Summary
Long-lasting disturbances in lipid and glucose metabolism present in metabolic syndrome (MetS) lead to serious cardiovascular diseases. The study was aimed to evaluate the effect of natural antioxidant vitamin E (VitE, 100 mg/kg/day, p.o.) on basal biochemical and physiological parameters characterizing MetS and on the changed function of the heart. Furthermore, the possible potentiation of VitE effect by synthetic pyridoindole antioxidant SMe1EC2 (SMe, 15 mg/kg/day, p.o.) was also tested. MetS was induced in hereditary hypertriglyceridemic rats (HTG) by the 5 weeks administration of high-fat fructose diet (HFFD: 1 % cholesterol, 7.5 % pork lard, 10 % fructose). The heart function was tested using Langendorff preparation under constant pressure. The functional parameters of isolated heart, dysrhythmias and evoked fibrillations were evaluated in conditions of ischemia-reperfusion. The HFFD increased body weight gain and serum levels of total cholesterol, low-density lipoproteins and blood glucose. The HFFD significantly increased heart flow and force of contraction, compared to standard diet (SD). During the reperfusion, the HFFD caused the increase of the ventricular premature beats number at the expense of decreasing the duration of serious dysrhythmias (ventricular tachycardias and fibrillations). The addition of VitE, SMe or their combination to the HFFD decreased body weight gain, depressed blood pressure, improved particular biochemical parameters. The combination of VitE and SMe suppressed the occurrence of serious dysrhythmias. Our data indicate that the HFFD-related disturbances led to alterations within pathophysiology in HTG rats. The results showed that a combination of antioxidants might have the potential to amend disorders accompanying MetS.
Keywords: Metabolic syndrome, HTG rats, Vitamin E, SMe1EC2, Langendorff preparation
Introduction
Modern sedentary lifestyle is linked to emergence of atherosclerosis leading to major cardiovascular disease [1–3]. Main causes of cardiovascular mortality are malign heart dysrhythmias and they represent a serious clinical problem [4]. More than half of the patients with ischemic heart disease die due to ventricular dysrhythmias and ventricular tachydysrhythmias, which are the most common and most severe forms of heart dysrhythmias [5].
Adult male hereditary hypertriglyceridemic (HTG) rats represent pre-diabetic animal model with mild metabolic disturbances, allowing to study cardiovascular and metabolic changes without obesity [6]. This model is characterized by hyperinsulinemia, mild hypertension, hypertriacylglycerolemia, postprandial hyperglycemia [7] and decreased levels of high density lipoproteins [8]. Addition of high-fat diet results in further aggravation of metabolic state of animals [9]. Long-lasting disturbances in lipid and glucose metabolism present in metabolic syndrome (MetS) lead to serious cardiovascular diseases. Inflammation and oxidative stress are closely related to MetS [10], therefore agents possessing both anti-inflammatory and anti-oxidative properties should have the potential in regulation of MetS.
The study was aimed to evaluate the effect of natural antioxidant vitamin E (VitE) on basal biochemical and physiological parameters characterizing MetS and on the changed function of the heart. The evaluation was focused on the reperfusion-induced dysrhythmias, using Langendorff preparation under constant pressure. Furthermore, the possible potentiation of VitE effect by synthetic pyridoindole antioxidant SMe1EC2 (SMe) was also tested.
Methods
Animals
All experimental procedures involving animals were approved by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, Center of Experimental Medicine, Slovak Academy of Sciences (IEPT CEM SAS), Animal Health and Animal Welfare Division of the State Veterinary and Food Administration of the Slovak Republic (the number of the permit 3853/18-221/3) and they conformed to Directive 2010/63/EU on protection of animals used for scientific purposes. Adult male HTG rats aged 16 weeks (n=50, weight 321.1±3.5 g at the onset of the experiment) from the Breeding Station of the Institute of Experimental Pharmacology and Toxicology (Dobra Voda, Slovakia) were used. The rats had free access to water and food and were kept on 12 h/12 h light/dark cycle and housed 5 animals per cage.
