Summary
During phototherapy of jaundiced newborns, vasodilation occurs in the skin circulation compensated by vasoconstriction in the renal and mesenteric circulation. Furthermore, there is a slight decrease in cardiac systolic volume, and blood pressure, as well as an increase in heart rate and discrete changes in the heart rate variability (HRV). The primary change during phototherapy is the skin vasodilation mediated by multiple mechanisms: 1) Passive vasodilation induced by direct skin heating effect of the body surface and subcutaneous blood vessels, modified by myogenic autoregulation. 2) Active vasodilation mediated via the mechanism provided by axon reflexes through nerve C-fibers and humoral mechanism via nitric oxide (NO) and endothelin 1 (ET-1). During and after phototherapy is a rise in the NO:ET-1 ratio. 3) Regulation of the skin circulation through the sympathetic nerves is unique, but their role in skin vasodilation during phototherapy was not studied. 4) Special mechanism is a photorelaxation independent of the skin heating. Melanopsin (opsin 4) – is thought to play a major role in systemic vascular photorelaxation. Signalling cascade of the photorelaxation is specific, independent of endothelium and NO. The increased skin blood flow during phototherapy is enabled by the restriction of blood flow in the renal and mesenteric circulation. An increase in heart rate indicates activation of the sympathetic system as is seen in the measures of the HRV. High-pressure, as well as low-pressure baroreflexes, may play important role in these adaptation responses. The integrated complex and specific mechanism responsible for the hemodynamic changes during phototherapy confirm adequate and functioning regulation of the neonatal cardiovascular system, including baroreflexes.
Keywords: Newborns, Phototherapy, Photorelaxation, Cardiovascular changes, Skin vasodilation, Baroreflexes
Introduction
Hyperbilirubinemia is the most commonly occurring metabolic complication of the neonatal period. In some neonates, hyperbilirubinemia reaches high values that threaten the infant with encephalopathy.
Phototherapy is currently the most effective non-invasive method of neonatal hyperbilirubinemia treatment. The main therapeutic principle of phototherapy is to accelerate the degradation and excretion of bilirubin by light. By exposing the skin and blood in vessels under the superficial layer of the skin to light with an appropriate wavelength and irradiance intensity, photochemical conversion of native bilirubin starts. This conversion results in the formation of several products – bilirubin photoisomers. Phototherapy products are more water-soluble and can be excreted into bile and urine, bypassing conjugation in the liver. In addition to those effects, bilirubin photoisomers compared to natural bilirubin are much less toxic [1,2].
The effectiveness of phototherapy depends, among other factors on the wavelength of the light, and the irradiance intensity. The most effective wavelength is 450–460 nm (corresponding to blue light). Light with a wavelength range of 400 to 520 nm and peaked at 450±20 nm, or in the range of 460–490 nm is recommended for neonatal phototherapy [3].
The child's position is supination and alternates with pronation, every two-three hours. The duration of phototherapy is up to several tens of hours depending on the severity of the hyperbilirubinemia and the phototherapy effectiveness. The child should be in a well-maintained thermoneutral environment, with regular feeding and increased fluid supply.
With proper indications and application, phototherapy is a relatively safe method. However, it has some side effects, among others, in the cardiovascular system.
Overview of the acute cardiovascular side effects of phototherapy
Phototherapy of jaundiced neonates is accompanied by vasodilation in the skin circulation, increasing blood flow (BF) [4,5,6]. The different results (increases of the BF by 40–220 %) obtained by these authors were due to the varying characteristics of the groups of newborns, different ambient and body temperature and methodologies used for skin BF measurements and phototherapy. However, it is evident that during neonatal phototherapy there is massive vasodilation in the skin and an increase in cutaneous blood flow.
Increased blood supply to the skin results in redistribution of blood. Yao et al. [7] and Pezzati et al. [8] found in the mesenteric circulation, a reduction of the postprandial increase in blood flow during phototherapy. Phototherapy was also accompanied by a significant reduction of blood flow velocity in the renal circulation and by a rise in renal vascular resistance [9].
Another important question was whether phototherapy of newborns significantly affects the blood flow in the cerebral and coronary circulation. Amato and Donati [10] found no significant changes in the characteristics of the blood flow through the brain. On the other hand, Benders et al. [11] found that the average velocity of blood flow in cerebral vessels increases after the initiation of phototherapy. Borenstein-Levin et al. [12] found no significant changes in velocity and flow in the coronary vessels during phototherapy. It seems that hyperperfusion of the skin during phototherapy does not occur at the expense of the cerebral or coronary perfusion.
During phototherapy, the venous return due to vasodilation in the cutaneous circulation may be reduced, but there is a lack of studies on this topic. There is a slight reduction in cardiac output (CO) due to a reduction in stroke volume (SV) as early as 30 min after the start of phototherapy [5,13]. In more recent work, Firouzi et al. [14] confirmed these results and found that phototherapy reduces left ventricular ejection fraction in preterm neonates.
