Abstract
BACKGROUND
The massive use of insecticides in public health has exerted selective pressure resulting in the development of resistance in Aedes aegypti to different insecticides in Venezuela. Between 2010 and 2020, the only insecticides available for vector control were the organophosphates (Ops) fenitrothion and temephos which were focally applied.
OBJECTIVES
To determine the state of insecticide resistance and to identify the possible biochemical and molecular mechanisms involved in three populations of Ae. aegypti from Venezuela.
METHODS
CDC bottle bioassays were conducted on Ae. aegypti collected between October 2019 and February 2020 in two hyperendemic localities for dengue in Aragua State and in a malaria endemic area in Bolívar State. Insecticide resistance mechanisms were studied using biochemical assays and polymerase chain reaction (PCR) to detect kdr mutations.
FINDINGS
Bioassays showed contrasting results among populations; Las Brisas was resistant to malathion, permethrin and deltamethrin, Urbanización 19 de Abril was resistant to permethrin and Nacupay to malathion. All populations showed significantly higher activity of mixed function oxidases and glutathione-S-transferases (GSTs) in comparison with the susceptible strain. The kdr mutations V410L, F1534C, and V1016I were detected in all populations, with F1534C at higher frequencies.
MAIN CONCLUSION
Insecticide resistance persists in three Ae. aegypti populations from Venezuela even in the relative absence of insecticide application.
Key words: Aedes aegypti, metabolic detoxification, kdr mutations, V410L, F1534C, V1016I
Aedes aegypti is the principal vector of several viruses including dengue, chikungunya, Zika and yellow fever. 1 This species originated in Africa and was originally a tree-hole forest mosquito, 2 spread to other continents through trade, and subsequently adapted to urban environments where the females oviposit in artificial containers. 3 More recently, Ae. aegypti has colonized rural areas throughout the world 4 , 5 , 6 and even remote isolated forest areas in southern Venezuela, 7 broadening the risk of arbovirus transmission.
According to World Health Organization (WHO), 1 dengue incidence has increased considerably in recent decades; most cases are asymptomatic or mild, or misdiagnosed as a febrile illness, and hence, the actual number of cases are under-reported. It has been estimated that 3.9 billion people are at risk of infection in 129 countries 8 with an estimated global burden of 390 million dengue infections per year. 9 At present, prevention and control of the transmission of dengue viruses is based heavily on vector control. Many efforts have been directed at the reduction of larval habitats and environmental sanitation with community participation, although these approaches can be challenging to sustain, especially in areas with socio-political-economic crises such as in Venezuela.
The use of chemical insecticides has been the main intervention for the control of Ae. aegypti in Venezuela for over six decades. Dichlorodiphenyltrichloroethane (DDT) was widely used from the 1950’s until the mid-1970’s for the control of Ae. aegypti (yellow fever) and anophelines (malaria). With the availability of the yellow fever vaccine and an efficient vaccination program, the use of DDT was discontinued in urban areas, but its use continued in rural areas for the control of vectors of malaria parasites until the mid-1990’s and was finally banned in 2001. 10
In 1989-1990, the first major outbreak of dengue and dengue hemorrhagic fever (DHF) was reported in Venezuela, with the first cases reported in Maracay, Aragua State and from there expanding to many urban areas in the country. 11 , 12 Subsequently, continuous outbreaks of dengue have been reported from Venezuela each year, with the simultaneous circulation of DENV1, DENV2, DENV3 and DENV4. 13 Although reduction of larval sources such as water tanks, cisterns, and used tires can help to suppress vector populations, its implementation has been sporadic while the use of insecticides has been extensive and intense. The primary insecticides used in Venezuela are organophosphates (OPs), such as temephos for larval control, and malathion in thermal foggers and ultra-low volume sprays for adult mosquito control. Additionally, pyrethroids were often incorporated into vector control programs. Since 2010, only fenitrothion (an OP) and temephos were available in limited quantities and applied in a focal manner: in and around houses with confirmed severe dengue cases.
The massive use of insecticides in public health has exerted selective pressure resulting in the development of resistance in Ae. aegypti to different insecticides in Venezuela as well as all over the world. 14 Resistance to DDT in Ae. aegypti was reported for the first time by Quarterman and Schoof 15 in Caracas, followed by a study conducted by Mouchet 16 in seven populations along the northern part of the country where resistance to DDT, dieldrin, and hexachlorocyclohexane (HCH) was reported. Mazzarri and Georghiou 17 detected resistance to OPs, carbamates, and pyrethroids in the states of Aragua and Falcón, even though control programs using these insecticides had only recently been implemented. Álvarez et al. 18 , 19 reported resistance to malathion, permethrin, and deltamethrin in populations of Ae. aegypti from western Venezuela, whereas Molina de Fernández et al. 20 showed for populations from different parts of the country that resistance to malathion was focal. More recently, Bastidas et al. 21 reported resistance to DDT in seven populations of Ae. aegypti from Aragua State, but all were susceptible to deltamethrin.
Resistance to insecticides is often due to an increase in metabolic activity and/or alteration of the insecticide target site. Three major groups of enzymes are often involved in metabolic resistance: carboxylesterases, cytochrome p450 monooxygenases, and glutathione-S-transferases (GSTs). In general, 22 esterases are associated with resistance to OPs, carbamates, and pyrethroids; the monooxygenases are involved in the metabolism of pyrethroids and detoxification of OPs and to lesser degree methyl-carbamates; and GSTs metabolize DDT to less toxic products and play a secondary role in resistance to pyrethroids and OPs.
Biochemical and synergistic assays conducted on Ae. aegypti in different parts of Venezuela reported high levels of esterase activity associated with resistance to temephos, malathion, chlorpyrifos, and pirimiphos methyl. 17 , 18 , 23 , 24 Other enzymes detected in populations of Ae. aegypti are overexpression of oxidases and GSTs associated with resistance to DDT. 21
Target site mechanisms involved in resistance to DDT and pyrethroids are mutations in the voltage-gated sodium channel (VGSC) of the nerve cell membrane. These mutations cause a reduction in the sodium channel sensitivity to the insecticides resulting in knockdown resistance (kdr) where the mosquito does not lose coordination immediately after exposure to the insecticide. Saavedra-Rodríguez et al. 25 reported very low frequencies of the mutations V1016I, I1011V, and I1011M among nine populations from Venezuela, whereas Álvarez et al. 26 found higher frequencies for the mutation F1534C than for the mutation V1016I in natural populations of Ae. aegypti from five states. Monitoring of insecticide resistance and the mechanisms involved are a priority within the vector control program due to hyperendemicity of dengue in urban areas and the risk of outbreaks extending to rural and remote areas. The objectives of the present study were to determine the state of insecticide resistance and to identify the possible biochemical and molecular mechanisms involved in three populations of Ae. aegypti from Venezuela: two sites hyperendemic for dengue, and the third located in a gold mining area which presented the risk of both Aedes-borne arboviruses as well as malaria.
