation, formal analysis, investigation, visualization, and writing—original draft, review, and editing.
M Di-Luoffo: data curation, formal analysis, supervision, validation, investigation, visualization, and writing—review and editing.
J Guillermet-Guibert: conceptualization, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Alessandri K, Sarangi BR, Gurchenkov VV, Sinha B, Kießling TR, Fetler L, Rico F, Scheuring S, Lamaze C, Simon A, et al. (2013) Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc Natl Acad Sci U S A 110: 14843–14848. 10.1073/pnas.1309482110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbazan J, Pérez-González C, Gómez-González M, Dedenon M, Richon S, Latorre E, Serra M, Mariani P, Descroix S, Sens P, et al. (2021) Cancer-associated fibroblasts actively compress cancer cells and modulate mechanotransduction. BioRxiv. 10.1101/2021.04.05.438443 (Preprint posted April 5, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschieri F, Le Devedec D, Tettarasar S, Elkhatib N, Montagnac G (2020) Frustration of endocytosis potentiates compression-induced receptor signaling. J Cell Sci 133: jcs239681. 10.1242/jcs.239681 [DOI] [PubMed] [Google Scholar]
- Berg IC, Mohagheghian E, Habing K, Wang N, Underhill GH (2021) Microtissue geometry and cell-generated forces drive patterning of liver progenitor cell differentiation in 3D. Adv Healthc Mater 10: e2100223. 10.1002/adhm.202100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle ST, Kular J, Nobis M, Ruszkiewicz A, Timpson P, Samuel MS (2020) Acute compressive stress activates RHO/ROCK-mediated cellular processes. Small GTPases 11: 354–370. 10.1080/21541248.2017.1413496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 173: 733–741. 10.1083/jcb.200601060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, et al. (2019) Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med 25: 628–640. 10.1038/s41591-019-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Alber M, Zartman JJ (2014) Sizing it up: The mechanical feedback hypothesis of organ growth regulation. Semin Cell Dev Biol 35: 73–81. 10.1016/j.semcdb.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang K-C, Meng Z (2021) Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front Cell Dev Biol 9: 673599. 10.3389/fcell.2021.673599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun MA, Cui Y, Elliott EE, Mo X, Otero JJ, Winter JO (2020) MicroRNA-mRNA interactions at low levels of compressive solid stress implicate mir-548 in increased glioblastoma cell motility. Sci Rep 10: 311. 10.1038/s41598-019-56983-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhu J, Wang Y-H, Hang C-H (2019) ROS-mediated mitochondrial dysfunction and ER stress contribute to compression-induced neuronal injury. Neuroscience 416: 268–280. 10.1016/j.neuroscience.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Appleby RN, Salter DM, Bader DA, Lee DA (2006) Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol 5: 192–201. 10.1007/s10237-006-0032-3 [DOI] [PubMed] [Google Scholar]
- Chronopoulos A, Thorpe SD, Cortes E, Lachowski D, Rice AJ, Mykuliak VV, Róg T, Lee DA, Hytönen VP, Del Río Hernández AE (2020) Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat Mater 19: 669–678. 10.1038/s41563-019-0567-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L, Welsh M (2013) VEGFA and tumour angiogenesis. J Intern Med 273: 114–127. 10.1111/joim.12019 [DOI] [PubMed] [Google Scholar]
- Cobbaut M, Karagil S, Bruno L, Diaz de la Loza MDC, Mackenzie FE, Stolinski M, Elbediwy A (2020) Dysfunctional mechanotransduction through the YAP/TAZ/Hippo pathway as a feature of chronic disease. Cells 9: 151. 10.3390/cells9010151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60. 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta I, McCollum D (2019) Control of cellular responses to mechanical cues through YAP/TAZ regulation. J Biol Chem 294: 17693–17706. 10.1074/jbc.REV119.007963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo RMS, Oba Y, Kuroda S, Tanaka E, Moriyama K (2014) RhoE regulates actin cytoskeleton organization in human periodontal ligament cells under mechanical stress. Arch Oral Biol 59: 187–192. 10.1016/j.archoralbio.2013.11.010 [DOI] [PubMed] [Google Scholar]