Changes in peripheral vascular resistance, blood redistribution and the decrease in cardiac output have effects on systemic blood pressure (BP). During phototherapy, there is a decrease in systolic, diastolic and mean blood pressure, together with a concomitant slight increase in heart rate (HR) [15,16]. Discrete changes in heart rate variability (HRV) are also present during phototherapy. These changes can be demonstrated by time analysis, Poincaré plots [17], as well as by nonlinear symbolic dynamics methods [18].
The mechanisms of cardiovascular changes during phototherapy
The primary cardiovascular change that subsequently causes adaptive reactions in the cardiovascular system is vasodilation in skin circulation. We assume that other changes in the cardiovascular system, such as the redistribution of blood from the mesenteric and renal circulation, as well as the increase in heart rate, are targeted for maintaining cardiac output and perfusion of the most important vital organs (heart, brain).
Mechanisms of vasodilation in the skin circulation
Skin vasodilation is mediated by a very complex physiological mechanism (Fig. 1).
Fig. 1.
Mechanisms involved in the skin vasodilation induced by thermal influx during neonatal phototherapy.
Vasodilation induced by local heat
Despite technical improvements in light sources for phototherapy (fluorescent bulbs, halogen lamps, light-emitting diodes – LEDs), the thermal influx, which heats the body surface, remains as a component of the applied light. The increase in body temperature depends on the type of light source, but more on the intensity of the irradiance, during conventional or in some cases intensive phototherapy [19].
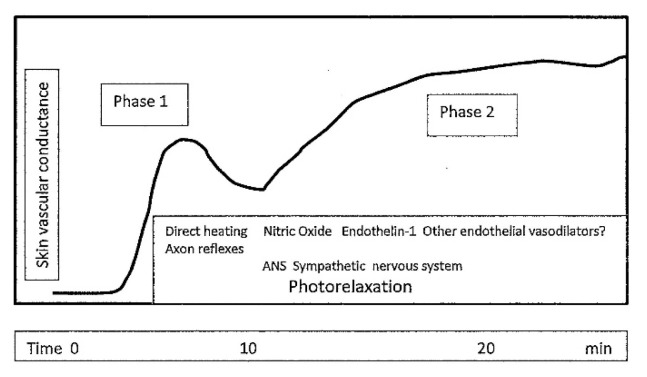
The vasodilatory response to local heating is biphasic [20,21,22]. (Fig. 2). Vasodilation starts already in the first minutes and is provided passively and actively.
Fig. 2.
The scheme of changes in skin vascular conductance during local warming (in healthy human adults). The first phase is due to the effect of direct local heating and axon reflexes, the second phase depends mainly on humoral regulation. Autonomic nervous system – sympathetic innervating skin vessels and photorelaxation can play a role during both phases. (Modified according to Charkoudian et al. [22].
Passive vasodilation relies on direct heating of the body surface and subcutaneous blood vessels. The diameter of the skin blood vessels is also influenced by myogenic autoregulation [23].
Neural and humoral mechanisms are involved to the active vasodilation. The initial rapid phase of the vasodilation relies on the local activity of sensory C-fibers nerves. The axon reflexes are mediated by vasodilatory substances such as Calcitonin Gene-Related Peptide (CGRP), substance P and neurokinin A [24].
The humoral mechanism starts later, in the second phase, and is provided mainly by endothelial substances nitric oxide (NO) and endothelin 1 (ET-1).
Nitric oxide (NO) is the most important vasodilatory substance produced by the endothelium. NO, once formed in endothelial cells, diffuses into the smooth muscle where it activates the enzyme guanylate cyclase with a consequent increase in the concentration of cyclic guanosine monophosphate (cGMP), which relaxes vascular smooth muscle. Nitric oxide (NO) is responsible for about 30 % of the active vasodilation to whole-body heat stress in adult humans [22,25].
Endothelin 1 induces vasoconstriction activating ETA and ETB2 receptors, through ETB1 vasodilation. In addition to its effect on the vessel diameter, ET-1 also co-regulates the production of its opponent NO. Stimulation of the ETB receptors in the endothelium increases the production of its opponent, NO, independently of mechanical stimulation by blood flow [26,27].
NO/ET-1 system is already active in the fetal and early postnatal period. ET-1 level is after birth, especially in premature newborns, even three times higher than later life and all three ET receptor subtypes are well developed at birth.
Like ET-1, the role of NO in the regulation of systemic vascular resistance is significantly greater in younger individuals. The NO level in the blood a. mesenterica superior as well as the response of this circulatory region to various NO-mediated vasodilatory stimuli are highest just in the neonatal period [28,29].