MATERIALS AND METHODS
Study sites - Aedes aegypti immature stages were collected between October 2019 and February 2020 in three localities (Fig. 1). Two were neighborhoods hyperendemic for dengue in the Maracay Metropolitan Area (estimated population: 1,212,981), 27 Aragua State and located 12.2 km apart: Las Brisas, Girardot Municipality (10º 17’ 3” N; 67º 24’ 21” W, altitude 470 m), with approximately 400 houses and a population of 1,600, and Urbanización 19 de Abril, Santiago Mariño Municipality (10º 13’ 24” N; 67º 30’ 56” W, altitude 436 m), with approximately 462 houses and a population of 1,848. The area is characterized by a mean annual temperature of 25.5ºC, 75% relative humidity and 910 mm of rainfall. 28 The third locality, Nacupay, El Callao Municipality, Bolívar State (7º 20’ 36” N; 61º 49’ 55” W, 167 m), is a gold mining neighborhood established around 1998 south of the town of El Callao, at the margins of the Yuruari River; this region is endemic for malaria with relative recent colonization by Ae. aegypti (< 20 years). Nacupay has about 180 houses and an estimated population of 720 and is characterized by an annual mean temperature of 26.2ºC, 81.9% relative humidity and annual rainfall of 1,276 mm. 29 The distance between Las Brisas and Nacupay is 950 km. The type of houses in the three locations are similar: brick, plastered and painted walls; galvanized sheet or concrete roofs; cement, tiled or granite floors. The relative location of study sites was plotted on a map based on images from Natural Earth raster (public domain: https://www.naturalearthdata.com). Between 2,000 and 3,000 larvae and pupae were collected from 200 L plastic containers used for water storage for domestic use in each locality and transported to the insectary of the Vector Control unit of the Directorate of Environmental Sanitation, Maracay for mosquito rearing and obtaining the next generations. Dry eggs (F1 from Aragua State and F6 from Bolívar State) were transported to the insectary at the US Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, USA and mosquitoes were reared at 27ºC and 80% relative humidity (RH). Females aged three to five days old and fed on a 10% sugar solution were used for bioassays.
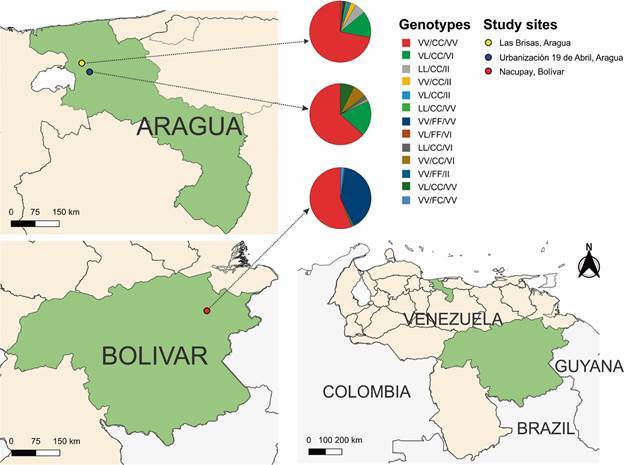
Fig. 1: relative location of study sites and genotype frequency for the kdr mutations V410L, F1534C, and V1016I in three populations of Aedes aegypti from Venezuela. Natural Earth raster (public domain: https://www.naturalearthdata.com).
Insecticide susceptibility tests - Bioassays were performed using the protocols described by CDC 30 at 24.8 - 25.8ºC and 17.5 - 20% RH. For each insecticide tested (Chem Service Inc, West Chester, PA), four replicates and one control were analyzed. Table I shows the insecticides tested for each Ae. aegypti strain, number of mosquitoes exposed, generation (F), diagnostic doses and percent mortality at 30 minutes exposure (diagnostic time). Malathion and deltamethrin bioassays for Las Brisas population were repeated on two consecutive days, and as results were similar, the data were pooled. WHO 31 criteria were used to determine the status for each population and each insecticide tested: R = resistant (mortality < 90%); C = resistance to be confirmed (mortality = 90-97%); S = susceptible (mortality ≥ 98%). Alive and dead mosquitoes were kept at -20ºC for molecular studies, and non-insecticide-exposed mosquitoes were stored at -20ºC for biochemical assays. Bioassays were not conducted using a susceptible reference strain.
TABLE I. Insecticides tested, diagnostic doses, number of mosquitoes exposed and percent mortality at the diagnostic time of 30 minutes for three Aedes aegypti populations from Venezuela.
| Locality | Insecticide tested | Diagnostic dose (µg/mL) | #Mosq exposed | generation (F) | Percent mortality | Status a |
| Las Brisas | Malathion | 50 | 214 | F1 | 78.5 | R |
| Malathion + PBO b | 50 | 100 | F2 | 93.0 | C | |
| Permethrin | 15 | 122 | F1 | 2.5 | R | |
| Permethrin 2X | 30 | 113 | F1 | 24.8 | R | |
| Permethrin + PBO b | 15 | 100 | F2 | 100 | S | |
| Deltamethrin | 10 | 201 | F1 | 52.7 | R | |
| Deltamethrin 2X | 20 | 105 | F1 | 100 | S | |
| Lambdacyhalothrin | 10 | 100 | F2 | 100 | S | |
| Urbanización 19 de Abril | Malathion | 50 | 112 | F2 | 98.2 | S |
| Permethrin | 15 | 104 | F2 | 74.0 | R | |
| Cypermethrin | 10 | 102 | F2 | 100 | S | |
| Nacupay | Malathion | 50 | 121 | F6 | 89.3 | R |
| Permethrin | 15 | 100 | F7 | 100 | S | |
| Deltamethrin | 10 | 100 | F7 | 100 | S | |
| Alphacypermethrin | 10 | 100 | F7 | 100 | S | |
| Lambdacyhalothrin | 10 | 100 | F7 | 100 | S |
a: R = resistance (mortality < 90%); C = resistance to be confirmed (mortality = 90-97%); S = susceptible (mortality ≥ 98%) 31 ; b: PBO = Piperonyl butoxide.
Synergist bioassays - The action of the synergist piperonyl butoxide (PBO), which inhibits oxidase activity, was investigated by exposing adult females of Las Brisas population 1 h prior to exposure to malathion or permethrin (Table I) following the CDC protocol. 30 Due to limited numbers of mosquitoes, we were unable to test for the inhibitory activity of additional synergists.
