Summary
Doxorubicin (DOX) is a cytostatic agent belonging to anthracycline group. Important role in mechanism associated with negative effects of DOX plays an oxidative stress. Heat shock proteins (HSPs) are part of mechanisms initiated in response to stressful stimuli and play an important role in cellular responses to oxidative stress through interaction with components of redox signaling. The present work was aimed to study the role of HSPs and autophagy in mechanisms underlying effects of sulforaphane (SFN), a potential activator of Nrf-2, on doxorubicin-induced toxicity in human kidney HEK293 cells. We investigated effects of SFN and DOX on proteins associated with regulation of heat shock response, redox signaling, and autophagy. Results show that SFN significantly reduced cytotoxic effects of DOX. The positive effects of SFN on DOX-induced changes were associated with up-regulation of Nrf-2 and HSP60 protein levels. In the case of another heat shock protein HSP40, SFN increased its levels when was administered alone but not in conditions when cells were exposed to the effects of DOX. Sulforaphane also reversed negative effects of DOX on activities of superoxide dismutases (SODs) and up-regulation of autophagy markers (LC3A/B-II, Atg5, and Atg12). In conclusion, the changes observed in HSP60 are of particular importance in terms of protecting cells from the effects of DOX. Finding that under conditions where SFN reduced cytotoxic effects of DOX were significantly increased protein levels of both Nrf-2 and HSP60 point to the role of HSP60 in mechanisms of redox signaling underlying effects of SFN on DOX-induced toxicity in HEK293 cells. Moreover, data confirmed an important role of autophagy in effects of SFN on DOX-induced toxicity.
Keywords: Doxorubicin, Heat Shock Proteins, Sulforaphane, Redox Signaling, Autophagy
Introduction
Doxorubicin (DOX) is a cytostatic agent belonging to the anthracycline group. Its use in cancer treatment is limited by its toxic effects on normal cells. The exact mechanism of its toxicity has not been fully elucidated yet, but the most frequently discussed is the increase of free radical levels [1]. Reactive oxygen and nitrogen species, which are produced at low levels, act as important signaling molecules. However, their excessive production can lead to oxidative damage of proteins, lipids, and DNA. Oxidative stress is one of the most common causes of pathological conditions in the body. It contributes to the development of many diseases such as inflammatory, cardiovascular, and neurodegenerative diseases or cancer [2–4].
Various antioxidants were studied for their effects on lowering of oxidative stress through modulation of Nrf-2 signaling pathway [5–7]. One of them is sulforaphane (SFN), a natural antioxidant found in various vegetables, especially broccoli. Previous studies have demonstrated several positive effects of SFN, such as antidiabetic, anticancer or cytoprotective [8,9]. Its involvement in the processes of protection of the heart [10], kidneys [11], and brain [12,13] from ischemic damage has also been documented. Mechanism of its action is primarily associated with activation of the Nrf-2 (Nuclear factor-erythroid factor 2-related factor 2) signaling pathway. This pathway is involved in elimination of excess of free radicals in the body through increased expression of antioxidant and detoxifying enzymes [14,15]. SFN may also play an important role in protecting cells from the DOX action. It has been found that SFN can activate the Nrf-2-related signaling pathway, thereby affecting the expression of the genes regulated by Nrf-2 after DOX administration [16,17]. SFN is also able to induce autophagy, which is associated with increased sensitivity of cancer cells to DOX treatment [16,18]. Several systems, including heat shock proteins (HSPs), are triggered during oxidative stress [19]. The family of HSPs includes multifunctional proteins that mainly ensure the stability and proper function of cellular proteins. They are part of mechanism called the heat shock response (HSR), which is triggered in response to stressful stimuli and ensures the maintenance of homeostasis in cells. HSPs expression is mediated by heat shock factors (HSFs). These transcription factors were found in six different forms in the human genome: HSF1, HSF2, HSF4, HSF5, HSFX (located on the X chromosome), and HSFY (located on the Y chromosome) [20]. HSF1 regulates genes encoding molecular chaperones and appears to play the most important role in HSR regulation. It exists interplay and feedback between HSF1 and HSPs. Under normal conditions HSP40, HSP70, and HSP90 serve as inhibitors of HSF1 and prevent translocation of this transcription factor into the nucleus [21]. On the other hand, during oxidative stress HSF1 increases the synthesis of HSPs [22].
A tight connection between HSPs and redox signaling exists. Free radicals are able to oxidize reactive protein residues, leading, in addition to the HSF1 activation, to the activation of other important transcription factors such as Nrf-2 [23]. Nrf-2 is a transcription factor normally maintained in the cytoplasm by its endogenous inhibitor Keap-1 (Kelch-like ECH-associated protein 1), where it is continuously degraded via ubiquitination [24]. Under pathological conditions (conditions of oxidative stress), Nrf-2 is translocated into the nucleus and through binding to the antioxidant-response element (ARE) sequence in the promoter region of Nrf-2 target genes is responsible for induction of their expression. In this way, Nrf-2 controls the expression of several antioxidant and detoxifying enzymes such as superoxide dismutase (SOD), heme oxygenase 1 (HO-1), catalase (CAT) or NAD(P)H quinone dehydrogenase 1 (NQO1) [25]. HSF1 has been shown to be involved in the activation of multiple genes together with Nrf-2, their joint involvement has been demonstrated in the regulation of HO-1 [26], HSP70 [27] or the adaptor protein for autophagy p62 [28].
