Summary Paragraph:
Direct molecular editing of heteroarene carbon-hydrogen (C–H) bonds through consecutive selective C–H functionalization has the potential to grant rapid access into diverse chemical space; a valuable but often challenging venture to achieve in medicinal chemistry1. Contrasting with electronically-biased heterocyclic C–H bonds2-9, remote benzocyclic C–H bonds on bicyclic aza-arenes are especially difficult to differentiate due to lack of intrinsic steric/electronic biases10-12. We herein report two conceptually distinct directing templates that enable the modular differentiation and functionalization of adjacent remote (C6 vs. C7) and positionally-similar positions (C3 vs. C7) on bicyclic aza-arenes through careful modulation of distance, geometry and previously unconsidered chirality in template design. This strategy enables direct C–H olefination, alkynylation, and allylation at adjacent C6 and C7 positions of quinolines in the presence of a competing C3 position that is spatially similar to C7. Notably, such site-selective, iterative, and late-stage C–H editing of quinoline-containing pharmacophores can be modularly performed in different orders to suit bespoke synthetic applications. This report, in combination with previously reported complementary methods, now fully establishes a unified late-stage ‘molecular editing’ strategy to directly modify bicyclic aza-arenes at any given site in different orders.
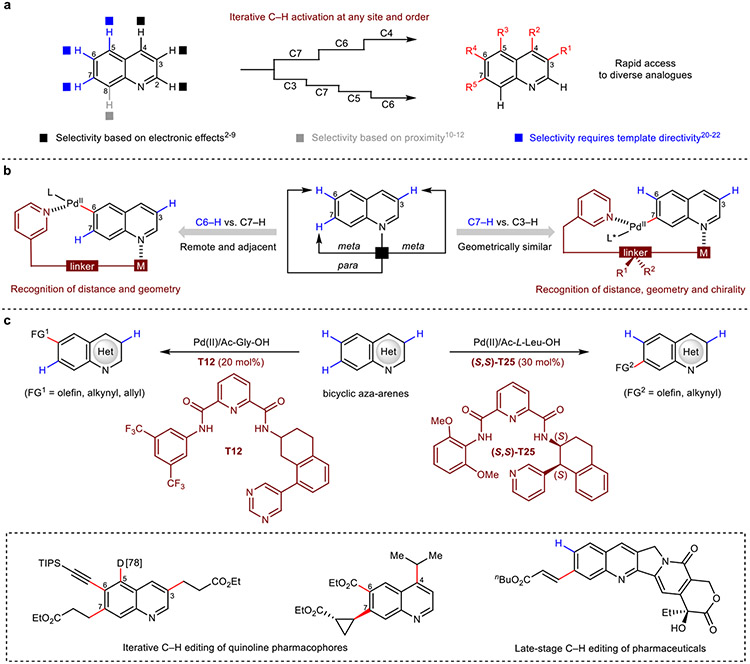
The efficient generation of diverse analogues with structural modifications at various sites forms a perennial synthetic challenge that underpins drug discovery. For a given molecular scaffold or pharmacophore, the selective and iterative activation of multiple inert C–H bonds at different sites represents the most direct strategy for the expeditious generation of structural diversity1,13. For example, functionalization with ten coupling partners at five different sites of quinoline scaffold could generate up to 100,000 structurally unique analogues using a unified editing strategy challenging through de novo synthesis (Fig. 1a). This notionally ideal ‘molecular editing’ approach, however, is marred by a lack of reliable methods for their late-stage selective functionalization, curtailing broader realization in a translational context14. As privileged pharmacophores for diverse biological targets, aza-arene heterocycles are particularly dominant within the realm of drug discovery. Within the azine component, leveraging a substrate’s intrinsic electronic properties have enabled the now-established site-selective functionalizations of C2–H3,4 and C4–H5,6,8,9 under a nucleophilic metallation regimen, and at C3–H2,7 via the corresponding electrophilic metallation process. In contrast, selective functionalization of multiple C–H bonds on the benzocyclic component of bicyclic aza-arene heterocycles remains to be realized. For these chemically-similar C–H bonds, leverage of proximity-driven effects to selectively direct the catalyst has been limited to benzocyclic C8–H bonds adjacent to Lewis basic heteroatoms10-12. Notably, the selective editing of remote positions, such as C5–C7 on quinoline-type scaffolds, remain inaccessible to established electronically-driven or substrate-directed approaches described above (Fig. 1a).
