INTRODUCTION
Preparticipation cardiovascular screening (PPCS) in young competitive athletes is performed to detect conditions associated with sudden cardiac death (SCD). Many medical societies and sports governing bodies recommend PPCS consisting of a focused history and physical examination (H&P) and 12-lead electrocardiogram (ECG).1-6 Initial ECG screening was criticized for high false-positive rates that led to substantial costs associated with secondary testing and unnecessary (temporary) restriction of athletes from participation. This led to substantial efforts by the scientific community to better understand the difference between physiologic and pathologic ECG findings in athletes. Beginning with the 2010 European Society of Cardiology (ESC) guidelines, several iterations of ECG interpretation standards have emerged, culminating in the most updated ECG interpretation criteria, the International Criteria.7-12 Since the initial publication of the International Criteria in 2017, multiple studies have shown improved diagnostic accuracy (improved specificity without compromising sensitivity) in different athletic populations. In this review, we present common pitfalls for ECG interpretation in athletes using the International Criteria and highlight future directions to consider in subsequent iterations of ECG screening standards.
EVOLUTION OF ELECTROCARDIOGRAM SCREENING CRITERIA IN ATHLETES
ECG screening among athletic populations first emerged with a New England Journal of Medicine publication by Corrado and colleagues on ECG screening for hypertrophic cardiomyopathy in young athletes.13 This was followed several years later by a broader and more systematic approach proposed by the ESC in 200510 with the publication of the first standardized ECG screening criteria in athletes, and multiple subsequent iterations have followed.7-12 The initial ESC 2005 criteria provided a list of “abnormal” ECG findings in athletes that warranted further evaluation. A series of National Collegiate Athletic Association articles using these criteria found high rates of abnormal ECGs and false-positive rates (>10%),14-17 which led to increased scrutiny of how ECG screening may lead to unnecessary secondary testing and significant costs on the medical system. The ESC subsequently proposed new criteria in 2010 that introduced the concept of “common/training-related” ECG patterns, in contrast to more concerning “uncommon/training-unrelated” findings.7 Although the incorporation of normal training-related ECG findings improved the diagnostic performance of ECG interpretation in athletes, studies using these criteria reported high rates of abnormal ECGs (3%–47%) and false-positive rates (5%–60%) depending on the patient population assessed.9,18-34 One of the notable features of these criteria was that they were derived from largely Caucasian cohorts and accordingly did not account for emerging data describing common repolarization abnormalities among Black athletes.35
The Seattle Criteria were created in 2013 to update interpretation standards inclusive of ethnic-specific ECG findings and provide a pragmatic approach for the sports medicine and cardiology communities.8 A major addition in this iteration was the recognition that convex (domed) ST elevations followed by T wave inversion (TWI) in V1 to V4 represents a normal, nonpathologic finding in Black athletes.35 The Seattle criteria also presented important changes in definitions of pathologic Q waves, TWI, ST-segment depressions, left axis deviation, right axis deviation, ventricular preexcitation, short QT, Brugada syndrome, nonspecific intraventricular conduction delay, and arrhythmias. These criteria significantly improved false-positive rates, reported between 2% and 22% depending on the population studied.9,19,25,26,30,32,36,37