Heat shock proteins are co-chaperones playing important role also in chaperone-mediated autophagy [29]. There is a direct interplay between autophagy and Nrf-2 signaling. They may regulate each other with a key role for the positive feedback loop p62-Keap-1-Nrf-2. Autophagy can activate Nrf-2 through a competitive interaction between p62 and Keap-1. The p62 contains a Keap-1 interacting region (KIR) motif that allows direct interaction of p62 with Keap-1 [30]. A direct link between autophagy and Nrf-2 signaling has also been documented in the cardiovascular system in heart failure, ischemic heart disease, cardiomyopathies or doxorubicin-induced cardiotoxicity [31–33]. The putative mechanism of the reciprocal action of HSPs and Nrf-2 in regulation of autophagy has not yet been elucidated, but there is a close link between these pathways in cellular responses to stress stimuli [34].
The aim of our study was to study the role of heat shock proteins in mechanisms underlying effects of sulforaphane on doxorubicin-induced toxicity in human kidney HEK293 cells. As part of this, we investigated the effects of both SFN and DOX on proteins associated with the regulation of heat shock response, redox signaling, and autophagy.
Materials and Methods
Materials
DL Sulforaphane (SFN), dimethyl sulfoxide (DMSO), and phenylmethylsulfonyl fluoride were purchased from Sigma Aldrich (USA), doxorubicin hydrochloride was obtained from Ebewe. Dithiothreitol (DTT) and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) were obtained from Serva (Heidelberg, Germany). Primary antibodies against Nrf-2 (ab89443) and Keap-1 (ab119403) were purchased from Abcam (Cambridge, UK). Antibodies against HSP60 (sc-13115), HSP70 (sc-66048), HSP90 (sc-13119) and GAPDH (sc-32233) were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibody recognizing HSP40 (#4871S), Beclin-1 (#3495S), LC3A/B (#12741), Atg5 (#12994), Atg12 (#4180), and secondary peroxidase-labeled anti-rabbit (#7074S) or anti-mouse (#7076S) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The Colorimetric SOD Assay kit (ab65354) was purchased from Abcam (Cambridge, UK).
Cell culture and treatment
In this study, we used HEK293 cells, an adherent cell line derived from human embryonic kidney cells [35]. Cells were cultured in RPMI 1640 complete medium supplemented with fetal bovine serum (10 % final concentration, Gibco, Thermo Fischer Scientific, Rockford, IL, USA) and penicillin-streptomycin (BioSera, France). Culturing was performed in a CO2 incubator (ESCO, Singapore) at 37 °C in an atmosphere containing 5 % CO2. Passaged cells were washed with serum-free RPMI 1640 medium and serum-free medium containing 0.25 % trypsin and 0.53 mM EDTA was used to release them from the Petri dish or plate. After 5 min of centrifugation at 125× g (centrifuge, Eppendorf Czech & Slovakia), were aliquots of cells resuspended for further cultivation in complete culture medium.
Determination of effects of sulforaphane and doxorubicin on viability of HEK293 cells
Firstly, we investigated the cytotoxic effects of DOX and SFN on HEK293 cells using MTT assay. For these experiments, we added 100 μl of cell suspension (104 cells) and 100 μl of medium containing DOX or SFN to a 96-well plate. To monitor cytotoxicity, we used DOX in the concentration range of 0–5 μmol/l and SFN in the concentration range of 0–40 μmol/l. After 21 h of culturing the cells in the presence of DOX or SFN, we determined their cytotoxicity using the MTT assay. Experiments were performed independently three times in triplicate.
Determination of the influence of sulforaphane on doxorubicin-induced cytotoxicity
To investigate the effect of SFN on the cytotoxicity of DOX, we precultured the cells for 2 h with 10 μmol/l sulforaphane [36,37] and then added DOX in the concentration range of 0–10 μmol/l. In control conditions, cells were before DOX treatment precultured for 2 h in the presence of 0.1 % DMSO (vehicle for SFN). After a further 21 h of cell culture, we determined the cell viability using the MTT assay. We analyzed the cytotoxicity of DOX (IC50 value for DOX) in the absence and presence of SFN. Experiments were performed independently three times in triplicate.
MTT assay to analyze cell viability
The impact of DOX and SFN on viability of HEK293 cells was determined using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay. After 21 h of culturing the cells in the presence of DOX and/or SFN we exposed cells for 3 h to 100 μl of 1 mg/ml MTT reagent in serum-free culture medium. After that, the formazan crystals were dissolved in 100 μl of dimethyl sulfoxide and the absorbance of each well was detected at 590 nm using a microplate (ELISA) reader (Labsystem).
Samples preparation
After the DOX and SFN culture experiments, the cells were also used to prepare samples for further biochemical analyses. To investigate the effect DOX and/or SFN we precultured the cells for 2 h with 10 μmol/l sulforaphane [36] and then added 2.5 μmol/l DOX or cell culture medium (control conditions without DOX). In control conditions without SFN were cells before DOX treatment precultured for 2 h in the presence of 0.1 % DMSO (vehicle for SFN). After a further 21 h of cell culture the cells on the plates were exposed to lysis solution (50 mM Tris-HCl, pH 7.40; 1 mM DTT; 1 mM EGTA; 1 mM PMSF). Subsequently, the obtained lysate was centrifuged for 10 min at 10000× g, at 4 °C. The supernatant obtained after this centrifugation was used for subsequent analyses. Protein concentrations in samples were determined by the Bradford method [38].