Design of the experiment
Standard rodent diet was produced by the certified producer of pellets at the Department of Toxicology and Breeding of Laboratory Animals, IEPT CEM SAS, Dobra Voda, Slovakia, which is registered under the number α SK 100089, code 6147. Composition of the SD: wheat, processed animal protein, oat, barley-corn, extruded lucerne, soybean extracted grit, wheat bran, wheat germs, mineral mix, vegetable oil, natrium chloride. Added vitamins per 1 kg: E 672 vitamin A – 20000 IU; E 671 vitamin D3 – 2000 IU; vitamin E – 70 mg; added amino acids per 1 kg: DL-methionine – 1.2 g; L-lysine – 0.8 g. Analytical components: 19.10 % nitrogen substances; 3.60 % fiber; 5.10 % oil and fat; 5.85 % ash; 9.10 % humidity.
Animals were divided into five experimental groups (n=10 rats/group) and fed 5 weeks with either standard diet (SD) or modified high-fat fructose diet (HFFD: 1 % cholesterol, 7.5 % pork lard, 10 % fructose). Control HTG rats were fed SD (group labeled SD). Another HTG groups were fed either HFFD (HFFD) or HFFD with addition of antioxidants, alone or in combination (HFFD – VitE; HFFD – SMe; HFFD – VitE+SMe). VitE (100 mg/kg/daily) or SMe (15 mg/kg/daily) were administered p.o.
The amount of consumed food was recorded each day as the difference between added and rest weight of food per cage, and recalculated to the consumed food in g/rat/day (mean ± SEM). The rat body weight was monitored once a week.
Determination of lipid profile
After 24-hour starvation of animals, the blood was collected from retroorbital plexus at the beginning (before starting dietary regime) and at the end of dietary experiment (after 5 weeks). ELISA diagnostics kits (Erba Lachema, CR) were used to determine the lipid profile from the blood serum. We measured levels of total cholesterol (Chol), low density lipoprotein (LDL), triacylglycerols (TG) and fasting glucose (Glu). The absorbances of the resulting colored compound was measured spectrophotometrically at 500 nm on LabSystems 352 Multiskan MS Microplate Reader (ThermoFisher Scientific, U.S.).
Blood pressure measurement
The systolic blood pressure (BP) of the animals was measured on the beginning and at the end of 5 weeks dietary experiment by non-invasive tail-cuff plethysmografic approach (ADInstruments, PowerLab, Australia) [11].
Functional studies
The functional parameters of isolated heart, dysrhythmias and evoked fibrillations were evaluated under the conditions of ischemia-reperfusion. The heart function was tested using Langendorff preparation under constant pressure [12]; ischemia/reperfusion occurred 20 min by stop-flow was followed by 25 min of reperfusion. Evaluation of coronary flow (F), left ventricular pressure (LVP), heart rate (HR), number of ventricular premature beats (VPB), time-duration of ventricular tachydysrhythmias (VT) and fibrillation (VF) were conducted.
Statistical evaluation
The data were statistically evaluated using GraphPad Prism 6 Software (La Jolla, USA). Data were expressed as means ± SEM. One-way analysis of variance (ANOVA) was used to evaluate the difference among all experimental groups (using the Bonferroni multiple comparison test).
The level of p<0.05 was considered as statistically significant difference. Different experimental groups were compared, thus different corresponding symbols were used to mark significancy as * vs. SD, as # vs. HFFD and § when comparing BP-end vs. BP-basal.
Results and Discussion
Previously, we have already reported that a high fat diet (1 % cholesterol, 7.5 % pork lard) with additional 10 % fructose can serve as proper model to induce experimental MetS [8]. In the present, the 5 weeks lasting experiment, HTG rats fed with HFFD developed several MetS-like features as dyslipidemia, increased fasting glucose level and increased number of ventricular premature beats (VPB). HTG rats fed with HFFD had weight gain significantly increased compared to SD (79.10±2.49 g vs. 46.90±2.15 g). Under these experimental conditions we tested effects of two antioxidants supplemented alone or in their combination.
