IMPORTANCE:
Critically ill patients frequently experience acute encephalopathy, often colloquially termed “altered mental status” (AMS); however, there are no consensus guidelines or criteria about performing lumbar puncture (LP) and advanced neuroimaging in medical ICU patients with unexplained encephalopathy.
OBJECTIVES:
We sought to characterize the yield of combined LP and brain MRI (bMRI) in such patients as determined by both the frequency of abnormal results and the therapeutic efficacy of these investigations, that is, how often results changed management.
DESIGN, SETTING, AND PARTICIPANTS:
Retrospective cohort study of medical ICU patients admitted to a tertiary academic center between 2012 and 2018 who had documented diagnoses of “AMS” and/or synonymous terms, no clear etiology of encephalopathy, and had undergone both LP and bMRI.
MAIN OUTCOMES AND MEASURES:
The primary outcome was the frequency of abnormal diagnostic testing results determined objectively for LP using cerebrospinal fluid (CSF) findings and subjectively for bMRI through team agreement on imaging findings deemed significant through retrospective chart review. We subjectively determined the frequency of therapeutic efficacy. Finally, we analyzed the effect of other clinical variables on the likelihood of discovering abnormal CSF and bMRI findings through chi-square tests and multivariate logistic regression.
RESULTS:
One hundred four patients met inclusion criteria. Fifty patients (48.1%) had an abnormal CSF profile or definitive microbiological or cytological data by LP, 44 patients (42.3%) had bMRI with significant abnormal findings, and 74 patients (71.2%) had abnormal results on at least one of these investigations. Few clinical variables were associated with the abnormal findings in either investigation. We judged 24.0% (25/104) of bMRI and 26.0% (27/104) of LPs to have therapeutic efficacy with moderate interobserver reliability.
CONCLUSIONS:
Determining when to perform combined LP and bMRI in ICU patients with unexplained acute encephalopathy must rely on clinical judgment. These investigations have a reasonable yield in this selected population.
Keywords: brain diseases, clinical decision-making, critical illness, neuroimaging, spinal puncture
KEY POINTS
Questions: In adult medical ICU patients with unexplained acute encephalopathy, how often does the combination of lumbar puncture (LP) and brain MRI (bMRI) yield useful information?
Findings: In this retrospective cohort study, we found that the combination of LP and bMRI revealed abnormal results in most patients with unexplained acute encephalopathy in the ICU. Approximately one in four LPs and bMRI scans had findings that changed management. Several clinical variables may increase the probability of discovering abnormal results in either investigation.
Meaning: Performing LP and bMRI in critically ill medical patients with unexplained acute encephalopathy yields actionable clinical data in at least one in four patients.
Acute encephalopathy frequently complicates critical illness (1–3) and may be described by clinicians under the syndromic moniker “altered mental status” (AMS) until a more precise diagnosis can be rendered (3, 4). Many ICU patients with acute encephalopathy ultimately prove to have delirium—a subtype of encephalopathy (3)—attributable to critical illness itself (1, 2) and some combination of putative delirium risk factors like age, pre-existing neurocognitive disorders, severity of illness, sleep deprivation, exposure to neurotropic medications, and premorbid alcohol abuse (5–7). In these delirious patients, treatment is largely supportive and aimed at underlying cause(s). However, primary neurologic disorders, such as meningoencephalitis, nonconvulsive seizures, and stroke, also commonly present with acute encephalopathic states like delirium. Because these conditions require specific management strategies, establishing a correct diagnosis is paramount.
Intensivists face challenges in determining which patients with acute encephalopathy merit a more exhaustive diagnostic evaluation, such as lumbar puncture (LP) and brain MRI (bMRI), to evaluate for primary neurologic disorders. For ICU patients who have not sustained head trauma or undergone recent neurosurgery, there are no universally accepted guidelines for obtaining LP. In previous single-center studies, the yield of LP primarily depended on the timing of the procedure relative to ICU admission and the pretest probability of neurologic disease (8–11). Further, among all hospitalized patients, nosocomial meningitis is rare (8, 12). With respect to bMRI, appropriate use criteria for acute encephalopathy are nebulous (13), and we are not aware of any studies specifically examining the yield of advanced neuroimaging in ICU patients who have no head trauma or recent neurosurgery.