Western blot analysis
For Western blot analysis, samples containing equivalent amounts of proteins in a single lane were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Before electrophoretic separation, isolated individual samples were mixed with reducing Laemmli sample buffer in a 1:1 ratio. These samples were heated for 5 min at 95 °C to denature the proteins. Proteins were separated by electrophoresis, the total amount of protein loaded into the gel was 15 μg. After electrophoretic separation, the proteins were transferred to nitrocellulose membranes, and after blocking nonspecific binding sites with 5 % non-fat milk in Tris Buffered Saline (TBS), the membranes were incubated overnight at 4 °C with the appropriate specific primary antibody. The corresponding peroxidase-labeled anti-rabbit or anti-mouse immunoglobulins (Cell Signaling Technology, Danvers, MA, USA) were used as secondary antibodies. Peroxidase reactions were detected by an enhanced chemiluminescence (ECL) system and quantified using Carestream software (version 5.0, Carestream Health, New Haven, CT, USA).
Determination of Superoxide Dismutase Activity
SOD activity in the samples was determined using a colorimetric superoxide dismutase activity assay kit (ab65354, Abcam, Cambridge, UK) according to the manufacturer's protocol. The assay kit uses the water-soluble tetrazolium salt WST-1, which, when reduced with a superoxide anion, produces a formazan dye. The higher the SOD activity in the sample, the less formazan dye is formed. The production of formazan was determined by measuring the absorbance at 450 nm.
Statistical evaluation
Results are presented as mean values ± standard error of the mean (SEM). We used one-factor ANOVA to detect statistically significant differences between groups. Differences between individual groups were determined using Tukey's post hoc test. Differences were considered significant at *p<0.05.
Results
Doxorubicin and sulforaphane cytotoxicity on HEK293 cells
The cytotoxicity of the substances used in further experiments was determined in culture experiments performed on HEK293 cells. Cytotoxicity was evaluated by determining the IC50 value ('inhibitory concentration'), which is a value indicating the concentration of the substance at which 50 % of the cells survive. In the case of DOX, we found the IC50 value to be 0.8 μM; in the case of SFN, we did not observe significant cytotoxic effects on HEK293 cells even at a concentration of 40 μM. Subsequently, we used the MTT assay to investigate how sulforaphane affects DOX-induced cytotoxicity. We found that preculture of cells with SFN at a concentration of 2.5 μM and also 10 μM significantly reduced the cytotoxic effects of DOX (Fig. 1). Therefore, we used SFN at a concentration of 10 μM in further biochemical analyses.
Fig. 1.
Effect of sulforaphane on changes in cytotoxicity of DOX. Quantitative analysis of changes in the IC50 value for DOX after precultivation with sulforaphane. The IC50 value indicates the concentration of the DOX at which 50 % of the cells survive. Statistical significance was analyzed by one-way ANOVA analysis. Each bar represents mean ± SEM. * p<0.05 compared to DOX. DOX-doxorubicin, DOX+SFN2.5 – doxorubicin with sulforaphane in a concentration of 2.5 μM, DOX+SFN10 – doxorubicin with sulforaphane in a concentration of 10 μM. Data were obtained from three independent experiments performed in triplicate.
Effect of doxorubicin and/or sulforaphane on Nrf-2 and Keap-1 in HEK293 kidney cells
Changes in Nrf-2 and Keap-1 protein levels after DOX and/or SFN administration were monitored by Western blot analysis using specific antibodies. We found that SFN significantly increased protein levels of Nrf-2. The increase was observed not only in the situation when SFN was administered alone but also in the conditions when cells were exposed to both SFN and DOX (Fig. 2A, C). In contrast to Nrf-2, application of SFN did not significantly influence the protein levels of Keap-1. However, the levels of Keap-1 were significantly increased after DOX administration. Precultivation of cells with SFN did not have a significant impact on DOX-induced changes in Keap-1 protein levels (Fig. 2B, C).
Fig. 2.
Effect of doxorubicin and/or sulforaphane on protein levels of Nrf-2 and Keap-1 in kidney HEK293 cells. (A) Quantitative analysis of changes in protein levels of Nrf-2 and (B) Keap-1. Values are expressed as the intensity of the reaction compared to the control (in %). (C) Western blot records showing the protein levels of Nrf-2, Keap-1, and housekeeping protein GAPDH. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, * p<0.05 compared to control, & p<0.05 compared to SFN, # p<0.05 compared to DOX. C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, DOX – 2.5 μM doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
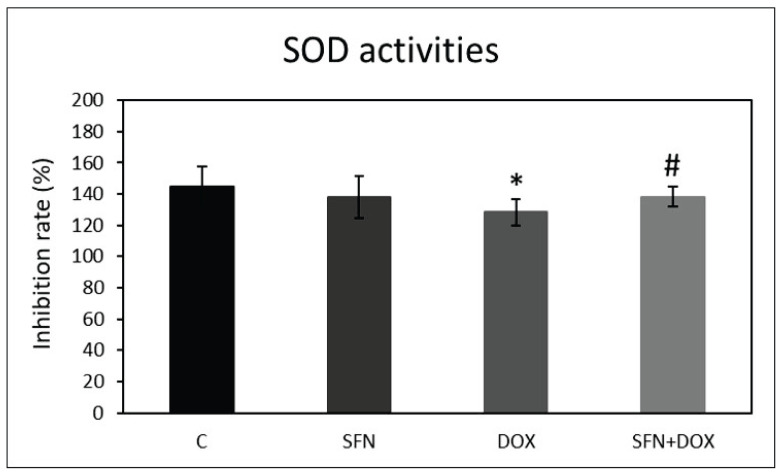
Effect of doxorubicin and/or sulforaphane on superoxide dismutase activities
We found that SFN up-regulated Nrf-2, a key component of redox signaling. We also investigated how SFN and DOX affects activities of superoxide dismutases (SODs), enzymes regulated by the Nrf-2 signaling pathway. Total SOD activity was determined using a commercially available SOD colorimetric assay kit. We found that DOX administration resulted in a significant reduction in SOD activity compared to the control group. SFN alone had no significant effect compared to control conditions. However, SFN had a positive effect on DOX-induced changes, which was associated with a significant increase in SOD activity when SFN was co-administered with DOX (Fig. 3).