Fig. 1. Molecular editing of heterocycles.
a, Molecular editing of quinoline. b, Challenges and solutions to C6 and C7-selective palladation. c, Catalytic C6 and C7-selective functionalizations (This work). M, metal; L, ligand; FG, functional group; TIPS, triisopropylsilyl; nBu, n-butyl.
We surmised that this eminent problem could be solved using reversibly-binding directing templates capable of selectively positioning the catalyst proximate to a target remote C–H bond via a macrocyclophanic pre-transition state15-19. In this context, bicyclic aza-arene scaffolds pose further obstacles for template-directed remote regioselection; in addition to suppressing functionalization at activated sites (C2–C4), the multiple adjacent, yet minutely inequivalent and unactivated remote benzocyclic positions (C5-C7) demand stringent regiochemical precision for their discrimination and selective activation. The feasibility of this template-directed approach for the activation of remote benzocyclic C–H bonds was first indicated in 2017, where stoichiometric template loadings to overcome deleterious azine binding have enabled the singular C5–H palladation and functionalization of quinoline20,21. In combination with norbornene relay, an indirect C6-selective arylation can also be realized based on this C5–H palladation, though this strategy only permits arylation with electron-deficient aryl iodides, requires a vacant C5 position, and fundamentally does not provide a solution for selective C6–H palladation and diverse functionalization necessary to achieve molecular editing22. The direct activation of C6 and C7 positions, as compared with the marginally more nucleophilic C5 and C8 positions, is particularly difficult both for the chemical inertness and electronic similarity of the two C–H bonds. Careful spatial analysis revealed subtle differences between C6 and C7–H in both distance (one bond difference) and geometry (meta vs. para), suggesting the possibility of precise template design to differentiate between these two C–H bonds. The same analysis also revealed that the sterically-similar and more activated C3–H possesses a similar distance (one bond difference) but identical geometry (meta vs. meta) to our desired C7–H bond. The latter challenge alluded that judicious spatial tuning of template distance and two-dimensional geometry may not be sufficient to impart selectivity for the remote C7 position. To address these pitfalls, we were further inspired by chiral catalyst-controlled regioselective functionalization of chiral polyols23-25. This led to the design of a chiral template, which upon interaction with a matched chiral catalyst, is capable of distinguishing between C3 and C7–H positions thereby providing the desired C7-selective functionalization (Fig. 1b).
We herein report two conceptually distinct directing templates that enable site-selective C6 and C7–H activation of bicyclic aza-arenes. These catalytic pyridine-based templates recruit the aza-arene substrate through N-coordination, enabling the directing arm to deliver the catalyst and precisely activate remote and adjacent C6 or C7–H bond (Fig. 1c). In parallel, we discovered that the use of a simple and readily prepared template chaperone (TC) can turn over the directing template, allowing it to be used catalytically for the first time (see Supplementary Information 2.3). Notably, chiral recognition is vital in the granular discrimination between competing C3 and C7–H bonds when differentiation via distance and geometry is insufficient. Thus, precise spatial and chiral recognition of a directing template now enables the iterative C–H editing of quinoline pharmacophores at any desired site and order.
