Abstract
Background
Same-day HIV testing and antiretroviral therapy (ART) initiation is being widely implemented. However, the optimal timing of ART among patients with tuberculosis (TB) symptoms is unknown. We hypothesized that same-day treatment (TB treatment for those diagnosed with TB; ART for those not diagnosed with TB) would be superior to standard care in this population.
Methods and findings
We conducted an open-label trial among adults with TB symptoms at initial HIV diagnosis at GHESKIO in Haiti; participants were recruited and randomized on the same day. Participants were randomized in a 1:1 ratio to same-day treatment (same-day TB testing with same-day TB treatment if TB diagnosed; same-day ART if TB not diagnosed) versus standard care (initiating TB treatment within 7 days and delaying ART to day 7 if TB not diagnosed). In both groups, ART was initiated 2 weeks after TB treatment. The primary outcome was retention in care with 48-week HIV-1 RNA <200 copies/mL, with intention to treat (ITT) analysis. From November 6, 2017 to January 16, 2020, 500 participants were randomized (250/group); the final study visit occurred on March 1, 2021. Baseline TB was diagnosed in 40 (16.0%) in the standard and 48 (19.2%) in the same-day group; all initiated TB treatment. In the standard group, 245 (98.0%) initiated ART at median of 9 days; 6 (2.4%) died, 15 (6.0%) missed the 48-week visit, and 229 (91.6%) attended the 48-week visit. Among all who were randomized, 220 (88.0%) received 48-week HIV-1 RNA testing; 168 had <200 copies/mL (among randomized: 67.2%; among tested: 76.4%). In the same-day group, 249 (99.6%) initiated ART at median of 0 days; 9 (3.6%) died, 23 (9.2%) missed the 48-week visit, and 218 (87.2%) attended the 48-week visit. Among all who were randomized, 211 (84.4%) received 48-week HIV-1 RNA; 152 had <200 copies/mL (among randomized: 60.8%; among tested: 72.0%). There was no difference between groups in the primary outcome (60.8% versus 67.2%; risk difference: −0.06; 95% CI [−0.15, 0.02]; p = 0.14). Two new grade 3 or 4 events were reported per group; none were judged to be related to the intervention. The main limitation of this study is that it was conducted at a single urban clinic, and the generalizability to other settings is uncertain.
Conclusions
In patients with TB symptoms at HIV diagnosis, we found that same-day treatment was not associated with superior retention and viral suppression. In this study, a short delay in ART initiation did not appear to compromise outcomes.
Trial registration
This study is registered with ClinicalTrials.gov NCT03154320.
In an open-label randomized trial from Haiti, Nancy Dorvil and colleagues investigate the effectiveness of same-day TB testing and treatment initiation in adults with TB symptoms at HIV diagnosis compared to standard care.
Author summary
Why was this study done?
HIV treatment guidelines worldwide recommend that antiretroviral therapy (ART) be initiated immediately or as soon as possible after HIV diagnosis, to improve linkage to care and treatment uptake.
A substantial proportion of patients present with symptoms of tuberculosis (TB) at HIV diagnosis. TB testing is ideally conducted prior to ART initiation in symptomatic patients.
The optimal timing of ART initiation for this patient population is unknown. From the health center perspective, a short delay in ART initiation facilitates TB testing. However, this strategy may result in extra visits for patients, which may result in loss to follow-up (LTFU).
What did the researchers do and find?
We conducted a randomized trial to assess the effectiveness of same-day TB testing and treatment initiation (TB medication if TB diagnosed; ART if TB not diagnosed) on the day of HIV diagnosis versus standard care, in adults with TB symptoms at HIV diagnosis in Haiti.
We found near universal ART initiation in both the same-day treatment and the standard groups.
Same-day treatment was not associated with higher rates of retention in care with viral suppression at 48 weeks after enrollment, compared to the standard group. This was the primary outcome of the study.
What do these findings mean?
Our findings indicate that both strategies are effective. Same-day TB testing and treatment initiation may be preferred at sites with this capacity, but short delays in treatment initiation do not appear to compromise outcomes.
An important caveat is that treatment was provided rapidly in the standard group, reflecting current standard practice.
This study is limited by being conducted at a single, large, urban HIV clinic in Haiti. Further study will be necessary to determine if these findings are reproducible in other settings.
Introduction
Initiating antiretroviral therapy (ART) on the day of HIV diagnosis (same-day ART) or as soon as possible afterwards is now recommended in treatment guidelines worldwide, with the goal of improving ART uptake and linkage to care and decreasing time to viral suppression [1–3]. However, a substantial proportion of patients worldwide report tuberculosis (TB) symptoms at HIV diagnosis, and the optimal timing of ART initiation in this group of patients is unknown [4].
The 2017 World Health Organization (WHO) guidelines recommended as a clinical consideration that ART should be briefly delayed while investigating for TB [5]. In 2021, the updated WHO guidelines stated that: “To delay ART in individuals with TB symptoms may result in harm because of delays in ART initiation and increase the risk of pretreatment loss to follow-up.” In response, the WHO guidelines were updated with a new clinical consideration for persons living with HIV (PLWH) and signs and symptoms suggesting TB to “initiate ART while rapidly investigating for TB, with close follow-up within 7 days to initiate TB treatment if TB is confirmed.” The 2021 WHO guidelines further state that research gaps include the impact of initiating ART among people with TB symptoms on mortality, TB and HIV outcomes, adverse events, immune reconstitution inflammatory syndrome (IRIS), retention in care and adherence [1]. A recent systematic review that was conducted to answer this research question found there was insufficient evidence about whether the presence of TB symptoms at HIV diagnosis should lead to ART deferral [6].
It is logistically challenging to complete a TB evaluation on the same day of HIV diagnosis, especially in lower-income settings, as the Xpert MTB/RIF assay (Cepheid, Sunnyvale, California, United States of America) has at least a 2-h turnaround time, and tests are often conducted in centralized laboratories, with further delays for specimen transport, testing backlog, and communication of test results. Furthermore, the sensitivity of 1 Xpert MTB/RIF test is about 80% in PLWH, and a single negative test does not rule out active TB [7].
