Abstract
Background:
Tobacco use among persons with Type II diabetes exponentially increases negative health consequences and mortality rates. It is especially troubling that diabetic persons who smoke have a greater difficulty with tobacco cessation as compared to non-diabetic smokers. Diabetes is a metabolic syndrome that consists of insulin resistance due to disruptions in insulin signaling. We have previously shown that insulin depletion enhances the motivational effects of nicotine.
Methods:
The present study expands our previous work by examining whether insulin resistance, produced by a high-fat diet (HFD) regimen, enhances the rewarding effects of nicotine, as measured by the conditioned place preference (CPP) paradigm. Rats were placed on either a regular diet (RD) or a HFD for 5 weeks, after which they were assessed for insulin resistance via blood glucose measurements after an insulin challenge. Rats then underwent a nicotine CPP study.
Results:
The findings revealed that HFD produced insulin resistant and non-insulin resistant animals. Interestingly, the magnitude of nicotine CPP was larger in insulin resistant rats versus RD rats. Nicotine CPP was absent in non-insulin resistant animals. A similar increase in body weight was observed in insulin resistant and non-insulin resistant rats as compared to RD rats. These findings suggest that neither the increased body weight nor the HFD per se in the insulin resistant rats contributed to the enhanced nicotine reward.
Conclusion:
These present study suggests that insulin resistant rats undergo unique neurobiological changes related to a disruption in insulin signaling that promotes the rewarding effects of nicotine.
Keywords: Tobacco, Diabetes, Insulin resistance, Drug use, Conditioned place preference, High fat diet
1. Introduction
The obesity epidemic has resulted in an exponential rise in the incidence of Type II diabetes (Buse et al., 2007). Diabetes increases the incidence of deleterious health conditions, such as hypertension, myocardial infarction, stroke and cancer (Buse et al., 2007). Tobacco use is a risk factor that further promotes the negative health consequences of Type II diabetes (Buse et al., 2007; Cho et al., 2009; Eliasson et al., 1997; Facchini et al., 1992; Gill et al., 2005). Unfortunately, the smoking cessation rates are significantly lower in diabetic versus non-diabetic persons (Gill et al., 2005; Solberg et al., 2004). The possibility exists that diabetic persons experience strong rewarding effects of nicotine, which may promote smoking initiation and continued use. These findings highlight the importance of research aimed at understanding the biological factors that promote tobacco use among persons with diabetes.
A hallmark feature of Type II diabetes is insulin resistance, which is defined by a reduction in sensitivity to insulin, leading to a disruption in insulin signaling. Insulin resistance in rodents is defined by a deficit in the ability of insulin to decrease plasma glucose levels following an insulin challenge (Inzucchi et al., 2012). Previous work has demonstrated that a high-fat diet (HFD) regimen produces insulin resistance in rodents (Ahren et al., 1999; Baladi et al., 2011; Buettner et al., 2006; Ramirez et al., 1990). The present study compared the rewarding effects of nicotine in HFD-induced insulin resistant and regular diet (RD) fed rats. Nicotine reward was assessed using the conditioned place preference (CPP) paradigm, which taps into drug-associated cues that modulate tobacco use. Based on the clinical literature, we hypothesized that insulin resistant rats would display enhanced rewarding effects of nicotine.
2. Methods
2.1. Subjects and diet
Adult Male Sprague-Dawley rats (Harlan, Inc.) weighing 350–400 g were pair-housed and maintained on a 12-h light/dark cycle with unrestricted access to food and water. The food was either a RD (Teklad 8604; 24.3% protein; 40.2% carbohydrate; 4.7% fat w/w) or a HFD (Bio-Serv F6387; 18.1% protein; 43% carbohydrate; 30% fat w/w). Rats were placed on either diet for 5 weeks. The body weights and blood glucose levels (BGL) were monitored weekly using an AlphaTRAK glucometer. All procedures were approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences.