- De Marzio M, Kılıç A, Maiorino E, Mitchel JA, Mwase C, O’Sullivan MJ, McGill M, Chase R, Fredberg JJ, Park J-A, et al. (2021) Genomic signatures of the unjamming transition in compressed human bronchial epithelial cells. Sci Adv 7: eabf1088. 10.1126/sciadv.abf1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Montel F, Vignjevic D, Prost J, Joanny J-F, Cappello G (2014) Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys J 107: 1821–1828. 10.1016/j.bpj.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Poterewicz G, Hoxha O, Choi J, Yoo W, Kayser J, Holt L, Hallatschek O (2017) SCWISh network is essential for survival under mechanical pressure. Proc Natl Acad Sci U S A 114: 13465–13470. 10.1073/pnas.1711204114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio I, Trushko A, Guillamat P, Blanch-Mercader C, Abuhattum S, Roux A (2022) Pressure and curvature control of the cell cycle in epithelia growing under spherical confinement. Cell Rep 40: 111227. 10.1016/j.celrep.2022.111227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Luoffo M, Ben-Meriem Z, Lefebvre P, Delarue M, Guillermet-Guibert J (2021) PI3K functions as a hub in mechanotransduction. Trends Biochem Sci 46: 878–888. 10.1016/j.tibs.2021.05.005 [DOI] [PubMed] [Google Scholar]
- Di-Luoffo M, Schmitter C, Barrere EC, Therville N, Chaouki M, D'Angelo R, Thibault B, Delarue M, Guillermet-Guibert J (2023) Compressive constraint promotes the cytotoxicity of PI3K signal targeted therapies in breast and pancreatic cancer cells. BioRxiv. 10.1101/2021.10.18.464825 (Preprint posted June 13, 2023). [DOI]
- Dolega ME, Delarue M, Ingremeau F, Prost J, Delon A, Cappello G (2017) Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nat Commun 8: 14056. 10.1038/ncomms14056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien C-B, Morcos PA, Rosenblatt J (2012) Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484: 546–549. 10.1038/nature10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, et al. (2015) Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523: 92–95. 10.1038/nature14329 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ (2008) Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem 283: 6735–6743. 10.1074/jbc.M708670200 [DOI] [PubMed] [Google Scholar]
- Ge Y, Li Y, Wang Z, Li L, Teng H, Jiang Q (2021) Effects of mechanical compression on chondrogenesis of human synovium-derived mesenchymal stem cells in agarose hydrogel. Front Bioeng Biotechnol 9: 697281. 10.3389/fbioe.2021.697281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Yu L, Lim CG, Goodley AS, Xiao X, Placone JK, Ferlin KM, Nguyen B-NB, Hsieh AH, Fisher JP (2016) Effect of dynamic culture and periodic compression on human mesenchymal stem cell proliferation and chondrogenesis. Ann Biomed Eng 44: 2103–2113. 10.1007/s10439-015-1510-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, et al. (2017) Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci U S A 114: E8618–E8627. 10.1073/pnas.1705179114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-K, Wouters W, Clark A, Herzog W (2012) Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res 30: 475–481. 10.1002/jor.21536 [DOI] [PubMed] [Google Scholar]
- Han X, Leng X, Zhao M, Wu M, Chen A, Hong G, Sun P (2017) Resveratrol increases nucleus pulposus matrix synthesis through activating the PI3K/Akt signaling pathway under mechanical compression in a disc organ culture. Biosci Rep 37: BSR20171319. 10.1042/BSR20171319 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haudenschild AK, Hsieh AH, Kapila S, Lotz JC (2009) Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 37: 492–502. 10.1007/s10439-008-9629-2 [DOI] [PubMed] [Google Scholar]
- He Y, Macarak EJ, Korostoff JM, Howard PS (2004) Compression and tension: Differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect Tissue Res 45: 28–39. 10.1080/03008200490278124 [DOI] [PubMed] [Google Scholar]