Thus, we sought to determine the utility of advanced diagnostic testing in medical ICU patients with unexplained acute encephalopathy. To increase the probability that diagnostic testing was being performed in situations of high clinical uncertainty, we studied patients who underwent both LP and bMRI during their ICU stay. The primary aim of our study was to establish how often LP and bMRI in this population revealed abnormal results. As secondary aims, we sought to determine whether specific patient characteristics and/or timing of these advanced diagnostic tests affected the likelihood of abnormal diagnostic results. As an exploratory aim, we sought to characterize the therapeutic efficacy of LPs, that is, how often findings probably or definitely affected treatment decisions. We hypothesized that combined LP and bMRI would result in therapeutically efficacious results in no more than 5% of patients with unexplained acute encephalopathy.
METHODS
Study Design and Inclusion Criteria
We conducted a retrospective cohort study of patients admitted to an academic medical ICU between July 1, 2010, and June 30, 2018. We included all adult patients 18 years old and older admitted to the medical ICU service who underwent both LP and bMRI during their index ICU admission. Furthermore, patients must have had “altered mental status” or a synonymous term documented in the medical record as the indication for both tests. Acceptable alternative patient descriptors for AMS included “comatose,” “delirious,” “encephalopathic,” “obtunded,” “unarousable,” or any other related term that the investigators felt reflected the concept of undifferentiated AMS. This study was initiated before the COVID-19 pandemic, and we decided to not expand our chart review beyond 2018 to avoid contamination by temporary changes inpatient populations and care practices that occurred during the pandemic.
Exclusion Criteria
We excluded patients for whom clear indications for LP (e.g., suspected meningitis, known leptomeningeal metastases) or bMRI (e.g., evaluation for stroke) were documented. We also excluded patients who underwent LP or bMRI before or after their care in the medical ICU service (e.g., in the emergency department, on a general ward). Further, we excluded patients with nondiagnostic studies: those with insufficient cerebrospinal fluid (CSF) for analysis, those with CSF rejected by the laboratory, those with insufficient image acquisition on bMRI, or a radiologist’s interpretation of bMRI as “nondiagnostic study” or synonymous term. Among those who underwent multiple LPs or bMRI scans under the care of the ICU service, only the first adequately diagnostic study was included for analysis.
Subject Protection and Subject Identification
Our institutional review deemed this study board (Medical College of Wisconsin/Froedtert project number 34644) exempt from full board review given its retrospective nature and low risk to subjects. Potential subjects were identified using an internal clinical records database and application of filters for inclusion criteria. Only patients who had been admitted to the ICU and who had undergone LP and bMRI during the same hospital admission were retrieved from the database. Investigators then manually screened all charts in the electronic health record to review inclusion and exclusion criteria.
Outcomes
Our primary outcome was the frequency of abnormal findings on LP or bMRI among medical ICU patients with unexplained acute encephalopathy. Our secondary outcome was identification of clinical variables that would potentially increase the yield of abnormal findings in these studies. Our exploratory outcome was the frequency that these investigations had therapeutic efficacy.
Definitions of Abnormal Findings and Therapeutic Efficacy
We defined abnormal findings on LP by the presence of pleocytosis (CSF WBC count > 5 per high-powered field), elevated CSF protein (at least 1.5× upper limit of normal), positive culture or polymerase chain reaction (PCR) test, or positive cytology. We also recorded LP opening pressure when available; however, given the infrequency with which the pressure was recorded, we did not include elevated opening pressure as an abnormal result.
An abnormal bMRI result was judged by group consensus of three investigators (M.E.N., A.G.S. [both internal medicine residents] and P.A.B. [a practicing medical intensivist]) after two investigators (M.E.N., P.A.B.) independently classified the study as “abnormal” or “negative for significant findings” based on whether new findings were present that could potentially be implicated in the patient’s encephalopathy. The two investigators (M.E.N., P.A.B.) had a Cohen’s kappa of 0.885 for their initial determinations of an abnormal study, indicating nearly perfect agreement, even before group adjudication.