Biochemical assays - To measure levels of detoxification enzymes, proteins, and the acetylcholinesterase insensitivity, assays were conducted according to published protocols. 32 - 37 All assays were conducted on 60 female mosquitoes each from Las Brisas, Urbanización 19 de Abril and Nacupay. Additionally, 30 female Rockefeller (susceptible strain) mosquitoes were run for comparison. Each individual whole mosquito was homogenized in 100 µL of KPO4 buffer (pH 7.2; pH adjusted with phosphoric acid) using a pestle and battery-operated homogenizer. The homogenate was then diluted to 2 mL. For all assays, each mosquito was run in triplicate. Plates were read using a SpectraMax M5e plate reader and SoftMax Pro 7 software (Molecular Devices, Sunnyvale, CA). Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
The acetylcholinesterase and insensitive acetylcholinesterase assays were run simultaneously on separate plates and the difference in color was compared between the two assays. For these assays, three solutions were made; ATCH (75 mg acetylthiocholine iodine dissolved in 10 mL acetone, and 90 mL KPO4 added), ATCH with propoxur (75 mg acetylthiocholine iodide, and 21 mg propoxur dissolved in 10 mL acetone, and 90 mL KPO4 buffer added), and DTNB [13 mg 5,5’-Dithiobis-(2-nitrobenzoic acid) dissolved in 100 mL KPO4 buffer]. For the insensitive acetylcholinesterase plate, each well contained 100 µL mosquito homogenate, and 100 µL ATCH with propoxur solution, and 100 µL DTNB solution. For the acetylcholinesterase plate, each well contained 100 µL mosquito homogenate, and 100 µL ATCH solution, and 100 µL DTNB solution. After 60 minutes incubation, absorbance values for each plate were read using a 414 nm filter.
The mixed-function oxidase (MFO) assay does not measure enzymatic activity, but rather quantifies the heme group mainly associated with mixed function oxidases, including cytochrome p450s. A solution of 3,3’,5,5’-tetra-methybenzidine (TMBZ) was prepared by dissolving 50 mg of TMBZ in 25 mL methanol and adding 75 mL of 0.25 M sodium acetate buffer (pH 5.0; pH adjusted with glacial acetic acid). Each well contained 100 µL of mosquito homogenate, 200 µL TMBZ solution, followed by 25 µL of 3% hydrogen peroxide. The positive control consisted of 10 µL of cytochrome C (0.01 mg/mL) and 90 µL of KPO4 buffer. After a 10-minute incubation, absorbance values were read using a 650 nm filter.
For GSTs, solutions of reduced glutathione (61 mg L-glutathione reduced and 100 mL KPO4 buffer) and cDNB (20 mg 1-chloro-2,4’-dinitrobenzene dissolved in 10 mL acetone, and 80 mL KPO4 buffer added) were made. To each well, 100 µL of mosquito homogenate (after centrifugation), 100 µL reduced glutathione solution, and 100 µL cDNB were added. The plate was read immediately (T0) and after 10 minutes incubation using a 340 nm filter.
For the protein assay, a solution of protein assay dye reagent concentrate (Bio-Rad) and dH2O (1:4) was made. Each well contained 20 µL mosquito homogenate (after centrifugation), 80 µL KPO4 buffer, and 200 µL protein dye solution. After 10 minutes incubation, absorbance values for each plate were read using a 595 nm filter. Based on the Bradford method of protein quantification, 38 a standard curve using bovine serum albumin was generated to quantify the total protein present in each mosquito. This total serves as an estimate for the size of each mosquito and corrects values obtained for all assays, apart from the acetylcholinesterase assay.
Kruskal-Wallis non-parametric tests were used to determine statistically significant differences in assay values by population. When differences were detected, Dunn’s Test was used for pairwise comparisons. Statistical analyses and figures were carried out using RStudio. 39 , 40 Results were considered statistically significant at p < 0.05.
kdr allele detection
DNA extraction - The Quanta Biosciences ExtractaTM Kit was used for DNA extraction from mosquitos of Las Brisas and Urbanización 19 de Abril (phenotype permethrin resistant), Nacupay (phenotype permethrin susceptible). Each mosquito was placed in a sterile 0.2 mL tube and 25 μL extraction buffer was added, followed by an incubation at 95ºC for 30 min in a C1000 Bio-Rad CFX 96 TouchTM Real-Time System thermocycler. At the end of the incubation, 25 μL of stabilization buffer was added. The concentration and quality of each DNA sample were determined in a NanoDropTM 2000/2000c spectrophotometer (Thermo Fisher Scientific).
Detection of mutations V410L, F1534C and V1016I and genotyping - Allele-specific polymerase chain reaction (PCR) was carried out in a C1000 Bio-Rad CFX 96 TouchTM Real-Time System thermocycler using the primers for V410L, V1016I and F1534C mutations (Table II) to determine the genotypes for each mutation by melting curve analysis following modified protocols from those described by Saavedra-Rodríguez et al. 25 , 41 and Yanola et al. 42
TABLE II. Primer sequencies used in kdr genotyping.
| Mutation | Primer name | Sequence (5’ - 3’) |
| V410L | Val410fw | GCG GGC AGG GCG GCG GGG GCG GGG CCA TCT TCT TGG GTT CGT TCT ACC GTG |
| Leu410fw | GCG GGC ATC TTC TTG GGT TCG TTC TAC CAT T | |
| 410rev | TTC TTC CTC GGC GGC CTC TT | |
| F1534C | Cys1534fw | GCG GGC AGG GCG GCG GGG GCG GGG CCT CTA CTT TGT GTT CTT CAT CAT GTG |
| Phe1534fw | GCG GGC TCT ACT TTG TGT TCT TCA TCA TAT T | |
| 1534rev | TCT GCT CGT TGA AGT TGT CGA T | |
| V1016I | Val1016fw | GCG GGC AGG GCG GCG GGG GCG GGG CCA CAA ATT GTT TCC CAC CCG CAC CGG |
| Ile1016fw | GCG GGC ACA AAT TGT TTC CCA CCC GCA CTG A | |
| 1016rev | TGA TGA ACC SGA ATT GGA CAA AAG C |
The methodology described by Saavedra-Rodríguez et al. 41 for the V410L mutation was modified as follows; the reaction mixture (20 μL) contained 9.9 μL of Quantabio PerfeCta SYBR Green Supermix (95054), 0.1 μL of each fw primer and 0.2 μL rev primer and 1.0 μL of DNA. The amplification consisted of 95ºC for 3 min (activation step), followed by 40 cycles at 95ºC for 10 s, 60ºC for 10 s and 72ºC for 30 s, and a final extension step of 10 s at 95ºC. The melting curves were determined from 65ºC to 95ºC, with an increase of 0.2ºC every 10 s, where a simple peak at 80ºC corresponded to the homozygous mutant type (Leu410/Leu410), two peaks at 80ºC and 83ºC corresponded to the heterozygote (Leu410/Val410) and a single peak at 83ºC corresponded to the homozygous wild type (Val410/Val410).