- Hogrebe NJ, Reinhardt JW, Gooch KJ (2017) Biomaterial microarchitecture: A potent regulator of individual cell behavior and multicellular organization. J Biomed Mater Res A 105: 640–661. 10.1002/jbm.a.35914 [DOI] [PubMed] [Google Scholar]
- Huang D, Peng Y, Li Z, Chen S, Deng X, Shao Z, Ma K (2020) Compression-induced senescence of nucleus pulposus cells by promoting mitophagy activation via the PINK1/PARKIN pathway. J Cell Mol Med 24: 5850–5864. 10.1111/jcmm.15256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen FM, Alvarez-Breckenridge CA, Kuter BM, Fink AL, Bihun IV, Lastrapes M, Penson T, Schmidt SP, Wojtkiewicz GR, Ning J, et al. (2019) The dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin Cancer Res 25: 3374–3383. 10.1158/1078-0432.CCR-18-3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Martin JD, Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16: 321–346. 10.1146/annurev-bioeng-071813-105259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, He D, Ma Y, Su J, Wu X, Cui S, Li Z, Zhou Y, Yu H, Liu Y (2021) Force-induced autophagy in periodontal ligament stem cells modulates M1 macrophage polarization via AKT signaling. Front Cell Dev Biol 9: 666631. 10.3389/fcell.2021.666631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Minia A, Pliaka V, Fotis C, Alexopoulos LG, Stylianopoulos T (2019. a) Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Sci Rep 9: 978. 10.1038/s41598-018-37425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Voutouri C, Minia A, Pliaka V, Fotis C, Alexopoulos LG, Stylianopoulos T (2019. b) Mechanical compression regulates brain cancer cell migration through MEK1/Erk1 pathway activation and GDF15 expression. Front Oncol 9: 992. 10.3389/fonc.2019.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Stylianopoulos T (2018) Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front Oncol 8: 55. 10.3389/fonc.2018.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Li R, Mills GB, Stylianopoulos T, Zervantonakis IK (2022) Mechanical stress signaling in pancreatic cancer cells triggers p38 MAPK- and JNK-dependent cytoskeleton remodeling and promotes cell migration via Rac1/cdc42/myosin II. Mol Cancer Res 20: 485–497. 10.1158/1541-7786.MCR-21-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X (2020) The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif 53: e12779. 10.1111/cpr.12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Gao M-Q, Kang S, Choi YP, Lee JH, Kim JE, Han HH, Mun SG, Cho NH (2017) Mechanical compression induces VEGFA overexpression in breast cancer via DNMT3A-dependent miR-9 downregulation. Cell Death Dis 8: e2646. 10.1038/cddis.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Sung JS, Jang Y, Cha YJ, Kang S, Han HH, Lee JH, Cho NH (2019) Compression-induced expression of glycolysis genes in CAFs correlates with EMT and angiogenesis gene expression in breast cancer. Commun Biol 2: 313. 10.1038/s42003-019-0553-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan B, Mitchel JA, O’Sullivan MJ, Park CY, Kim JH, Cole WC, Butler JP, Park J-A (2018) Airway epithelial compression promotes airway smooth muscle proliferation and contraction. Am J Physiol Lung Cell Mol Physiol 315: L645–L652. 10.1152/ajplung.00261.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MLLF, Barbosa LER (2019) Endoscopic stent in malignant colonic obstruction: The risk of tumor seeding. J Coloproctol 39: 357–364. 10.1016/j.jcol.2019.05.001 [DOI] [Google Scholar]
- Li P, Liang Z, Hou G, Song L, Zhang R, Gan Y, Zhang C, Xu Y, Zhou Q (2017) N-Cadherin-mediated activation of PI3K/Akt-GSK-3β signaling attenuates nucleus pulposus cell apoptosis under high-magnitude compression. Cell Physiol Biochem 44: 229–239. 10.1159/000484649 [DOI] [PubMed] [Google Scholar]