Finally, because abnormal results on LP or bMRI may not have inherent diagnostic value, we evaluated the therapeutic efficacy of LP and bMRI, which we defined as results that changed clinical management. Two reviewers (M.E.N., P.A.B.) independently rated therapeutic efficacy on 5-point Likert scales for 40 patients. Cohen’s kappa was 0.64 for dichotomized ratings (score of 3 or higher) of therapeutic efficacy between the two reviewers. Because this kappa indicated moderate agreement (14), the remaining determinations of therapeutic efficacy were performed by a single investigator (M.E.N.).
Sample Size Calculation
We hypothesized that the therapeutic efficacy of LP or bMRI would approximate 5% among patients meeting inclusion criteria. To achieve 95% CI that the yield was 5% with a 95% CI of ± 2.5%, we sought a sample size of 215 patients.
Potential Explanatory Variables
For each patient, we collected key demographic and clinical variables that we hypothesized could explain the diagnostic yield of LP and bMRI. We chose many variables based on risk factors for primary ICU-acquired delirium (5–7) and previous studies examining the yield of LP (8–12) in hospitalized patients. Other variables were hypothesized by the team to be associated with yield based on clinical experience or known pathophysiologic mechanisms (e.g. immunocompromising condition increasing the risk of meningitis, personal history of malignancy increasing the risk of metastatic disease or leptomeningeal carcinomatosis, atrial fibrillation increasing the risk of multifocal cardioembolic stroke, etc). These variables are included in Table 1.
TABLE 1.
Frequency of Patient Demographics, Clinical Characteristics, and Other Clinical Variables Hypothesized to Affect Diagnostic Yield of Either Lumbar Puncture or Brain MRI in Unexplained Altered Mental Status
| Clinical Variable | Yes, n (%) | No, n (%) |
|---|---|---|
| Patient demographics and characteristics | ||
| Age > 65 yr | 36 (34.6) | 68 (65.4) |
| Male sex | 52 (50.0) | 52 (50.0) |
| ICU length of stay > 2 d before LP | 51 (49.0) | 53 (51.0) |
| ICU length of stay > 2 d before bMRI | 63 (60.6) | 41 (39.4) |
| History of immunocompromising conditions | 44 (42.3) | 60 (57.7) |
| Malignancy in past 5 yr | 26 (25.0) | 78 (75.0) |
| Underlying neurocognitive disorder | 14 (13.5) | 90 (86.5) |
| Dementia | 5 (4.8) | 99 (95.2) |
| Previous brain injury or stroke | 16 (15.4) | 88 (84.6) |
| Congenital or developmental delay | 4 (3.8) | 100 (96.2) |
| Atrial fibrillation | 14 (13.5) | 90 (86.5) |
| Ventriculoperitoneal shunt | 2 (1.9) | 102 (98.1) |
| Other clinical variables | ||
| Electroencephalogram within 48 hr before or after LP or bMRI | 83 (79.8) | 21 (20.2) |
| CT performed before bMRI and/or LP | 97 (93.3) | 7 (6.7) |
| CT performed within 1 wk before bMRI | 82 (78.8) | 22 (21.2) |
| Continuous sedation infusion of at least 12 continuous hours in the previous 72 hr before LP or MRI | 50 (48.1) | 54 (51.9) |
| Active use of therapeutic anticoagulation | 17 (16.3) | 87 (83.7) |
| Variables specific to LP | ||
| Fevers within 24 hr preceding LP | 39 (37.5) | 65 (62.5) |
| Antibacterial use within past 48 hr before LP | 90 (86.5) | 14 (13.5) |
| Antifungal use within past 48 hr before LP | 29 (27.9) | 75 (72.1) |
| Antiviral use within past 48 hr before LP | 33 (31.7) | 71 (68.3) |
| Variables specific to bMRI | ||
| Focal neurologic sign on physical examination before bMRI | 18 (17.3) | 86 (82.7) |
| Significant hypertension (systolic blood pressure > 180 mm Hg or diastolic blood pressure > 120 mm Hg) within 48 hr preceding bMRI | 22 (21.2) | 82 (78.8) |
bMRI = brain MRI, LP = lumbar puncture.