2.2. Insulin resistance determination
After continuous exposure to the RD or HFD for 5 weeks, an insulin challenge test was performed. For the insulin challenge test, the rats were fasted overnight. The following morning, blood was collected from the tail vein of non-restrained animals and baseline BGLs were taken. The animals then received regular insulin (Humulin-R; 0.75 U/kg; i.p.) and BGLs were re-measured 15, 30, 60, 120 and 180 min later. According to the results of the BGLs obtained from the insulin challenge, the HFD fed rats were categorized into insulin resistant or non-insulin resistant groups.
2.3. CPP procedures
The CPP apparatus consisted of two distinct conditioning chambers. One of the chambers had black and white horizontally striped walls and a metal rod floor, whereas the adjacent chamber had solid white walls and a metal mesh floor. The tactile and visual cues were chosen to produce a biased apparatus. All rats were handled for 3 days prior to the start of conditioning. An 8-day procedure was used, consisting of a pre-conditioning test day, six conditioning days, and a post-conditioning test day. On the pre-conditioning test, time spent in each chamber was recorded for 15 min. During conditioning, rats were injected with saline or nicotine (0.2 mg/kg, free base, s.c.) and were placed into their initially non-preferred side for 30 min. The 0.2 mg/kg dose of nicotine was chosen because it produces a reliable CPP in rats (Le Foll and Goldberg, 2005; Torres et al., 2008). On the post-conditioning test, the amount of time spent in each chamber was re-assessed for 15 min. CPP data were expressed as a difference in time spent on the drug-paired side between preconditioning and postconditioning test days. An increase in the difference in time spent on the drug-paired side between nicotine conditioned groups with their respective saline conditioned groups qualified as a CPP.
2.4. Statistical analysis
Two-way ANOVAs were used to analyze the BGLs and CPP data, while a one-way ANOVA was used to analyze body weight. Newman–Keuls analyses were performed to assess group differences with a criteria of p < 0.05. At the completion of the 5-week diet regimen, 12 rats were placed in the RD group, 12 rats were placed in the HFD non-insulin resistant group and 11 rats were placed in the HFD insulin resistant group.
3. Results
3.1. Insulin resistance and body weight
Following an insulin challenge, rats that received a HFD displayed significant alterations in BGLs (Diet main effect: F(2,163) = 72.47, p < 0.001) (Fig. 1A). The pattern of changes in BGL revealed that HFD consumption produced two subsets of rats. The first subset, the HFD insulin resistant rats, exhibited increased baseline BGLs versus RD rats and a blunted decrease in response to insulin, indicative of insulin resistance. The second subset, the HFD non-insulin resistant rats, exhibited baseline blood glucose levels similar to control rats and displayed a progressive drop in BGLs in response to an insulin injection, indicative of rats that were non-insulin resistant. The average body weights of the animals in each treatment group were similar at the time they were assigned to their diet regimens. Rats assigned to the RD group had an average weight of 371 g and rats placed in the HFD group had an average body weight of 374 g. After 5 weeks of access, body weights of all HFD fed rats were significantly elevated as compared to RD rats (Diet main effect: F(2,34) = 11.93, p < 0.001) (Fig. 1B).
Fig. 1.
