Abstract
Background:
As the drug-related overdose crisis and COVID-19 pandemic continue, communities need increased access to medications for opioid use disorder (MOUD) (i.e., buprenorphine and methadone). Disparities in the type of MOUD prescribed or administered by racial and ethnic categories are well described in the outpatient clinical environment. It is unknown, however, if these disparities persist when MOUD is provided in acute care hospitals.
Methods:
This study assessed differences in the delivery of buprenorphine versus methadone during acute medical or surgical hospitalizations for veterans with opioid use disorder (OUD) by racial categories (Black Non-Hispanic or Latino vs. White Non-Hispanic or Latino). Data were obtained retrospectively from the Veterans Health Administration (VHA) for federal fiscal year 2017. We built logistic regression models, adjusted for individual and hospital-related covariates, and calculated the predicted probabilities of MOUD delivery by racial categories.
Results:
The study cohort (n = 1,313 unique patients; N = 107 VHA hospitals) had a mean age of 57 (range 23 to 87 years), was predominantly male (96%), and composed entirely of Black (29%) or White (71%) patients. White patients were 11% more likely than Black patients to receive buprenorphine than methadone during hospitalization (p = 0.010; 95% CI: 2.7%, 20.0%). Among patients on MOUD prior to hospitalization, White patients were 21% more likely than Black patients to receive buprenorphine (p = 0.000; 95% CI: 9.8%, 31.5%). Among patients newly initiated on MOUD during hospitalization, there were no differences by racial categories.
Conclusion:
We observed disparities in the delivery of buprenorphine versus methadone during hospitalization by racial categories. The observed differences in hospital-based MOUD delivery may be influenced by MOUD received prior to hospitalization within the racialized outpatient addiction treatment system. The VHA and health systems more broadly must address all aspects of racism that contribute to inequitable MOUD access throughout all clinical contexts.
1. Introduction
As the United States (U.S.) drug-related overdose crisis continues, low-barrier and on-demand access to medication for opioid use disorder (MOUD) is critical. First-line MOUD includes methadone and buprenorphine. However, uptake of these life-saving treatments1 are sub-optimal.2,3 Prescribers and researchers often view methadone and buprenorphine as relatively equivalent medications when dosed properly,4 however, there are significant differences in how these medications are accessed due to policies. Opioid treatment programs (OTPs) are the only facilities (except for hospitals) allowed to administer methadone for OUD. OTPs must abide by federal (42 Code of Federal Regulations 8) and state regulatory requirements, which dictate many aspects of care (e.g., take-home medication privileges, urine drug screening).5 Patients must visit OTPs, often daily, to receive their methadone. In contrast, patients most often access buprenorphine in the outpatient context through a written prescription from an office-based prescriber with the medication dispensed through the pharmacy system. Although both medications are federally regulated (e.g., buprenorphine prescribing panel limits), methadone regulations5 limit the ability to create individualized treatment plans, to meet patient specific needs, and can impact employment opportunities or personal relationships.6
Long-standing oppressive forces (e.g., racism and classism)7–9 inform the system design differences between methadone and buprenorphine. The methadone system was established, in part, to address White political concerns related to crime and increased heroin use in New York City in the late 1960s, specifically targeting predominantly Black inner-city communities.8,9 In contrast, the buprenorphine X-waiver system was designed with the intention to increase treatment access for the suburban substance user (e.g., predominantly White communities).8,10,11 The consequences of racialized treatment system design are reflected in contemporary MOUD access inequities. For example, in New York City, across zip code areas, buprenorphine receipt was negatively correlated with poverty, as well as Black Non-Hispanic, and Hispanic demographics, while methadone use was positively correlated with poverty and percent of people who were Hispanic.12 Similarly, for pregnant people with OUD, Black Non-Hispanic women and Hispanic women had a lower likelihood than White Non-Hispanic women of receiving buprenorphine as compared to methadone.13 A 2016 cross-sectional analysis of all 3,142 counties in the U.S. observed that counties with highly segregated Black and Hispanic or Latino communities had more methadone facilities per capita compared to highly segregated White communities, which had more buprenorphine availability per capita.14
The Veterans Health Administration (VHA), the largest integrated health system in the U.S., provides a unique opportunity for improving access to MOUD.15 In an idealized state, MOUD delivery would occur across the clinical continuum, providing patients with OUD numerous touchpoints to receive the “right care, in the right place, at the right time”16 including seamless transitions of care between outpatient and inpatient settings. Unfortunately, the reality of achieving seamlessly integrated care across clinical environments is challenging.17 Prior research demonstrates that hospitalization is a “reachable moment” from the perspective of patients with substance use disorders (SUD) and hospital-based addiction treatment providers18,19 and acute care settings can create opportunities to facilitate linkage to community-based treatment upon discharge.20,21 There is, however, scant literature on MOUD access during hospitalization. Prior research suggests that buprenorphine or methadone delivery during hospitalization is inadequate.22 An analysis of hospitalized veterans with OUD determined that delivery of any type of opioid agonist therapy (i.e., buprenorphine, methadone, or non-specific agonist therapy) during admission was infrequent (i.e., 15% of the study cohort).22 When adjusted for individual and hospital-level covariates using multilevel logistic regression, administration of opioid agonist therapy during hospitalization was not associated with racial or ethnic categories.22 This study, a focused analysis of prior work,22 examined differences in the delivery of MOUD type (buprenorphine vs. methadone) between racial categories (Black Non-Hispanic or Latino vs. White Non-Hispanic or Latino) for hospitalized patients with OUD. We hypothesized that White patients would have increased odds of receiving buprenorphine versus methadone as compared with Black patients.