After the publication of the Seattle Criteria, subsequent studies showed that isolated voltage criteria for atrial enlargement and axis deviation correlated poorly with underlying cardiac disorders in asymptomatic athletes.38 This recognition led to publication of the Refined Criteria in 2014, which included a borderline group of ECG patterns (left atrial enlargement, right atrial enlargement, left axis deviation, right axis deviation, right ventricular hypertrophy, Black athlete repolarization pattern), whereby the presence of 2 or more borderline ECG findings warranted further evaluation.9 With this change, the Refined Criteria once again lowered the reported false-positive rate to 3% to 16%.9,25,30,39
The International Criteria are the most recent iteration of ECG interpretation guidelines in athletes and were created in 2017 by an international panel of experts in cardiology and sports medicine.11 Notable changes in the International Criteria included a change in the definition for pathologic Q waves, recognition of juvenile TWI in V1 to V3 as a normal finding in athletes aged younger than 16 years, and addition of epsilon waves and TWI 1 mm or greater in V5 or V6 alone to the “abnormal” category.40 Multiple large-scale screening studies have assessed the diagnostic accuracy of the International Criteria and have reported lower false-positive rates ranging from 1.3% to 6.8%.25,26,41-43 Notably, one of the higher reported false-positive rates (6.8%) was reported in a 2019 study, by McClean and colleagues, which included 1304 Arab and Black athletes in Qatar, highlighting the need for ongoing study to better refine ECG criteria across diverse populations.26 In contrast, Hyde and colleagues reported a false-positive rate of only 1.3% among 5258 college athletes with application of the International Criteria by sports cardiology experts.41 Several subsequent studies have been performed in unique demographic populations and sporting disciplines to further characterize the utility of the International Criteria in specific athlete cohorts and have identified populations where the ECG criteria perform well and others that may require further refinement.22,25,26,41-73
COMMON PITFALLS
Although the publication of the International Criteria has significantly reduced false-positive rates for ECG interpretation in athletes, there are still ECG patterns that are frequently misclassified by clinicians, particularly those without experience in the interpretation of athlete ECGs. Multiple previous studies have shown that physicians with limited experience in ECG interpretation in athletes will incorrectly classify a large proportion of normal ECGs in athletes as abnormal,74-77 which can lead to downstream costs from secondary testing and unnecessary sport restriction and psychosocial burden on athletes.77 However, when physicians are instructed to use a standardized ECG interpretation tool, there is improved accuracy.60,75
In studies which have compared local ECG interpretation to an expert overread, ECG findings that are commonly misinterpreted by local providers as abnormal include: LVH (left ventricular hypertrophy), nonpathologic TWI, isolated axis deviation, IVCD less than 140 milliseconds, RBBB in isolation, misinterpreted accessory pathway, nonpathologic rhythm variants, PVCs (<2 per strip), first-degree AV block, and J-waves.45,47 In contrast, ECG findings classified as normal by local providers but readjudicated as abnormal by expert overread include: pathologic TWI, biatrial enlargement, pathologic Q waves, pathologic ST-segment depressions, and atrial tachyarrhythmias.45,47 These studies highlight the importance of continual medical education for clinicians using ECG in the cardiovascular care of athletes. This education can occur via in-person educational courses with content experts, online training courses (https://uwsportscardiology.org/e-academy/), or other educational materials. An overview of commonly misclassified ECG abnormalities using the International Criteria for ECG interpretation is presented in Table 1. Examples of ECGs commonly misclassified are presented in Figs. 1-4.
Table 1.