From the health center perspective, a short delay to conduct TB testing is more feasible than same-day TB testing prior to ART initiation in many settings. However, this strategy may result in added visits for patients to return for test results and ART initiation, which may be associated with pretreatment loss to follow-up (LTFU). Data on the consequences of short delays in ART initiation are limited. In the clinical trials of same-day ART versus standard care, the study populations either excluded or included a minority of patients with TB symptoms [8–11]. Furthermore, these trials were conducted several years ago, when the standard of care included multiple visits prior to ART initiation. Consequently, participants in the comparison groups generally initiated ART weeks to months after HIV diagnosis, while the current standard of care has shifted to avoid delays in ART initiation.
We conducted a randomized trial to determine whether same-day TB testing and treatment initiation (TB medication or ART) was associated with superior outcomes, compared with standard care (initiating TB treatment within 7 days and delaying ART to day 7 if TB not diagnosed), in adults with TB symptoms at HIV diagnosis. In a prior study of same-day ART versus standard care, which was conducted by our research team in persons newly diagnosed with early HIV infection in Haiti, we found that same-day ART improved rates of retention in care with viral suppression [8]. In addition to making treatment initiation logistically easier for patients, we attributed the benefits of same-day ART to an increased sense of hope, optimism, and overall connectedness to the healthcare system. Based on the results of our prior study, we hypothesized that same-day treatment would improve the primary outcome of retention with viral suppression at 48 weeks after enrollment.
Methods
Study design and setting
We conducted an open-label, randomized trial of same-day treatment versus standard care for patients who presented with symptoms of TB at HIV diagnosis at the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO) in Port-au-Prince, Haiti. The primary study endpoint was the proportion of participants in each group who were retained in care with 48-week HIV-1 RNA <200 copies/mL.
GHESKIO is a Haitian nongovernmental organization and the largest provider of HIV and TB treatment in the Caribbean. HIV care at GHESKIO is largely funded by the US President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. All care is provided free of charge. The adult HIV prevalence in Haiti is an estimated 1.8% and the estimated incidence of TB is 168/100,000 [12,13]. Haiti is ranked 163 out of 191 countries on the Human Development Index, adjacent to Nigeria, Rwanda, Benin, and Uganda [14]. Throughout the study period, there was widespread gang violence, kidnapping, and civil unrest in Port-au-Prince, and the US State Department ranked Haiti “Level 4: Do not Travel.”
Ethics statement
The study was approved by the institutional review boards at GHESKIO, Mass General Brigham, Florida International University, and Weill Cornell Medical College. Written informed consent was obtained from all participants.
Study participants
Participants were recruited if they tested positive for HIV at GHESKIO and reported at least 1 TB symptom on a routinely conducted questionnaire, which was conducted in accordance with GHESKIO standard care [15]. Patients were eligible for study inclusion if they were infected with HIV-1, ≥18 years of age, able to provide informed consent, and reported cough, fever, and/or night sweats of any duration and/or weight loss, which was confirmed by the study physician. Initially, enrollment was limited to patients who reported loss of at least 10% of body weight, but in April 2018, this was changed to any reported weight loss, because most patients were unaware of their exact change in weight.
Patients were excluded if they had received ART previously, failed to demonstrate preparedness on an ART readiness questionnaire, were treated for TB in the prior year, were pregnant or breastfeeding, had active drug or alcohol use, or a mental condition that would interfere with their ability to adhere to study requirements, or planned to transfer care during the study period. Patients were also excluded if they presented with symptoms consistent with WHO stage 4 neurologic disease (cryptococcal meningitis, tuberculosis meningitis, central nervous system toxoplasmosis, HIV encephalopathy, or progressive multifocal leukoencephalopathy), or WHO “danger signs” of temperature >39 degrees Celsius, pulse >120 beats/minute, respiratory rate >30, or inability to walk unaided. Before undergoing any study procedure, all participants provided written informed consent.
Randomization and masking
Participants were randomized in a 1:1 ratio using a computer-generated random-number list in the GHESKIO Data Management Unit. A data manager who had no other involvement in study procedures conducted randomization by using a computer-number generator, using permuted blocks with variable block sizes from 2 to 6. The data manager transmitted participant allocation to the study physician. Participants, site personnel, and investigators were not masked to group assignment.
Study procedures
Participants were enrolled and randomized on the day of HIV diagnosis. In both groups, specimens were collected for creatinine, alanine transaminase (ALT), aspartate transaminase (AST), complete blood count (CBC), and CD4 count testing (FACS Count, Becton Dickinson, Franklin Lakes, New Jersey, USA); same-day results were not available. Participants also received a digital chest radiograph and provided a spot sputum specimen for Xpert Ultra (Cepheid, Sunnyvale, California, USA) and liquid mycobacterial culture (Mycobacteria Growth Indicator Tube [MGIT], BACTEC, Beckton Dickinson, Franklin Lakes, New Jersey, USA). Specimens were refrigerated upon collection and transported to the central GHESKIO Biosafety Level 3 (BSL-3) laboratory across Port-au-Prince for testing. Key study interventions are illustrated in Fig 1.
Fig 1. Study intervention for the standard and same-day treatment groups.