2. Material and Methods
2.1. Study Definitions and Conceptual Framework
In alignment with new standards on publishing manuscripts on racial health inequities23 we provide definitions of racial categories and racism. Racial categories do not represent inherent biological or genetic differences24 but are a sociologic construct, which “captures the impact of racism.”25 Racism is “a system (consisting of structures, policies, practices, and norms) that structures opportunity and assigns value based on phenotype, or the way people look … it unfairly disadvantages some individuals and communities.”26 Racism exists in society within multilevel contexts: internalized, personally mediated, and institutionalized.25
The Kilbourne conceptual framework27 outlines three phases of health disparities research: 1) detecting (e.g., measuring disparities); 2) understanding (e.g., identifying determinants at the individual, provider, clinical encounter, and health system level); and 3) reducing (e.g., interventions to reduce disparities). Our research study exists within the first (detecting) and second phases (understanding); thus, we use the Kilbourne et al (2006) health disparities definition: “as observed clinically and statistically significant differences in health outcomes or health care use between socially distinct vulnerable and less vulnerable populations that are not explained by the effects of selection bias.”27
2.2. Study Design and Cohort
We conducted a retrospective analysis of electronic health record and administrative data from hospitalized adult veterans (18 years of age and older) within the continental U.S. with an OUD diagnosis within the year preceding the discharge date of index hospitalization in federal fiscal year 2017.22 Priest and colleagues22 and Priest17 describe details on data extraction methods, facility inclusion, and study variable definitions (e.g., OUD ICD-10 codes). There were two time periods for this study: a) pre-period (the 30 days prior to hospitalization) and b) hospitalization. Patients were included in this study if they received at least one dose of buprenorphine or methadone while hospitalized. Patients were excluded if: 1) they were a race or ethnicity other than Black Non-Hispanic or Latino or White Non-Hispanic or Latino (this exclusion included those with unknown racial or ethnic categories), 2) they received buprenorphine or methadone for pain in the pre-period (determined by formulation of buprenorphine or methadone), 3) they received naltrexone or concurrent naltrexone plus a first-line MOUD in the pre-period or during hospitalization, 4) they received a non-specific MOUD during hospitalization, or 5) they received more than one type of MOUD during hospitalization (See Figure 1). The Veterans Affairs Portland Health Care System Institutional Review Board approved this study.
Figure 1.
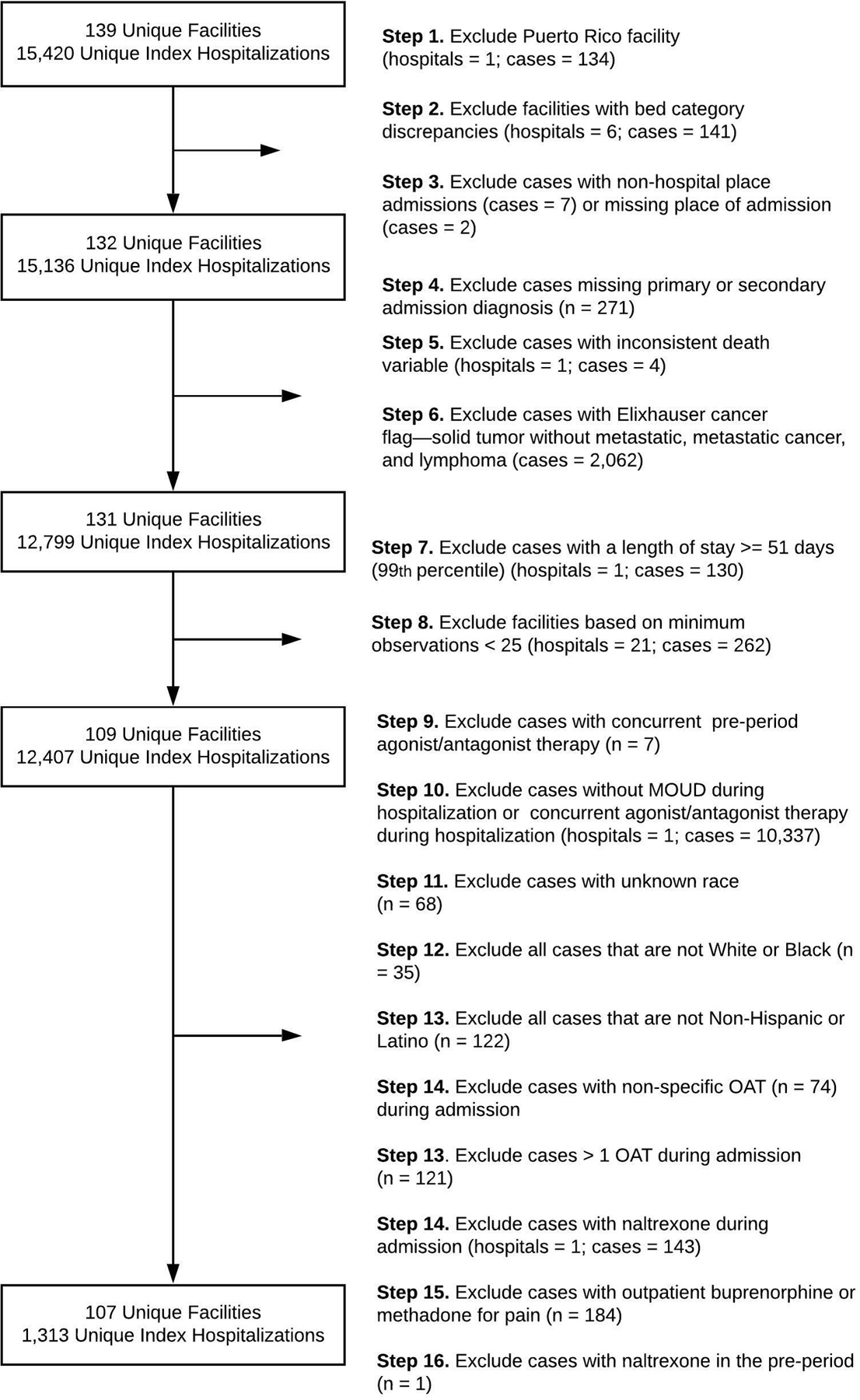
Study Cohort Selection Diagram. This figure reflects the changes in the study cohort from the existing dataset published Priest and colleagues.