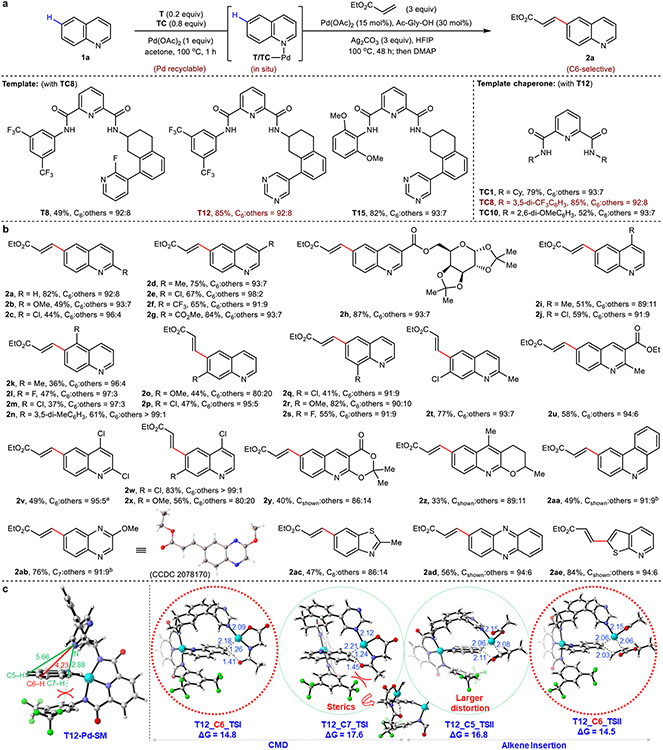
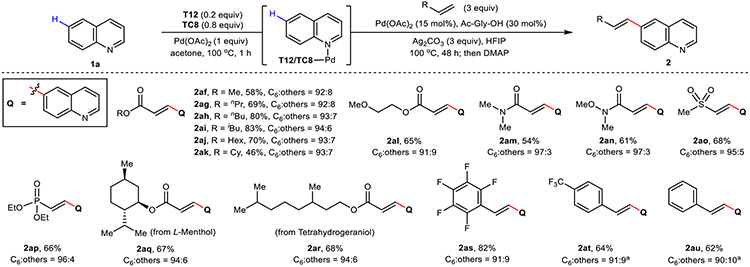
Using quinoline 1a as the model substrate, we initially targeted the development of a selective C6–H olefination reaction. Considering that our previous C5 template is used in stoichiometric amounts, a key objective at the outset of our studies was the catalytic use of our templates. This was solved by an easily synthetized symmetrical template chaperone TC8 (two steps, no chromatography) to mask the quinoline nitrogen and facilitate product turnover from the directing template. An initial hit was found through a systematic screen of linker length in the presence of 2-fluoro-3-phenylpyridyl motif as the directing group (T3, see Supplementary Information Table S1)26. A rigidified analogue of T3 bearing an alicyclic two-carbon spacer (T8) was next pursued, which to our delight, gave a marked improvement to both yield and C6 selectivity. Further tuning of the directing motif (T9 to T12, Table S1) showed that yields were improved using 3-phenylpyrimidyl-bearing T12 (Fig. 2a), while an assessment of the left-hand portion of the template and template chaperone scaffolds gave no noticeable improvements in reactivity and selectivity. The optimal result with T12 and TC8, both bearing 3,5-ditrifluoromethylphenyl side arms, likely arises from their structural homology, improving the efficacy of substrate/product exchange in the reaction. In all cases, the incorporated palladium within the directing template and template chaperones can be easily recovered as the TC-Pd-MeCN complex, and recycled with no loss in reaction efficacy (see Supplementary Information 2.9).
Fig. 2. C6 (and related)-selective C–H olefination reactions of quinoline and other heterocycles.
a, Selected optimization of directing template and template chaperone scaffolds. b, Scope of aza-arenes. c, DFT analysis rationalizes the observed C6 selectivity of quinoline 1a for template T12. Optimization yield and selectivity of 2a are determined by 1H NMR analysis. All yields are isolated yields for reaction scope. Bond lengths are denoted in Å. aUsing T8 (0.2 equiv). bUsing T15 (0.2 equiv) and TC10 (0.8 equiv). Cy, cyclohexyl; SM, starting material; TS, transition state.
With optimized template and conditions in hand, we next evaluated the reaction scope with respects to quinoline and related heterocycles (2a to 2ae, Fig. 2b). Various electron-donating and electron-withdrawing groups were compatible in the reaction showing little difference in yield or selectivity (2a to 2s), confirming the power of our proximity-driven directing approach in overriding inherent electronic preferences. A series of multiply-substituted and polycyclic quinolines were also well tolerated (2t to 2aa), and we were pleased to find that the reaction tolerates a variety of aza-arenes; quinoxaline (2ab), benzothiophene (2ac), phenazine (2ad), and thieno[2,3-b]pyridine (2ae) all afforded the desired products in good yields and high selectivities. Next, we examined the scope of coupling partners with unsubstituted quinoline under the standard conditions (Extended Data Fig. 1). A variety of acrylates (2af to 2al), vinylamides (2am and 2an), vinylsulfone (2ao), vinylphosphonate (2ap), styrenes (2as to 2au) and more complex terpenoid-derived acrylate coupling partners (2aq, from L-menthol; 2ar, from tetrahydrogeraniol) were well tolerated, delivering the corresponding products in good to excellent yields and selectivities.