Common pitfalls of electrocardiogram interpretation in athletes using the International Criteria
| ECG Abnormality | Common Pitfalls |
|---|---|
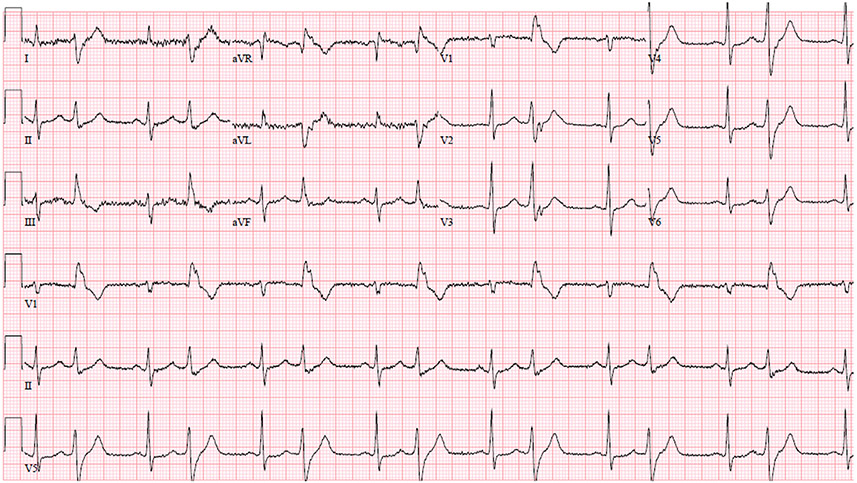
| Inferior TWI | Classified as abnormal with TWI in lead III plus aVF or lead II. Per the International Criteria, TWI in lead III is not considered abnormal; thus, abnormal inferior TWI requires TWI in both lead II and aVF (Fig. 1) |
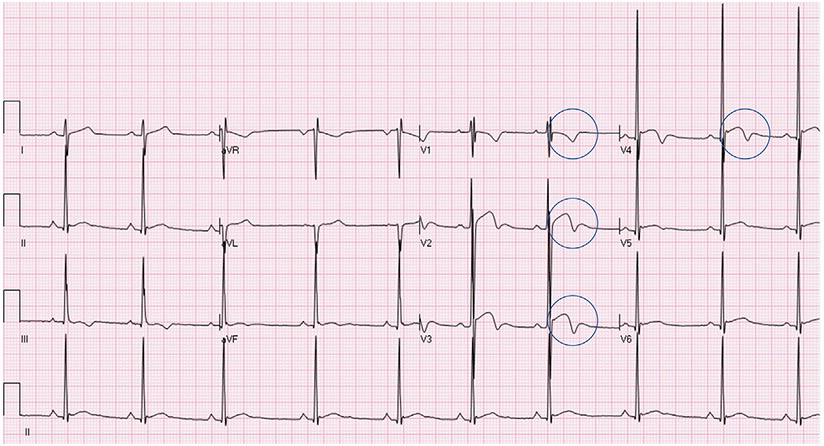
| Black athlete repolarization pattern | ECGs are classified as abnormal, which have a physiologic Black athlete repolarization pattern consisting of J-point elevation with convex ST-segment elevation and TWI confined to V1–V4 (Fig. 2). Extension of TWI into V5 is an abnormal finding and not part of the Black athlete repolarization pattern |
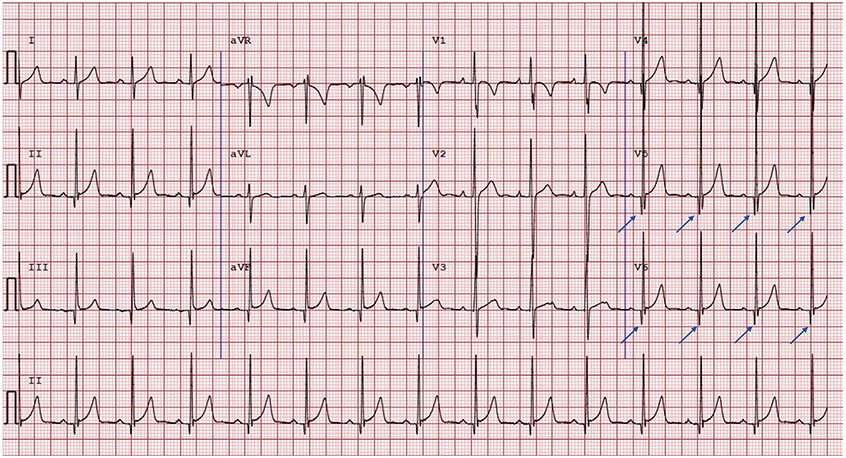
| Pathologic Q waves | Classified as abnormal when the Q wave is long and thin but does not meet newer criteria including a Q/R > 0.25 or Q wave >40 ms duration (Fig. 3) |
| Borderline ECG findings | Athletes with 1 borderline ECG finding (eg, axis deviation, atrial enlargement, or complete RBBB) are flagged as abnormal when the International Criteria requires 2 borderline findings (Fig. 4) |
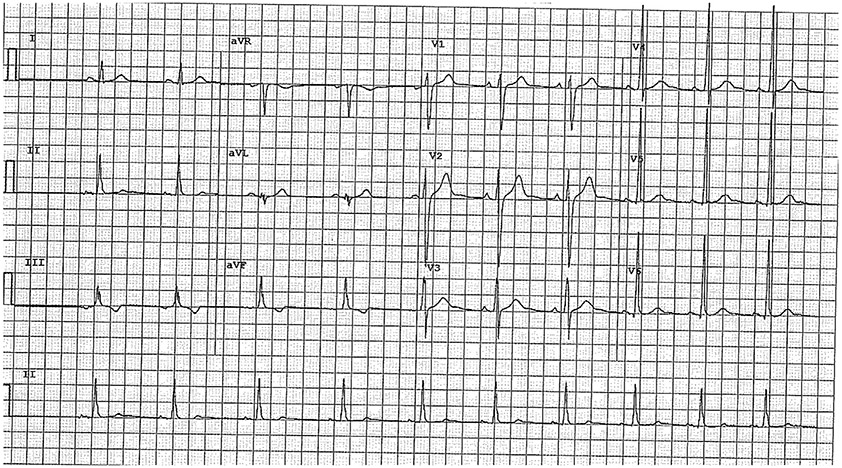
Fig. 1.