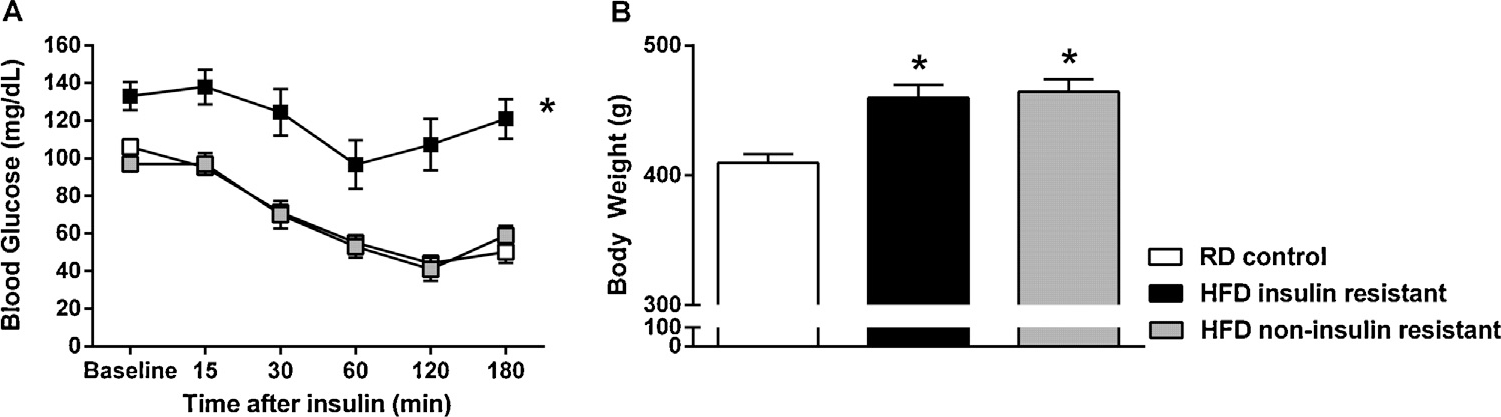
Mean (±S.E.M) blood glucose levels and body weight in HFD and RD treated rats. Panel A: Plasma blood glucose levels were measured prior to and at timed intervals following an injection of insulin (0.75 U/kg, i.p.). Panel B: Fasting body weights (g) taken just prior to the insulin challenge. n = 8–12 rats/group. *Represents differences from RD control group (p < 0.05).
3.2. CPP
Nicotine produced CPP in both RD and HFD insulin resistant rats (drug × diet interaction: F(2,35) = 3.266, p < 0.05). Furthermore, HFD insulin resistant rats spent significantly more time on the nicotine-paired side as compared to the HFD non-insulin resistant and RD rats (p < 0.05). Interestingly, HFD non-insulin resistant rats did not display nicotine CPP (Fig. 2).
Fig. 2.
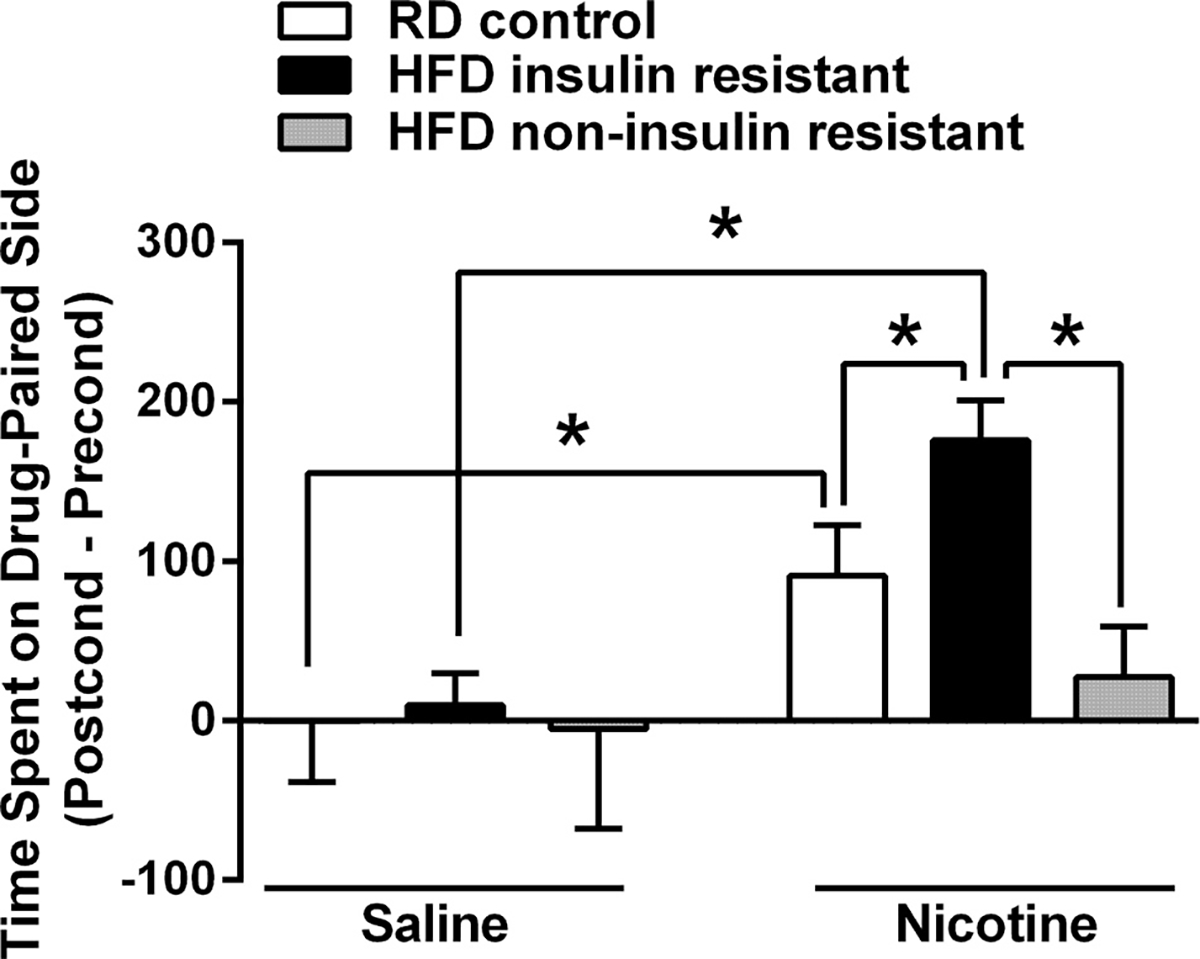
Mean (±S.E.M) difference in time spent (s) in the drug-paired chamber between preconditioning and postconditioning test days for rats conditioned with saline or nicotine (0.2 mg/kg) in the CPP paradigm. HFD insulin resistant rats exhibited greater rewarding effects of nicotine as compared to RD controls, whereas HFD non-insulin resistant rats did not exhibit any rewarding effects of nicotine. n = 6–9 rats/group. *Represents differences between indicated groups (p < 0.05).
4. Discussion
The present study revealed that consumption of HFD resulted in the development of two subgroups of rats, those that were insulin resistant and those that were non-insulin resistant, in response to an insulin challenge. Given that not all overweight humans develop insulin resistance and Type II diabetes, this distribution among HFD rats lends validity to this rodent model of insulin resistance. The major finding of the present study is that insulin resistant rats displayed elevated nicotine reward. This is consistent with our previous finding that rats depleted of insulin display a suppression of dopamine systems along with an increase in nicotine self-administration behavior (O’Dell et al., 2013). Taken together, these studies suggest that a disruption in insulin signaling via insulin resistance or a depletion of insulin alters neural systems in a manner that promotes the rewarding effects of nicotine.