For the F1534C mutation, the modified methodology described by Yanola et al. 42 was followed; the reaction mixture (18 μL) contained 9 μL of Quantabio PerfeCta SYBR Green Supermix (95054), 0.65 μL of the primer Cys1534fw, 0.6 μL of each of Phe1534fw and F1534rev primers and 2 μL of the DNA. The amplification consisted of 95ºC for 3 min, followed by 37 cycles at 95ºC for 10 s, 57ºC for 30 s and 72ºC for 30 s, and 95ºC for 10 s. The melting curves were determined from 65ºC to 95ºC, with an increase of 0.5ºC every 5 s, where a simple peak at 82ºC corresponded to the homozygous mutant type (Cys1534/Cys1534), two peaks at 78ºC and 82ºC corresponded to the heterozygote (Phe1534/Cys1534) and a single peak at 78ºC corresponded to the homozygous wild type (Phe1534/Phe1534).
The Saavedra-Rodríguez et al. 25 modified methodology was followed for the V1016I mutation; the reaction mixture (18 μL) contained 8 μL of Quantabio PerfeCta SYBR Green Supermix (95054), 0.34 μL of primer Val1016fw and 0.4 μL of each of primers Ile1016fw and 1016rev plus 2 μL of DNA. The amplification consisted of 95ºC for 3 min, followed by 35 cycles at 95ºC for 10 s, 60ºC for 10 s and 72ºC for 30 s, and a final extension step of 10 s at 95ºC. The melting curves were determined from 65ºC to 95ºC, with an increase of 0.2ºC every 10 s, where a simple peak at 76ºC corresponded to the homozygous mutant type (Ile1016/Ile1016), two peaks at 76ºC and 83ºC corresponded to the heterozygote (Val1016/Ile1016) and a single peak at 83ºC corresponded to the homozygous wild type (Val1016/Val1016). Positive and negative (ddH2O) controls were run for all PCR assays.
Allele frequencies and linkage disequilibrium analysis - The allele frequency of each mutation (p) was calculated as the sum of two times the number of mutant homozygotes and the number of heterozygotes, divided by 2n, where n is the number of mosquitoes analyzed. The 95% confidence interval (CI) was calculated using the following formula:
The HardyWeinberg package 43 , 44 was used to calculate the Hardy-Weinberg equilibrium (HWE). The HWE was expressed as Wright’s inbreeding coefficient FIS 45 and a χ2 test was used to test the null hypothesis FIS = 0 [degrees of freedom (df) = 1].
RESULTS
Bioassays - Bioassays showed that the population from Las Brisas was resistant to malathion, permethrin and deltamethrin (Table I), although mortality increased following pre-exposure to the synergist PBO before exposing to permethrin or doubling the concentration for deltamethrin; PBO pre-exposure still showed possible resistance for malathion but suggested that enzymes other than oxidases (e.g., esterases), play a key role in resistance. The population of Urbanización 19 de Abril (F2) was resistant only to permethrin while Nacupay (F6) showed resistance to malathion.
Biochemical assays - Ten percent (6 of 60) of Las Brisas samples, and 1.7% (1 of 59) of Urbanización 19 de Abril samples were higher than the 30% cut-off point for acetylcholinesterase activity remaining in the presence of propoxur (Fig. 2A).
Fig. 2: boxplots of biochemical assay results for Aedes aegypti populations. (A) Acetylcholinesterase. (B) Mixed Function Oxidases. (C) Glutathione-S-transferases. (D) Protein.
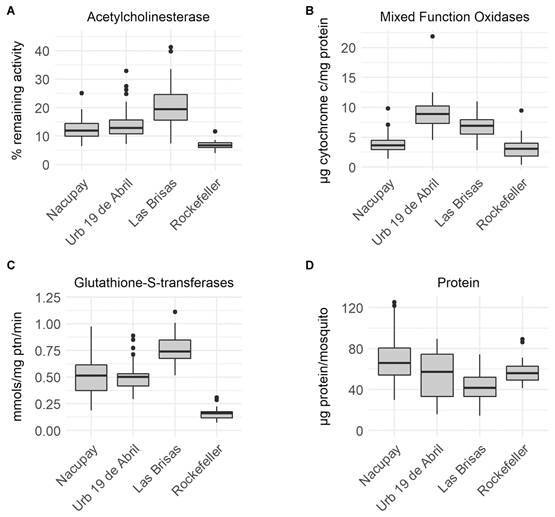
Oxidase levels between Rockefeller (median 3.04 µg cytochrome c/mg protein) and Nacupay (median 3.64 µg cytochrome c/mg protein) were not significantly different. However, oxidase levels in Urbanización 19 Abril (median 8.90 µg cytochrome c/mg protein) and Las Brisas (median 6.92 µg cytochrome c/mg protein) were significantly higher than Rockefeller (p < 0.001). Pairwise comparisons showed significant differences between Nacupay and the two other Venezuelan populations (p < 0.001) (Fig. 2B).
Glutathione-S-transferase levels were significantly different (p < 0.001) between Rockefeller (median 0.16 mmols/mg protein/min) and all three Venezuelan populations (Urbanización 19 Abril - median 0.50 mmols/mg protein/min; Las Brisas - median 0.74 mmols/mg protein/min; Nacupay - median 0.52 mmols/mg protein/min). All pairwise comparisons between the Venezuelan populations were also statistically significant (p < 0.001) except Nacupay and Urbanización 19 Abril (Fig. 2C).
Protein levels were significantly higher (p = 0.016) in Rockefeller (median 56 µg/mosquito) than Las Brisas (41.5 µg/mosquito). However, protein levels between Rockefeller and Urbanización 19 Abril (median 57.2 µg/mosquito) and Nacupay (66.0 µg/mosquito) were not significantly different. All pairwise comparisons between the Venezuelan populations were significantly different (p < 0.05).
Although esterase assays were conducted, the results were inconsistent likely due to faulty chemicals and therefore are not reported. Due to constraints on obtaining more mosquitos it was impossible to repeat the assays.
Kdr allele detection - Table III shows the populations, sample sizes and numbers of mosquitoes of each genotype, the frequency of mutant alleles, the 95% confidence interval around that frequency, the inbreeding coefficient (FIS) and the significance (p) of the χ2 test.
TABLE III. Genotype, mutant allele frequency, 95% confidence interval (CI), inbreeding coefficient (FIS), chi-square significance (p) for mutations V410L, F1534C, and V1016I in three populations of Aedes aegypti from Venezuela.
| Mutation | Population | N | Genotype | kdr Frequency (95% CI) | Hardy Weinberg Disequilibrium | |||
| Mutant allele | Heterozygote | Wild type | FIS | p-value | ||||
| V410L | LL | VL | VV | |||||
| Las Brisas a | 72 | 5 | 12 | 55 | 0.15 (0.10-0.22) | 0.36 | 0.0025 | |
| Urbanización 19 de Abril a | 154 | 3 | 40 | 111 | 0.15 (0.11-0.19) | -0.02 | 0.7826 | |
| Nacupay b | 96 | 0 | 1 | 95 | 0.01 (0.00-0.03) | -0.01 | 0.9591 | |
| F1534C | CC | FC | FF | |||||
| Las Brisas a | 72 | 70 | 0 | 2 | 0.97 (0.93-0.99) | 1 | < 0.0001 | |
| Urbanización 19 de Abril a | 155 | 155 | 0 | 0 | 1.00 (0.99-1.00) | NA | NA | |
| Nacupay b | 96 | 55 | 2 | 39 | 0.58 (0.51-0.65) | 0.96 | < 0.0001 | |
| V1016I | II | VI | VV | |||||
| Las Brisas a | 72 | 8 | 11 | 53 | 0.19 (0.13-0.26) | 0.50 | < 0.0001 | |
| Urbanización 19 de Abril a | 151 | 3 | 41 | 107 | 0.16 (0.12-0.20) | -0.03 | 0.684 | |
| Nacupay b | 96 | 1 | 2 | 93 | 0.02 (0.01-0.05) | 0.49 | < 0.0001 | |
a: Las Brisas and Urbanización 19 de Abril phenotype = permethrin resistant; b: Nacupay phenotype = permethrin susceptible.