- Li S, Hua W, Wang K, Gao Y, Chen S, Liu W, Song Y, Wu X, Tu J, Kang L, et al. (2018) Autophagy attenuates compression-induced apoptosis of human nucleus pulposus cells via MEK/ERK/NRF1/Atg7 signaling pathways during intervertebral disc degeneration. Exp Cell Res 370: 87–97. 10.1016/j.yexcr.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Li CW, Lau YT, Lam KL, Chan BP (2020) Mechanically induced formation and maturation of 3D-matrix adhesions (3DMAs) in human mesenchymal stem cells. Biomaterials 258: 120292. 10.1016/j.biomaterials.2020.120292 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen M, Hu J, Sheng R, Lin Q, He X, Guo M (2021. a) Volumetric compression induces intracellular crowding to control intestinal organoid growth via Wnt/β-catenin signaling. Cell Stem Cell 28: 63–78.e7. 10.1016/j.stem.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang J, Deng X, Huang D, Shao Z, Ma K (2021. b) Compression stress induces nucleus pulposus cell autophagy by inhibition of the PI3K/AKT/mTOR pathway and activation of the JNK pathway. Connect Tissue Res 62: 337–349. 10.1080/03008207.2020.1736578 [DOI] [PubMed] [Google Scholar]
- Lim Lam VK, Hin Wong JY, Chew SY, Chan BP (2021) Rac1-GTPase regulates compression-induced actin protrusions (CAPs) of mesenchymal stem cells in 3D collagen micro-tissues. Biomaterials 274: 120829. 10.1016/j.biomaterials.2021.120829 [DOI] [PubMed] [Google Scholar]
- Lin H, Peng Y, Li J, Wang Z, Chen S, Qing X, Pu F, Lei M, Shao Z (2021) Reactive oxygen species regulate endoplasmic reticulum stress and ER-mitochondrial Ca2+ crosstalk to promote programmed necrosis of rat nucleus pulposus cells under compression. Oxid Med Cell Longev 2021: 8810698. 10.1155/2021/8810698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-S, Lee OK (2014) In search of the pivot point of mechanotransduction: Mechanosensing of stem cells. Cell Transplant 23: 1–11. 10.3727/096368912X659925 [DOI] [PubMed] [Google Scholar]
- Lomakin AJ, Cattin CJ, Cuvelier D, Alraies Z, Molina M, Nader GPF, Srivastava N, Sáez PJ, Garcia-Arcos JM, Zhitnyak IY, et al. (2020) The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370: eaba2894. 10.1126/science.aba2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Xue S-L, De Vos WH, Redondo-Morata L, Surin M, Lafont F, Hannezo E, Gabriele S (2021) Cell monolayers sense curvature by exploiting active mechanics and nuclear mechanoadaptation. Nat Phys 17: 1382–1390. 10.1038/s41567-021-01374-1 [DOI] [Google Scholar]
- Luo M, Cai G, Ho KKY, Wen K, Tong Z, Deng L, Liu AP (2022) Compression enhances invasive phenotype and matrix degradation of breast Cancer cells via Piezo1 activation. BMC Mol Cell Biol 23: 1. 10.1186/s12860-021-00401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K-G, Shao Z-W, Yang S-H, Wang J, Wang B-C, Xiong L-M, Wu Q, Chen S-F (2013) Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthritis Cartilage 21: 2030–2038. 10.1016/j.joca.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Mary G, Malgras B, Perez JE, Nagle I, Luciani N, Pimpie C, Asnacios A, Pocard M, Reffay M, Wilhelm C (2022) Magnetic compression of tumor spheroids increases cell proliferation in vitro and cancer progression in vivo. Cancers (Basel) 14: 366. 10.3390/cancers14020366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey AE, Doxtater KA, Yallapu MM, Chauhan SC (2020) Biophysical changes caused by altered MUC13 expression in pancreatic cancer cells. Micron 130: 102822. 10.1016/j.micron.2019.102822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchen CN, Zignego DL, June RK (2017) Metabolic responses induced by compression of chondrocytes in variable-stiffness microenvironments. J Biomech 64: 49–58. 10.1016/j.jbiomech.2017.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikura T, Miyata S (2019) Effect of mechanical compression on invasion process of malignant melanoma using in vitro three-dimensional cell culture device. Micromachines (Basel) 10: E666. 10.3390/mi10100666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Gupta VK, Lee H-P, Lee JY, Wisdom KM, Varma S, Flaum EM, Davis C, West RB, Chaudhuri O (2019) Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27Kip1 signaling axis. Sci Adv 5: eaaw6171. 10.1126/sciadv.aaw6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ho-Bouldoires TH, Sollier K, Zamfirov L, Broders-Bondon F, Mitrossilis D, Bermeo S, Guerin CL, Chipont A, Champenois G, Leclère R, et al. (2022) Ret kinase-mediated mechanical induction of colon stem cells by tumor growth pressure stimulates cancer progression in vivo. Commun Biol 5: 137. 