Common Pitfalls—Inferior TWIs. Example of an ECG with isolated inferior TWIs in leads III and aVF. This ECG is considered normal per the International criteria. Given TWI in III is considered normal, inferior TWI need to be present in both II and aVF to be considered abnormal.
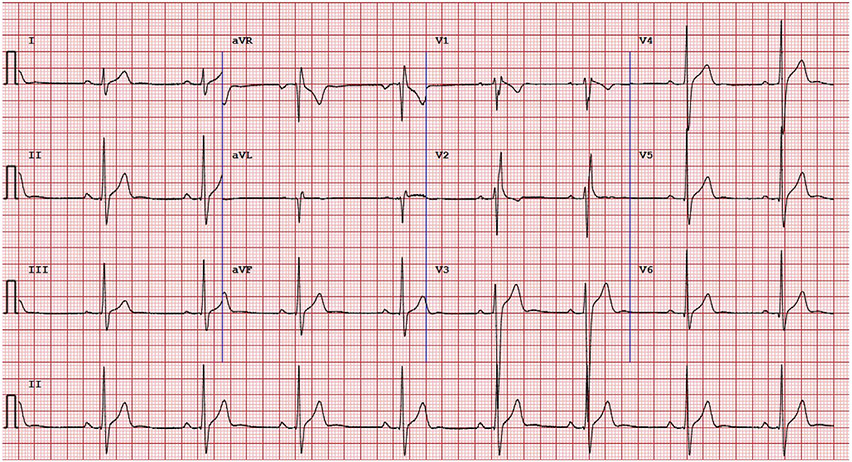
Fig. 4.
Common Pitfalls—Isolated right bundle branch block. Example of ECG with isolated complete right bundle branch block. QRS axis does not meet International Criteria threshold for right axis deviation (≥120°). This ECG would be considered as normal per the International Criteria given that only one borderline criteria is present and there are no other abnormal findings.
FUTURE DIRECTIONS
Although the International Criteria outperform all previous ECG interpretation standards in athletes, there is scope for improvement as new evidence emerges (Table 2). Ideally, ECG interpretation criteria would be individualized for the demographic and sport of each athlete. However, making the criteria too complex also limits the ease of use and application in everyday practice. Therefore, trade-offs are needed that maximize sensitivity/specificity in unique populations but also maintain user-friendly criteria, particularly when applied outside of expert centers. In the following sections, we present future considerations for subsequent ECG interpretation standards.
Table 2.