Therefore, some researchers have also focused their research on NO and ET-1 in explaining hemodynamic changes during phototherapy of newborns. Ergenekon et al. [30] found an increased urinary NO concentration in preterm neonates during phototherapy. Similarly, Abu Faddan et al. [31] after 24 h of phototherapy found a significant rise in serum level of NO, but also together with ET-1. However, the NO concentration increased more, thus the NO:ET ratio increased significantly. The authors suggest that during phototherapy, the dynamic balance between NO and ET-1 is shifted to the NO side, leading to vasodilation.
The simultaneous increase of NO and ET-1 during phototherapy is not surprising. It may be mediated by the above-mentioned feedback mechanism via ETB receptors. Opposing effects of NO and ET-1 on vascular wall tone are responsible for the dynamic balance, and under physiological conditions, it helps to regulate blood flow adequate to requirements.
Blood vessels in the skin may be affected by prostaglandins (PGs) synthesis. In addition to local effects, PGs could also affect more distant vessels, such as the ductus arteriosus (DA). It has been hypothesized that during phototherapy, PGs level increases and this causes a slowing down closure or reopening of the duct arteriosus [13]. However, more recent studies have not confirmed these suggestions [32,33].
Other endothelial vasodilators could be involved in the vasodilation like carbon monoxide (CO) and/or hydrogen sulfide (H2S). Unlike NO and CO, H2S does vasodilation via activation of ATP-sensitive potassium channels in vascular smooth muscle. H2S by functioning through other pathways completes the effects of NO and CO even in neonates [34]. The role of these gaseous mediators in vasodilation has not yet been studied during phototherapy.
Neural regulation of skin circulation
The skin vessel diameter is under unique control, through two populations of sympathetic nerves: adrenergic vasoconstrictor and “cholinergic” vasodilatory system. Both systems innervate non-glabrous skin, glabrous areas (palms, soles, lips) are innervated only by the vasoconstrictor sympathetic system [35].
Sympathetic vasoconstrictory adrenergic system cooperates with co-transmitters – neuropeptide Y (NPY) and ATP [23]. This system can produce two qualitatively different effects – vasoconstriction or vasodilation by changing its activity. Withdrawal of the activity of these nerves is responsible for 10–20 % of the cutaneous vasodilation during hyperthermia [35]. We can hypothesize that during phototherapy in newborns the reduced tonic activity in the adrenergic sympathetic system facilitates vasodilation.
The sympathetic “cholinergic” vasodilatory system is responsible for vasodilation as well as for sweating. There were presumed as neurotransmitters acetylcholine and bradykinin. More recent works point to the involvement of VIP or/a pituitary adenylate-cyclase-activating peptide (PACAP) as neurotransmitters [25,36,37].
Neural regulation of skin circulation is responsible for 80–90 % of vasodilation in a hot environment in adults [22]. During phototherapy in newborns, the contribution of the nerves to the skin vasodilation has not been studied.
Light-induce vasodilation – photorelaxation
When the phototherapy technique (e.g. LEDs) without a significant increase in skin/body temperature is used, an increase in skin blood flow does remain but is smaller. This suggests that there are also other special mechanisms of vasodilation independent of the skin/body heating during phototherapy. This special mechanism is photorelaxation of skin vessels.
The reversible relaxation of blood vessels by light was demonstrated by Furchgott et al. [38]. In both in vitro and in vivo animal experiments, they found that light can induce vasodilation. The effect depended on the initial muscle tone and both the intensity and the wavelength of the light used.
Photorelaxation is wavelength-specific, with a maximal response at ~430–460 nm. Blue light (wavelength 455 nm) acts more effectively on the vessel's diameter [39]. Interestingly, this is the wavelength range that is used in phototherapy of neonates with hyperbilirubinemia.
Currently, light receptors in blood vessels and their signalling pathways are being intensively studied. In the retina, photopigments – opsins (Opn1, Opn2) are present for visual perception. Research has focused on so-called “atypical opsins”, which are found not only in the eye but also in extraocular, often deep-located tissues, e.g. in the coronary arteries or airways [40,41].
Of these “no-visual, atypical opsins”, the most studied is opsin4 (Opn4) – melanopsin. Melanopsin belongs to the family of G-protein coupled membrane receptors (GPCRs) designed to detect a variety of physical, chemical, and biological signals [42]. In the eye, melanopsin is found in ganglion cells (ipRGCs – intrinsically photosensitive retinal ganglion cells). These cells make up about 1 % of all RGCs. However, they are also found in the lens, m. sphincter pupilae, brain, neurons, and adipocytes. Melanopsin in these structures is important in the control of circadian rhythm, in the maintenance of attention and wakefulness, and for the pupillary reflex [43].
Melanopsin has also been demonstrated in arteries of the systemic circulation. Sikka et al. [39] using PCR, showed that Opn4 is also expressed in blood vessels and is thought to play a major role in photorelaxation. The blood vessels of Opn4−/− mice do not show photorelaxation, and the photorelaxation is also inhibited by an Opn4-specific inhibitor.