Monitored biochemical parameters of lipid profile and BP measured in HTG rats are listed in Table 1. Administration of the HFFD to the rats enhanced signs of MetS manifested as increased not only body weight gain but also the heart weight. Administration of antioxidants did not affect these parameters. The heart weight/body weight ratio did not change among the groups. The liver weight was increased in HFFD rats compared to SD. The treatment did not affect this further (data not shown).
Table 1.
Biochemical parameters of lipid profile (total cholesterol – Chol; low density lipoprotein – LDL; blood glucose – Glu; triacylglycerols – TG) and blood pressure (BP) measured in hereditary hypertriglyceridemic (HTG) rats. BP measured at the beginning of the experiment (BP-basal) and at the end of 5 weeks dietary intervention (BP-end).
| Chol (mmol/l) | LDL (mmol/l) | Glu (mmol/l) | TG (mmol/l) | BP-basal (mm Hg) | BP-end (mm Hg) | |
|---|---|---|---|---|---|---|
| SD | 1.48±0.08 | 0.52±0.07 | 7.34±0.25 | 1.53±0.19 | 146.34±1.60 | 151.12±1.65 |
| HFFD | 3.96±0.33*** | 2.62±0.38*** | 8.30±0.16** | 1.84±0.28 | 142.06±2.28 | 153.82±1.96§§ |
| HFFD+SMe | 3.41±0.23*** | 2.24±0.25*** | 8.00±0.38 | 1.67±0.16 | 141.27±2.16 | 139.48±2.83*,# |
| HFFD+VitE | 3.31±0.18*** | 2.20±0.27***,# | 7.68±0.39 | 1.56±0.14 | 143.22±1.86 | 140.68±2.03*,# |
| HFFD+SMe+VitE | 3.30±0.33*** | 2.62±0.40*** | 7.66±0.69 | 1.75±0.19 | 143.60±1.76 | 130.96±2.15**,##,§§§ |
SD – standard diet; HFFD – high fat fructose diet; vitE – vitamin E; SMe – synthetic pyridoindole antioxidant SMe1EC2; Data are expressed as mean values ± SEM. The significant differences marked as * p<0.05; ** p<0.01; *** p<0.001; * vs. SD; # p<0.05; ## p<0.01; # vs. HFFD; §§ p<0.01; §§§ p<0.001; § BP-end vs. BP-basal.
Serum levels of Chol, LDL and Glu were increased compared to the SD experimental group. As already HTG rats have increased blood pressure, HFFD induced only minimal nonsignificant additional effect. There were not noted distinct changes within systolic BP between HFFD and SD groups, however, the administration of the tested antioxidants (VitE, SMe, VitE+SMe) led to significant decrease of the BP at the end of 5 weeks dietary regime. The effect of the antioxidant supplementation on particular biochemical parameters on HTG rats was already proven previously [8,9].
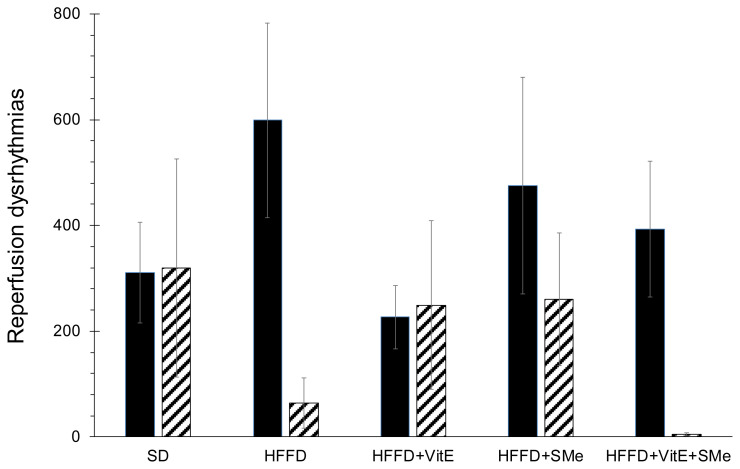
Functional studies on isolated heart showed that the HFFD significantly (*p<0.047) increased the coronary flow and the force of contraction, compared to SD. The evoked fibrillation was not influenced by HFFD (data not shown). During the reperfusion, the HFFD caused the increase of the VPB number at the expense of decreasing the duration of serious dysrhythmias (VT and VF; Fig. 1). The supplementation of the HFFD with VitE, either alone or in combination with SMe, decreased body weight gain, depressed blood