Future directions of electrocardiogram interpretation in athletes
| ECG Parameters | Future Considerations |
|---|---|
| Age/sex/geographic origin | Optimization of ECG criteria in diverse populations |
| PVC morphology | Consideration of the frequency of PVCs in conjunction with PVC morphology as “benign” or “malignant” (eg, 1 malignant PVC vs multiple benign PVCs warrants additional investigation) |
| Low QRS voltage | Consideration of adding low QRS voltage criteria given association with ARVC, myocarditis, nonischemic LV scar, and infiltrative myocardial diseases |
| QRS fragmentation | Consideration of QRS fragmentation as a potential borderline finding because this parameter has been associated with multiple pathologic cardiovascular conditions in the general population |
| ST-segment depression morphology | Consideration of ST-segment depression morphology (eg, horizontal or downsloping [but not upsloping] warrants additional investigation) |
| Borderline ECG findings | Further understanding on which combination of findings predict underlying pathologic condition |
| Beat-to-beat variation | Guidance on how to interpret abnormal ECG findings if only present in a subset of beats in any specific lead (eg, abnormal if >50% of beats) |
Race/Ethnicity/Geographic Origin-Specific Electrocardiogram Criteria
The first race-specific ECG criterion was the Black athlete repolarization pattern (convex/“domed” ST elevations followed by TWI in V1–V4) included in the 2013 Seattle Criteria, given the absence of pathologic findings in studies performing comprehensive cardiovascular testing in athletes with this pattern.11,35 Although the recognition of this pattern reduced false-positive rates, the use of race to delineate all Black athletes has recently been challenged. In a study by Riding and colleagues involving 1698 mixed sport athletes, the authors observed significant differences in benign TWI patterns (V1–V4) in Black athletes based on geographic origin (Middle African 11.8%, West African 5.3%, African-American/Caribbean 2.4%, East African 1.5%, North African 0%).72 The authors conclude that because there is heterogeneity in the prevalence of benign TWI patterns between the athlete cohorts, larger subgroups based on geographic origin should be studied before it is concluded that this repolarization pattern can be generalized to all Black athletes.
This repolarization pattern has also been characterized in non-Black athletes. Calore and colleagues compared anterior TWI in 80 athletes (66% Black) to 153 patients with hypertrophic or arrhythmogenic cardiomyopathy.78 Cardiomyopathy was completely excluded in athletes with a combination of J-point elevation 1 mm or greater and TWI not extending beyond V4, regardless of race. These findings require additional investigation in larger cohorts of athletes with different race/ethnicity and geographic origin.
Since the publication of the International Criteria in 2017, multiple subsequent studies assessed these criteria in populations of athletes from all over the world including the United States,41,42,44-49 the United Kingdom,22,25,50-52 the Netherlands,53 Macedonia,54 Poland,55 Italy,43,56-58 Switzerland,43,59,60 China,61 Malaysia,62-64 Pacific Islands,65,66 Ecuador,67 Argentina,68 Qatar,26,72,79 Ghana,69,73 Cameroon,70 and Nigeria.71 Within these, rates of abnormal ECGs based on the International Criteria have varied widely. Athlete cohorts with high rates of abnormal ECGs have included Ghanian male soccer players (23.3%),73 Malaysian male soccer players (20%),64 United States National Basketball Association male players (15.6%),46 Cameroonian male ultramarathoners (13.6%),70 Middle African male athletes from the Qatar Olympic Committee (11.9%),72 United States national team female soccer players (11.5%),44 and Caucasian male professional cyclists (9.3%).59 In contrast, populations with low rates of abnormal ECGs include US collegiate athletes (1.5%–2.1%)41,42,45,48 and UK soccer players (1.8%).25
Given this persistent heterogeneity, it is clear that the generation of high-quality data from a diverse collection of source populations is required, with an emphasis on data defining not only race but geographic origin when possible. Such data will allow future iterations of ECG interpretation criteria to more explicitly consider race/ethnicity and geographic origins in the creation of specific recommendations.