In general, the frequency for the mutant alleles for F1534C (CC) varied between 1 (Urbanización 19 de Abril) and 0.58 (Nacupay) while very low frequencies were found for V410L (LL) (0.01-0.15) and V1016I (II) (0.02-0.19) in all populations. Fig. 1 shows the genotype frequency in the three study sites. Double (CC/II and LL/CC) and triple mutations (LL/CC/II) were present only in the populations from Maracay with frequencies less than 3% and 6% respectively. Forty percent of mosquitoes from Nacupay were wild type for the three kdr alleles (VV/FF/VV). For V410L, there was deficiency of homozygotes (LL) (FIS < 0) for the populations from Nacupay and Urbanización 19 de Abril while for Las Brisas there was excess of homozygotes (FIS > 0). For F1534C, an excess of homozygotes (CC) was detected in all populations analyzed with fixation detected in Urbanización 19 de Abril. Although at very low frequency, the mutation V1016I had excess of homozygotes (II) in Las Brisas and Nacupay and deficiency of homozygotes in Urbanización 19 de Abril. There was HWE only for the mutant alleles LL of the populations Nacupay and Urbanización 19 de Abril and II of Urbanización 19 de Abril.
DISCUSSION
The Venezuelan vector control program has relied mainly on the use of insecticides, with the introduction of DDT in 1946 for the control of Anopheles mosquitoes, 46 and later for the control of Ae. aegypti in urban areas, followed by the introduction of the OPs temephos and malathion in 1970 against larvae and adults of Ae. aegypti, 17 , 47 and pyrethroids in 1990. 17 The intensive and extensive use of these insecticides in public health and agriculture has exerted selective pressure on the mosquito populations, resulting in the development of resistance to various insecticides in Ae. aegypti and other mosquito vectors throughout the world. 48 , 49 , 50 , 51 The present study reflects this phenomenon, in particular the population from Las Brisas, a neighborhood of Maracay city, where resistance to malathion, permethrin, and deltamethrin was detected (Table I). This city has consistently presented a high prevalence of dengue which has historically required intensive use of malathion and pyrethroids, although the vector control program has had very limited activities since 2010; the use of malathion was discontinued and instead only fenitrothion and temephos were used on a very restrictive basis. Resistance to malathion has been reported previously from the Maracay area 17 , 52 , 53 as well as from other regions in Venezuela associated with metabolic resistance due to high levels of α- and ꞵ-esterases. 17 , 18 , 24 , 52 Although we could not determine the levels of esterases in the present study, the moderate reversion of mortality with the use of the synergist PBO (Table I) suggests that enzymes such as oxidases (MFOs) might be partially involved in the metabolism of malathion. Elevated activity of GSTs was also detected in this population (Fig. 2). These mechanisms were also present, but to a lesser extent, in the population from Urbanización 19 de Abril which was susceptible to malathion (Table I). This suggests caution towards the future use of malathion in these localities, given the resistance detected in two of the sites and the detection of mechanisms associated with OP resistance in the third.
Although resistance is a focal phenomenon, it is widespread in Venezuela and has evolved rapidly. 18 , 19 , 21 , 52 - 54 The first report of resistance to pyrethroids in Venezuela was to permethrin in mosquitoes collected in Maracay and Coro during 1992. 17 A few years later, Pérez and Molina de Fernández 55 detected resistance to lambdacyhalothrin in three populations from Aragua State, while a population from Girardot was also resistant to deltamethrin and a population from Mario Briceño Iragorry to cyfluthrin, with MFOs implicated as the primary mechanism of resistance. In the present study, resistance to permethrin and deltamethrin in populations of Ae. aegypti from Maracay was detected, even though these insecticides have not been used by the local control programs since 2010. Rodríguez et al. 54 associated resistance to deltamethrin in Ae. aegypti from Venezuela and other Latin American countries with the activity of esterases, and GSTs, as well as cytochrome oxidases. 56 We found significantly high levels of GSTs in all the populations, particularly the one from Las Brisas, which could be contributing to pyrethroid resistance (Fig. 2C). The levels of MFOs were significantly higher in the populations from Maracay (Las Brisas and Urbanización 19 de Abril) compared to the more recently established neighborhood (Nacupay) and susceptible (Rockefeller) strains, hence suggesting a role of MFOs in the pyrethroid resistance detected in the Maracay populations (Table I).
It is important to point out that resistance to pyrethroids might be due, to some degree, to cross-resistance with DDT. 57 , 58 Both compounds share the same target site, the VGSC, and kdr mutations in this gene have been associated with changes in sensitivity in several insect groups, including Anopheles 59 , 60 and Ae. aegypti mosquitoes. 58 , 61 , 62 , 63 The present study also detected point mutations in the gene coding for the VGSC (V1016I, F1534C, V410L), which have been previously reported in this species. 25 , 41 , 42 , 64 The kdr mutations could be an additional mechanism contributing to pyrethroid resistance in the populations evaluated (Table III).
The kdr mutation V1016I was reported for the first time in four populations from Venezuela by Saavedra-Rodríguez et al. 25 The V1016I mutation was also identified in mosquitoes collected in western and central Venezuela between 2008 and 2012 26 and La Pedrera, Maracay, during 2012-2013, 21 the latter located within 2 km from Las Brisas. In general, the frequencies reported for the mutant genotype were low (Table III), although higher than those reported previously by Saavedra-Rodríguez et al. 25 Nevertheless, so far this mutation per se has not been confirmed to reduce VGSC sensitivity to pyrethroids. 41 The kdr mutation F1534C has a wide geographic distribution in Asia, Africa, and Latin America. 14 It has been demonstrated that this mutation reduces the VGSC sensitivity to pyrethroids 65 and usually the mutant type has much higher frequencies than other co-occurrent mutations such as V1016I and V410L. 26 , 66 , 67 , 68 In the present study, frequencies varied from 0.58 in Nacupay (population susceptible to Type I and Type II pyrethroids) to fixation with a frequency of 1.0 in Urbanización 19 de Abril (population resistant to permethrin but susceptible to cypermethrin) (Tables I, III, Fig. 1). The kdr mutation V410L was reported for the first time in Ae. aegypti from Brazil and confirmed reduced VGSC sensitivity to pyrethroids. 64 The present study reports for the first time the presence of this mutation in Ae. aegypti populations from Venezuela. The frequencies observed were very low, particularly in the Nacupay pyrethroid-susceptible population (Tables I, III). Although at low frequencies, the strains from Las Brisas and Urbanización 19 de Abril had triple (LL/CC/II) and/or double (LL/CC or CC/II) homozygous mutants (Fig. 1). Although kdr alleles contribute to pyrethroid resistance, the elevated levels of metabolic enzymes might also play a key role.