Fig. 3.
Effect of doxorubicin and/or sulforaphane on SOD activities in kidney HEK293 cells. SOD activities were determined using colorimetric assay kit (Abcam). The data represents the percentage of inhibition of superoxide production by SOD activity. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, * p<0.05 compared to vehicle control, # p<0.05 compared to DOX. C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, 2.5 μM DOX – doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
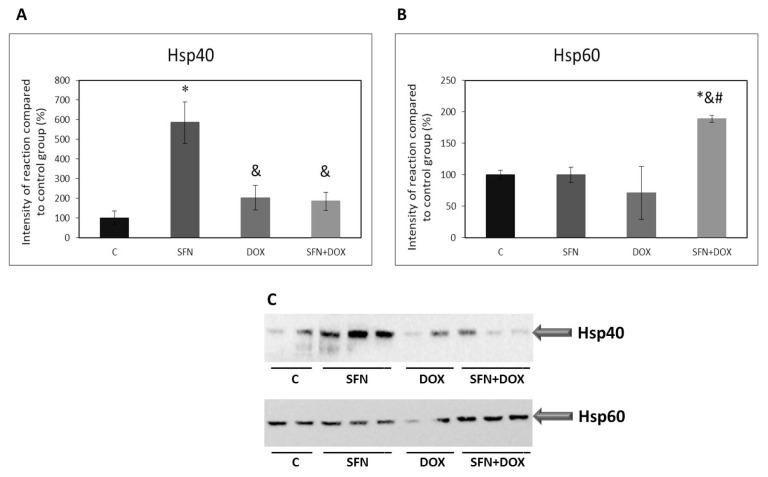
Effect of doxorubicin and/or sulforaphane on protein levels of heat shock proteins in HEK293 kidney cells
Heat shock proteins (HSPs) are activated in cellular responses to stress stimuli. They have several functions in the cells and they can serve also as co-chaperones in chaperones mediated autophagy (CMA). We investigated how DOX administration affects protein levels of HSP40, HSP60, HSP70, and HSP90, and how these effects of DOX are modulated after SFN admi-nistration. We found that DOX alone had no significant effects on the monitored HSPs. Significant changes occurred after SFN administration, namely in the protein levels of HSP40 and HSP60 (Fig. 4A, B, C), suggesting a specific role of these HSPs in the cellular responses to administration of SFN alone or in combination with DOX. While in the case of HSP40 sulforaphane stimulated an increase of protein levels under control conditions (Fig. 4A, C), for HSP60 sulforaphane increased the levels of this protein in the situation where cells were also exposed to DOX (Fig. 4B, C).
Fig. 4.
Effect of doxorubicin and/or sulforaphane on protein levels of HSP40 and HSP60 in HEK293 cells. (A) Quantitative analysis of changes in protein levels of HSP40 and (B) HSP60. Values are expressed as the intensity of the reaction compared to the control (in %). (C) Western blot record showing the protein levels of HSP40 and HSP60. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, * p<0.05 compared to vehicle control, & p<0.05 compared to SFN, # p<0.05 compared to DOX. C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, DOX – 2.5 μM doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
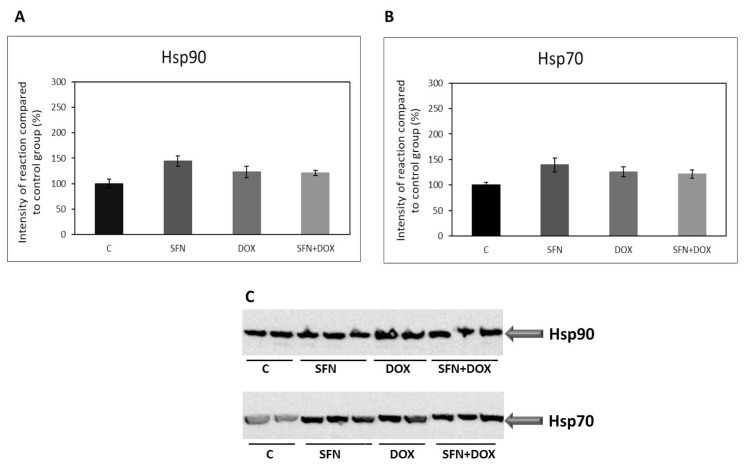
In the case of heat shock proteins HSP70 and HSP90, we did not observe any significant changes in their protein levels after SFN and/or DOX treatment (Fig. 5A, B, C).
Fig. 5.