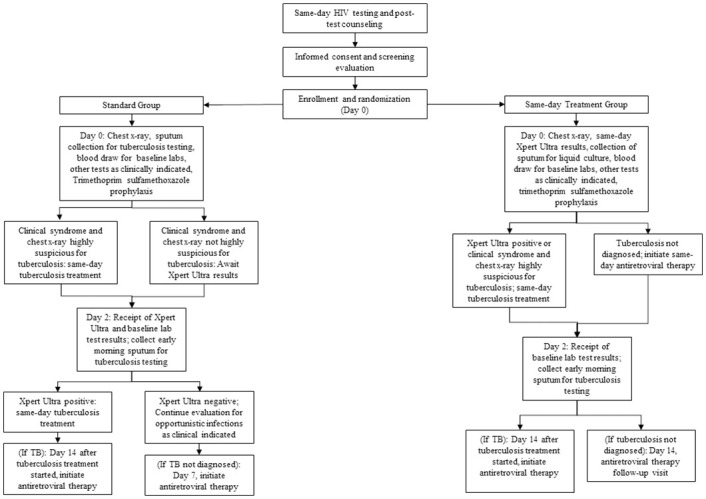
In the standard group, participants with a clinical syndrome and chest radiograph that were highly suspicious for TB initiated same-day TB treatment, without Xpert Ultra results. The remaining participants received neither ART nor TB medication on day 0. All were discharged home with co-trimoxazole prophylaxis, as well as symptomatic therapy as clinically indicated. On day 2, participants brought an early morning specimen to the laboratory for a second Xpert Ultra test and liquid mycobacterial culture and received the results of their first Xpert Ultra and baseline laboratory tests. Those with positive Xpert Ultra tests initiated same-day treatment; those with negative tests underwent continued evaluation for other opportunistic infections (OIs) as clinically indicated. On day 7, those who had not initiated TB treatment started ART; initially this was day 10, but in April 2018 it was changed to day 7 to reflect changes in standard of care. Those with TB initiated ART after completing 2 weeks of TB treatment, in accordance with WHO guidelines [1].
The same-day group received same-day (day 0) evaluation for TB, including Xpert Ultra results. This required immediate transport of sputum specimens to the central lab, prompt testing, and a phone call to study staff with test results. The study physician reviewed all clinical data and made a same-day determination about whether each participant had TB. All participants were discharged from the day 0 visit with either TB medication or ART, as well as co-trimoxazole prophylaxis, and symptomatic therapy as clinically indicated. On day 2, participants brought an early morning specimen to the laboratory for a second Xpert Ultra test and liquid mycobacterial culture, and received the results of their baseline laboratory tests, as for the standard group. Those with TB initiated ART after completing 2 weeks of TB treatment [1].
Other than the timing of availability of Xpert Ultra test results, and the timing of TB and ART treatment initiation, the same care was provided to both groups. First-line ART included efavirenz (EFV), tenofovir disoproxil fumarate (TDF), and lamivudine (3TC) until December 2018, when dolutegravir (DTG) became available. During 2019, all participants on EFV-based regimens were switched to DTG-based regimens, regardless of viral load results, in accordance with national guidelines. TB was treated with standard therapy in accordance with WHO guidelines [16]. Prophylactic isoniazid was given to all participants who were not diagnosed with active TB. Participants with CD4 count <100 cells/mm3 were treated with 5 days of empiric azithromycin and those diagnosed with TB and CD4 count <100 cells/mm3 were prescribed prophylactic prednisone, in accordance with the REALITY and PredART study interventions [17,18]. Follow-up visits were scheduled at weeks 4, 8, 12, 24, 36, and 48, as was standard of care during the study period. HIV-1 RNA testing was conducted at weeks 24 and 48, in accordance with national guidelines. At every study visit, participants were queried about symptoms of IRIS using published criteria and definitions [19,20].
Retention activities were similar to those provided for all PLWH at GHESKIO. Community health workers (CHWs) made reminder phone calls in advance of each visit, and after missed visits, with home visits for participants who could not be reached by phone. Participants received a transportation subsidy of 100 Haitian gourdes ($US 1.00) and a phone card (valued at 100 Haitian gourdes) at each visit. Participants were counseled about the importance of adhering to therapy and of returning to clinic when they had symptoms.
Outcomes
The primary outcome was the proportion of participants retained in care with HIV-1 RNA <200 copies/mL at 48 weeks after enrollment, with intention to treat (ITT) analysis, with a prespecified window of +/− 12 weeks; missing test results were considered virologic failures. Secondary outcomes included: 48-week HIV-1 RNA cut-offs of <50 copies/mL and <1,000 copies/mL; all-cause mortality, new Division of AIDS Grade 3 or 4 adverse events that were at least a 1-day increase above baseline, adherence as measured by medication possession ratio (proportion of days ART was dispensed within the study period), virologic failure with switch to second-line ART, and TB diagnosis after ART initiation (all throughout the 48-week study period); incidence of IRIS during the 12 weeks after ART initiation; and sensitivity of spot and early morning Xpert Ultra. Bacteriologically confirmed (BAC+) TB required confirmation by molecular testing or culture. Empiric TB was defined as symptoms and chest radiograph consistent with TB, without BAC+ confirmation. Participants were considered retained if they attended the 48-week visit. LTFU was defined as lost to care without known death; those who returned for at least 1 visit after missing the 48-week visit were classified as returned to care. Deaths were defined by family report; death certificates are not available in Haiti. Transfer was defined as documented transfer to an outside clinic.
Statistical analysis
The prespecified target sample size was 600. For the primary outcome, a sample size of 388 was calculated to provide at least 80% power to detect an absolute difference of 14% in the proportion of participants with 48-week HIV-1 RNA <200 copies/mL (51% in the standard and 65% in the same-day treatment group), assuming a (two-sided) significance level of 0.05, using the Chi-square test; these estimates were based on a prior study of same-day ART conducted by our team at GHESKIO [8]. The target sample size was inflated to 600 to provide 80% power to detect an absolute difference in a secondary outcome of 48-week mortality of 6% (10% in the standard and 4% in the same-day treatment group), assuming a LTFU rate of 5% and significance level of 0.05. Enrollment was stopped on January 16, 2020, after 500 participants had been enrolled, at the recommendation of the independent Data Safety Monitoring Board (DSMB), which met twice yearly to review study progress and participant safety. The target enrollment for the primary outcome (n = 388) had already been surpassed, and the secondary outcome of mortality was much lower than expected in the standard group (2.4% in the standard versus 3.6% in the same-day group). Though stopping rules had not been prespecified, the DSMB determined that it would be futile to achieve a detectable mortality difference with the addition of the remaining 100 participants.
Baseline characteristics were summarized using simple frequencies and proportions for categorical variables and medians with interquartile ranges (IQRs) for continuous variables by treatment group. We compared the proportion of participants with 48-week HIV-1 RNA <200 copies/mL (primary endpoint) and binary secondary outcomes using the Chi-square test (Fisher exact test was used instead for sparse data). For all comparisons, we presented unadjusted risk difference (RD) with 95% confidence interval (CI) and p-value. CIs were computed by using a Wilson score interval.