Age-Specific Electrocardiogram Criteria
Previous iterations of ECG screening criteria have been developed for the screening of asymptomatic athletes aged older than 12 years and younger than 35 years.8,11 More research is needed to understand if the International Criteria can be applied to younger athletes aged less than 12 years or if specific modifications of the criteria are needed. Preliminary studies have shown that these ECG screening criteria may also be effective in Master’s athletes (age >35 years).53 As the International Criteria have been specifically curated for athletes aged 35 years or younger, extrapolation to older populations requires additional study and consideration.
Sex-Specific Electrocardiogram Criteria
Although it is well established that female athletes have different ECG features compared with male athletes,80 the only sex-specific recommendation in the International Criteria pertains to outpoints for an abnormal corrected QT segment (QTc), defined in female athletes as 480 milliseconds or greater and in male athletes as 470 milliseconds or greater. Of specific interest in the screening setting is that female athletes, especially female endurance athletes, frequently have a higher percentage of anterior TWI (V1–V3). In studies assessing sex-based differences in ECG patterns, the prevalence of anterior TWI in female athletes ranges from 2% to 9%,25,44,81-83 and anterior TWI in this population are unlikely to represent underlying cardiac pathologic condition.25,44,83 Therefore, the presence of anterior TWI in female athletes may be a normal finding not warranting additional investigation. It has also been speculated that ECG lead placement may differ between male and female athletes due to the presence of breast tissue, particularly in the setting of large screening events in which ECGs are often not performed in completely private environments. Added consideration of sex differences in the interpretation of anterior TWI thus represents an important focus for future research.
Low QRS Voltage
Low QRS voltage is typically defined as a QRS amplitude of less than 0.5 mV in all 6 limb leads or less than 1 mV in the precordial leads.84 Other definitions including the total sum of limb leads less than 3.0 mV have also been used. However, low voltage should also be considered in those with significant interval decreases in QRS voltage on 2 consecutive ECGs, in which case, the difference may suggest interval development of a pathologic condition. Although low voltage can be secondary to many cardiac and noncardiac causes (eg, obesity, emphysema), in competitive athletes important causes of SCD, which may demonstrate low QRS voltage, include arrhythmogenic right ventricular cardiomyopathy (ARVC),85 myocarditis,86 nonischemic LV scar, and infiltrative cardiac diseases. These conditions have increased electrical impedance where replacement fibrosis and the loss of electrically active myocardial mass lead to low QRS voltages.
Recent studies have suggested that a low voltage QRS can help differentiate ARVC from electrocardiographic remodeling in athletes.87,88 In a study by Brosnan and colleagues of 100 healthy athletes matched with 100 ARVC patients both with TWI in at least 2 anterior ECG leads (V1-V4), the ARVC patients had a greater prevalence of low voltage in the limb leads (21% vs 1 %, P < .001), as well as more frequent precordial TWI beyond V3 (34% vs 8%, P < .001), inferior TWI (31% vs 3%, P < .001), and PVCs (18% vs 0%, P < .001).88 The authors conclude that low QRS voltages may be an additional finding to differentiate healthy athletes from those with ARVC. A subsequent study by Finocchiaro and colleagues replicated this finding, comparing 162 patients with ARVC to 129 young controls with anterior TWI, again demonstrating an increased prevalence of low limb lead QRS voltage in the ARVC patients compared with controls (15% vs 4%, P = .01).87 Among Olympic athletes (n = 516), Mango and colleagues found low QRS amplitude, defined here as either QRS amplitude of less than 0.5 mV in all 6 limb leads or less than 1 mV in the precordial leads, to be present in 4% of athletes but did not find any significant associations with pathologic condition.89 In another recent study, Zorzi and colleagues compared the prevalence of low QRS voltage in the limb leads (all <0.5 mV) between Italian athletes (n = 2229), Black athletes (n = 1115), general population patients (n = 1115), and patients with known arrhythmogenic cardiomyopathy (AC) or nonischemic LV scar (NILVS, n = 58).90 The key finding of this article was a low prevalence of low QRS voltage in athletes compared with the AC and NILVS patients (1.1% vs 12%). In addition, the authors also noted that 2/5 (40%) athletes with low QRS and exercise-induced ventricular arrhythmias were found to have a cardiomyopathy on cardiac MRI (1 AC, 1 NILVS). The authors therefore conclude that low QRS voltage should be considered in future iterations of ECG screening criteria.