Photorelaxation has also been demonstrated in the pulmonary vasculature (mice, rats), where Opn4 in co-modulation with opsin3 (Opn3) provides pulmonary photovasodilation [44] and in the porcine coronary vasculature [40]. Opsin3 was also found in airway smooth muscles and mediates their photorelaxation [41,45].
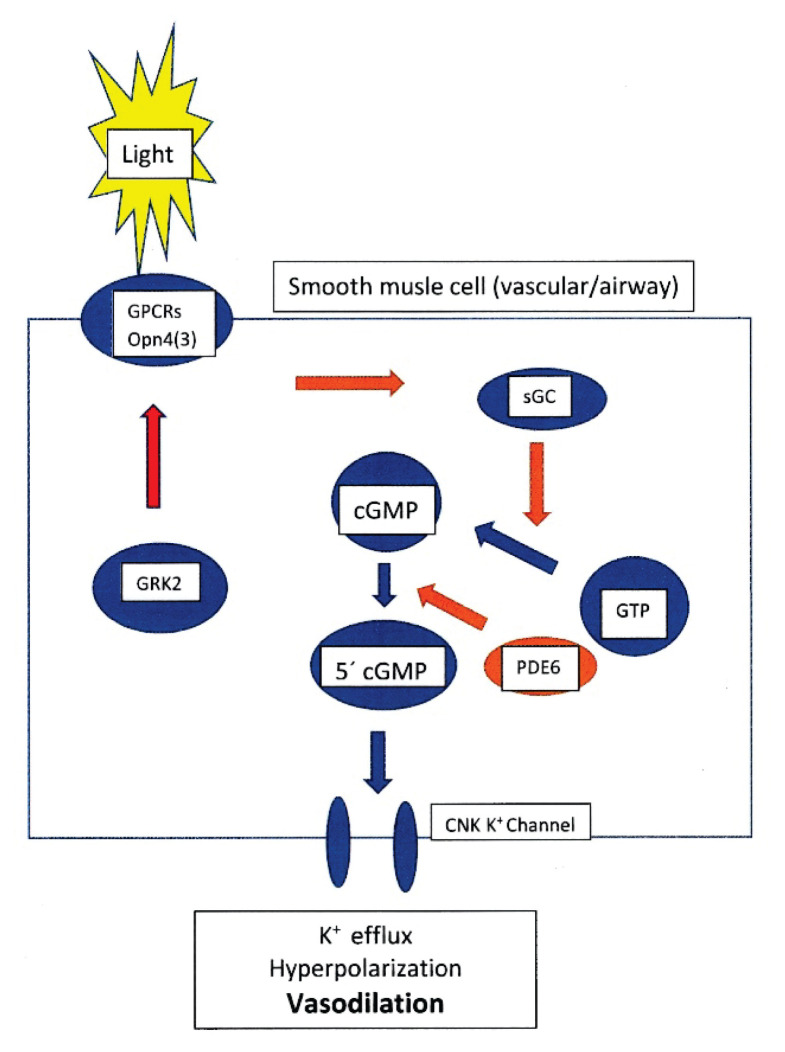
The melanopsin signalling pathway in blood vessels is specific, different from the signalling cascade in the retina, sphincter pupilae, or white adipocytes. It does not involve protein kinase G and is independent of endothelium and NO. The pathway involves soluble guanylate cyclase (sGC) and phosphodiesterase 6 (PDE6) [39,42].
The first part of the melanopsin signalling pathway (Fig. 3) is its activation by light, which results in the photochemical isomerization of 11-cis-retinal to all-trans-retinal. This is an extremely rapid process lasting 60–100 fs (10−15 s). After activation of the regulatory G proteins [46], the cascade continues by soluble guanylate cyclase activation. Following activation of guanylate cyclase (sGC), the intracellular level of cyclic guanosine monophosphate (cGMP) increases. Increased level of cGMP activates a specific phosphodiesterase (PDE), which cleaves cGMP to produce 5′cGMP. The 5′cGMP thus generated is involved in the regulation of K+-channels in vascular smooth muscle cells.
Fig. 3.
Simplified scheme of suggested signal transduction mechanism of the melanopsin (Opn4) and/or opsin 3 (Opn3) in photorelaxation of the vascular/airway smooth muscle cells. (Modified according to Sikka et al. [39], Barreto Ortiz et al. [44]). GPCRs – G protein coupled receptors opsin 4/3; sGC – soluble guanylate cyclase; GTP – Guanosine-5′-triphosphate; cGMP – cyclic guanosine monophosphate; PDE6 – phosphodiesterase 6; 5′cGMP – Guanosine 3’,5’-cyclic monophosphate. GRK2 – G protein receptor kinase (βARK 1 – beta-adrenergic receptor kinase) modulates opsins activity and inhibits the activation of the opsins (Opn4, 3).