For all analyses, an intention-to-treat approach was used, in which all participants were analyzed according to their assigned group. We did all analyses with SAS version 9.4. The study was registered with ClinicalTrials.gov NCT03154320.
Results
A total of 1,738 adults (age ≥18 years) without known current TB treatment or pregnancy were diagnosed with HIV from November 6, 2017 to January 16, 2020. Of these, 669 (38.5%) reported at least 1 TB symptom during routine symptom screening and were informed about the study. Ninety-three patients were excluded prior to screening because they were not interested in study participation (n = 50), not accepting of HIV diagnosis (n = 23), or had a severe untreated co-morbidity (n = 20). Of the 576 patients referred for screening, 500 were recruited, enrolled, and randomize, on the same day (n = 250 per group)—see Fig 2. All 250 participants in each group were included in the analysis. The final study visit occurred on March 1, 2021.
Fig 2. CONSORT Diagram: Screening, randomization, and analysis populations.
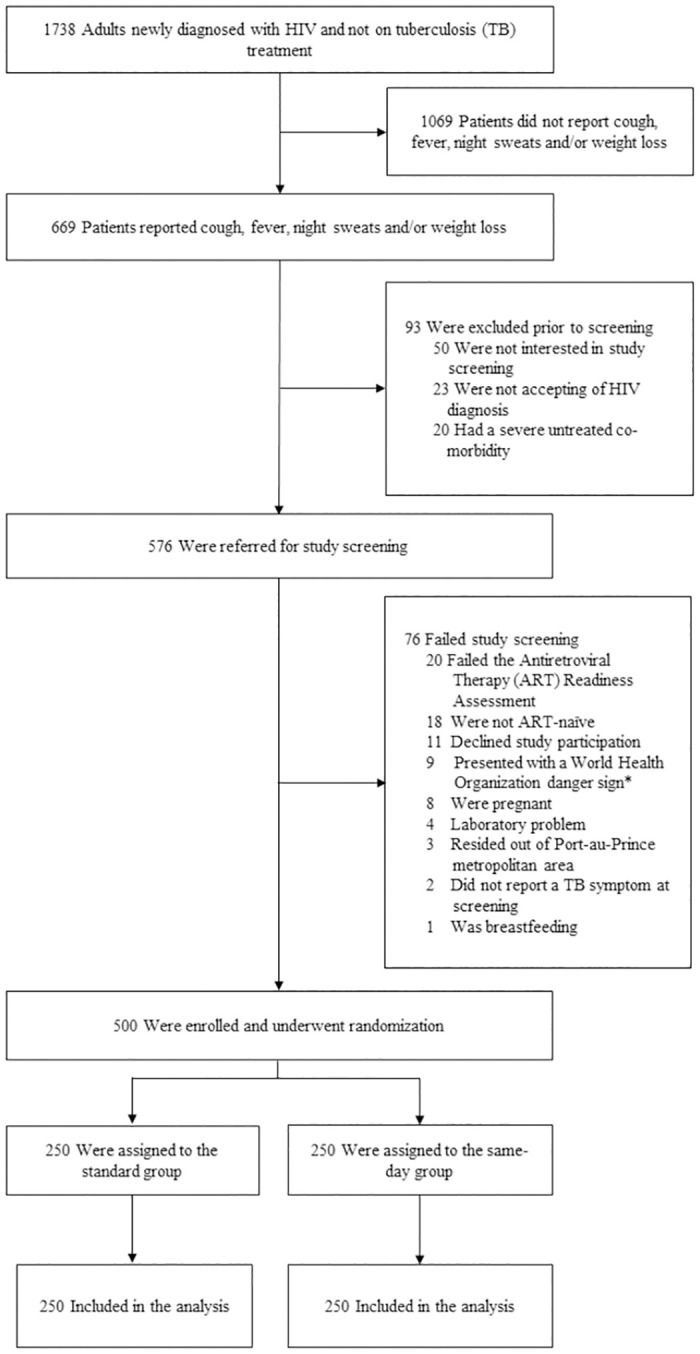
*World Health Organization danger signs include respiratory rate >30/minute, fever >39° C, pulse >120 minute, and unable to walk unaided.
The median age of enrolled participants in the total cohort was 37 years (IQR: 30, 45), 234 (46.8%) were female, and 372 (74.4%) were living on <$US 1.00 per day (see Table 1). Two hundred (40.0%) participants reported cough, 194 (38.8%) reported fever, 72 (14.4%) reported night sweats, and 491 (98.2%) reported weight loss at study screening. Median body mass index (BMI) was 20.6 (IQR: 18.7, 22.9) and median CD4 count was 274 (IQR: 128, 426); 101 (20.2%) had CD4 count <100 cells/mm3. Forty (16.0%) participants in the standard group, and 48 (19.2%) in the same-day treatment group were diagnosed with baseline TB. Of these, 32 (80.0%) in the standard and 36 (75.0%) in the same-day treatment groups had BAC+ TB.
Table 1. Baseline characteristics of study participants by group.