2.3. Measures
2.3.1. Primary Outcome.
Our primary outcome measure was type of MOUD received during hospitalization (buprenorphine vs. methadone). Pharmacotherapy variable definitions are in the Table 1 footnotes.
Table 1.
Patient and Hospital Characteristics by Racial Categories
| All | Black | White | Statistical Significance | |
|---|---|---|---|---|
| Total | 100% (n = 1,313) | 29.7% (n = 285) | 70.73% (n = 928) | |
| a Age | 60.0 median 56.5 mean Range: 23, 87 |
63.0 median 63.1 mean Range: 30, 87 |
58.0 median 53.8 mean Range: 23, 82 |
*** |
| b Gender | M: 95.5% (1,254) F: 4.5% (59) |
M: 97.7% (376) F: 2.3% (9) |
M: 94.6% (878) F: 5.4% (50) |
* |
| b MOUD During Admission | ||||
| Buprenorphine2 | 44.0 % (578) | 30.4% (117) | 49.7% (461) | *** |
| Methadone3 | 56.0% (735) | 69.6% (268) | 50.3% (467) | |
| b Received MOUD Prior to Hospitalization4 | 47.8% (627) | 44.4% (171) | 45.6% (456) | N.S. |
| b Received Opioids Prior to Hospitalization5 | 8.1% (106) | 8.8% (34) | 7.8% (72) | N.S. |
| b Received Opioids During Hospitalization6 | 33.5% (438) | 32.2% (124) | 33.8% (314) | N.S. |
| b OUD-Related Infection During Admission7 | 8.5% (111) | 8.3% (32) | 8.5% (79) | N.S. |
| b OUD-Related Diagnosis During Admission8 | 31.0% (407) | 27.8% (107) | 32.3% (300) | N.S. |
| b Co-Occurring Psychiatric Disorder9 | 67.6% (887) | 54.3% (209) | 73.1% (678) | *** |
| b Co-Occurring Substance Use Disorder10 | 52.3% (687) | 45.7% (176) | 55.1% (511) | ** |
| a Length of Stay (days) | 5.0 Median 7.6 Mean Range: 2, 50 |
5.0 Median 8.3 Mean Range: 2, 46 |
5.0 Median 7.3 Mean Range: 1, 50 |
* |
| b Received ICU Services During Admission | 16.1% (211) | 14.8% (57) | 16.6% (154) | N.S. |
| b Received Surgical Services During Admission | 5.3% (70) | 4.4% (17) | 5.7% (53) | N.S. |
| b Hospital Size | ||||
| Small: 1 to 49 beds | 7.3% (96) | 3.1% (12) | 9.0% (84) | *** |
| Medium: 50 to 99 beds | 28.3% (371) | 19.7% (76) | 31.8% (295) | |
| Large: 100+ beds | 64.4% (846) | 77.1% (297) | 59.2% (549) | |
| b Hospital Location | ||||
| Midwest | 27.0% (270) | 38.4% (148) | 22.3% (207) | *** |
| Northeast | 25.1% (329) | 20.5% (79) | 26.9% (250) | |
| South | 29.6% (388) | 32.5% (125) | 28.3% (263) | |
| West | 18.4% (241) | 8.6% (33) | 22.4% (208) |
Table Notes.
Kruskal-Wallis Rank Sum Test
Pearson chi-square test
statistical significance: p < 0.05*; p < 0.01**; p < 0.001***
N.S. = Non-significant; MOUD = medication for opioid use disorder
This includes buprenorphine sublingual tablet (n = 38), buprenorphine/naloxone film sublingual (n = 66), buprenorphine/naloxone sublingual tablet (n = 491), buprenorphine injection (n = 1), buprenorphine patch (n = 2), J0571 buprenorphine oral 1 mg (n = 1). Patients could receive more than one formulation of the same product.
This includes methadone injection (n = 1), methadone solution concentration (n = 91), methadone solution oral (n = 74), methadone tablet (n = 510), methadone unknown formulation (n = 22), methadone tablet effervescence (n = 42), methadone tablet oral (n = 63), S0109 methadone oral 5 mg (n = 4). Patients could receive more than one formulation of the same product.
This includes buprenorphine buccal film (n = 1), buprenorphine sublingual tablet (n = 9), buprenorphine/naloxone sublingual film (n = 54), buprenorphine/naloxone sublingual tablet (n = 321), VHA opioid treatment program stop code visits (n = 286), H0033 oral medication and administration direct observation code (n = 9), J0575 buprenorphine/naloxone over 10 mg (n = 1), J0571 buprenorphine oral 1 mg (n = 1), S0109 methadone oral 5 mg (n = 3). Patients could receive more than one formulation.
This includes codeine (n = 3), fentanyl (n = 2), hydrocodone (n = 37), hydromorphone (n = 3), morphine (n = 11), oxycodone (n = 30), and tramadol (n = 36). Patients could receive more than one type of opioid and formulation. We excluded buprenorphine and methadone formulations for pain from this variable.
This includes codeine (n = 3), fentanyl (n = 44), hydrocodone (n = 82), hydromorphone (n = 161), morphine (n = 133), oxycodone (n = 208), meperidine (n = 1), and tramadol (n = 75).