10.1038/s42003-022-03079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nia HT, Datta M, Seano G, Zhang S, Ho WW, Roberge S, Huang P, Munn LL, Jain RK (2020) In vivo compression and imaging in mouse brain to measure the effects of solid stress. Nat Protoc 15: 2321–2340. 10.1038/s41596-020-0328-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgaard C, Vind AC, Stonadge A, Kjøbsted R, Snieckute G, Antas P, Blasius M, Reinert MS, Del Val AM, Bekker-Jensen DB, et al. (2022) ZAKβ is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle. EMBO J 41: e111650. 10.15252/embj.2022111650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott JM, Dean IS, Mouw JK, Weaver VM (2018) Feeling stress: The mechanics of cancer progression and aggression. Front Cell Dev Biol 6: 17. 10.3389/fcell.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Li P, Zhang R, Xu Y, Song L, Zhou Q (2017) Role of p38-MAPK pathway in the effects of high-magnitude compression on nucleus pulposus cell senescence in a disc perfusion culture. Biosci Rep 37: BSR20170718. 10.1042/BSR20170718 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park J-A, Tschumperlin DJ (2009) Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol 41: 459–466. 10.1165/rcmb.2008-0195OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Burckhardt CJ, Lazcano R, Solis LM, Isogai T, Li L, Chen CS, Gao B, Minna JD, Bachoo R, et al. (2020) Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578: 621–626. 10.1038/s41586-020-1998-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Kumar S (2012) Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A 109: 10334–10339. 10.1073/pnas.1118073109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly N, Knight MR, Thuleau P, Graziana A, Muto S, Ranjeva R, Mazars C (2001) The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 30: 413–421. 10.1054/ceca.2001.0250 [DOI] [PubMed] [Google Scholar]
- Pelaez D, Arita N, Cheung HS (2012) Extracellular signal-regulated kinase (ERK) dictates osteogenic and/or chondrogenic lineage commitment of mesenchymal stem cells under dynamic compression. Biochem Biophys Res Commun 417: 1286–1291. 10.1016/j.bbrc.2011.12.131 [DOI] [PubMed] [Google Scholar]
- Poillet-Perez L, White E (2019) Role of tumor and host autophagy in cancer metabolism. Genes Dev 33: 610–619. 10.1101/gad.325514.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman I, De Croos JA, Pilliar R, Kandel RA (2010) Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium 48: 232–242. 10.1016/j.ceca.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Ren K, Ma Y, Huang Y, Liang W, Liu F, Wang Q, Cui W, Liu Z, Yin G, Fan W (2011) Periodic mechanical stress activates MEK1/2-ERK1/2 mitogenic signals in rat chondrocytes through Src and PLCγ1. Braz J Med Biol Res 44: 1231–1242. 10.1590/s0100-879x2011007500150 [DOI] [PubMed] [Google Scholar]
- Rizzuti IF, Mascheroni P, Arcucci S, Ben-Mériem Z, Prunet A, Barentin C, Rivière C, Delanoë-Ayari H, Hatzikirou H, Guillermet-Guibert J, et al. (2020) Mechanical control of cell proliferation increases resistance to chemotherapeutic agents. Phys Rev Lett 125: 128103. 10.1103/PhysRevLett.125.128103 [DOI] [PubMed] [Google Scholar]
- Schreivogel S, Kuchibhotla V, Knaus P, Duda GN, Petersen A (2019) Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J Tissue Eng Regen Med 13: 1992–2008. 10.1002/term.2948 [DOI] [PubMed] [Google Scholar]
- Sheridan C (2019) Pancreatic cancer provides testbed for first mechanotherapeutics. Nat Biotechnol 37: 829–831. 10.1038/d41587-019-00019-2 [DOI] [PubMed] [Google Scholar]