Data Analyses
We performed statistical analyses using XLSTAT (Addinsoft, New York, NY) in Microsoft Excel (Microsoft, Redmond, WA) for chi-square analyses and Stata Statistical Software Release 16.0 (StataCorp LLC, College Station, TX) for multivariate logistic regression.
For each hypothesized explanatory variable, we performed a chi-square test against the primary outcomes of abnormal results on LP and bMRI. Given our anticipated sample size, we considered p value of less than 0.05 as a statistically significant variable for chi-square tests. We then performed subsequent backward-stepwise multivariable logistic regression analysis on candidate variables. Because our sample size was smaller than expected, we set a threshold p value of less than 0.10 in backward-stepwise elimination to create multivariable logistic regression models for both LP and bMRI.
RESULTS
We identified 146 patients during the study period that had a medical ICU admission with both LP and bMRI performed during the same hospitalization. Among these, 104 patients met inclusion criteria. Patients were excluded due to diagnostic studies being obtained outside of the ICU (29 patients), diagnostic studies being incomplete or nondiagnostic (five patients), lack of documentation of AMS (four patients), or clear documentation for alternative indications for study (four patients).
In our cohort, mean patient age was 56.2 years with an interquartile range of 39.9–72.5 years. Male and female patients were equally represented (52 males, 50%). The mean ICU length of stay was 16.1 days with a median of 12 days. For posthospitalization disposition, 33 patients either expired in-hospital or were discharged to hospice (31.7%); 43 were discharged to acute inpatient rehabilitation, skilled nursing facility, or long-term acute care hospital (41.3%); 22 were discharged alive home (21.1%); the remaining three patients were discharged to other hospitals or had an unknown disposition.
LPs were slightly more often performed within 2 days of ICU admission (53 LPs, or 51.0%). However, bMRI scans were more frequently performed 2 days or more after ICU admission (63 studies, or 60.6%). Nearly all patients (97 patients, 93.6%) had undergone CT of the head during their emergency department course, hospital admission, or ICU course before LP and bMRI. Most patients (89 patients, 85.6%) who underwent LP had antibiotic use within 48 hours preceding LP. The frequencies of all patient demographics and characteristics and clinical variables of interest are elaborated in Table 1.
Frequency of Abnormal Results and Therapeutic Efficacy
Abnormal results were found in 48.1% of LPs (50/104 patients [95% CI, 38.5–57.7%]) and 42.3% of bMRI scans (44/104 patients; 95% CI, 32.8–51.8%). Twenty patients (19.2% [95% CI, 11.6–26.8%]) had abnormal results on both LP and bMRI. Thus, the combined crude “yield”—that is, at least one investigation revealing abnormal results—was 71.2% (74/104 patients [95% CI, 62.5–79.9%]).
Twenty-seven of 104 LPs (26.0% [95% CI, 17.4–34.5%]) and 25 of 104 bMRI scans (24.0% [95% CI, 15.7%–32.4%]) were determined to have therapeutic efficacy, that is, led to probable or definitive change in treatment or management. Forty-two patients (40.4% [95% CI, 31.0–49.8%]) had therapeutically efficacious results on at least one of these investigations.
Among the 50 LPs with abnormal findings, only 34.0% (17/50 CSF analyses) demonstrated abnormal cytology (three cases) or culture or PCR evidence of CNS infection (14 cases). The remaining 66.0% (33/50 CSF analyses) were only considered abnormal based on pleocytosis or elevated CSF protein.
Abnormal bMRI results most frequently included restricted diffusion (26 studies), findings suggestive of meningitis or encephalitis (seven studies), hyperintensities with multiple diagnostic considerations (seven studies), and new hemorrhage or microhemorrhage (six studies), and progression of known metastatic disease (two studies). Other abnormal findings limited to a single bMRI result included new brain mass with mass effect, enlarging cystic hygromas with mass effect, fungal sinusitis, new dural enhancement, and critical vascular stenoses. Note that some studies had multiple abnormal findings.