Density functional theory (DFT) studies were conducted to understand the origin of C6-selectivity in our catalytic templates. Analysis of the concerted metalation-deprotonation (CMD) step immediately excluded a C7-selective pathway because of higher energies incurred by repulsive interactions between the template phenyl ring and ligand acetyl groups (Fig. 2c). The analysis also suggested that the initial C–H metalation was likely unselective between C5 and C6, which was corroborated by observing unselective substrate deuterium incorporation (see Supplementary Information 2.14). A larger template distortion required to access the C5 position results in a lower energy barrier for the C6-selective alkene insertion step relative to C5. Therefore, a combination of a more favored C–H activation (disfavoring C7) and alkene insertion steps (disfavoring C5) give rise to the observed C6 selectivity for template T12.
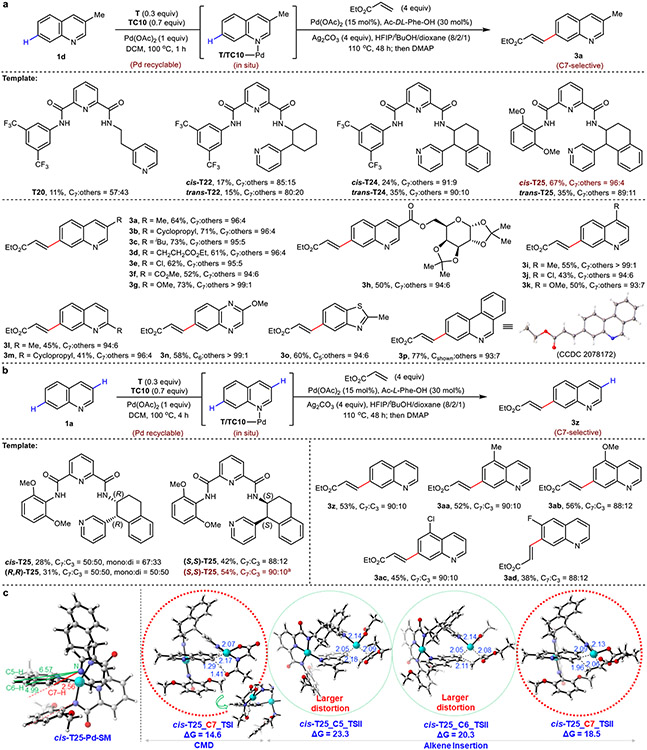
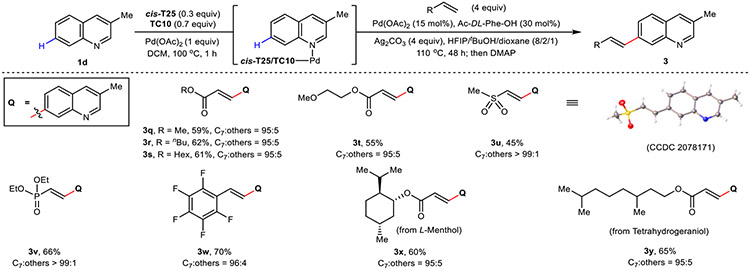
The success of our C6-selective catalytic template prompted us to investigate whether C7-selectivity was also feasible through judicious spatial optimization. Our study commenced with 3-methylquinoline 1d bearing no C–H bond at the C3, wherein an initial hit was found using a template bearing a two-carbon spacer to the directing 3-pyridyl motif (T20, Fig. 3a). Template rigidification (T22 and T24, Fig. 3a) improved yield and selectivity, with cis- and trans-T2A notably delivered the product 3a in high selectivity. Systematic tuning of the template’s left arm identified that a 2,6-dimethoxyphenyl motif provided markedly improved C7 yield and selectivity, with the best C7-selective template (cis-T25, Fig. 3a) afforded 3a in 67% NMR yield with 96:4 selectivity. Using racemic cis-T25 and Ac-DL-Phe-OH ligand, a range of C3-, C4-, and C2-substituted quinolines smoothly afforded the C7-olefinated products (3a to 3m) in good yield and excellent selectivity (Fig. 3a). Other pharmaceutically important heterocycles (quinoxaline, benzothiophene, and phenanthridine) were also compatible, generating distally-olefinated products 3n to 3p in a site-selective manner. Additionally, a diverse set of olefinic coupling partners were competent using 1d as the substrate, successfully reacting with acrylates (3q to 3t, 3x and 3y), vinyl sulfone (3u), vinyl phosphonate (3v) and styrene (3w) in moderate to good yields with excellent C7-selectivity (Extended Data Fig. 2).