This includes endocarditis (n = 11), osteomyelitis (n = 52), bacteremia (n = 29), discitis (n = 6), septic arthritis (n = 10), brain abscess (n = 7), joint infection (n = 5), empyema (n = 4), and lung abscess (n = 6).
This includes a primary and/or secondary ICD-10 OUD diagnosis code during hospitalization: Primary: F11.10 (n = 3), F11.20 (n = 29), F11.21 (n = 1), F11.220 (n = 3), F11.229 (n = 1), F11.23 (n = 72), F11.24 (n = 7), F11.251 (n = 1), F11.259 (n = 1), T40.1X1 (n = 4), T40.1X2 (n = 1), T40.2X1 (n = 3), T40.2X2 (n = 1), T40.3X1 (n = 1), T40.604 (n = 1). Secondary: F11.10 (n = 10), F11.20 (n = 232), F.11.21 (n = 2), F.11.23 (n =33), F.11.24 (n = 1), F11.29 (n = 1), F11.90 (n = 3), F11.93 (n = 2), T40.2X1 (n = 1), T40.2X4 (n = 1), and T40.3X1 (n = 1).
This includes psychiatric disorder diagnoses within 365 days prior to index hospitalization: adjustment disorder (n = 36), anxiety disorder (n = 375), mood disorder (n = 673), non-mood psychotic disorder (n = 92), PTSD (n = 434), and self-harm (n = 39).
This includes co-occurring substance use disorders within 365 days prior to the index hospitalization: alcohol use disorder (n = 480), cannabis use disorder (n = 208), cocaine use disorder (n = 67), hallucinogen use disorder (n = 4), nicotine dependence (n = 53), other psychoactive use disorders (n = 276), other stimulant related disorders (n = 141), other substance use disorders (n = 40), sedative hypnotic disorder (n = 66), and inhalant use disorder (n = 8).
2.3.2. Independent Variable.
The independent variable of interest was racial category (Black vs. White). There were too few individuals with other race or ethnicity identities for reliable analysis.
2.3.2. Covariates.
We selected covariates demonstrated to be associated with type of MOUD received based on prior research,17,22 data availability, and expertise of the authorship team and adjusted for the following patient-related characteristics: age, birth sex (male/female), receipt of any MOUD in the pre-period (buprenorphine, methadone, >1 type of agonist therapy, non-specific agonist therapy) (yes/no), receipt of opioids in the pre-period (yes/no), receipt of opioids during hospitalization (yes/no), OUD-related infection during hospitalization (yes/no), primary or secondary OUD-related diagnosis during hospitalization (yes/no), co-occurring psychiatric diagnosis prior to hospitalization (yes/no), co-occurring SUD diagnosis prior to hospitalization (yes/no), hospital length of stay (number of days), receipt of ICU services during hospitalization (yes/no), and receipt of surgical services during hospitalization (yes/no). We adjusted for the following hospital covariates for each patient’s hospitalization: hospital size (small [1 to 49 beds], medium [50 to 99 beds], large [≥100 beds]) and regional location (Midwest, Northeast, South, and West).
2.4. Statistical Analyses
We used Stata 15.128 for bivariate analyses, logistic regression modeling, and calculated predicted probabilities. For data management, coding, and descriptive statistics we used R Studio29 and open source packages.30–34 To prepare our logistic regression model, we examined Pearson’s correlation coefficients to assess for co-linearity of model covariates35 at a threshold of 80%, and no associations between covariates reached that level. A Hosmer-Lemeshow test evaluated model goodness-of-fit36; the model fit the data well. We graphically evaluated linearity in the log-odds of the outcome variables with each continuous covariate (age, length of stay) using a LOWESS scatter plot37 at two different bandwidths (0.8, 0.4), and found that LOWESS plots were approximately linear. A clustered sandwich estimator of variance addressed the multilevel structure of the data (i.e., patients within hospitals).38 We report predicted probabilities and 95% confidence intervals. See supplemental materials for regression outputs (adjusted odds ratios, standard error, p-values [alpha value threshold of 0.05], and 95% confidence intervals) and predicted probability tables.
2.4.1. Sensitivity Analyses.
We conducted three sensitivity analyses. The first analysis excluded hospitals with fewer than 10 patients. We hypothesized that hospitals with infrequent OUD patients could have a different clinical practice as compared with hospitals with more OUD patients. The second analysis excluded patients who did not receive MOUD in the pre-period (i.e., included patients who received MOUD pre-hospitalization and during hospitalization) and the third analysis excluded patients who received MOUD in the pre-period (i.e., included patients who received MOUD only during hospitalization), respectively. We hypothesized that these two groups of patients could have different access to MOUD type during admission by race (Black vs. White), and other unmeasurable elements. When the primary outcome results differed significantly in magnitude or direction from the sensitivity analyses, we reported the results alongside our primary analysis.
3. Results
3.1. Patient and Hospitalization-Related Characteristics: Descriptive Statistics
The study cohort included 1,313 unique patients with index hospitalizations from 107 hospitals from the VHA acute care hospitals in the continental U.S. Patients had a mean age of 57 years (range 23 to 87 years), were predominantly male (n = 1,254; 95.6%) and composed entirely of Black (n = 385; 29.3%) or White (n = 928; 70.7%) patients. Eight percent of patients filled prescriptions for opioids pre-hospitalization (n = 106). Co-occurring SUDs (n = 687; 52.3%) and other psychiatric diagnoses (n = 887; 67.6%) were common. Length of hospital stay ranged from 2 to 50 days, with a median of 5 days and a mean of 8 days. Hospitalizations occurred most often in large facilities (n = 846; 64.4%) distributed throughout four U.S. regions: Midwest (n = 355; 27.0%); Northeast (n = 329; 25.1%); South (n = 388; 29.6%); and the West (n = 241; 18.4%). Reasons for hospitalization were heterogenous, with 540 unique primary ICD-10 diagnosis codes and 465 unique secondary ICD-10 diagnosis codes. See supplemental materials for top 10 most common primary and secondary diagnoses. See Table 1 for characteristics by racial categories and Table 2 for characteristics by MOUD type.