It is important to point out that in the present study different generations were assayed. For the populations from Maracay (Las Brisas and Urbanización 19 de Abril), F1 and F2 were used, while for Nacupay we had F6 and F7. This fact might partly explain the susceptibility of Ae. aegypti in this area to all pyrethroids, as the loss of resistance can occur after several generations of colonization. However, this population notably remained resistant to malathion. It has been shown in laboratory studies that phenotypic resistance to pyrethroids can be lost after several generations free of insecticide pressure. 69 , 70 , 71 Also, the Nacupay population showed the lower frequencies of the kdr alleles F1534C and I1016V. Nevertheless, results from the literature are inconsistent when analyzing the effect of colonization on the frequency of mutant kdr alleles. In fact, Vera-Maloof et al. 71 showed that the frequencies of the alleles F1534C and I1016V declined after eight generations free from pyrethroid pressure although the rate and pattern of decline varied among strains. Grossman et al. 70 showed significant loss of phenotypic resistance after ten generations while the double-mutant haplotype (CC/II) did not vary significantly over time.
The present study shows that despite the absence of strong insecticide pressure due to the very limited vector control operations since 2010, resistance persisted in Ae. aegypti populations. Similar results were found in Brazil 72 where the use of pyrethroids for the control of Ae. aegypti was discontinued in 2000 due to high levels of resistance; those authors attributed the persistence of pyrethroid resistance, in part, to the personal use of insecticides by householders and private companies. This does not seem to be the case for Venezuela, as the availability of insecticides for personal use has been very limited and expensive due to the economic crisis and the private importation of insecticide was banned.
As stated earlier, insecticide resistance is a focal phenomenon: Molina de Fernandez et al. 20 showed that within a few kms Ae. aegypti susceptibility to insecticides varied. Furthermore, spatial and seasonal fluctuations in insecticide resistance can be based on variation in the biology and genetics of vector populations. 73 , 74 , 75 , 76 The results of the present study as well as those of Bastidas et al. 21 indicate that resistance can be maintained for longer periods than expected in the absence of known insecticide pressure. One possible explanation is the limited gene flow among Ae. aegypti populations in Venezuela even though dispersion of Ae. aegypti eggs/adults can be easily carried out by different means of transportation. 77
Insecticide resistance mutations have emerged independently all over the world, and the complexities of metabolic resistance implicate the overexpression of multiple genes. 78 The increasing reports of insecticide resistance in Ae. aegypti populations all over the world is a clear signal to vector control programs and public health authorities about the importance of the rational use of insecticides coupled with alternative strategies. Also, there is an urgent need for investment in infrastructure for a reliable supply of piped water and solid waste disposal. A longitudinal study conducted in six municipalities of the Metropolitan Area of Maracay showed that all immature Aedes indices (house, container and Breteau) were very high and significantly related to the lack of reliable water supply, 79 and irregular service of garbage collection and disposal was significantly related with an increase in dengue cases. 80
As insecticide resistance continues to grow, the need for successful vector-borne disease control will require well-resourced, effective, and sustainable interventions and infrastructure that is less permissive of mosquito breeding.
ACKNOWLEDGEMENTS
To Enrique Páez and José Domingo Mora, Dirección de Control de Vectores, MPPSalud for valuable discussion; Janet McAllister for comments on results and data analysis; and Miguel A Zúñiga for preparing the map.
REFERENCES
- 1.WHO Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-denguehttps://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- 2.Moore M, Sylla M, Goss L, Burugu MW, Sang R, Kamau LW. Dual African origins of global Aedes aegypti s l. populations revealed by mitochondrial DNA. PLoS Negl Trop Dis. 2013;7:e2175. doi: 10.1371/journal.pntd.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti - A Review. Mem Inst Oswaldo Cruz. 2013 doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katyal R, Kumar K, Gill KS. Breeding of Aedes aegypti and its impact on dengue/DHF in rural areas. Dengue Bulletin. 1997;21:93–95. [Google Scholar]
- 5.Tsuda Y, Suwonkerd W, Chawprom S, Prajakwong S, Takagi M. Differential spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Control Assoc. 2006;22(2):222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard HJ, Olano VA, Jaramillo JF, Matiz MI, Sarmiento D, Stenström TA. A cross-sectional survey of Aedes aegypti immature abundance in urban and rural household containers in central Colombia. Parasit Vectors. 2017;10:356–356. doi: 10.1186/s13071-017-2295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Palis Y, Guzmán H, Espinoza J, Cárdenas L, Bevilacqua M, Medina D. Primer registro de Aedes (Stegomyia) aegypti (L ) en areas remotas del estado Bolívar. Bol Malariol Salud Ambient. 2011;51(1):89–91. [Google Scholar]
- 8.Brady OJ, Gething PW, Bhatt S, Messina JP, Browntein JS, Hoen AG. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venezuela Ley N° 55. Ley sobre Sustancias, Materiales y Desechos Peligrosos. Gaceta oficial de la República Bolivariana de Venezuela. 2001 [Google Scholar]
- 11.PAHO - Pan American Health Organization Dengue haemorrhagic fever in Venezuela. Epidemiol Bull. 1990;11:7–9. [PubMed] [Google Scholar]
- 12.Unknown author Dengue haemorrhagic fever in Venezuela. Regional Office for South-East Asia. 1991 https://apps.who.int/iris/handle/10665/146024 [Google Scholar]
- 13.Rodríguez-Roche R, Villegas E, Cook S, Kim PP, Hinojosa Y, Rosario D. Population structure of the dengue viruses, Aragua, Venezuela, 2006-2007 Insights into dengue evolution under hyperendemic transmission. Infect Genet Evol. 2012;12:332–344. doi: 10.1016/j.meegid.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quarterman KD, Schoof HF. The status of insecticide resistance in arthropods of public health importance in 1956. Am J Trop Med Hyg. 1958;7:74–83. doi: 10.4269/ajtmh.1958.7.74. [DOI] [PubMed] [Google Scholar]