Fig. 3. C7 (and related)-selective C–H olefination reactions of quinoline and other heterocycles.
a, Selected template optimization for C7-olefination of 1d and scope of heterocyclic substituted substrates. b, Selected condition optimization for C7-olefination of 1a and scope of benzocyclic substituted substrates. c, DFT analysis rationalizes the observed C7 selectivity of 3-methylquinoline 1d for template cis-T25. Optimization yield and selectivity are determined by 1H NMR analysis. All yields are isolated yields for reaction scope. Bond lengths are denoted in Å. aUsing Pd(MeCN)2Cl2 (15 mol%) and Ac-L-Leu-OH (30 mol%).
However, subjecting unsubstituted quinoline la with racemic cis-T25 under our optimized conditions gave a disappointing 50:50 mixture of products at the C3 and C7 positions in 56% total yield (Fig. 3b). This outcome restricts the order of iterative C–H activation sequence and affirms initial analyses indicating the similar spatial (distance and geometry) positioning of the C3 and C7–H bonds relative to the anchoring azine nitrogen; an observation supported by DFT calculations (see Supplementary Information Fig. S12). Further inspired by the use of chiral catalysts to control site-selective modifications in chiral polyol-containing natural products23,24, we wondered if a matched combination of an enantiopure directing template and a chiral catalyst could distinguish between these two highly similar positions. Thus, C7-selectivity was re-evaluated against unsubstituted quinoline 1a in the presence of enantiopure (R,R)- and (S,S)-T25 with Ac-L-Phe-OH as the chiral ligand. Gratifyingly, the use of (S,S)-T25 matches the chiral ligand, providing 3z with high C7 selectivity (C7:C3 = 88:12). The mismatched combination of (R,R)-T25 with Ac-L-Phe-OH gave mixture products (C7:C3 = 50:50). Further optimization of (S,S)-T25 with chiral ligands afforded 3z in 54% yield and selectivity (C7:C3 = 90:10) (Fig. 3b). These results indicate that competing C–H bonds that are spatially (distance and geometrically) similar can be further distinguished through matched chirality-recognition. Under the optimal conditions, C5- and C6-substituted quinolines, which gave no selectivity in the presence of racemic cis-T25 and ligand, provided 3aa to 3ad in moderate yields and high C7 selectivities (Fig. 3b).
To rationalize the observed C7-selectivity of chiral template (S,S)-T25, H/D exchange experiments were conducted with unsubstituted quinoline 1a. These experiments demonstrated that deuterium incorporation at the C7 position is more favored in the presence of the matched chiral template/ligand combination (see Supplementary Information 2.14). As these chiral ligands are known to participate in the CMD transition state, our DFT analysis specifically focused on this crucial step with chiral template (S,S)-T25 and ligands Ac-L-Phe-OH or Ac-D-Phe-OH. Despite extensive investigation, our obtained free energy profiles did not fully explain the high C7-selectivity that was experimentally observed. On the other hand, the observed C7-selectivity of 3-methylquinoline 1d with racemic template cis-T25 was fully consistent with DFT analysis (Fig. 3c). Cis-T25 gave the lowest energy transition state for all key steps at C7 compared to other positions (see Supplementary Information Fig. S8). Further inspection revealed that increased template distortion was required to access both C5 and C6 positions, leaving C7 as the sole favorable pathway for this template.