Once K+ channels open and K+ escapes from the cell, the membrane hyperpolarizes with subsequent relaxation of the vascular wall – vasodilation [39,42,44].
The role of melanopsin in the vasculature is yet to be proven in the ontogenetic development of vasculature. In mice, it influences postnatal regression of embryonic hyaloid vasculature and retinal vessel formation. Lack of melanopsin in mice results in the persistence of hyaloid blood vessels and a reduced number of neurons in the retina [47]. Other putative functions of vascular melanopsin and their potential therapeutic use are still speculative.
We hypothesize that photorelaxation by melanopsin activation may also play an important role in phototherapy due to the wavelengths of light applied. However, this hypothesis needs direct evidence of melanopsin in the skin vessels of neonates.
Generalized effects in the cardiovascular system during phototherapy mediated via the autonomic nervous system
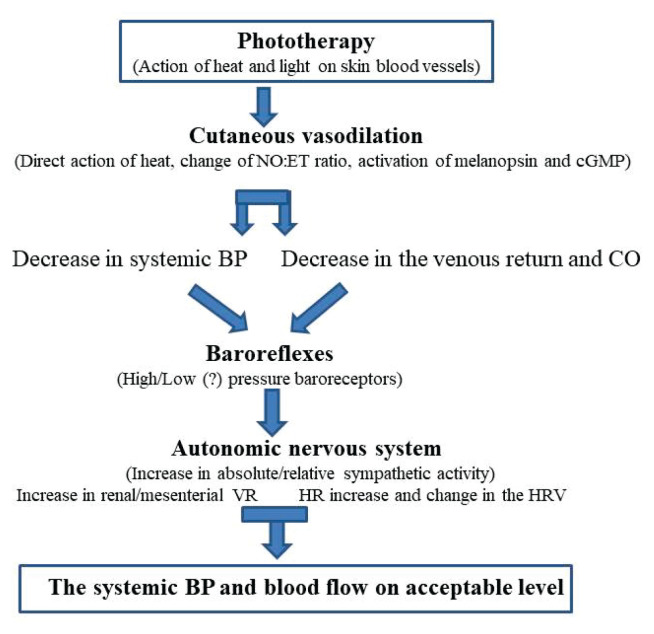
Redistribution of blood and increase in blood flow in the cutaneous circulation is made by its transfer from the renal and mesenteric circulations. Baroreflexes are the most likely mechanism for this redistribution. They induce vasoconstriction in these regional circulations already at a slight decrease in systemic blood pressure (Fig. 4).
Fig. 4.
Scheme of cardiovascular effects and some of their mechanisms during phototherapy of jaundiced newborns. NO – nitric oxide, ET – endothelin 1, cGMP – cyclic guanosine monophosphate, BP – systemic blood pressure, CO – cardiac output, VR – vascular resistance, HR – heart rate, HRV – heart rate variability.
Compensatory physiological reactions to the blood pressure fall could be mediated through high-pressure as well as by low-pressure baroreflexes. The baroreflexes help to maintain blood pressure at physiological levels, more in mature newborns regarding higher baroreflex sensitivity. Baroreflexes are already functional in premature newborns. Several authors [48,49] have found that baroreflex sensitivity (BRS) in neonates is low, but increases markedly with gestational and postnatal age. Also, Haskova et al. [50] and Javorka et al. [51] found positive correlations in preterm neonates of baroreflex sensitivity (BRS) with gestational and postconceptional age as well as with birth and current weight.
Cardiac output (CO) is slightly decreased. The cause of this decrease could be not only in the redistribution of blood and reduction of venous return, but also in reduction of motor activity of the infant [5]. There may also be a role of the higher increased nitric oxide production, which can act on the sinoatrial node [52]. Although the reduction in CO is small, it may further impair the peripheral tissue perfusion (e.g. in the splanchnic and renal circulation) mainly in the smallest premature infants or pathological newborns.
The drop in systemic blood pressure [15] could be the result of massive skin vasodilation, a decrease in venous return and cardiac output. The increased level of nitric oxide during phototherapy may also contribute to the blood pressure decrease. A negative correlation was found between mean systemic blood pressure and serum levels of NO (and a positive correlation between ET-1 and mean BP) after phototherapy in newborns [53].
The significant increase in heart rate during phototherapy may be produced by a complex mechanism helping to maintain cardiac output, pressure and blood flow. Heart rate can be increased by raising body temperature during phototherapy by direct influence on the sinoatrial node, as well as through stimulation of peripheral and central thermoreceptors.
The decrease in systemic blood pressure through high-pressure baroreceptors facilitates the tachycardic response during phototherapy [15]. Functioning baroreflexes in newborns may alter activity of the sympathetic pathway regulating the heart, as we have seen in the assessment of the heart rate variability (HRV) by dynamic symbolics [18].
We can assume that low-pressure baroreceptors in the right atrium are also involved in this response, when venous return is reduced during phototherapy.