- 16.Mouchet J. La resistance aux insecticides chez Aedes aegypti et les espaces voisines. Bull WHO. 1967;36:569–577. [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzarri MB, Georghiou GP. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti (L) from Venezuela. J Am Mosq Control Assoc. 1995;11:315–322. [PubMed] [Google Scholar]
- 18.Álvarez L, Ponce G, Oviedo M, López B, Flores AE. Resistance to malathion and deltamethrin in Aedes aegypti (Diptera Culicidae) from Western Venezuela. J Med Entomol. 2013;50(5):1031–1039. doi: 10.1603/me12254. [DOI] [PubMed] [Google Scholar]
- 19.González LA, García GP, Suárez AF. Mecanismos de resistencia a la permetrina en dos poblaciones de Aedes aegypti del occidente de Venezuela. Bol Malariol Salud Ambient. 2016;56(1):43–52. [Google Scholar]
- 20.Molina de Fernández D.Bastidas D.Figueroa L Malatión vs Aedes aegypti (Linnaeus) (Diptera: Culicidae) de diferentes regiones de Venezuela. Bol Malariol Salud Ambient. 2013;53:44–55. [Google Scholar]
- 21.Bastidas D. Molina de Fernández D.Ferrer E Caracterización de la resistencia a los insecticidas DDT y Lambdacialotrina asociados con la mutación kdr V1016I en algunas cepas de Aedes aegypti (Linnaeus 1762) (Diptera Culicidae) de Venezuela. Bol Malariol Salud Ambient. 2018;58:16–31. [Google Scholar]
- 22.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 23.Bisset J, Rodríguez M. Molina de Fernández D.Díaz C.Soca A Esterasas elevadas como mecanismo de resistencia a insecticidas organofosforados en cepas de Aedes aegypti. Rev Cubana Med Trop. 2001;53:37–43. [PubMed] [Google Scholar]
- 24.Bastidas D, Figueroa L, Pérez E. Molina de Fernández D Estado de la resistencia a insecticidas organosintéticos de Aedes aegypti de Coro, estado Falcón, Venezuela. Bol Malariol Salud Ambient. 2015;50:173–183. [Google Scholar]
- 25.Saavedra-Rodríguez K, Urdaneta L, Rajatileka S, Moulton M, Flores A, Fernández I. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007;16:785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 26.Álvarez L, Ponce G, Oviedo M, Briceño A, Flores A. Mecanismos asociados a la resistencia al derribo "kdr" a la deltametrina en Aedes aegypti del occidente de Venezuela. Bol Malariol Salud Ambient. 2014;54:58–67. [Google Scholar]
- 27.INE XIV Censo Nacional de Población y Vivienda. 2014. https://www.ine.gov.ve/documentos/Demografoia/CensoPoblacionyVivienda/pdf/aragua.pdf
- 28.Wheather Atlas Clima y previsión meteorológica mensual Maracay, Venezuela. https://www.weather-atlas.com/es/Venezuela/maracay-clima
- 29.Wheather Atlas Clima y previsión meteorológica mensual El Callao, Venezuela. https://www.weather-atlas.com/es/Venezuela/el-callao-clima
- 30.CDC Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. US Department of Health and Human Services. 2011 [Google Scholar]
- 31.WHO - World Health Organization . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2. Geneva: World Health Organization; 2016. [Google Scholar]
- 32.Brogdon WG. Mosquito protein microassay I. Protein determinations from small portions of single-mosquito homogenates. Comp Biochem Physiol B. 1984;79:457–459. doi: 10.1016/0305-0491(84)90405-x. [DOI] [PubMed] [Google Scholar]
- 33.Brogdon WG. Microassay of acetylcholinesterase activity in small portions of single mosquito homogenates. Comp Biochem Physiol C Comp Pharmacol Toxicol. 1988;90:145–150. doi: 10.1016/0742-8413(88)90110-7. [DOI] [PubMed] [Google Scholar]
- 34.Brogdon WG, Barber AM. Fenitrothion-deltamethrin cross resistance conferred by esterases in Guatemalan Anopheles albimanus. Pestic Biochem Physiol. 1990;3:130–139. [Google Scholar]
- 35.Brogdon WG, Barber AM. Microplate assay of glutathione S-transferase activity for resistance detection in single mosquito triturates. Comp Biochem Physiol B. 1990;96:339–342. doi: 10.1016/0305-0491(90)90385-7. [DOI] [PubMed] [Google Scholar]
- 36.Brogdon WG, McAllister JC, Vulule J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J Am Mosq Control Assoc. 1997;13:233–237. [PubMed] [Google Scholar]
- 37.WHO Techniques to detect insecticide resistance mechanisms (field and laboratory manual) https://apps.who.int/iris/handle/10665/83780
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2020 https://www.R-project.org/ [Google Scholar]
- 40.RStudio Team RStudio: integrated development for R. RStudio. 2020 http://www.rstudio.com/ [Google Scholar]
- 41.Saavedra-Rodríguez K, Vera-Maloof F, Campbell C, García-Rejón J, Lenhart A, Penilla P. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Sci Rep. 2018;8:6747–6747. doi: 10.1038/s41598-018-25222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara LA. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop Med Int Health. 2011;16(4):501–509. doi: 10.1111/j.1365-3156.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 43.Graffelman J, Morales-Camarena J. Graphical tests for Hardy-Weinberg Equilibrium based on the ternary plot. Hum Hered. 2008;65(2):77–84. doi: 10.1159/000108939. [DOI] [PubMed] [Google Scholar]
- 44.Graffelman J. Exploring diallelic genetic markers the Hardy-Weinberg Package. J Stat Software. 2015;64(3):1–23. [Google Scholar]
- 45.Wright S. Systems of mating II. The effects of inbreeding on the genetic composition of a population. Genetics. 1921;6:124–143. doi: 10.1093/genetics/6.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabaldon A. The nation-wide campaign against malaria in Venezuela. Trans R Soc Trop Med Hyg. 1949;43(2):113–132. doi: 10.1016/0035-9203(49)90037-1. [DOI] [PubMed] [Google Scholar]
- 47.Georghiou GP, Wirth M, Tran H, Saume E, Knudsen AB. Potential for organophosphate resistance in Aedes aegypti (Diptera Culicidae) in the Caribbean area and neighboring countries. J Med Entomol. 1987;24:29O–294. doi: 10.1093/jmedent/24.3.290. [DOI] [PubMed] [Google Scholar]
- 48.Ranson H, Burhani J, Lumjuan N, Black WC. Insecticide resistance in dengue vectors. TropIKA.net. 2010 http://journal.tropika.net [Google Scholar]
- 49.Vontas J, Kioulos E, Pavlidi N, Morou E, della Torre A, Ranson H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pest Bioch Phys. 2012;104:126–131. [Google Scholar]
- 50.Liu N. Insecticide resistance in mosquitoes impact, mechanisms, and research directions. Ann Rev Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 51.Gan SJ, Leong YQ, Barhanuddin MFH, Wong ST, Wong SF, Mak JW. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia a review. Parasit Vectors. 2021;14:315–315. doi: 10.1186/s13071-021-04785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodríguez MM, Bisset JA. Molina de Fernández D.Lauzón L.Soca A Detection of insecticide resistance in Aedes aegypti (Diptera Culicidae) from Cuba and Venezuela. J Med Entomol. 2001;38(5):623–628. doi: 10.1603/0022-2585-38.5.623. [DOI] [PubMed] [Google Scholar]