In chi-square analyses, none of the hypothesized explanatory variables was significantly associated with abnormal findings on LP. Only the presence of systemic anticoagulation was associated with abnormal bMRI results (χ2 = 4.177, p < 0.05).
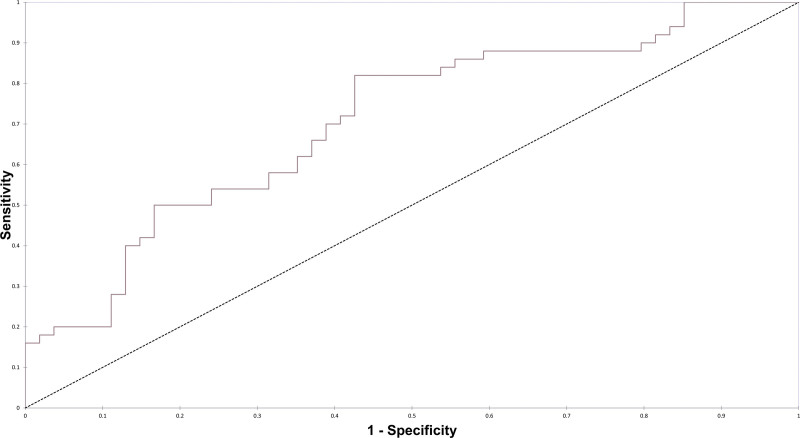
We then constructed multivariable logistic analyses using backward-stepwise approach to identify candidate variables potentially associated with abnormal results (p < 0.10). In this multivariable model, LP performed more than 2 days after ICU admission (odds ratio [OR] = 0.43 [95% CI, 0.18–1.03]), underlying cognitive deficit (OR= 0.23 [95% CI, 0.060–0.85]), history of malignancy (OR = 0.44 [95% CI, 0.17–1.17]) and antibacterial use within the past 48 hours (OR = 4.71 hr [95% CI, 1.25–17.8 hr]) were identified as potentially useful markers of abnormal results. The area under the receiver operating curve (AUROC) for the LP model using these four variables was 0.707 (Fig. 1).
Figure 1.
Receiver operating curve for multivariate logistic regression model of lumbar puncture diagnostic yield with the area under the receiver operating curve of 0.707.
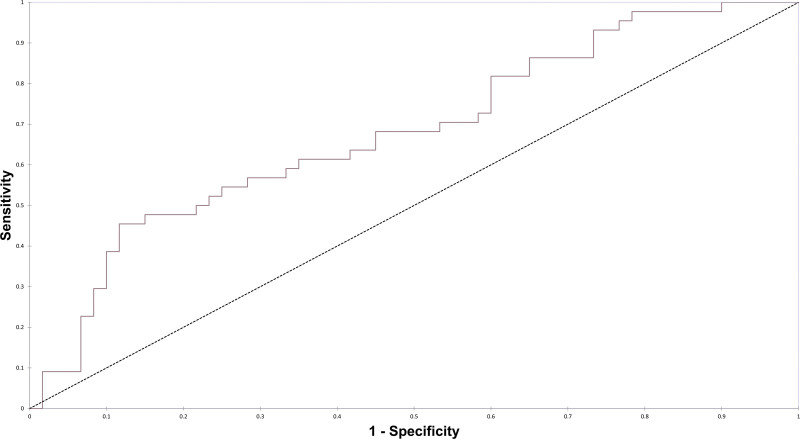
For bMRI, we constructed a similar backward-stepwise logistic regression model. In this model, electroencephalogram (EEG) within the past 48 hours (OR = 0.39 hr [95% CI, 0.12–0.97 hr]), active use of anticoagulation (OR = 3.99 [95% CI, 1.26–12.6]), and age over 65 years (OR = 2.45 yr [95% CI, 0.99–6.07 yr]) emerged as the variables most strongly associated with abnormal results on bMRI in the multivariate model, and the AUROC for the bMRI model using these three variables was 0.676 (Fig. 2).