Another possible explanation is the direct action of NO on the cardiac pacemaker, increasing heart rate [54]. El-Masry et al. [53] found a positive correlation between serum ET-1 and NO levels and heart rate after phototherapy in preterm and term newborns, but it is unclear whether this is a direct causal relationship or based on other physiological changes. The final effect of the NO on heart rate may be modified by generalized action of NO on autonomic nervous activity. It is known that NO exerts a tonic enhancing effect on human cardiac vagal control in adult humans [48].
The described changes in cardiovascular parameters during phototherapy do not usually jeopardize mature newborns. The existence of a few serious cardiovascular complications during phototherapy in mature newborns suggest that physiological newborns (apart from hyperbilirubinemia) are admirably well equipped to handle even such a specific therapeutic challenge. The situation is different in very premature newborns, who already have primarily lower baseline blood pressure values, increased heart rate and lower baroreflex sensitivity. It appears that in this group of infants, especially in haemodynamically unstable neonates (incipient sepsis, etc.), phototherapy can lead to severe hypotension and other serious or even fatal complications.
Conclusions
During phototherapy of newborns with hyperbilirubinemia, a massive vasodilation occurs in the skin circulation compensated by vasoconstriction in the renal and mesenteric circulations, without markedly affecting the coronary and cerebral circulations. Furthermore, there is a decrease in systemic blood pressure, an increase in heart rate and changes in heart rate variability indicating raising sympathetic activity.
The mechanism of these changes is very complex and involves operations from the molecular to the systemic level. Functional changes in the cardiovascular system during phototherapy confirm adequate cardiovascular regulation in newborns, including special regulation of the skin circulation. These changes are important in maintaining optimal vital functions in this specific environmental and therapeutical conditions.
Acknowledgements
This work was supported by project VEGA No. 1/0283/21.
Funding Statement
This work was supported by project VEGA No. 1/0283/21.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1. Hansen TWR. Phototherapy for neonatal jaundice--therapeutic effects on more than one level? Semin Perinatol. 2010;34:231–34. doi: 10.1053/j.semperi.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 2. Dvořák A, Pospíšilová K, Žížalová K, Capková N, Muchová L, Vecka M, Vrzáčková N, Křížová J, Zelenka J, Vítek L. The effects of bilirubin and lumirubin on metabolic and oxidative stress markers. Front Pharmacol. 2021;12:567001. doi: 10.3389/fphar.2021.567001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhutani VK. Committee on Fetus and Newborn; American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011;128:e1046–e1052. doi: 10.1542/peds.2011-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu PYK, Wong WH, Hodgman JE, Levan N. Changes in blood flow in the skin and muscle with phototherapy. Pediatr Res. 1974;8:257–62. doi: 10.1203/00006450-197404000-00007. [DOI] [PubMed] [Google Scholar]
- 5. Walther FJ, Wu PY, Siassi B. Cardiac output changes in newborns with hyperbilirubinemia treated with phototherapy. Pediatrics. 1985;76:918–921. doi: 10.1542/peds.76.6.918. [DOI] [PubMed] [Google Scholar]
- 6. Jahnukainen T, Lindqvist A, Jalonen J, Kero P, Valimaki I. Responsiveness of cutaneous vasculature to thermal stimulation during phototherapy in neonatal jaundice. Eur J Pediatr. 1999;158:757–760. doi: 10.1007/s004310051195. [DOI] [PubMed] [Google Scholar]
- 7. Yao AC, Martinussen M, Johansen OJ, Brubakk AM. Phototherapy associated changes in mesenteric blood flow response to feeding in term neonates. J Pediatr. 1994;124:309–312. doi: 10.1016/S0022-3476(94)70325-6. [DOI] [PubMed] [Google Scholar]
- 8. Pezzati M, Biagiotti R, Vangi V, Lombardi E, Wiechmann L, Rubaltelli FF. Changes in mesenteric blood flow response to feeding: conventional versus fibre-optic phototherapy. Pediatrics. 2000;105:350–353. doi: 10.1542/peds.105.2.350. [DOI] [PubMed] [Google Scholar]
- 9. Benders MJ, Van Bel F, van de Bor M. The effect of phototherapy on renal blood flow velocity in preterm infants. Biol Neonate. 1998;73:228–34. doi: 10.1159/000013981. [DOI] [PubMed] [Google Scholar]
- 10. Amato M, Donati F. Cerebral blood flow velocity in term infants treated with phototherapy. Brain Dev. 1991;13:417–419. doi: 10.1016/S0387-7604(12)80039-8. [DOI] [PubMed] [Google Scholar]