| Standard group (n = 250) | Same-day treatment group (n = 250) | |
|---|---|---|
| Female—no. (%) | 117 (46·8) | 117 (46·8) |
| Age at enrollment (years)—median (IQR) | 37·0 (30·0, 45·0) | 36·0 (30·0, 45·0) |
| Income—no. (%) | ||
| <$US 1.00 per day | 190 (76·0) | 182 (72·8) |
| ≥$US 1.00 per day | 58 (23·2) | 65 (26·0) |
| Missing or unknown | 2 (0·8) | 3 (1·2) |
| Education level—no. (%) | ||
| None | 46 (18·4) | 38 (15·2) |
| Some primary | 95 (38·0) | 101 (40·4) |
| At least some secondary | 107 (42·8) | 107 (42·8) |
| Missing or unknown | 2 (0·8) | 4 (1·6) |
| Marital status—no. (%) | ||
| Single | 64 (25·6) | 70 (28·0) |
| Married | 137 (54·8) | 124 (49·6) |
| Formerly married | 47 (18·8) | 53 (21·2) |
| Missing or unknown | 2 (0·8) | 3 (1·2) |
| TB symptoms—no. (%) | ||
| Reported cough | 100 (40·0) | 100 (40·0) |
| Reported fever | 96 (38·4) | 98 (39·2) |
| Reported night sweats | 34 (13·6) | 38 (15·2) |
| Reported weight loss | 243 (97·2) | 248 (99·2) |
| BMI—median (IQR) | 21·0 (18·8, 23·5) | 20·3 (18·5, 22·7) |
| CD4 count (cells/mm3)—median (IQR) | 293 (157, 447) | 228 (114, 398) |
| CD4 count category—no. (%) | ||
| <100 cells/mm3 | 43 (17·2) | 58 (23·2) |
| 100 to 349 cells/mm3 | 109 (43·6) | 116 (46·4) |
| 350 to 499 cells/mm3 | 48 (19·2) | 36 (14·4) |
| ≥500 cells/mm3 | 47 (18·8) | 38 (15·2) |
| Missing | 3 (0·6) | 2 (0·4) |
| Hemoglobin (g/dl)—median (IQR) | 12·0 (10·2, 13·6) | 11·7 (10·0, 13·4) |
| TB status at HIV diagnosis—no. (%) | ||
| Bacteriologically confirmed TB | 32 (12·8) | 36 (14·4) |
| Empirically diagnosed TB | 8 (3·2) | 12 (4·8) |
| TB not diagnosed | 210 (84·0) | 202 (80·8) |
BMI, body mass index; IQR, interquartile range; TB, tuberculosis.
Among participants with BAC+ TB, 64.7% (44/68) had a positive Xpert Ultra test on the spot specimen; 72.1% (49/68) had a positive Xpert Ultra test on the early-morning specimen; 80.1% (55/68) had at least 1 positive Xpert Ultra; 97.1% (66/68) were culture-positive; and 2 (2.9%) were Xpert Ultra positive but culture-negative. An additional 18 participants were empirically diagnosed with pulmonary TB, with negative sputum testing, and 2 participants were diagnosed with extrapulmonary TB.
Baseline TB prevalence varied by reported symptoms. Among participants in both groups who reported cough, fever, night sweats, or weight loss, 35.0% (70/200), 33.0% (64/194), 44.4% (32/72), and 17.3% (85/491), respectively, were diagnosed with TB. Among participants in both groups who reported only weight loss, 3.8% (9/239) were diagnosed with TB.
In the standard group, 33 of 40 (82.5%) participants diagnosed with baseline TB initiated treatment prior to ART initiation; of these, 30 (90.1%) initiated TB treatment within 7 days of enrollment, and all 33 initiated ART at a median time of 14 days (IQR: 13, 14) after TB treatment. Seven (17.5%) participants (with negative Xpert Ultra and positive mycobacterial cultures) initiated ART prior to TB diagnosis; TB treatment was initiated within a range of 14 to 128 days after ART. Among the 210 participants who were not diagnosed with baseline TB, 205 (97.6%) initiated ART at a median time of 7 days (IQR: 7, 10). Of these, 110 (53.7%) initiated ART within 7 days, 83 (40.5%) from 8 to 14 days and 12 (5.8%) >14 days after HIV diagnosis. Among all participants randomized to the standard group (regardless of TB status), ART was initiated a median of 9 days (IQR: 7, 12) after enrollment.
In the same-day treatment group, 37 of 48 (77.1%) participants diagnosed with baseline TB initiated TB treatment on the day of HIV diagnosis; of these, 36 (97.3%) initiated ART at a median time of 14 days (IQR: 14, 15) after TB treatment, and one was LTFU prior to starting ART. Eleven (22.9%) participants initiated ART prior to TB treatment. Of these, 5 had negative spot but positive early morning Xpert Ultra tests and initiated TB treatment within a range of 7 to 18 days after ART; 6 had negative Xpert Ultra tests, but positive mycobacterial cultures, and initiated TB treatment within a range of 37 to 89 days after ART. All 202 participants who were not diagnosed with baseline TB initiated ART on the day of HIV diagnosis. Among all participants randomized to the same-day treatment group (regardless of TB status), ART was initiated at a median time of 0 days (IQR: 0, 0) after enrollment.
Among the 250 participants in the standard group, 229 (91.6%) attended the 48-week visit, and 220 (88.0%) received HIV-1 RNA testing with the 48-week visit window; of these, 168 (76.4% of those tested; 67.2% of those randomized) had HIV-1 RNA <200 copies/mL (see Table 2). Among the 250 participants in the same-day treatment group, 218 (87.2%) attended the 48-week visit, and 211 (84.4%) received HIV-1 RNA testing within the 48-week visit window; of these, 152 (72.0% of those tested; 60.8% of those randomized) had HIV-1 RNA <200 copies/mL. There was no difference in the primary outcome (retained in care with 48-week HIV-1 RNA <200 copies/mL) between the same-day and standard groups (RD: −0.06; 95% CI: −0.15, 0.02; p = 0.14).
Table 2. Primary and secondary study outcomes by group.
| Outcome | Standard group (n = 250) | Same-day treatment group (n = 250) | RD (95% CI) | p-Value |
|---|---|---|---|---|
| Primary outcome | ||||
| 48-week HIV-1 RNA <200 copies/mL* | 168 (67·2) | 152 (60·8) | −0·06 (−0·15, 0·02) | 0·14 |
| Secondary outcomes | ||||
| 48-week HIV-1 RNA <50 copies/mL* | 159 (63·6) | 137 (54·8) | −0·09 (−0·17, −0·002) | 0·045 |
| 48-week HIV-1 RNA <1,000 copies/mL* | 176 (70·4) | 159 (63·6) | −0·07 (−0·15, 0·01) | 0·11 |
| Forty-eight-week outcomes** | ||||
| Attended 48-week study visit* | 229 (91·6) | 218 (87·2) | −0·04 (−0·10, 0·01) | 0·11 |
| Died | 6 (2·4) | 9 (3·6) | 0·01 (−0·02, 0·05) | 0·43 |
| Lost to follow-up | 10 (4·0) | 14 (5·6) | 0·02 (−0·02, 0·06) | 0·40 |
| Missed 48-week visit due to gap in care, with subsequent return to care | 4 (1·6) | 6 (2·4) | 0·01 (−0·02, 0·04) | 0·52 |
| Transferred | 1 (0·4) | 3 (1·2) | 0·01 (-0·01, 0·03) | 0·62 |
*Nine participants in the standard treatment and 7 in the same-day group attended 48-week visit without HIV-1 RNA testing.