In recent years, numerous studies have assessed the effects of insulin in reward processing, particularly for palatable foods in healthy rats. These studies found that intracranial application of acute doses of insulin to rats suppresses the dopamine system and decreases food reward (Figlewicz et al., 2008, 2006; Mebel et al., 2012; Sipols et al., 2002). On the other hand, deletion of insulin receptors in healthy rats increases rewarding effects of palatable foods (Könner et al., 2011). These studies, along with our present findings suggest that the effect of insulin is to reduce reward processing, whereas disruption of insulin signaling increases reward processing. The effects of insulin and its signaling machinery may alter reward processing by working to reduce dopamine release (O’Dell et al., 2013), as well as changes in the density and function of dopamine transporter and receptors (O’Dell et al., 2013; Williams et al., 2007). However, the precise mechanism by which insulin resistance enhances nicotine reward processing requires further examination
Another finding of this report is that HFD fed rats that did not develop insulin resistance, also did not display nicotine CPP. This is consistent with a report showing that the rewarding effects of nicotine are diminished in mice, as well as humans that consume a HFD (Blendy et al., 2005). In support of this hypothesis, we observed in our study that the RD control rats that were not insulin resistant developed nicotine CPP. Therefore, the possibility exists that consumption of HFD altered the reward circuitry in a manner that suppressed nicotine reward, via mechanisms independent of insulin signaling. Altogether, a distinct pattern emerged following the HFD regimen, such that HFD-induced disruption of insulin signaling resulted in an enhanced nicotine reward processing, whereas HFD consumption in the absence of compromised insulin signaling resulted in attenuated nicotine reward processing.