pressure, improved particular biochemical parameters. The combination of VitE and SMe also suppressed the occurrence of serious dysrhythmias VT and VF. We aimed to support expected positive effect of VitE with the effect of synthetic pyridoindole antioxidant SMe1EC2, as the last one was effective even in the monotherapy of experimental MetS [13,14]. Novel derivative SMe1EC2 is pharmaco-logically practicable antioxidant drug that belongs to the group of substituted pyridoindoles such are active substances and highly effective scavengers of reactive oxygen species [15]. Our aforementioned findings are in good agreement with previous studies investigating the potential of SMe in terms of improved cardiac function by elevating the left ventricular developed pressure and cardiac contractility [16]. Under similar experimental design, on HTG rats fed with HFFD and supplemented by SMe, there were observed significantly improved coronary flow and decreased sensitivity of hearts to electrically induced ventricular fibrillation [17]. Recently, there was tested on HTG rats the supplementation of combination of hypolipidemic and cardioprotective atorvastatin with plant extract silymarin, known for its antioxidant and anti-inflammatory actions. This concept of combining the administration of synthetic drug supported by natural antioxidant, potentiated the hypolipidemic effect, reduced ectopic lipid accumulation, improved glucose metabolism, and increased antioxidant and anti-inflammatory actions [18]. Our results showed that combination of VitE and SMe leads to a reduction of the number of serious dysrhythmias (VT and VF) and moreover, the blood pressure at the end of 5 weeks lasting experiment had the lowest value in the group with a combination of SMe and VitE.
Fig. 1.
Reperfusion dysrhythmias observed after 5 weeks of dietary experiment on hereditary hypertriglyceridemic (HTG) rats. SD – standard diet; HFFD – high fat fructose diet; vitE – vitamin E; SMe – synthetic pyridoindole antioxidant SMe1EC2; VPB – the number of ventricular premature beats [n]; VT – time-duration of ventricular tachydysrhythmias [s]; VF – time-duration of ventricular fibrillations [s]; Black full columns – VPB; Dashed columns – VT+VF; Data are expressed as mean values ± SEM.
Conclusions
Our data indicate that the HFFD-related disturbances led to alterations within pathophysiology in HTG rats. The results showed that a combination of antioxidants might have the potential to amend disorders accompanying MetS. In the HTG rats, the HFFD induced disturbances resembling MetS leading to the injured heart capability to resist reperfusion dysrhythmias. The results showed that a combination of antioxidants might have the potential to amend these disorders.
Acknowledgements
This study work was supported by grants: VEGA No 2/0120/19, VEGA No 2/0104/21, APVV-18-0336, and EU project ITMS 2014 + 313021Y920.