Effect of doxorubicin and/or sulforaphane on protein levels of HSP90 and HSP70 in kidney HEK293 cells. (A) Quantitative analysis of changes in protein levels of HSP90 and (B) HSP70. Values are expressed as the intensity of the reaction compared to the control (in %). (C) Western blot record showing the protein levels of HSP90 and HSP70. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, DOX – 2.5 μM doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
Effect of doxorubicin and/or sulforaphane on protein levels of Beclin-1 and LC3A/B-II in HEK293 kidney cells
To monitor changes in proteins associated with autophagy, we focused on the Beclin-1 and LC3A/B-II. These proteins are essential for autophagosome formation and thus important for proper autophagy function. We found that DOX administration, similarly to Keap-1, significantly increased Beclin-1 protein levels compared to the control group. However, SFN administration had no significant effect on the levels of this protein (Fig. 6A, C). Also in case of LC3A/B-II, we observed its elevated levels after DOX administration. But when SFN was co-administered with DOX, there was a significant decrease in its protein levels (Fig. 6B, C).
Fig. 6.
Effect of doxorubicin and/or sulforaphane on protein levels of Beclin-1 and LC3A/B-II in HEK293 cells. (A) Quantitative analysis of changes in protein levels of Beclin-1 and (B) LC3A/B-II. Values are expressed as the intensity of the reaction compared to the control (in %). (C) Western blot records showing the protein levels of Beclin-1 and LC3A/B-II. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, * p<0.05 compared to vehicle control, & p<0.05 compared to SFN, # p<0.05 compared to DOX. C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, DOX – 2.5 μM doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
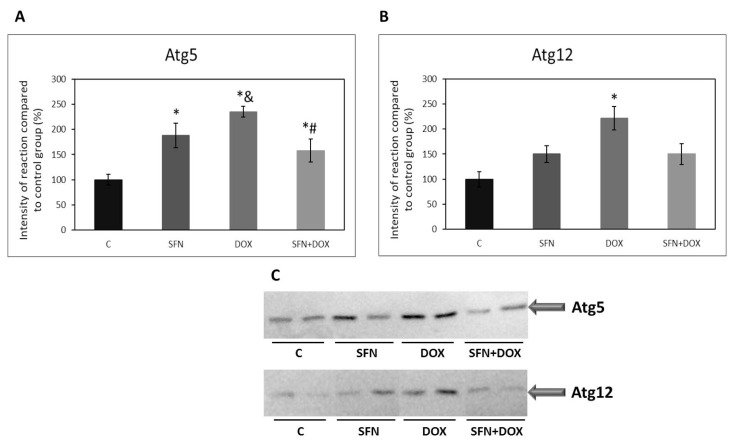
Effect of doxorubicin and/or sulforaphane on protein levels of autophagy related proteins in HEK293 kidney cells
Autophagy related proteins Atg5 and Atg12 are a part of complex which is responsible for elongation of the phagophore in the autophagy pathway. We investigated how DOX administration affects the levels of these proteins and whether SFN influences possible effect of DOX. We found that, similarly to other studied autophagic proteins, there was a significant increase in the levels of both Atg5 and Atg12 after DOX administration compared to the control group. In the case of Atg5, there was a significant decrease in its protein levels after SFN co-administration with DOX. A similar trend we observed for Atg12, but the observed change was not significant. (Fig. 7A, B, C).
Fig. 7.
Effect of doxorubicin and/or sulforaphane on protein levels of Atg5 and Atg12 in HEK293 cells. (A) Quantitative analysis of changes in protein levels of Atg5 and (B) Atg12. Values are expressed as the intensity of the reaction compared to the control (in %). (C) Western blot records showing the protein levels of Atg5 and Atg12. Statistical significance was analyzed by one-way ANOVA. Each bar represents mean ± SEM, n=4–6, * p<0.05 compared to vehicle control, & p<0.05 compared to SFN, # p<0.05 compared to DOX. C – Control (0.1 % DMSO), SFN – 10 μM sulforaphane, DOX – 2.5 μM doxorubicin, SFN+DOX – sulforaphane + doxorubicin.
Discussion
Heat shock proteins play important roles in processes regulating protein and cell membrane stability and function, in the repair of misfolded proteins caused by stress conditions, as well as in autophagy [29]. In our study, we investigated the possible involvement of HSPs in responses of HEK293 cells to the effects of DOX. However, we did not observe significant changes in monitored HSPs after exposure of HEK293 cells to the action of DOX. Nevertheless, we found significant changes in heat shock protein levels in HEK293 cells after sulforaphane treatment.
SFN is a natural isothiocyanate compound found in various cruciferous vegetables, especially broccoli, and its antioxidant, anti-inflammatory, and anticancer effects have been documented [39,40]. In our experimental model we demonstrated protective role of SFN on doxorubicin action in HEK293 kidney cells. We found reduction of DOX-induced cytotoxicity after SFN treatment at a concentration as low as 2.5 μM. Similarly to our findings, SFN has been shown to play a positive role in the prevention of doxorubicin-induced toxicity in cardiac H9c2 cells [41]. To date, only a small number of studies have confirmed the direct effect of SFN on individual heat shock proteins. Important role of SFN has been demonstrated in increasing proteosome activity, which was associated with activation of the heat shock response and induction of HSP27 expression [42]. Sulforaphane was also found to directly interact with specific amino acid residues of HSP90 and inhibit its function [43]. However, the exact mechanisms by which sulforaphane may influence the heat shock response are not yet fully elucidated. In our study, sulforaphane alone stimulated an increase in HSP40 levels. The changes observed in HSP60 levels seemed to be of particular importance in terms of protecting cells from the effects of DOX treatment. Here, we found that under conditions where SFN reduced the cytotoxic effects of DOX on cells was a significant increase in HSP60 protein levels. This finding is in agreement with results of studies documenting possible role of some HSPs in mechanisms of protection of cells from DOX-induced damage [44,45].