Table 2.
Patient and Hospital Characteristics by MOUD Type Received During Hospitalization
| Buprenorphine | Methadone | Statistical Significance | |
|---|---|---|---|
| Total | 44.0% (578) | 56.0% (735) | |
| a Age | 57.0 median 53.3 mean Range: 24, 85 |
62.0 median 59.0 mean Range: 23, 87 |
*** |
| b Gender | M: 95.0% (549) F: 5.0% (29) |
M: 95.9% (705) F: 4.1% (30) |
N.S. |
| b Race | |||
| Black/Non-Hispanic or Latino | 20.2% (117) | 36.5% (268) | *** |
| White/Non-Hispanic or Latino | 79.8% (461) | 63.5% (467) | |
| b Received MOUD Prior to Admission | 68.5% (396) | 31.4% (231) | *** |
| b Received Opioids Prior to Admission | 7.8% (45) | 8.3% (61) | N.S. |
| b Received Opioids During Admission | 22.7% (137) | 40.5% (322) | *** |
| b OUD-Related Infection During Admission | 4.8% (28) | 11.3% (83) | *** |
| b OUD-Related Diagnosis During Admission | 34.8% (201) | 28.0% (206) | * |
| b Co-Occurring Psychiatric Disorder | 75.6% (437) | 61.2% (450) | *** |
| b Co-Occurring Substance Use Disorder | 59.5% (344) | 46.7% (343) | *** |
| a Length of Stay (days) | 5.0 Median 6.7 Mean Range: 2 – 47 |
5.0 Median 8.3 Mean Range: 2 – 50 |
*** |
| b Received ICU Services During Admission | 14.7% (85) | 17.1% (126) | N.S. |
| b Received Surgical Services During Admission | 4.0% (23) | 6.6% (47) | * |
| b Hospital Size | |||
| Small: 1 to 49 beds | 11.2% (65) | 4.2% (31) | *** |
| Medium: 50 to 99 beds | 27.3% (158) | 29.0% (213) | |
| Large: 100+ beds | 61.4% (355) | 66.8% (491) | |
| b Hospital Location | |||
| Midwest | 27.0% (156) | 27.1% (199) | ** |
| Northeast | 23.9% (138) | 26.0% (191) | |
| South | 33.2% (192) | 26.7% (196) | |
| West | 15.9% (92) | 20.3% (149) |
Table Notes.
Kruskal-Wallis Rank Sum Test
Pearson chi-square test
statistical significance: p < 0.05*; p < 0.01**; p < 0.001***
N.S. = Non-significant; MOUD = medication for opioid use disorder; See Table 1 footnotes for additional details.
3.2. MOUD Receipt: Descriptive Statistics
Across all patients, in unadjusted analyses, methadone (n = 735; 56.0%) was received more often than buprenorphine (n = 578; 44.0%) during hospitalization. Black patients received methadone 70% of the time (n = 268) and buprenorphine 30% of the time (n = 117). In contrast, White patients received methadone (n = 467) and buprenorphine (n = 461) essentially equally during hospitalization. In the pre-period, nearly half of patients (n = 627; 47.8%) received MOUD. For this group of patients, 63% received buprenorphine (n = 396) and 37% received methadone (n = 231) while hospitalized. Over half of patients in the study sample initiated MOUD during hospitalization (n = 686, 52.2%): 27% received buprenorphine (n = 182) versus 74% methadone (n = 504). See supplemental materials for the characteristics of the pre-period and initiated during hospitalization MOUD groups.
3.3. Logistic Regression and Predicated Probabilities: Primary Outcome
In the final logistic regression model, after adjusting for covariates, White patients had increased odds of receiving buprenorphine versus methadone as compared to Black patients (Adjusted Odds Ratio [aOR]: 1.81; p = 0.012; 95% Confidence Interval [CI]: 1.14 to 2.86). Using this model, we calculated predicted probabilities and observed that White patients were 11.1% more likely than Black patients to receive buprenorphine versus methadone during hospitalization (p = 0.010; 95% CI: 2.7%, 20.0%).
In our first sensitivity analysis (excluding hospitals with less than 10 patients) there was no substantive difference in predicted probabilities. In our second sensitivity analysis, which included patients who received MOUD in the pre-period and during hospitalization, the predicted probabilities increased: White patients were 21% more likely than Black patients to receive buprenorphine (p = 0.000; 95% CI: 9.8%, 31.5%). In our third sensitivity analysis, among patients who only received MOUD during hospitalization but not in the pre-period, the predicted probabilities of MOUD receipt by racial category were no longer statistically significant. Regression outputs for each sensitivity analysis and the predicted probabilities are in the supplemental materials.
4. Discussion
Our study characterized the differential delivery of buprenorphine and methadone by racial categories during hospitalization in the VHA for patients with OUD. Among the entire study population, when we adjusted for individual and hospital-related characteristics, White patients were 11% more likely than Black patients to receive buprenorphine than methadone during hospitalization. Using our sensitivity analyses, we explored elements that could be associated with the observed differences from the primary analysis. For patients who had received MOUD in the pre-period and during hospitalization (n = 627), the predicted probability of buprenorphine receipt for White patients as compared to Black patients increased to 21%. In contrast, when we only included patients initiated on MOUD during hospitalization (n = 686) there was no longer a difference in MOUD type received during hospitalization by racial categories.