Figure 2.
Receiver operating curve for multivariate logistic regression model of brain MRI diagnostic yield with the area under the receiver operating curve of 0.676.
DISCUSSION
In this retrospective study of patients with unexplained acute encephalopathy admitted to a medical ICU, we found that greater than 70% had abnormal findings on LP or bMRI. The therapeutic efficacy, or the frequency with which results changed management, was 24.0% for bMRI and 26.0% for LP.
Disappointingly, we were not able to identify clinical variables that were strongly associated with abnormal findings. The AUROC for both multivariate models suggests an improvement in accuracy over a “coin flip,” but ultimately the associations we describe are probably not robust enough to guide clinicians who are considering performing these investigations. Because the candidate variables for improving the crude “yield” of LP all have face validity, these variables might prove more strongly associated with diagnostic yield in a larger cohort with increased power to detect differences. For LP results, the frequency of abnormal findings and therapeutic efficacy were also likely affected by the high use of empirical antibiotics before performing the procedure. Given that many of the abnormal findings on bMRI included evidence of new ischemic infarcts or hemorrhage, the association between therapeutic anticoagulation and abnormal findings on bMRI results was not surprising. Whether EEG was already performed may reflect diagnostic uncertainty and thus is unlikely to be useful, even if the association were more robust in a larger sample size.
Together, these findings continue to support that physicians must rely on clinical judgment, rather than specific variables, to determine the necessity of LP and bMRI. Clinicians seeking guidance on when to obtain these studies in unexplained acute encephalopathy could consider the test-treatment threshold approach to clinical decision-making (15). In this approach, clinicians obtain further diagnostic data when diseases are considered probable enough that they cannot be excluded by existing clinical data, but not so likely that empiric treatment is indicated irrespective of diagnostic data.
Our study suggests that actionable clinical information that changes management will be discovered in one in every four ICU patients with unexplained encephalopathy who undergo these neurodiagnostic tests. This return is reasonable when considering the risks, inconveniences, and costs incurred through testing. Overall, complication rates of LP are low in contemporary studies and are further attenuated through ultrasound guidance (16–18). Risks of bMRI are limited to the potential complications inpatient transport to the radiology department and into the MRI scanner, which are overall low but not completely negligible (19, 20). Our data might suggest that these tests are indicated for all cases of otherwise unexplained encephalopathy among ICU patients. This interpretation; however, should be tempered with caution as abnormal findings, particularly for LP, are not necessarily therapeutically or prognostically useful. Furthermore, our cohort undoubtedly was enhanced by an unquantifiable selection bias and confounding by indication.
Our study has other significant limitations. As a retrospective study, our study could not capture all clinical variables that may have factored into decisions to obtain LPs and bMRI scans; these include admission and secondary diagnoses, findings on CT of the head, and EEG. Our study was also underpowered a priori given our gross underestimate of therapeutic efficacy, and our sample size was even smaller than anticipated for the study period. Our study also relies heavily on adequacy of documentation in the electronic health record, particularly for determining whether unexplained AMS was the primary indication for performing these tests. We also cannot exclude the possibility that AMS was erroneously given as the indication for testing when other diagnoses were suspected; for example, 18 patients in this cohort had focal neurologic findings, and bMRI may have actually been ordered to seek out a focal lesion. Further, this study analyzed patients in one academic center’s medical ICU where local practices, clinical culture, and patient populations may not be representative of other ICUs. Finally, although we strived to reduce bias in ascertaining therapeutic efficacy, our process relied on team members without expertise in neurocritical care and a definition lacking robust external validity.
In conclusion, our study suggests that performing LP and bMRI in medical ICU patients with unexplained acute encephalopathy frequently demonstrates abnormalities. Each of these diagnostic tests has therapeutic efficacy in approximately one in four such patients. We identified several clinical variables associated with finding abnormalities in LP and bMRI, but these associations should not supplant clinical judgment.