The activity of HSPs under oxidative stress may also be closely linked to the Nrf-2 signaling pathway [23]. HSPs are activated by cellular responses to stress stimuli by the action of transcription factor HSF1, which may also contribute to the regulation of transcription of some genes together with Nrf-2 [24,25]. The effect of SFN is likely mediated by affecting the Nrf-2 signaling pathway [14,40]. This is confirmed also by our study, where application of sulforaphane alone, but also in combination with doxorubicin, resulted in an increase in Nrf-2 protein levels in HEK293 cells. SFN has been shown to realize its positive role in the prevention of negative effects induced by DOX through increased Nrf-2 expression also in H9c2 cells [41]. Interestingly, we found that Keap-1 protein was not affected after SFN application. This finding is in contrast to results of studies documenting changes in Keap-1 protein levels after SFN treatment [46,47]. Keap-1 is the major but not the exclusive regulator of Nrf-2. Nrf-2 can be regulated by multiple signaling pathways and proteins under conditions of oxidative stress such as Akt kinase signaling pathway proteins. It has been found that SFN can affect Nrf-2 indirectly through activation of Akt kinase signaling, leading to inhibition of GSK-3β kinase-mediated proteosomal degradation of Nrf-2 [6]. Moreover, it has not yet been fully clarified whether SFN can form a stable product with Keap-1 and thus influence Keap-1 function directly. Therefore, the ability of SFN to affect Nrf-2 independently of Keap-1 and also the not very stable direct binding between SFN and Keap-1 could explain the fact that we failed to observe changes in Keap-1 protein levels after SFN application.
A properly functioning Nrf-2 pathway controls the expression of several antioxidant and detoxifying enzymes. Superoxide dismutase is one of the antioxidant enzymes whose expression is directly regulated by Nrf-2 and plays a very important role in the response mechanisms of animal cells to pathological stimuli [23]. The link between the effect of DOX and SOD activity is documented by our data obtained in the HEK293 cell model, where we found inhibition of SOD activity after DOX treatment. Here, we also demonstrated a direct relationship between SOD and the Nrf-2 pathway, which was documented by an increase in SOD activity after treatment with the Nrf-2 activator, sulforaphane. It has been found that DOX caused a significant decrease in SOD activities also in H9c2 cardiac cells and this negative effect of DOX on SOD was significantly prevented by SFN [41].
It is known that autophagy proper function is essential to ensure the antioxidant response mediated by the Nrf-2 signaling pathway [48]. Also some heat shock proteins are important co-chaperones in chaperone-mediated autophagy [29]. In consequence of SFN application we observed significant changes in HSP40 and HSP60. Therefore, we monitored changes in levels of several proteins (LC3A/B-II, Atg5, Atg15, and Beclin-1) playing essential role in autophagosome formation and the proper functioning of autophagy [49]. However, we were unable to demonstrate sufficiently the involvement of autophagy in the processes associated with the effects of SFN alone on HEK293 cells, as the protein levels of several autophagy markers (Beclin-1, LC3A/B-II, and Atg12) were not significantly altered after SFN treatment. The only exception was Atg5 where we observed increased levels of this protein after exposure of cells to the effects of SFN. In contrast to SFN, we found that doxorubicin administration significantly increased the protein levels of all investigated autophagy markers. This points to the involvement of autophagy in the processes associated with the effects of DOX on HEK293 cells. Induction of autophagy after DOX treatment was observed also in other experimental studies [50,51]. Moreover, our data showed that the effects of SFN on DOX-mediated cellular injury in HEK293 cells involve modulation of processes associated with autophagy induction. This was documented by reversal of DOX-induced effects on autophagy markers (LC3A/B-II, Atg5, and Atg12) by SFN.
Conclusions
Our results suggest the possible involvement of heat shock proteins and autophagy in the effects of sulforaphane on the cytotoxic effects of doxorubicin. However, the role of the distinct types of HSPs seems to be different. The changes observed in HSP60 are of particular importance in terms of protecting cells from the effects of DOX, on the other hand, the role of HSP40 seems to be more important in the mechanisms of action of sulforaphane alone. The finding that under conditions in which SFN reduced the cytotoxic effects of DOX, both Nrf-2 and HSP60 protein levels were significantly increased suggests a role for HSP60 in the mechanisms of redox signaling underlying the effects of SFN on DOX-induced toxicity in HEK293 cells. Up-regulation of autophagy markers after DOX treatment points to the involvement of autophagy in its cytotoxic effects. The reversal of DOX-induced up-regulation of autophagy markers (LC3A/B-II, Atg5, and Atg12) in consequence of SFN application also indicates that in mechanisms of SFN action on DOX-mediated cellular injury in HEK293 cells play important role modulation of processes associated with autophagy induction. In conclusion, our findings may contribute to elucidate the interconnections between heat shock response, redox signaling, and autophagy under conditions of oxidative stress. However, further studies are needed to understand the exact mechanism of the interplay between these pathways.
Acknowledgements
This work was funded by VEGA SR grant No. 2/0179/21 and grant of Agency for Research and Development APVV-18-0548.