**These are mutually exclusive outcomes which include all 500 randomized participants.
CI, confidence interval; RD, risk difference.
Among the 220 participants in the standard and 211 in the same-day treatment group who received 48-week viral load testing, 159 in the standard group had HIV-1 RNA <50 copies/mL (72.3% of those tested; 63.6% of those randomized), compared with 137 in the same-day group (64.9% of those tested; 54.8% of those randomized). The same-day group was less likely than the standard group to achieve 48-week HIV-1 RNA <50 copies/mL (54.8% versus 63.6%; RD: −0.09; 95% CI: −0.17, −0.002)—see Table 2. With an HIV-1 RNA cut-off of 1,000 copies/mL, 176 in the standard group (80.0% of those tested; 70.4% of those randomized) and 159 in same-day treatment group (75.4% of those tested; 63.6% of those randomized) achieved viral suppression; there was no difference between the same-day and standard groups (63.6% versus 70.4%; RD: −0.07; 95% CI: −0.15, 0.01). There was also no difference in the timing of the 48-week visit between groups (median time of 333 days [IQR: 328 to 344] in the standard group and 336 days [IQR: 329 to 348; p = 0.35] in the same-day group).
In the standard group, 21 participants (8.4%) were not retained in ART care at 48 weeks after enrollment. A total of 6 (2.4%) died, 10 (4.0%) were LTFU, 4 (1.6%) had a gap in care during the 48-week visit window with subsequent return to care, and 1 (0.4%) was transferred (see Table 2). In the same-day treatment group, 32 (12.8%) participants were not retained in ART care at 48 weeks after enrollment. A total of 9 (3.6%) died, 14 (5.6%) were LTFU, 6 (2.4%) had a gap in care during the 48-week visit window with subsequent return to care, and 3 (1.2%) were transferred. There were no differences between groups in retention (87.2% versus 91.6%; RD: −0.04; 95% CI: −0.10, 0.01) or mortality (3.6% versus 2.4%; RD: +0.01; 95% CI: −0.02, 0.05).
Three (1.2%) participants in the standard group were diagnosed with incident TB, 2 of these were treated empirically for pulmonary TB and 1 for extrapulmonary TB. One (0.04%) participant in the same-day group was diagnosed with incident TB (Xpert Ultra/culture-positive tuberculous lymphadenitis). No participant in either group was diagnosed with either unmasking or paradoxical IRIS during the first 12 weeks after ART initiation.
Among the 6 participants in the standard group who died during the study period, 2 deaths were attributed to incident TB, 1 to mycobacterial avium complex, 1 to advanced AIDS with OI of unclear etiology, 1 to tear gas inhalation during a street protest, and 1 cause of death was unknown. Among the 9 participants who died in the same-day treatment group, 3 were attributed to advanced AIDS with OI of unclear etiology, 1 to advanced AIDS and gastroenteritis, 1 to Kaposi’s sarcoma diagnosed after enrollment, 1 to cancer, 1 to renal failure, and 2 were unknown.
Among the participants who received 48-week viral load testing, 61.8% (136/220) in the standard and 65.9% (139/211) in the same-day group had adherence ≥90% by pharmacy refill; there were no differences in adherence between the 2 groups (RD: 0.04; 95% CI: −0.05, 0.13). Among participants with 48-week HIV-1 RNA ≥200 copies/mL, adherence was <90% among 76.9% (40/52) in the standard and 57.6% (34/59) in the same-day treatment group.
Among the 245 participants in the standard group who initiated ART, 70 (28.6%) received only an EFV-based regimen, 83 (33.9%) initiated EFV and switched to a DTG-based regimen due to drug availability, 90 (36.7%) received only a DTG-based regimen, and 2 (0.8%) initiated EFV and switched to a second-line PI-based regimen. Among the 249 participants in the same-day treatment group who initiated ART, these numbers were 73 (29.3%), 75 (30.1%), 94 (37.8%), and 7 (2.8%), respectively. There was no difference between groups in switches to second-line ART (2.8% versus 0.8%; RD: 0.02; 95% CI: −0.004, 0.04; p = 0.18). Two participants per group (0.08%) developed a new Grade 3 or 4 adverse event that was at least a one-grade increase from baseline. In the standard group, 1 participant developed Grade 4 increase in creatinine and 1 developed Grade 4 anemia. In the same-day group, 1 participant developed a Grade 3 increase in creatinine and 1 developed Grade 4 anemia. None of these adverse events were judged by the investigators to be related to the intervention.
Discussion
The results of this randomized trial show that in patients with TB symptoms at initial HIV diagnosis, a short delay in treatment initiation does not appear to compromise outcomes, compared with same-day treatment. The findings of this study do not confirm our hypothesis that same-day TB assessment and same-day treatment with TB medication or ART would improve retention with viral suppression in this patient population. We did not detect a statistically significant difference between groups in any primary or secondary outcome, except that the standard group was more likely to achieve 48-week viral suppression at the cut-off of <50 copies/mL. An important caveat is that treatment was provided rapidly in the standard group, with median time to ART initiation of only 7 days in those not diagnosed with TB, which meets WHO criteria for rapid ART initiation [21]. Our findings indicate that either strategy—same-day TB testing with initiation of same-day treatment or a short delay in ART initiation for TB assessment—is associated with near-universal initiation of ART and high levels of retention in care. The determination of which strategy is preferred will depend on outcomes other than those reported in this study, including patient costs and service delivery efficiency.
In prior randomized trials which found superior rates of retention and viral suppression with same-day ART, including a study conducted at our site in Haiti, the majority of standard group participants delayed ART initiation for weeks to months after HIV diagnosis [8,9,11,22]. In contrast, in this study delays in treatment initiation were minimized for all participants, reflecting current practice, and the standard group achieved rates of viral suppression that were numerically similar or higher than those of the same-day ART groups in previous trials, which ranged from 44% to 64% [8–11].