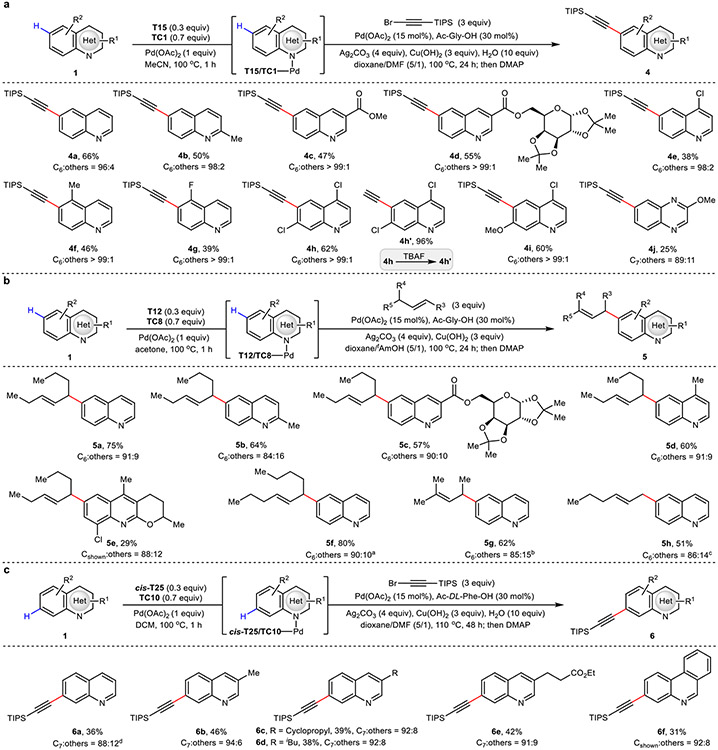
The scope of transformation intercepted from this catalytic directed remote C–H palladation was broadened through achieving the site-selective C–H alkynylation and allylation of aza-arenes (4a to 4j, 5a to 5h, 6a to 6f, Extended Data Fig. 3); representing versatile linchpins for further diversification27-29. Uniformly, high site-selectivity was obtained for the template-assisted C6 and C7-selective alkynylation reactions. The corresponding allylation reactions were similarly effective, giving comparatively higher reactivity albeit with slightly reduced C6-selectivity. In all cases, a range of substitutions were tolerated, signaling the robustness of this catalytic template strategy for direct remote functionalization.
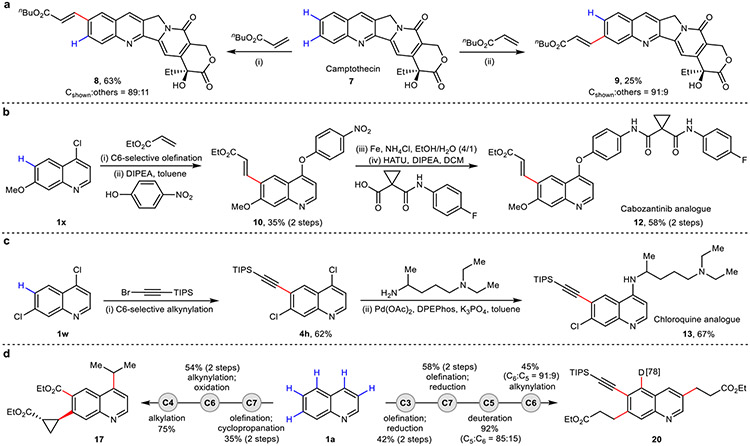
The applicability of this method in a drug discovery context was first exemplified by the divergent late-stage site-selective C–H functionalization of the anticancer natural product camptothecin (Fig. 4a)30. Subjecting camptothecin to our optimized C6-selective template generated novel analogue 8 in 63% yield, while the corresponding C7-selective template generated its regioisomer 9 in 25% yield. Successful C–H editing of key pharmacophores was also demonstrated, providing novel analogues of anticancer agent cabozantinib 1231,32 (Fig. 4b) and antimalarial agent chloroquine 1333 (Fig. 4c). Finally, we were eager to address the ultimate challenge of executing sequential site-selective late-stage ‘molecular editing’ in any desired order on a quinoline scaffold; its feasibility demonstrated by successful iterative C–H activations to access products 17 and 20 bearing diverse substitutions (Fig. 4d).
Fig. 4. Synthetic applications.
a, Late-stage remote site-selective C–H modification of camptothecin. Reaction conditions are provided in Supplementary Information 2.10. b, Synthesis of cabozantinib analogue through C6–H olefination. c, Synthesis of chloroquine analogue through C6–H alkynylation. d, Molecular editing of quinoline through iterative C–H activation in different orders. Reaction conditions are provided in Supplementary Information 2.13. Deuterium incorporation is shown in square brackets.