- Song F, Jiang D, Wang T, Wang Y, Lou Y, Zhang Y, Ma H, Kang Y (2017) Mechanical stress regulates osteogenesis and adipogenesis of rat mesenchymal stem cells through PI3K/Akt/GSK-3β/β-catenin signaling pathway. Biomed Res Int 2017: 6027402. 10.1155/2017/6027402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, et al. (2012) Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A 109: 15101–15108. 10.1073/pnas.1213353109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, Jain RK (2013) Coevolution of solid stress and interstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res 73: 3833–3841. 10.1158/0008-5472.CAN-12-4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Ishihara S, Mizutani T, Kawabata K, Haga H (2015) Compressive stress induces dephosphorylation of the myosin regulatory light chain via RhoA phosphorylation by the adenylyl cyclase/protein kinase A signaling pathway. PLoS One 10: e0117937. 10.1371/journal.pone.0117937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranian C, Fankhauser L, Harter PN, Ratcliffe CDH, Zeiner PS, Messmer JM, Hoffmann DC, Frey K, Westphal D, Ronellenfitsch MW, et al. (2022) The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol 24: 213–225. 10.1093/neuonc/noab159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault B, Ramos-Delgado F, Pons-Tostivint E, Therville N, Cintas C, Arcucci S, Cassant-Sourdy S, Reyes-Castellanos G, Tosolini M, Villard AV, et al. (2021) Pancreatic cancer intrinsic PI3Kα activity accelerates metastasis and rewires macrophage component. EMBO Mol Med 13: e13502. 10.15252/emmm.202013502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyjanova J, Flores-Cortez E, Reichner JS, Franck C (2015) Matrix confinement plays a pivotal role in regulating neutrophil-generated tractions, speed, and integrin utilization. J Biol Chem 290: 3752–3763. 10.1074/jbc.M114.619643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushko A, Di Meglio I, Merzouki A, Blanch-Mercader C, Abuhattum S, Guck J, Alessandri K, Nassoy P, Kruse K, Chopard B, et al. (2020) Buckling of an epithelium growing under spherical confinement. Dev Cell 54: 655–668.e6. 10.1016/j.devcel.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL (2012) Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A 109: 911–916. 10.1073/pnas.1118910109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uroz M, Wistorf S, Serra-Picamal X, Conte V, Sales-Pardo M, Roca-Cusachs P, Guimerà R, Trepat X (2018) Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat Cell Biol 20: 646–654. 10.1038/s41556-018-0107-2 [DOI] [PubMed] [Google Scholar]
- Venturini V, Pezzano F, Català Castro F, Häkkinen H-M, Jiménez-Delgado S, Colomer-Rosell M, Marro M, Tolosa-Ramon Q, Paz-López S, Valverde MA, et al. (2020) The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370: eaba2644. 10.1126/science.aba2644 [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127. 10.1126/science.7684161 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang J, Bai D, Song J, Ye R, Zhao Z, Lei L, Hao J, Jiang C, Fang S, et al. (2013) Cell proliferation is promoted by compressive stress during early stage of chondrogenic differentiation of rat BMSCs. J Cell Physiol 228: 1935–1942. 10.1002/jcp.24359 [DOI] [PubMed] [Google Scholar]
- Wise GE, King GJ (2008) Mechanisms of tooth Eruption and orthodontic tooth movement. J Dent Res 87: 414–434. 10.1177/154405910808700509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MO, Nishida K, Bavington C, Godolphin JL, Dunne E, Walmsley S, Jobanputra P, Nuki G, Salter DM (1997) Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: Evidence of a role for alpha 5 beta 1 integrin as a chondrocyte mechanoreceptor. J Orthop Res 15: 742–747. 10.1002/jor.1100150517 [DOI] [PubMed] [Google Scholar]
- Xiong F, Ma W, Bénazéraf B, Mahadevan L, Pourquié O (2020) Mechanical coupling coordinates the co-elongation of axial and paraxial tissues in avian Embryos. Dev Cell 55: 354–366.e5. 10.1016/j.devcel.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D-L, Li H-T, Liu S-H (2020) TIMP3/TGF-β1 axis regulates mechanical loading-induced chondrocyte degeneration and angiogenesis. Mol Med Rep 22: 2637–2644. 10.3892/mmr.2020.11386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Zeng X-L, Ni J-H, Huang X-F (2013) Comparison of the biological response of osteoblasts after tension and compression. Eur J Orthod 35: 59–65. 10.1093/ejo/cjr016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