Footnotes
All authors made substantial contributions to the study design, data analysis, and interpretation. Drs. Nothem and Salazar contributed equally to data acquisition. All authors contributed to the drafting and revising of the manuscript for intellectual content and approved this version. All authors agree to be accountable for all aspects of the work.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ely EW, Inouye SK, Bernard GR, et al. : Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286:2703–2710 [DOI] [PubMed] [Google Scholar]
- 2.Ouimet S, Kavanagh BP, Gottfried SB, et al. : Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007; 33:66–73 [DOI] [PubMed] [Google Scholar]
- 3.Slooter AJC, Otte WM, Devlin JW, et al. : Updated nomenclature of delirium and acute encephalopathy: Statement of ten Societies. Intensive Care Med 2020; 46:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrouz R, Godoy DA, Azarpazhooh MR, et al. : Altered mental status in the neurocritical care unit. J Crit Care 2015; 30:1272–1277 [DOI] [PubMed] [Google Scholar]
- 5.Maldonado JR: Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018; 33:1428–1457 [DOI] [PubMed] [Google Scholar]
- 6.Zaal IJ, Devlin JW, Peelen LM, et al. : A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015; 43:40–47 [DOI] [PubMed] [Google Scholar]
- 7.Mehta S, Cook D, Devlin JW, et al. ; SLEAP Investigators: Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 2015; 43:557–566 [DOI] [PubMed] [Google Scholar]
- 8.Metersky ML, Williams A, Rafanan AL: Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis 1997; 25:285–288 [DOI] [PubMed] [Google Scholar]
- 9.Jackson WL, Jr, Shorr AF: The yield of lumbar puncture to exclude nosocomial meningitis as aetiology for mental status changes in the medical intensive care unit. Anaesth Intensive Care 2006; 34:21–24 [DOI] [PubMed] [Google Scholar]
- 10.Adelson-Mitty J, Fink MP, Lisbon A: The value of lumbar puncture in the evaluation of critically ill, non-immunosuppressed, surgical patients: A retrospective analysis of 70 cases. Intensive Care Med 1997; 23:749–752 [DOI] [PubMed] [Google Scholar]
- 11.Khasawneh FA, Smalligan RD, Mohamad TN, et al. : Lumbar puncture for suspected meningitis after intensive care unit admission is likely to change management. Hosp Pract (1995) 2011; 39:141–145 [DOI] [PubMed] [Google Scholar]
- 12.Durand ML, Calderwood SB, Weber DJ, et al. : Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 1993; 328:21–28 [DOI] [PubMed] [Google Scholar]
- 13.Luttrull MD, Boulter DJ, Kirsch CFE, et al. ; Expert Panel on Neurological Imaging: ACR Appropriateness Criteria® Acute Mental Status Change, Delirium, and New Onset Psychosis. J Am Coll Radiol 2019; 16:S26–S37 [DOI] [PubMed] [Google Scholar]
- 14.McHugh ML: Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012; 22:276–282 [PMC free article] [PubMed] [Google Scholar]
- 15.Pauker SG, Kassirer JP: The threshold approach to clinical decision making. N Engl J Med 1980; 302:1109–1117 [DOI] [PubMed] [Google Scholar]
- 16.Millington SJ, Silva Restrepo M, Koenig S: Better with ultrasound: Lumbar puncture. Chest 2018; 154:1223–1229 [DOI] [PubMed] [Google Scholar]
- 17.Wolfe KS, Kress JP: Risk of Procedural Hemorrhage. Chest 2016; 150:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodilsen J, Mariager T, Vestergaard HH, et al. : Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA 2020; 324:1419–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahner D, Nikolic A, Marhofer P, et al. : Incidence of complications in intrahospital transport of critically ill patients—experience in an Austrian university hospital. Wien Klin Wochenschr 2007; 119:412–416 [DOI] [PubMed] [Google Scholar]
- 20.Schwebel C, Clec’h C, Magne S, et al. ; OUTCOMEREA Study Group: Safety of intrahospital transport in ventilated critically ill patients: a multicenter cohort study. Crit Care Med 2013; 41:1919–1928 [DOI] [PubMed] [Google Scholar]