In summary, a unified catalytic remote-directing template strategy allowed for precise differentiation of remote and adjacent C6 and C7–H bonds, as well as similar C3 and C7–H bonds of a pharmaceutically-relevant bicyclic aza-arene scaffold. The modularity of C6/C7 functionalization described herein, combined with previously reported methods, completes the suite of reactions required to edit all C–H bonds within bicyclic aza-arene scaffolds in different orders. Notably, the realization of C7–H selective activation over C3–H also established chiral recognition as an effective means in fine-tuning remote site-selectivity between positionally similar C–H bonds, complementing previously employed distance and geometric parameters in directing template design.
Extended Data
Extended Data Fig. 1. Additional olefin scope for C6 (and related)-selective C–H olefination reactions of quinoline and other heterocycles.
All yields are isolated yields. aUsing conditions in Extended Data Fig. 3b. nPr, n-propyl; iBu, isobutyl; Hex, n-hexyl.
Extended Data Fig. 2. Additional olefin scope for C7 (and related)-selective C–H olefination reactions of quinoline and other heterocycles.
All yields are isolated yields.
Extended Data Fig. 3. Site-selective C–H alkynylation and allylation of aza-arenes.
a, C6 (and related)-selective C–H alkynylation of aza-arenes. b, C6 (and related)-selective C–H allylation of aza-arenes. c, C7 (and related)-selective C–H alkynylation of aza-arenes. All yields are isolated yields. aUsing trans-decene (3 equiv). bUsing trans-4-methyl-2-pentene (3 equiv). cUsing 1-hexene (3 equiv). dUsing (S,S)-T25 (0.3 equiv), Pd(OAc)2 (20 mol%), Ac-L-Phe-OH (40 mol%), alkynylation reagent (4 equiv), 100 °C. TBAF, tetra-n-butylammonium fluoride.
Supplementary Material
Acknowledgements
We gratefully acknowledge The Scripps Research Institute and the NIH (National Institute of General Medical Sciences grant R01 GM102265) for their financial support. Computations were performed on the Hoffman2 cluster at UCLA and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the NSF (OCI-1053575). We are grateful for financial support of the UCLA work from the NSF (CHE-1764328 to KNH) and the NSF under the NSF Center for Selective C–H Functionalization (CHE-1700982). Dr. Jason Chen, Brittany Sanchez, and Emily Sturgell are acknowledged for their assistance with LC-MS analysis. We thank Dr. Milan Gembicky, Dr. Jake Bailey and the UCSD Crystallography Facility for X-ray crystallographic analysis.
Footnotes
Competing interests J.-Q.Y. and Z.F. are inventors on a patent application related to this work (US Patent application 63/334,828) filed by The Scripps Research Institute. The authors declare no other competing interests.
Supplementary information The online version contains supplementary material available at
Data availability
The data supporting the findings of this study are available within the paper, its Supplementary Information, and free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/structures) under reference numbers CCDC 2078170-2078173, 2132680.
References
- 1.Wencel-Delord J & Glorius F C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem 5, 369–375 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Takagi J, Sato K, Hartwig JT, Ishiyama T & Miyaura N Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 43, 5649–5651 (2002). [Google Scholar]
- 3.Nakao Y, Kanyiva KS & Hiyama T A strategy for C–H activation of pyridines: direct C-2 selective alkenylation of pyridines by nickel/lewis acid catalysis. J. Am. Chem. Soc 130, 2448–2449 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Berman AM, Lewis JC, Bergman RG & Ellman JA Rh(I)-catalyzed direct arylation of pyridines and quinolines. J. Am. Chem. Soc 130, 14926–14927 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakao Y, Yamada Y, Kashihara N & Hiyama T Selective C-4 alkylation of pyridine by nickel/lewis acid catalysis. J. Am. Chem. Soc 132, 13666–13668 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Tsai C-C et al. Bimetallic nickel aluminun mediated para-selective alkenylation of pyridine: direct observation of η2,η1-pyridine Ni(0)-Al(III) intermediates prior to C–H bond activation. J. Am. Chem. Soc 132, 11887–11889 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ye M, Gao G-L & Yu J-Q Ligand-promoted C-3 selective C–H olefination of pyridines with Pd catalysts. J. Am. Chem. Soc 133, 6964–6967 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, du Jourdin XM & Knochel P Transition-metal-free BF3-mediated regioselective direct alkylation and arylation of functionalized pyridines using Grignard or organozinc reagents. J. Am. Chem. Soc 135, 4958–4961 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S, Saga Y, Andou T, Matsunaga S & Kanai M Cobalt-catalyzed C-4 selective alkylation of quinolines. Adv. Synth. Catal 356, 401–405 (2014). [Google Scholar]