Our findings may suggest that the primary driver of disparities in MOUD receipt during hospitalization was related to the care received prior to hospitalization. Importantly, we are not suggesting that racism (internalized, personally mediated, and institutionalized)25,26 does not impact hospital-based care, but instead that institutionalized racism in the design of an outpatient MOUD delivery system that may perpetuate and exacerbate inequities within the hospital-context. Literature describes differences based on racial and ethnic categories in access to MOUD,39–41 differential receipt of MOUD (buprenorphine vs. methadone),42 and differences in buprenorphine retention43 suggesting that a racialized MOUD delivery system may exist within the VHA. Within the VHA, for example, Black race was found to be negatively associated with the odds of receiving buprenorphine as compared to methadone in a 2012 cohort of veterans.42
4.1. Practice, Policy, System, and Research Implications
Ideally, MOUD-related practice, policy, and delivery systems are patient-informed and patient-centered. Core elements of patient-centered care for persons with SUDs include shared-decision making and individualized care.44 It appears, based on our research and prior studies, that the current racialized system does not facilitate individualized care equitably among people with different racial categories. Inequitable MOUD access may have implications for patient-related recovery experiences and clinical outcomes. Scheduling an intake appointment at a methadone clinic, for example, can be logistically cumbersome; over a third of patients admitted to methadone treatment experienced a delay in treatment entry45 and delays were associated with Black racial category.45 The logistical inflexibility of methadone dosing versus outpatient buprenorphine prescribing could impact employment opportunities, in turn impacting long-term outcomes. Patients, themselves, may have more negative perceptions of methadone,46 in turn, impacting their self-efficacy and subsequent recovery trajectories. Moreover, Black, Latino/Latina, American Indian/Alaska Native patients with OUD have an increased risk of experiencing racial discrimination within the medical setting as compared with White patients.47
Hospitals are a touchpoint within the broader OUD care continuum.17 Multiple nested elements (individual, organizational, system-level, and policies)48 inform access to MOUD within the hospital and community-based treatment. Our study, and prior research, illustrates the importance of efforts targeting interventions (programmatic and policy) focused on the outpatient context to decrease MOUD access inequities. To address the racialized design of the outpatient MOUD system, interventions should focus on changing policies which contribute to treatment inequities. Interventions could involve policy and system reform of OTPs (e.g., methadone liberalization through the distribution of methadone through the pharmacy system or primary care)49,50 or further deregulation of buprenorphine by completely eliminating federal enrollment requirements and patient panel limitations.51 In addition to MOUD-related policy changes, Jordan and colleagues52 suggest developing new models of clinical care (e.g., integrated services to address whole-person health) and implementing culturally sensitive treatment services, such as the delivery of addiction-related treatment or interventions in churches within Black communities.53 Decisionmakers should also consider broader health equity policies that may impact access to MOUD, such as increasing health insurance coverage through Medicaid expansion, which is associated with increases in buprenorphine prescriptions.54 Policymakers should also consider interventions outside of the healthcare delivery system, such as ending the “war on drugs”55 through the decriminalization of substance possession or the provision of safe supply.56
Future research should include patients in the design of research studies on hospital-based MOUD delivery. Quantitative approaches could explore racial disparities in MOUD access within the hospital context among non-VHA health systems or other integrated health systems. In concert with recent calls to action for developing SUD research agendas that address structural racism and violence,57 researchers could qualitatively explore from the patient perspective how multilevel racism impacts their access to MOUD. In the COVID-19 era, advances in treatment delivery (e.g., telemedicine) may widen disparities in care.58 The consequences of these delivery changes should be assessed. Finally, there is a continued need to identify quantitative and qualitative approaches for capturing the multilevel mechanisms of racism at the individual (e.g., Perceived Racism Scale, the Index of Race-Related Stress)59 and community-level (e.g., redlining index of mortgage discrimination, index of dissimilarity).60
4.2. Study Limitations
There are several limitations to this study. The health services delivery context (the VHA) and the veteran-based cohort constrain study generalizability. This was an analysis of an existing dataset22; our study design and cohort were constrained by prior research questions.61 It is not possible to identify the exact mechanism by which racism impacts the observed inequities in MOUD delivery within these data. It is possible that Black patients in our study experienced any combination of multilevel mechanisms of racism62 when receiving differential care inside and outside the hospital, which we were unable to capture, and future research should explore drivers of access inequity. There are inherent issues with the collection of racial/ethnic category data. Prior research demonstrates that assigned race in the electronic health record may be a biased towards whiteness, meaning, that Black patients are more likely to be labeled White versus Black when asked to self-report their race.63 Thus, the differences we observed may be smaller due to racial/ethnic category misclassification. Due to demographics and size of the study cohort we were unable to include other racial and ethnic categories in our statistical analyses. The exclusion of other racial and ethnic categories has implications from a data justice perspective.64 We were unable to assess the impact of the racialized MOUD delivery system on different groups of minoritized patients. Moreover, racial and ethnic categories are not a static construct, and may reflect changes in socio-political and economic contexts over time.65 This is important to consider as the temporality of when a veteran’s racial or ethnic identity was documented in the VHA system is unknown but likely differs among the cohort. There are likely additional confounding patient-related characteristics that could influence access to hospital-based MOUD, such as housing insecurity,66 specific co-occurring SUDs,66 partner substance use,66 or geographic residence (e.g., long drive times to OTPs for people residing in rural settings).67 We did not have access to data on broader health system factors, such as hospital affiliation with OTPs and community-based access to outpatient services.48 Finally, we were unable to capture patient MOUD preference.
5. Conclusions
Racial and ethnic disparities in access to buprenorphine and methadone in the outpatient context are well-described across different patient populations (e.g., urban residence, pregnancy, veterans) and other clinical contexts (e.g., outpatient). We observed that differential MOUD delivery persisted by racial categories within the acute care context, likely driven, in part, by care received in the racialized outpatient MOUD delivery system as articulated by our sensitivity analyses. These inequities in care may have implications for patient recovery-related experiences, such as starting or continuing treatment. The VHA, and hospital leaders more broadly, should investigate and address multilevel racism that may be informing differential delivery of buprenorphine and methadone between Black and White patients inside and outside the hospital.