In contrast to our approach, in which all participants received at least 1 Xpert Ultra result prior to ART initiation, participants in the intervention group in the Simplified Algorithm for Treatment Eligibility (SLATE) II study with nonserious TB symptoms and a negative urine point-of-care lipoarabinomannan antigen of mycobacteria (LAM) test were eligible for same-day ART, with next-day tracing to contact those with positive Xpert MTB/RIF results [10]. Rates of rapid ART initiation and 8-month retention were higher in the intervention group, with no reported serious TB-related adverse events; however, only 3 of the 7 confirmed TB cases in the intervention group initiated same-day ART.
Eighteen percent of participants in our study cohort were diagnosed with TB at baseline. Though a small number started ART prior to TB diagnosis, and about 20% presented with CD4 counts <100 cells/mm3, no cases of IRIS were detected during the first 12 weeks after ART initiation [19,20]. This provides some reassurance about initiating ART after only a single negative Xpert Ultra test. However, participants with symptoms and chest radiographs that were highly suggestive of TB were initiated on TB treatment, regardless of sputum testing results. Furthermore, prophylactic prednisone was prescribed for participants with TB and CD4 count <100 cells/mm3.
The implications of our study findings are that a short delay in treatment initiation does not appear to be associated with inferior outcomes. Within the model of differentiated service delivery, patients do not all have the same needs. Same-day ART is ideal for many patients, and may be preferred at sites that can perform same-day Xpert MTB/RIF testing, which may eliminate the need for a return visit for test results. In contrast, in large sites such as ours, with centralized laboratories, the provision of same-day Xpert MTB/RIF results may be logistically complicated. An alternative approach would be initiating ART while Xpert results are pending, but we agree with the systematic review which concluded that there is insufficient evidence to recommend this strategy in settings with high burdens of TB [6]. Only a small number of patients with TB have been included in same-day ART studies, and nearly all received EFV-based regimens [6,9–11,23]. However, DTG is associated with faster improvements in immune function. ART-mediated immune restoration may cause pulmonary dysfunction, even in patients on TB treatment without clinical evidence of TB-IRIS; initiating DTG-based ART in the presence of untreated TB could potentially provoke even greater declines in pulmonary function [24,25]. Post-TB lung disease may contribute to the higher long-term mortality reported in patients cured of TB, compared with control populations [26,27].
It is critical to emphasize that the GHESKIO approach includes more than just ART initiation, as other successful programs have also described [8–11,22,23,28]. GHESKIO staff are trained in building a relationship with patients, including creating a welcoming environment, minimizing stigma, and explaining the purpose of testing for TB and other conditions, and the importance of treatment adherence. Moreover, all patients who are diagnosed with HIV at GHESKIO—regardless of study participation—are prescribed co-trimoxazole prophylaxis at a minimum on the day of HIV diagnosis. GHESKIO also provides medications free of charge for relief of common symptoms, such as cough and fever. In this way, every patient who is newly diagnosed with HIV leaves GHESKIO with at least 1 medication and support for their treatment adherence—as well as a hopeful message that HIV is a treatable disease.
Nearly two-thirds of the total study cohort achieved the primary outcome of retention in care with 48-week viral suppression. Retention rates were high, but viral suppression rates were suboptimal in both groups, though similar to global estimates from the International epidemiology Databases to Evaluate AIDS (IeDEA) consortium [29]. It is noteworthy that Haiti suffered from severe political instability, civil unrest, and gang-related violence throughout the study period and there were added disruptions from the COVID-19 pandemic [30]. In this context, it was not always feasible for participants to receive ART refills in a timely manner. Furthermore, first-line ART included an EFV-based regimen for the first year of study enrollment, and EFV resistance has been reported in over 20% of ART-naïve patients at GHESKIO [31].
Our study was conducted among nonpregnant, ART-naïve participants in a large urban clinic, which may limit the generalizability of our findings. Moreover, the quality of care which is provided at GHESKIO is high, as evidenced by the rapid time to ART initiation in both groups in this study. It is also noteworthy that we utilized a more aggressive diagnostic approach for TB than is generally provided in usual care, which may have contributed to our lack of TB-IRIS. In addition, all participants received medication for prophylaxis of OIs or symptomatic relief on the day of HIV diagnosis, which may have contributed to our high retention rate in the standard group. However, we note the prescription of non-ART medications on the day of HIV diagnosis is common practice in many settings. We also used an ART Readiness Assessment for eligibility in the study. It is possible that patients who failed this assessment would have preferentially benefited from the same-day treatment intervention, but we note that <5% of patients failed this assessment.
In conclusion, our results indicate that same-day treatment does not appear to be associated with superior outcomes, compared with standard care, for patients who present with TB symptoms at HIV diagnosis. Our findings indicate that either strategy—same-day TB testing with initiation of same-day treatment or a short delay in ART initiation for TB assessment—is associated with high rates of ART initiation and retention in care.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(DOC)
Acknowledgments
We thank the patients who participated in this study and the GHESKIO staff who cared for them. We also thank the members of the Data Safety Monitoring Board, the Community Advisory Board, and the ethics committees for their expertise and advice.
Abbreviations
- ALT
alanine transaminase
- ART
antiretroviral therapy
- AST
aspartate transaminase
- BMI
body mass index
- CBC
complete blood count
- CHW
community health worker
- CI
confidence interval
- DTG
dolutegravir
- EFV
efavirenz
- IQR
interquartile range
- IRIS
immune reconstitution inflammatory syndrome
- ITT
intention to treat
- LTFU
loss to follow-up
- OI
opportunistic infection
- PEPFAR
US President’s Emergency Plan for AIDS Relief
- PLWH
persons living with HIV
- RD
risk difference
- TB
tuberculosis
- TDF
tenofovir disoproxil fumarate
- WHO
World Health Organization
Data Availability
All relevant data are within the paper and its Supporting information files. We have included a de-identified dataset as supporting information.