- 10.Kwak J, Kim M & Chang S Rh(NHC)-catalyzed direct and selective arylation of quinolines at the 8-position. J. Am. Chem. Soc 133, 3780–3783 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Konishi S et al. Site-selective C–H borylation of quinolines at the C8 position catalyzed by a silica-supported phosphane-iridium system. Chem. Asian. J 9, 434–438 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Murai M, Nishinaka N & Takai K Iridium-catalyzed sequential silylation and borylation of heteroarenes cased on regioselective C–H bond activation. Angew. Chem. Int. Ed 57, 5843–5847 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Das S, Incarvito CD, Crabtree RH & Brudvig GW Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science 312, 1941–1943 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Szpilman AM & Carreira EM Probing the biology of natural products: molecular editing by diverted total synthesis. Angew. Chem. Int. Ed 49, 9592–9628 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Leow D, Li G, Mei T-S & Yu J-Q Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuninobu Y, Ida H, Nishi M & Kanai M A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem 7, 712–717 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Davis HJ, Mihai MT, Phipps RJ, Ion pair-directed regiocontrol in transition-metal catalysis: a meta-selective C–H borylation of aromatic quaternary ammonium salts. J. Am. Chem. Soc 138, 12759–12762 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Hoque ME, Bisht R, Haidar C & Chattopadhyay B Noncovalent interactions in Ir-catalyzed C–H activation: L-shaped ligand for para-selective borylation of aromatic esters. J. Am. Chem. Soc 139, 7745–7748 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Zhang T et al. A directive Ni catalyst overrides conventional site selectivity in pyridine C–H alkenylation. Nat. Chem 13, 1207–1213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Tanaka K & Yu J-Q Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature 543, 538–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishna K et al. Coordination assisted distal C–H alkylation of fused heterocycles. Angew. Chem. Int. Ed 58, 13808–13812 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Shi H et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. Nat. Chem 12, 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CA & Miller SJ Site-selective derivatization and remodeling of erythromycin A by using simple peptide-based chiral catalysts. Angew. Chem. Int. Ed 45, 5616–5619 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Tay J-H et al. Regiodivergent glycosylations of 6-deoxy-erythronolide B and oleandomycin-derived macrolactones enabled by chiral acid catalysis. J. Am. Chem. Soc 139, 8570–8578 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimakos V & Taylor MS Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem. Rev 118, 11457–11517 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Chu L et al. Remote meta-C–H activation using a pyridine-based template: achieving site-selectivity via the recognition of distance and geometry. ACS Cent. Sci 1, 394–399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerchen A et al. Non-directed cross-dehydrogenative (hetero)arylation of allylic C(sp3)–H bonds enabled by C–H activation. Angew. Chem. Int. Ed 57, 15248–15252 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Fu L, Zhang Z, Chen P, Lin Z & Liu G Enantioselective copper-catalyzed alkynylation of benzylic C–H bonds via radical relay. J. Am. Chem. Soc 142, 12493–12500 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Porey S et al. Alkyne linchpin strategy for drug: pharmacophore conjugation: experimental and computational realization of a meta-selective inverse sonogashira coupling. J. Am. Chem. Soc 142, 3762–3774 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Pan P et al. Structure-based drug design and identification of H2O-soluble and low toxic hexacyclic camptothecin derivatives with improved efficacy in cancer and lethal inflammation models in vivo. J. Med. Chem 61, 8613–8624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krajewska J, Olczyk T & Jarzab B Cabozantinib for the treatment of progressive metastatic medullary thyroid cancer. Expert Rev. Clin. Pharmacol 9, 69–79 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Personeni N, Rimassa L, Pressiani T, Smiroldo V & Santoro A Cabozantinib for the treatment of hepatocellular carcinoma. Expert Rev. Anticancer Ther 19, 847–855 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Hwang JY et al. Synthesis and evaluation of 7-substituted 4-aminoquinoline analogues for antimalarial activity. J. Med. Chem 54, 7084–7093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper, its Supplementary Information, and free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/structures) under reference numbers CCDC 2078170-2078173, 2132680.