Supplementary Material
Funding:
This work was supported by the National Institute on Drug Abuse [F30 DA044700; F30 DA052972; UG1DA015815], the Greenlick Family Scholarship, the OHSU MD/PhD Program, National Center for Advancing Translational Sciences [TL1TR002371], U.S. Department of Veterans Affairs Health Services Research & Development [I01HX002518], and the University of Pittsburgh Department Psychiatry Emerging Star Award. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosure Statement: None of the authors report a conflict of interest. The views expressed in this article are those of the authors’ and do not necessarily represent the views of the U.S. Department of Veterans Affairs, the U.S. Government, or any of our funding agencies.
References
- 1.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beetham T, Saloner B, Gaye M, Wakeman SE, Frank RG, Barnett ML. Therapies offered at residential addiction treatment programs in the United States. JAMA 2020;324(8):804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch AB, Greenfield SF, Reif S, Normand S-LT, Huskamp HA. Outpatient care for opioid use disorder among the commercially insured: Use of medication and psychosocial treatment. J Subst Abuse Treat 2020;115:108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;2:CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse Mental Health Services Administration. Federal guidelines for opioid treatment programs In. Vol Vol HHS Publication; No.(SMA) PEP 15-FEDGUIDEOTP2015. [Google Scholar]
- 6.National Academies of Sciences, Engineering, and Medicine. Medications for Opioid Use Disorder Save Lives Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 7.Priest KC. Commentary on Jin et al. (2020): Regulatory and allocative policies inform access to opioid agonist therapy. Addiction 2020;115(12):2255–2256. [DOI] [PubMed] [Google Scholar]
- 8.Hansen H, Roberts SK. Two tiers of biomedicalization: Methadone, buprenorphine, and the racial politics of addiction treatment. Advances in Medical Sociology 2012;14(12):79–102. [Google Scholar]
- 9.James K, Jordan A. The opioid crisis in black communities. The Journal of Law, Medicine & Ethics 2018;46(2):404–421. [DOI] [PubMed] [Google Scholar]
- 10.Hansen H, Parker C, Netherland J. Race as a Ghost Variable in (White) Opioid Research. Science, Technology, & Human Values 2020:0162243920912812. [Google Scholar]
- 11.Netherland J, Hansen HB. The war on drugs that wasn’t: Wasted whiteness,“dirty doctors,” and race in media coverage of prescription opioid misuse. Culture, Medicine, and Psychiatry 2016;40(4):664–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen H, Siegel CE, Case BG, Bertollo DN, DiRocco D, Galanter M. Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. The Journal of Behavioral Health Services & Research 2013;40(3):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of Racial and Ethnic Disparities in the Use of Medication to Treat Opioid Use Disorder Among Pregnant Women in Massachusetts. JAMA Network Open 2020;3(5):e205734–e205734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BD. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA network open 2020;3(4):e203711–e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priest KC, McCarty D, Lovejoy TI. Expanding access to medications for opioid use disorder: Program and policy approaches from outside the Veterans Health Administration. J Gen Intern Med 2020;35(Suppl 3):886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutherford PA, Provost LP, Kotagal UR, Luther K, Anderson A. Achieving hospital-wide patient flow. IHI White Paper Cambridge: Institute for Healthcare Improvement 2017. [Google Scholar]
- 17.Priest KC. Hospital-based services for opioid use disorder: A study of supply-side attributes. Dissertations and Theses 2019;Paper 4829. [Google Scholar]
- 18.Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med 2017;12(5):339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: A qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med 2017;32(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Internal Medicine 2014;174(8):1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Englander H, Dobbertin K, Lind BK, et al. Inpatient Addiction Medicine Consultation and Post-Hospital Substance Use Disorder Treatment Engagement: a Propensity-Matched Analysis. J Gen Intern Med 2019;34(12):2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: A pragmatic retrospective cohort analysis. J Gen Intern Med 2020;35(8):2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog 2020;10. [Google Scholar]
- 24.Kaplan JB, Bennett T. Use of Race and Ethnicity in Biomedical Publication. JAMA 2003;289(20):2709–2716. [DOI] [PubMed] [Google Scholar]
- 25.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health 2000;90(8):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones CP. Confronting institutionalized racism. Phylon (1960-) 2002:7–22. [Google Scholar]
- 27.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health 2006;96(12):2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stata: Statsitics/Data Analysis [computer program] StatCorp LLC; 2017. [Google Scholar]
- 29.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 30.Wickham H Welcome to the tidyverse. Journal of Open Source Software 2019;4(43):1686. [Google Scholar]
- 31.Wickham H plyr: The split-apply-combine strategy for data analysis. Journal of Statistical Software 2011;40(1). [Google Scholar]
- 32.Fox J, Weisberg S. An [R] Companion to Applied Regression, Third Edition Vol 2. Thousand Oaks, CA: SAGE Publications; 2019. [Google Scholar]
- 33.dplyr: A Grammar of Data Manipulation. [computer program] Version R package version; 0.8.32019. [Google Scholar]
- 34.icd: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes [computer program] 2019.