Funding Statement
This study was funded by a grant from the National Institute of Allergy and Infectious Diseases (R01AI131998; primary investigator: SK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. World Health Organization. March 2021. Accessed July 1, 2022. https://www.who.int/publications/i/item/9789240022232. [PubMed]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adultadolescent-arv.pdf. Accessed July 1, 2022.
- 3.Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Centers for Disease Control and Prevention, National Institutes for Health, the HIV Medicine Association, and the Infectious Disease Society of America. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed July 1, 2022.
- 4.Hamada Y, Lujan J, Schenkel K, Ford N, Getahun H. Sensitivity and specificity of WHO’s recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5(9):e515–e523. doi: 10.1016/S2352-3018(18)30137-1 [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. World Health Organization, 2017. Accessed July 1, 2022. http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. [PubMed]
- 6.Burke RM, Rickman HM, Singh V, Kalua T, Labhardt ND, Hosseinipour M, et al. Same-day antiretroviral therapy initiation for people living with HIV who have tuberculosis symptoms: a systematic review. HIV Med. 2022;23(1):4–15. doi: 10.1111/hiv.13169 [DOI] [PubMed] [Google Scholar]
- 7.Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;6:CD009593. doi: 10.1002/14651858.CD009593.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig SP, Dorvil N, Devieux JG, Hedt-Gauthier BL, Riviere C, Faustin M, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med. 2017;14(7):e1002357. doi: 10.1371/journal.pmed.1002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Initiating Antiretroviral Therapy for HIV at a Patient’s First Clinic Visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016;13(5):e1002015. doi: 10.1371/journal.pmed.1002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maskew M, Brennan AT, Fox MP, Vezi L, Venter WDF, Ehrenkranz P, et al. A clinical algorithm for same-day HIV treatment initiation in settings with high TB symptom prevalence in South Africa: The SLATE II individually randomized clinical trial. PLoS Med. 2020;17(8):e1003226. doi: 10.1371/journal.pmed.1003226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labhardt ND, Ringera I, Lejone TI, Klimkait T, Muhairwe J, Amstutz A, et al. Effect of Offering Same-Day ART vs Usual Health Facility Referral During Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults With HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA. 2018;319(11):1103–1112. doi: 10.1001/jama.2018.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS Country Fact Sheets. Haiti 2021. Accessed September 1, 2022. https://www.unaids.org/en/regionscountries/countries/haiti.
- 13.Global Tuberculosis Program, World Health Organization, Country Profile. Haiti. 2021. Accessed September 1, 2022. https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22HT%22.
- 14.United Nations Development Program, Human Development Reports, 2022. Accessed March 15, 2023. https://hdr.undp.org/data-center/country-insights#/ranks.
- 15.Rivera VR, Lu L, Ocheretina O, Jean Juste MA, Julma P, Archange D, et al. Diagnostic yield of active case finding for tuberculosis at human immunodeficiency virus testing in Haiti. Int J Tuberc Lung Dis. 2019;23(11):1217–1222. doi: 10.5588/ijtld.18.0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Operation Handbook on Tuberculosis, Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment. May 2022. Accessed July 1, 2022. https://www.who.int/publications/i/item/9789240050761. [PubMed]
- 17.Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med. 2017;377(3):233–245. doi: 10.1056/NEJMoa1615822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meintjes G, Stek C, Blumenthal L, Thienemann F, Schutz C, Buyze J, et al. Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N Engl J Med. 2018;379(20):1915–1925. doi: 10.1056/NEJMoa1800762 [DOI] [PubMed] [Google Scholar]
- 19.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, Moodley P, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49(9):1424–1432. doi: 10.1086/630208 [DOI] [PubMed] [Google Scholar]
- 21.Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach, July 2021. March 1, 2023. https://www.who.int/publications/i/item/9789240031593. [PubMed]
- 22.Amanyire G, Semitala FC, Namusobya J, Katuramu R, Kampiire L, Wallenta J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539–e548. doi: 10.1016/S2352-3018(16)30090-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen S, Maskew M, Larson BA, Brennan AT, Tsikhutsu I, Fox MP, et al. Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya. PLoS Med. 2019;16(9):e1002912. doi: 10.1371/journal.pmed.1002912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auld SC, Maenetje P, Ravimohan S, Weissman D, Ncube I, Mlotshwa M, et al. Declines in Lung Function After Antiretroviral Therapy Initiation in Adults With Human Immunodeficiency Virus and Tuberculosis: A Potential Manifestation of Respiratory Immune Reconstitution Inflammatory Syndrome. Clin Infect Dis. 2020;70(8):1750–1753. doi: 10.1093/cid/ciz733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravimohan S, Auld SC, Maenetje P, Ratsela N, Mlotshwa M, Ncube I, et al. Lung Injury on Antiretroviral Therapy in Adults With Human Immunodeficiency Virus/Tuberculosis. Clin Infect Dis. 2020;70(9):1845–1854. doi: 10.1093/cid/ciz560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph Y, Yao Z, Dua A, Severe P, Collins SE, Bang H, et al. Long-term mortality after tuberculosis treatment among persons living with HIV in Haiti. J Int AIDS Soc. 2021;24(7):e25721. doi: 10.1002/jia2.25721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(10):1129–1137. doi: 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 28.Maskew M, Brennan AT, Venter WDF, Fox MP, Vezi L, Rosen S. Retention in care and viral suppression after same-day ART initiation: One-year outcomes of the SLATE I and II individually randomized clinical trials in South Africa. J Int AIDS Soc. 2021;24(10):e25825. doi: 10.1002/jia2.25825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han WM, Law MG, Egger M, Wools-Kaloustian K, Moore R, McGowan C, et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV. 2021;8(12):e766–e775. doi: 10.1016/S2352-3018(21)00265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haiti Travel Advisory. Travel.State.Gov. US Department of State. Accessed December 1, 2021. https://travel.state.gov/content/travel/en/traveladvisories/traveladvisories/haiti-travel-advisory.html.
- 31.Koenig S, Wu J, Pierre S. HIV drug resistance (HIVDR) in Haiti: Impact on future guidelines. AIDS 2020 virtual meeting. July 2020. Abstract PEB0258.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting information files. We have included a de-identified dataset as supporting information.