The present study has several important clinical implications. Our results suggest that persons with insulin resistance may be more vulnerable to tobacco use and more resistant to quit smoking due to strong rewarding effects of nicotine. Thus, the possibility exists that greater rewarding effects of nicotine may promote tobacco use initiation among persons with diabetes. Our results also imply that the latter effect is likely associated with changes in the cellular response to insulin within the brain reward circuitry. Our findings also suggest that proper normalization of insulin signaling in persons with diabetes may play a critical role in reducing tobacco initiation and promoting cessation. Further research is needed to understand the mechanisms by which disruptions in insulin signaling alter reward processing. These findings will lead to better tobacco cessation strategies that will reduce the high mortality rates among persons with diabetes.
Acknowledgment
We thank Ms. Fatemeh Almasarweh for assisting with animal husbandry, management of rodent diets and data acquisition.
Role of funding source
This project was supported by funds provided by the Western University of Health Sciences and a grant from the American Diabetes Association (7-12-BS-135).
Footnotes
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
References
- Ahren B, Gudbjartsson T, Al-Amin AN,Martensson H,Myrsen-Axcrona U, Karlsson S, Mulder H, Sundler F, 1999. Islet perturbations in rats fed a high-fat diet. Pancreas 18, 75–83. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP, 2011. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology (Berl.) 217, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C, 2005. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology (Berl.) 180, 306–315. [DOI] [PubMed] [Google Scholar]
- Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC, 2006. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 36, 485–501. [DOI] [PubMed] [Google Scholar]
- Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr., Grimm RH Jr., Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD, 2007. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am. J. Cardiol. 99, 21i–33i. [DOI] [PubMed] [Google Scholar]
- Cho NH, Chan JC, Jang HC, Lim S, Kim HL, Choi SH, 2009. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin. Endocrinol. Oxf. 71, 679–685. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Attvall S, Taskinen MR, Smith U, 1997. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur. J. Clin. Invest. 27, 450–456. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM, 1992. Insulin resistance and cigarette smoking. Lancet 339, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ, 2008. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R388–R394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW, 2006. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol. Behav. 89, 611–616. [DOI] [PubMed] [Google Scholar]
- Gill GV, Morgan C, MacFarlane IA, 2005. Awareness and use of smoking cessation treatments among diabetic patients. Diabet. Med. 22, 658–660. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR, 2012. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55, 1577–1596. [DOI] [PubMed] [Google Scholar]
- Könner AC, Hess S, Tovar S, Mesaros A, Sanchez-Lasheras C, Evers N, Verhagen LA, Bronneke HS, Kleinridders A, Hampel B, Kloppenburg P, Bruning JC, 2011. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 13, 720–728. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, 2005. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl.) 178, 481–492. [DOI] [PubMed] [Google Scholar]
- Mebel DM,Wong JC, Dong YJ, Borgland SL, 2012. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 36, 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Natividad LA, Pipkin JA, Roman F, Torres I, Jurado J, Torres OV, Friedman TC, Tenayuca JM, Nazarian A, 2013. Enhanced nicotine self-administration and suppressed dopaminergic systems in a rat model of diabetes. Addict. Biol, 10.1111/adb.12074 (ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R, Lopez JM, Bedoya FJ, Goberna R, 1990. Effects of high-carbohydrate or high-fat diet on carbohydrate metabolism and insulin secretion in the normal rat. Diabetes Res. 15, 179–183. [PubMed] [Google Scholar]
- Sipols AJ, Bayer J, Bennett R, Figlewicz DP, 2002. Intraventricular insulin decreases kappa opioid-mediated sucrose intake in rats. Peptides 23, 2181–2187. [DOI] [PubMed] [Google Scholar]
- Solberg LI, Desai JR, O’Connor PJ, Bishop DB, Devlin HM, 2004. Diabetic patients who smoke: are they different? Ann. Fam. Med. 2, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE, 2008. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav. 90, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A, 2007. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 5, e274. [DOI] [PMC free article] [PubMed] [Google Scholar]