- 35.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013;36(1):27–46. [Google Scholar]
- 36.Fagerland MW, Hosmer DW. A generalized Hosmer–Lemeshow goodness-of-fit test for multinomial logistic regression models. The Stata Journal 2012;12(3):447–453. [Google Scholar]
- 37.Andersen R Nonparametric methods for modeling nonlinearity in regression analysis. Annual Review of Sociology 2009;35:67–85. [Google Scholar]
- 38.Rogers W Regression standard errors in clustered samples. Stata technical bulletin 1994;3(13). [Google Scholar]
- 39.Manhapra A, Stefanovics E, Rosenheck R. Initiating opioid agonist treatment for opioid use disorder nationally in the Veterans Health Administration: Who gets what? Substance abuse 2019;41(1):110–120. [DOI] [PubMed] [Google Scholar]
- 40.Finlay AK, Harris AH, Timko C, et al. Disparities in access to medications for opioid use disorder in the Veterans Health Administration. J Addict Med 2021;15(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essien UR, Sileanu FE, Zhao X, et al. Racial/Ethnic Differences in the Medical Treatment of Opioid Use Disorders Within the VA Healthcare System Following Non-Fatal Opioid Overdose. J Gen Intern Med 2020;35(5):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manhapra A, Quinones L, Rosenheck R. Characteristics of veterans receiving buprenorphine vs. methadone for opioid use disorder nationally in the Veterans Health Administration. Drug Alcohol Depend 2016;160:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manhapra A, Petrakis I, Rosenheck R. Three‐year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. The American journal on addictions 2017;26(6):572–580. [DOI] [PubMed] [Google Scholar]
- 44.Marchand K, Beaumont S, Westfall J, et al. Conceptualizing patient-centered care for substance use disorder treatment: findings from a systematic scoping review. Substance Abuse Treatment, Prevention, and Policy 2019;14(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gryczynski J, Schwartz RP, Salkever DS, Mitchell SG, Jaffe JH. Patterns in admission delays to outpatient methadone treatment in the United States. J Subst Abuse Treat 2011;41(4):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cioe K, Biondi BE, Easly R, Simard A, Zheng X, Springer SA. A systematic review of patients’ and providers’ perspectives of medications for treatment of opioid use disorder. J Subst Abuse Treat 2020;119:108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pro G, Zaller N. Interaction effects in the association between methadone maintenance therapy and experiences of racial discrimination in US healthcare settings. PLoS ONE 2020;15(2):e0228755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priest KC, Englander H, McCarty D. “Now hospital leaders are paying attention”: A qualitative study of internal and external factors influencing addiction consult services. J Subst Abuse Treat 2020;110:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samet JH, Botticelli M, Bharel M. Methadone in Primary Care — One Small Step for Congress, One Giant Leap for Addiction Treatment. N Engl J Med 2018;379(1):7–8. [DOI] [PubMed] [Google Scholar]
- 50.Calcaterra S, Bach P, Chadi A, et al. Methadone matters: what the United States can learn from the global effort to treat opioid addiction. J Gen Intern Med 2019;34(6):1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.HHS Releases New Buprenorphine Practice Guidelines, Expanding Access to Treatment for Opioid Use Disorder [press release] 2021.
- 52.Jordan A, Mathis ML, Isom J. Achieving Mental Health Equity: Addictions. Psychiatric Clinics 2020;43(3):487–500. [DOI] [PubMed] [Google Scholar]
- 53.Jordan A, Babuscio T, Nich C, Carroll KM. A feasibility study providing substance use treatment in the Black church. J Subst Abuse Treat 2021;124:108218. [DOI] [PubMed] [Google Scholar]
- 54.Wen H, Hockenberry JM, Borders TF, Druss BG. Impact of Medicaid Expansion on Medicaid-covered Utilization of Buprenorphine for Opioid Use Disorder Treatment. Med Care 2017;55(4):336–341. [DOI] [PubMed] [Google Scholar]
- 55.Earp BD, Lewis J, Hart CL. Racial Justice Requires Ending the War on Drugs. The American Journal of Bioethics 2020:1–29. [DOI] [PubMed] [Google Scholar]
- 56.Culbert L, Fumano D. B.C. to offer Canada’s first, permanent safe drug supply in response to overdose crisis. Vancouver Sun 2021. [Google Scholar]
- 57.Bluthenthal RN. Structural racism and violence as social determinants of health: Conceptual, methodological and intervention challenges. Drug Alcohol Depend 2021:108681. [DOI] [PubMed]
- 58.Glied S, Lleras-Muney A. Technological innovation and inequality in health. Demography 2008;45(3):741–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atkins R Instruments measuring perceived racism/racial discrimination: review and critique of factor analytic techniques. International journal of health services : planning, administration, evaluation 2014;44(4):711–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groos M, Wallace M, Hardeman R, Theall KP. Measuring inequity: a systematic review of methods used to quantify structural racism. Journal of Health Disparities Research and Practice 2018;11(2):13. [Google Scholar]
- 61.Cheng HG, Phillips MR. Secondary analysis of existing data: opportunities and implementation. Shanghai archives of psychiatry 2014;26(6):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Academies of Sciences E, Medicine. Communities in action: Pathways to health equity National Academies Press; 2017. [PubMed] [Google Scholar]
- 63.Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of Race, Ethnicity, and Language Preference in an Electronic Health Record. J Gen Intern Med 2015;30(6):719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King C, Englander H, Priest KC, Korthuis PT, McPherson S. Addressing Missing Data in Substance Use Research: A Review and Data Justice-based Approach. J Addict Med 2020;14(6):454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nobles M History counts: a comparative analysis of racial/color categorization in US and Brazilian censuses. Am J Public Health 2000;90(11):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Englander H, King C, Nicolaidis C, et al. Predictors of Opioid and Alcohol Pharmacotherapy Initiation at Hospital Discharge Among Patients Seen by an Inpatient Addiction Consult Service. J Addict Med 2020;14(5):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joudrey PJ, Chadi N, Roy P, et al. Pharmacy-based methadone dispensing and drive time to methadone treatment in five states within the United States: A cross-sectional study. Drug Alcohol Depend 2020;211:107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