Funding Statement
This work was funded by VEGA SR grant No. 2/0179/21 and grant of Agency for Research and Development APVV-18-0548.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1. Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, Lobato NS, Tostes RC. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br J Pharmacol. 2017;174:3527–3541. doi: 10.1111/bph.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yi G, Din Jud, Zhao F, Liu X. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020;11:2725–737. doi: 10.1039/C9FO01466G. [DOI] [PubMed] [Google Scholar]
- 4. Xia M-H, Yan X-Y, Zhou L, Xu L, Zhang L-C, Yi H-W, Su J. p62 suppressed VK3-induced oxidative damage through Keap1/Nrf2 pathway in human ovarian cancer cells. J Cancer. 2020;11:1299–1307. doi: 10.7150/jca.34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y, Chen Q, Hao Q, Bao D, Hou D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep. 2017;7:10114. doi: 10.1038/s41598-017-10693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xin Y, Bai Y, Jiang X, Zhou S, Wang Y, Wintergerst KA, Cui T, Ji H, Tan Y, Cai L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3β/Fyn pathway. Redox Biol. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebrahimpour S, Shahidi SB, Abbasi M, Tavakoli Z, Esmaeili A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci Rep. 2020;10:15957. doi: 10.1038/s41598-020-71971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Opthalmol Vis Sci. 2005;46:979–987. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- 9. Qazi A, Pal J, Maitah M, Fulciniti M, Pelluru D, Nanjappa P, Lee S, et al. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: potential use in chemoprevention and as adjuvant in chemotherapy. Transl Oncol. 2010;3:389–399. doi: 10.1593/tlo.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silva-Palacios A, Ostolga-Chavarría M, Sánchez-Garibay C, Rojas-Morales P, Galván-Arzate S, Buelna-Chontal M, Pavón N, et al. Sulforaphane protects from myocardial ischemia-reperfusion damage through the balanced activation of Nrf2/AhR. Free Radic Biol Med. 2019;143:331–340. doi: 10.1016/j.freeradbiomed.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 11. Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214–223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 12. Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 13. Kapoor S, Kala D, Svoboda J, Daněk J, Faridová A, Brnoliaková Z, Mikulecká A, Folbergrová J, Otáhal J. The effect of sulforaphane on perinatal hypoxic-ischemic brain injury in rats. Physiol Res. 2022;71:401–441. doi: 10.33549/physiolres.934878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh P, Sharma R, McElhanon K, Allen CD, Megyesi JK, Beneš H, Singh SP. Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radic Biol Med. 2015;86:90–101. doi: 10.1016/j.freeradbiomed.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dovinova I, Kvandova M, Balis P, Gresova L, Majzunova M, Horakova L, Chan J, Barancik M. The role of Nrf2 and PPARγ in the improvement of oxidative stress in hypertension and cardiovascular diseases. Physiol Res. 2020;69(Suppl 4):S541–S553. doi: 10.33549/physiolres.934612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bose C, Awasthi S, Sharma R, Beneš H, Hauer-Jensen M, Boerma M, Singh SP. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS One. 2018;13:e0193918. doi: 10.1371/journal.pone.0193918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–22. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang F, Wang F, Liu Y, Wang S, Li X, Huang Y, Xia Y, Cao C. Sulforaphane induces autophagy by inhibition of HDAC6-mediated PTEN activation in triple negative breast cancer cells. Life Sci. 2018;213:149–157. doi: 10.1016/j.lfs.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 19. Reeg S, Jung T, Castro JP, Davies KJA, Henze A, Grune T. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic Biol Med. 2016;99:153–166. doi: 10.1016/j.freeradbiomed.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomez-Pastor R, Burchfiel ET, Thiele DJ. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:4–19. doi: 10.1038/nrm.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klumpen E, Hoffschröer N, Zeis B, Gigengack U, Dohmen E, Paul RJ. Reactive oxygen species (ROS) and the heat stress response of Daphnia pulex: ROS-mediated activation of hypoxia-inducible factor 1 (HIF-1) and heat shock factor 1 (HSF-1) and the clustered expression of stress genes. Biol Cell. 2017;109:39–64. doi: 10.1111/boc.201600017. [DOI] [PubMed] [Google Scholar]
- 22. Kovács Sigmond, Hotzi Bohár, Fazekas Deák, Vellai Barna. HSF1Base: A Comprehensive Database of HSF1 (Heat Shock Factor 1) Target Genes. Int J Mol Sci. 2019;20:5815. doi: 10.3390/ijms20225815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDuffee AT, Senisterra G, Huntley S, Lepock JR, Sekhar KR, Meredith MJ, Borrelli MJ, et al. Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J Cell Physiol. 1997;171:143–151. doi: 10.1002/(SICI)1097-4652(199705)171:2<143::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24. McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–1600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 25. Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets. 2018;18:538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 26. Akagi R, Kubo T, Hatori Y, Miyamoto T, Inouye S. Heme oxygenase-1 induction by heat shock in rat hepatoma cell line is regulated by the coordinated function of HSF1, NRF2 and BACH1. J Biochem. 2021;170:501–510. doi: 10.1093/jb/mvab065. [DOI] [PubMed] [Google Scholar]