- 53.Pérez E. Molina de Fernández D Resistencia focal a insecticidas organosintéticos en Aedes aegypti (Linneaus, 1762) (Díptera Culicidae) de diferentes municipios del estado Aragua, Venezuela. Bol Malariol Salud Ambient. 2009;49:143–150. [Google Scholar]
- 54.Rodríguez MM, Bisset JA. Molina de Fernández D Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007;23(4):420–429. doi: 10.2987/5588.1. [DOI] [PubMed] [Google Scholar]
- 55.Pérez EE. Molina de Fernández D Resistance of Aedes aegypti to pyretroides in municipalities of Aragua state, Venezuela. J Am Mosq Control Assoc. 2001;17(3):166–180. [Google Scholar]
- 56.Berge JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos Trans R Soc Lond B Biol Sci. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hemingway J, Boddington RG, Harris J. Mechanisms of insecticide resistance in Aedes aegypti (L ) (Diptera: Culicidae) from Puerto Rico. Bull Entomol Res. 1989;79:123–130. [Google Scholar]
- 58.Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17:87–94. doi: 10.1046/j.1365-2915.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 59.Martínez-Tórres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 60.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 61.Prapanthadara L, Promtet N, Koottathep S, Somboon P, Suwonkerd W, McCarroll L. Mechanisms of DDT and permethrin resistance in Aedes aegypti from Chiang Mai, Thailand. Dengue Bull. 2002;26:185–189. [Google Scholar]
- 62.Smith LB, Kasai S, Scott JG. Voltage-sensitive sodium channel mutations S989P + V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines. Pest Manag Sci. 2018;74(3):737–745. doi: 10.1002/ps.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva JJ, Kouam CN, Scott JG. Levels of cross-resistance to pyrethroids conferred by the Vssc knockdown resistance allele 410L+1016I+1534C in Aedes aegypti. PLoS Negl Trop Dis. 2021;15(7):e0009549. doi: 10.1371/journal.pntd.0009549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haddi K, Tomé HVV, Du Y, Valbor WR, Nomura Y, Martins GF. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti a potential challenge for mosquito control. Sci Rep. 2017;7:46549–46549. doi: 10.1038/srep46549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Nat Acad Sci USA. 2013;110:11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodríguez MM, Ruíz A, Piedra L, Gutiérrez G, Rey J, Cruz M. Multiple insecticide resistance in Aedes aegypti (Diptera Culicidae) from Boyeros municipality, Cuba and associated mechanisms. Acta Trop. 2020;212:105680–105680. doi: 10.1016/j.actatropica.2020.105680. [DOI] [PubMed] [Google Scholar]
- 67.Itokawa K, Futurani S, Tokaoka A, Maekawa Y, Sawabe K, Komagata O. A first, naturally occurring substitution at the second pyrethroid receptor of voltage-gated sodium channel of Aedes aegypti. Pest Manag Sci. 2021;77:2887–2893. doi: 10.1002/ps.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granada Y, Mejía-Jaramillo AM, Zuluaga S, Triana-Chávez O. Molecular surveillance of resistance to pyrethroids insecticides in Colombian Aedes aegypti populations. PLoS Negl Trop Dis. 2021;15(12):e0010001. doi: 10.1371/journal.pntd.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brito LP, Linss JG, Lima-Camara TN, Bolinato TA, Peixoto AA, Lima JBP. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS One. 2013;8(4):e60878. doi: 10.1371/journal.pone.0060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossman MK, Uc-Puc V, Rodriguez J, Cutler DJ, Morran LT, Manrique-Saide P. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biol Lett. 2018;14:20180022–20180022. doi: 10.1098/rsbl.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vera-Maloof FZ, Saavedra-Rodríguez K, Penilla-Navarro RP, Rodríguez-Ramírez AD, Dzul F, Manrique-Saide P. Loss of pyrethroid resistance in newly established laboratory colonies of Aedes aegypti. PLoS Negl Trop Dis. 2020;14(3):e0007753. doi: 10.1371/journal.pntd.0007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macoris MdL, Martins AJ, Andrighetti MTM, Lima JBP, Valle D. Pyrethroid resistance persists after ten years without usage against Aedes aegypti in governmental campaigns lessons from São Paulo State, Brazil. PLoS Negl Trop Dis. 2018;12(3):e0006390. doi: 10.1371/journal.pntd.0006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s s. from Uganda. Am J Trop Med Hyg. 2010;82:566–573. doi: 10.4269/ajtmh.2010.08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saavedra-Rodríguez K, Beaty M, Lozano-Fuentes S, Denham S, García-Rejón J, Reyes-Solís G. Local evolution of pyrethroid resistance offsets gene flow among Aedes aegypti collections in Yucatán State, México. Am J Trop Med Hyg. 2015;92(1):201–209. doi: 10.4269/ajtmh.14-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deming R, Manrique-Saide P, Barreiro AM, Cardeña EUK, Che-Mendoza A, Jones B. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasit Vectors. 2016;9:63–63. doi: 10.1186/s13071-016-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott ML, Hribar LJ, Leal AL, McAllister J. Characterization of pyrethroid resistance mechanisms in Aedes aegypti from the Florida Keys. Am J Trop Med Hyg. 2021;104(3):1111–1122. doi: 10.4269/ajtmh.19-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrera F, Urdaneta L, Rivero J, Zoghbi N, Ruíz J, Carrasquel G. Population genetic structure of the dengue mosquito Aedes aegypti in Venezuela. Mem Inst Oswaldo Cruz. 2006;101(6):625–633. doi: 10.1590/s0074-02762006000600008. [DOI] [PubMed] [Google Scholar]
- 78.Faucon F, Gaude T, Dusfour I, Navratil V, Corbel V, Juntarajumnong W. In the hunt for genomic markers of metabolic resistance to pyrethroids in the mosquito Aedes aegypti an integrated next-generation sequencing approach. PLoS Negl Trop Dis. 2017;11(4):e0005526. doi: 10.1371/journal.pntd.0005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rubio-Palis Y, Guzmán H, Sánchez V, Pérez-Ybarra LM. Fluctuaciones poblaciones de Aedes aegypti (Diptera Culicidae) y casuística de dengue en seis municipios del estado Aragua, Venezuela. Bol Malariol Salud Ambient. 2017;57:1–16. [Google Scholar]
- 80.Monsalve NC, Rubio-Palis Y, Pérez ME. Modelaje Bayesiano espacio-temporal de factores asociados con la incidencia del dengue en el área metropolitana de Maracay, Venezuela. Bol Malariol Salud Ambient. 2010;50:59–72. [Google Scholar]