Funding Statement
This study work was supported by grants: VEGA No 2/0120/19, VEGA No 2/0104/21, APVV-18-0336, and EU project ITMS 2014 + 313021Y920.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1. Ahmed MH, Blaha JM, Nasir K, Rivera JJ, Blumenthal SR. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109:288–95. doi: 10.1016/j.amjcard.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 2. de Souza MFM, Gawriszewski VP, Orduňez P, Sanhueza A, Espinal MA. Cardiovascular disease mortality in the Americas: current trends and disparities. Heart. 2012;98:1207–1212. doi: 10.1136/heartjnl-2012-301828. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go SA. American Heart Association Statistics Committee. Heart disease and stroke statistics - 2016 update. A report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, Assi HI. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23:786. doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayés de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Hearth J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 6. Zicha J, Pechánová O, Cacányiová S, Cebová M, Kristek F, Török J, Simko F, Dobesová Z, Kunes J. Hereditary hypertriglyceridemic rat: a suitable model of cardiovascular disease and metabolic syndrome? Physiol Res. 2006;55(Suppl 1):S49–S63. doi: 10.33549/physiolres.930000.55.S1.49. [DOI] [PubMed] [Google Scholar]
- 7. Stolba P, Dobesová Z, Husek P, Opltová H, Zicha J, Vrána A, Kunes J. The hypertriglyceridemic rat as a genetic model of hypertension and diabetes. Life Sci. 1992;51:733–740. doi: 10.1016/0024-3205(92)90482-5. [DOI] [PubMed] [Google Scholar]
- 8. Kaprinay B, Lipták B, Slovák L, Švík K, Knezl V, Sotníková R, Gáspárová Z. Hypertriglyceridemic rats fed high fat diet as a model of metabolic syndrome. Physiol Res. 2016;65(Suppl 4):S515–S518. doi: 10.33549/physiolres.933524. [DOI] [PubMed] [Google Scholar]
- 9. Michalikova D, Tyukos Kaprinay B, Brnoliakova Z, Sasvariova M, Krenek P, Babiak E, Frimmel K, et al. Impact of improving eating habits and rosmarinic acid supplementation on rat vascular and neuronal system in the metabolic syndrome model. Br J Nutr. 2020;20:1–11. doi: 10.1017/S000711452000327X. [DOI] [PubMed] [Google Scholar]
- 10. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36:14–0. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 11. Lipták B, Kaprinay B, Gáspárová Z. A rat-friendly modification of the non-invasive tail-cuff to record blood pressure. Lab Animal. 2017;46:251–53. doi: 10.1038/laban.1272. [DOI] [PubMed] [Google Scholar]
- 12. Brosková Z, Knezl V. Protective effect of novel pyridoindole derivatives on ischemia/reperfusion injury of the isolated rat heart. Pharmacol Res. 2011;63:967–974. doi: 10.1016/S1734-1140(11)70612-0. [DOI] [PubMed] [Google Scholar]
- 13. Bezek Š, Brnoliaková Z, Sotníková R, Knezl V, Paulovičová E, Navarová J, Bauer V. Monotherapy of experimental metabolic syndrome: I. Efficacy and safety. Interdiscip Toxicol. 2017;10:81–85. doi: 10.1515/intox-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knezl V, Sotnikova R, Brnoliakova Z, Stankovicova T, Bauer V, Bezek Š. Monotherapy of experimental metabolic syndrome: II. Study of cardiovascular effects. Interdiscip Toxicol. 2017;10:86–92. doi: 10.1515/intox-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kovacikova L, Majekova M, Stefek M. Substituted pyridoindoles as biological antioxidants: drug design, chemical synthesis, and biological activity. Methods Mol Biol. 2015;1208:313–327. doi: 10.1007/978-1-4939-1441-8_23. [DOI] [PubMed] [Google Scholar]
- 16. Liptak B, Knezl V, Gasparova Z. Anti-arrhythmic and cardio-protective effects of atorvastatin and a potent pyridoindole derivative on isolated hearts from rats with metabolic syndrome. Bratisl Lek Listy. 2019;120:200–206. doi: 10.4149/BLL_2019_034. [DOI] [PubMed] [Google Scholar]
- 17. Salvaras L, Kovacic T, Janega P, Liptak B, Sasvariova M, Michalikova D, Tyukos Kaprinay B, et al. Synthetic pyridoindole and rutin affect upregulation of endothelial nitric oxide synthase and heart function in rats fed a high-fat-fructose diet. Physiol Res. 2021;70:851–863. doi: 10.33549/physiolres.934670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marková I, Malínská H, Hüttl M, Miklánková D, Oliyarnyk O, Poruba M, Rácová Z, Kazdová L, Večeřa R. The combination of atorvastatin with silymarin enhances hypolipidemic, antioxidant and anti-inflammatory effects in a rat model of metabolic syndrome. Physiol Res. 2021;70:33–43. doi: 10.33549/physiolres.934587. [DOI] [PMC free article] [PubMed] [Google Scholar]