- 27. Almeida DV, da Silva Nornberg BF, Geracitano LA, Barros DM, Monserrat JM, Marins LF. Induction of phase II enzymes and hsp70 genes by copper sulfate through the electrophile-responsive element (EpRE): insights obtained from a transgenic zebrafish model carrying an orthologous EpRE sequence of mammalian origin. Fish Physiol Biochem. 2010;36:347–353. doi: 10.1007/s10695-008-9299-x. [DOI] [PubMed] [Google Scholar]
- 28. Samarasinghe B, Wales CTK, Taylor FR, Jacobs AT. Heat shock factor 1 confers resistance to Hsp90 inhibitors through p62/SQSTM1 expression and promotion of autophagic flux. Biochem Pharmacol. 2014;87:445–455. doi: 10.1016/j.bcp.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dou J, Su P, Xu C, Wen Z, Mao Z, Li W. Targeting Hsc70-based autophagy to eliminate amyloid β oligomers. Biochem Biophys Res Commun. 2020;524:923–928. doi: 10.1016/j.bbrc.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–23. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 31. Sun B, Xu Y, Liu ZY, Meng WX, Yang H. Autophagy assuages myocardial infarction through Nrf2 signaling activation-mediated reactive oxygen species clear. Eur Rev Med Pharmacol Sci. 2020;24:7381–7390. doi: 10.26355/eurrev_202007_21906. [DOI] [PubMed] [Google Scholar]
- 32. Zang H, Wu W, Qi L, Tan W, Nagarkatti P, Nagarkatti M, Wang X, Cui T. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020;69:2720–734. doi: 10.2337/db19-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan JA, Zhang Y, et al. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine. 2021;69:103456. doi: 10.1016/j.ebiom.2021.103456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dayalan Naidu S, Dikovskaya D, Gaurilcikaite E, Knatko EV, Healy ZR, Mohan H, Koh G, et al. Transcription factors NRF2 and HSF1 have opposing functions in autophagy. Sci Rep. 2017;7:11023. doi: 10.1038/s41598-017-11262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 36. Li W, Trieu J, Blazev R, Parker BL, Murphy KT, Swiderski K, Lynch GS. Sulforaphane attenuates cancer cell-induced atrophy of C2C12 myotubes. Am J Physiol Cell Physiol. 2023;324:C205–C221. doi: 10.1152/ajpcell.00025.2022. [DOI] [PubMed] [Google Scholar]
- 37. Bai Y, Chen Q, Sun YP, Wang X, Lv L, Zhang LP, Liu JS, Zhao S, Wang XL. Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 upregulation. Cardiovasc Ther. 2017;35:e12277. doi: 10.1111/1755-5922.12277. [DOI] [PubMed] [Google Scholar]
- 38. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39. Li S, Yang H, Chen X. Protective effects of sulforaphane on diabetic retinopathy: activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp Anim. 2019;68:221–31. doi: 10.1538/expanim.18-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, et al. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutr Metab (Lond) 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bai Y, Chen Q, Sun YP, Wang X, Lv L, Zhang LP, Liu JS, Zhao S, Wang XL. Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 upregulation. Cardiovasc Ther. 2017;35:e12277. doi: 10.1111/1755-5922.12277. [DOI] [PubMed] [Google Scholar]
- 42. Gan N, Wu YC, Brunet M, Garrido C, Chung FL, Dai C, Mi L. Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J Biol Chem. 2010;285:35528–35536. doi: 10.1074/jbc.M110.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Karagöz GE, Seo YH, Zhang T, Jiang Y, Yu Y, Duarte AMS, et al. Sulforaphane inhibits pancreatic cancer through disrupting Hsp90-p50Cdc37 complex and direct interactions with amino acids residues of Hsp90. J Nutr Biochem. 2012;23:1617–1626. doi: 10.1016/j.jnutbio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gabrielson K, Bedja D, Pin S, Tsao A, Gama L, Yuan B, Muratore N. Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Res. 2007;67:1436–1441. doi: 10.1158/0008-5472.CAN-06-3721. [DOI] [PubMed] [Google Scholar]
- 45. Jing L, Jiang JR, Liu DM, Sheng JW, Zhang WF, Li ZJ, Wei LY. Structural characterization and antioxidant activity of polysaccharides from Athyrium multidentatum (Doll.) ching in D-galactose-induced aging mice via PI3K/AKT pathway. Molecules. 2019;24:3364. doi: 10.3390/molecules24183364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nordgren KKS, Wallace KB. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol Appl Pharmacol. 2014;274:107–116. doi: 10.1016/j.taap.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 47. Li B, Kim DS, Yadav RK, Kim HR, Chae HJ. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int J Mol Med. 2015;36:53–64. doi: 10.3892/ijmm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Copple IM, Lister A, Obeng AD, Kitteringham NR, Jenkins RE, Layfield R, Foster BJ, Goldring CE, Park BK. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J Biol Chem. 2010;285:16782–6788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu K, Yuan Y, Wen J, Chen D, Zhu W, Ouyang Z, Wang W. LncRNA Sox2OT-V7 promotes doxorubicin-induced autophagy and chemoresistance in osteosarcoma via tumor-suppressive miR-142/miR-22. Aging (Albany NY) 2020;12:6644–6666. doi: 10.18632/aging.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang J, Zha Y, Jiao Y, Li Y, Zhang S. Protective role of cezanne in doxorubicin-induced cardiotoxicity by inhibiting autophagy, apoptosis and oxidative stress. Toxicology. 2023;485:153426. doi: 10.1016/j.tox.2023.153426. [DOI] [PubMed] [Google Scholar]