- 11. Benders MJ. The effect of phototherapy on cerebral blood flow velocity in preterm infants. Acta Paediatr. 1998;87:786–792. doi: 10.1111/j.1651-2227.1998.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 12. Borenstein-Levin L, Sharif D, Amshalom A, Riskin A, Hemo M, Khalil A, Bader D, Kugelman A. Effects of Phototherapy on Coronary Blood Flow in Healthy Neonates: A Pilot Study. Neonatology. 2016;110:75–82. doi: 10.1159/000444244. [DOI] [PubMed] [Google Scholar]
- 13. Benders MJ, Van Bel F, van de Bor M. Cardiac output and ductal reopening during phototherapy in preterm infants. Acta Paediatr. 1999;88:1014–1019. doi: 10.1111/j.1651-2227.1999.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 14. Firouzi M, Sherkatolabbasieh H, Nezami A, Shafizadeh S. Effect of phototherapy on stroke volume in newborn infants with jaundice. J Pediatr Intensive Care. 2020;9:207–09. doi: 10.1055/s-0040-1708556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Javorka K, Zavarská L’. Zmeny systémového tlaku krvi a kardiorespiračných parametrov u nedonosených novorodencov počas fototerapie. (Article in Slovak) Cesk Pediatr. 1990;45:230–232. [PubMed] [Google Scholar]
- 16. Nandrážiová L, Javorka K, Czippelová B, Mat’ašová K. Zmeny tlaku krvi a niektorých d’alších parametrov počas fototerapie donosených novorodencov. (Article in Slovak) Česko-Slovenská Pediat. 2019;74:449–457. [Google Scholar]
- 17. Weissman A, Berkowitz E, Smolkin T, Blazer S. Effect of phototherapy on neonatal heart rate variability and complexity. Neonatology. 2009;95:41–46. doi: 10.1159/000151754. [DOI] [PubMed] [Google Scholar]
- 18. Uhríková Z, Zibolen M, Javorka K, Chládeková L, Javorka M. Hyperbilirubinemia phototherapy in newborns: Effect on cardiac autonomic control. Early Hum Dev. 2015;91:351–356. doi: 10.1016/j.earlhumdev.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 19. Aydemir O, Soysaldı E, Kale Y, Kavurt S, Bas AY, Demirel N. Body temperature changes of newborns under fluorescent versus LED phototherapy. Ind J Pediatr. 2014;81:751–4. doi: 10.1007/s12098-013-1209-2. [DOI] [PubMed] [Google Scholar]
- 20. Pergola PE, Kellog DL, JR, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 21. Minson CT, Berry LT, Joyner MI. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 22. Charkoudian N. Skin blood flow in adult human thermoregulation:how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 23. Cracowski J-L, Roustit M. Human skin microcirculation. Compr Physiol. 2020;10:1105–1154. doi: 10.1002/cphy.c190008. [DOI] [PubMed] [Google Scholar]
- 24. Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/S0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 25. Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- 26. Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ETA and ETB receptors in cardiovascular disease. Ann Rev Pharmacol Toxicol. 2007;47:731–759. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu G-S, Wu H, WB-Q, Huang R-Z, Zhao L-H, Wen Y. Effect of phototherapy on blood endothelin and nitric oxide levels: clinical significance in preterm infants. World J Pediatr. 2008;4:31–35. doi: 10.1007/s12519-008-0006-x. [DOI] [PubMed] [Google Scholar]
- 28. Reber KM, Su BY, Clark KR, Clark KR, Pohlman DL, Miller CE, Nowicki PT. Developmental expression of eNOS in postnatal swine mesenteric artery. Am J Physiol. 2002;283:G1328–G1335. doi: 10.1152/ajpgi.00067.2002. [DOI] [PubMed] [Google Scholar]
- 29. Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: Contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 30. Ergenekon E, Gücüyener K, Dursun H, Erbaş D, Oztürk G, Koç E, Atalay Y. Nitric oxide production in newborns under phototherapy. Nitric Oxide. 2002;6:69–72. doi: 10.1006/niox.2001.0364. [DOI] [PubMed] [Google Scholar]
- 31. Abu Faddan NH, Abd El-Aziz NHR, Abd El-Azeem HG, Shreif T. Effect of phototherapy on blood levels of endothelin-1 and nitric oxide in hyberbilirubinemic newborn infants. e-J Neonat Res. 2014;4:14–20. [Google Scholar]
- 32. Surmeli-Onay O, Yurdakok M, Karagoz T, Erkekoglu P, Ertugrul I, Takci S, Giray BK, Aykan HH, Korkmaz A, Yigit S. A new approach to an old hypothesis; phototherapy does not affect ductal patency via PGE2 and PGI2. J Matern Fetal Neonatal Med. 2015;28:16–2. doi: 10.3109/14767058.2014.899575. [DOI] [PubMed] [Google Scholar]
- 33. Yurdakök M. Phototherapy in the newborn: what's new? J Pediatr Neonat Individual Med. 2015;4:e040255. doi: 10.7363/040255. [DOI] [Google Scholar]
- 34.Wright IM, Dyson RM. Microcirculation of the Newborn. In: LENASI H, editor. Microcirculation Revisited. From Molecules to Clinical Practice. London: IntechOpen; 2016. pp. 81–100. https://www.intechopen.com/chapters/51870, https://doi.org/10.5772/64584. [Google Scholar]
- 35.Johnson JM, Proppe DW. Cardiovascular adjustment to heat stress. In: FREGLZ MI, BLATTEIS CM, editors. Handbook of Physiology. I. Oxford University Press; New York: 1996. pp. 215–243. Section 4: Environmental Physiology. [DOI] [Google Scholar]
- 36. Kellog DL, JR, Liu Y, McAllister K, Friel C, Pergola PE. Bradykinin does not mediate cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 2002;93:1215–1221. doi: 10.1152/japplphysiol.01142.2001. [DOI] [PubMed] [Google Scholar]
- 37. Kellog DL, Jr, Zhao JL, Wu Y, Johnson JM. VIP/PACAP receptor mediation of cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 2010;109:95–100. doi: 10.1152/japplphysiol.01187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Furchgott RF, Sleator WJ, McCaman MW, Elchlepp J. Relaxation of arterial strips by light and the influence of drugs on this photodynamic effect. J Pharmacol Exp Ther. 1955;113:122. [Google Scholar]
- 39. Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, Steppan J, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci U S A. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Batenburg WW, Kappers MHW, Eikmann MJ, Ramzan SNA, de Vries R, Danser AHJ. Light-induced vs. bradykinin-induced relaxation of coronary arteries: do S-nitrosothiols act as endothelium-derived hyperpolarizing factors? J Hypertens. 2009;27:1631–1640. doi: 10.1097/HJH.0b013e32832bff54. [DOI] [PubMed] [Google Scholar]
- 41. Yim PD, Gallos G, Perez-Zoghbi JF, Zhang Y, Xu D, Wu A, Berkowitz DE, Emala CW. Airway smooth muscle photorelaxation via opsin receptor activation. Am J Physiol Lung Cell Mol Physiol. 2019;316:L82–L93. doi: 10.1152/ajplung.00135.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stachurska A, Tadeusz Sarna T. Regulation of melanopsin signalling: key interactions of the nonvisual photopigment. Photochem Photobiol. 2019;95:83–94. doi: 10.1111/php.12995. [DOI] [PubMed] [Google Scholar]
- 43. Wahl S, Engelhardt M, Schaupp P, Lappe Ch, Ivanov I. The inner clock - blue light sets the human rhythm. J Biophotonics. 2019;12:e201900102. doi: 10.1002/jbio.201900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, Chen J, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G-protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L93–L106. doi: 10.1152/ajplung.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu AD, Dan W, Zhang Yi, Vemaraju S, Upton BA, Lang BA, Buhr ED, Berkowitz DE, Gallos G, Emala CW, Yim PD. Opsin 3-Gαs promotes airway smooth muscle relaxation modulated by G protein receptor kinase 2. Am J Resp Cell Mol Biol. 2021;64:59–68. doi: 10.1165/rcmb.2020-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qiu X, Kumbalasin T, Carlson SM, Wong V, Krishna I, Provencio, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 47. Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–46. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gournay V, Drouin E, Rozé JC. Development of baroreflex control of heart rate in preterm and full term infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F151–F154. doi: 10.1136/fn.86.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yiallourou SR, Sands SA, Walker AM, Horne RSC. Postnatal development of baroreflex sensitivity in infancy. J Physiol. 2010;588:2193–203. doi: 10.1113/jphysiol.2010.187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haskova K, Javorka M, Czippelova B, Zibolen M, Javorka K. Baroreflex sensitivity in premature infants - relation to the parameters characterizing intrauterine and postnatal condition. Physiol Res. 2017;66(Suppl 2):S257–S264. doi: 10.33549/physiolres.933681. [DOI] [PubMed] [Google Scholar]
- 51. Javorka K, Haskova K, Czippelova B, Zibolen M, Javorka M. Baroreflex sensitivity and blood pressure in premature infants - Dependence on gestational age, postnatal age and sex. Physiol Res. 2021;70(Suppl 3):S349–S356. doi: 10.33549/physiolres.934829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–69. doi: 10.1161/01.HYP.36.2.264. [DOI] [PubMed] [Google Scholar]
- 53. El-Masry HMA, Hassan AA, Hashim AM, Aladawy MAA, Abdelwahab AAA. Evaluation of serum endothelin-1 (ET-1) and nitric oxide (NO) levels in unconjugated hyperbilirubinemic neonates. Egypt J Hospit Med. 2020;81:1858–1865. doi: 10.21608/ejhm.2020.121012. [DOI] [Google Scholar]
- 54. Musialek P, Lei M, Brown HF, Paterson DJ, Casadei B. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f) Circ Res. 1997;81:60–68. doi: 10.1161/01.RES.81.1.60. [DOI] [PubMed] [Google Scholar]