Low QRS voltage has also been found in patients with myocarditis in the general population.91 Additional study is required to define the clinical implications of low QRS voltage and to determine whether it merits inclusion in future iterations of ECG interpretation criteria.
QRS Fragmentation
Fragmentation of a narrow QRS is defined as the presence of an additional R wave (R′), notching in the nadir of the S wave or the presence of greater than 1 R′ in 2 contiguous leads (Fig. 5).92 Conversely, fragmentation of a wide complex QRS has been defined as greater than 2 R waves (R″), more than 2 notches in the R wave, or more than 2 notches in the downstroke or upstroke of the S wave.92 Previous studies have shown that QRS fragmentation is associated with and often predicts poor prognosis in many cardiac diseases including chronic coronary artery disease (myocardial scar),93 dilated cardiomyopathy,94 ARVC,95 cardiac sarcoidosis,96 and Brugada syndrome.97 Of specific interest in the athletic populations would be if flagging QRS fragmentation aids in the detection of cardiomyopathies during PPCS.
Fig. 5..
QRS fragmentation. Example of ECG with QRS fragmentation. Red arrows indicate examples of QRS fragmentation.
Although QRS fragmentation has been associated with ARVC, the diagnostic performance of this finding seems to be limited. Notably, this ECG abnormality has not been included in the current or previous diagnostic criteria for ARVC.98,99
Limited studies have assessed the utility of QRS fragmentation in the diagnosis of underlying cardiac disorders in athletes. Recent study by Ollitrault and colleagues demonstrated that QRS fragmentation in V1 (fQRSV1) was more common in athletes than nonathletes (22% vs 5%, P < .001). Within this group, athletes with fQRSV1 (n = 26) showed significant structural differences compared with athletes without fQRSV1 (n = 93), including greater indexed right ventricular outflow tract (RVOT) dimensions, indexed RV basal diameter, tricuspid annular planar systolic excursion, indexed LV end diastolic diameter, and indexed LV mass.100 The authors therefore conclude that fQRSV1 is common among healthy athletes and may be considered a sign of RV remodeling, although this study did not provide any broader clinical or genetic correlation. Although this study suggests QRS fragmentation is common in healthy athletes and may not be a good distinguisher of disease, this study focused on QRS fragmentation in lead V1 only and larger scale studies are needed because earlier studies comparing ECG findings in athletes to patients with ARVC have not reported the presence or absence of QRS fragmentation.87,88 Although QRS fragmentation is easily recognizable, it remains unknown if it would provide additive diagnostic value above and beyond the current criteria.
Premature Ventricular Contractions
The current International Criteria recommend further evaluation for all athletes who have 2 or greater premature ventricular contractions (PVCs) on a 10-second ECG.11 Although PVCs can be a marker of myocardial disease, the chosen cutoff is arbitrary and does not consider PVC morphology, which can be an important diagnostic and prognostic marker.101
The morphology of PVCs can help to identify the anatomic origin of the ectopic beats, which has important implications for the likelihood of underlying cardiovascular disease. In athletes, infundibular right ventricular outflow tract and left ventricular outflow tract (RVOT and LVOT) and fascicular (left anterior and posterior) PVC origins are frequently seen and usually considered to be benign. RVOT PVCs are characterized by an LBBB pattern, inferior axis, and late precordial transition (R/s = 1 after V3; Fig. 6), whereas LVOT PVCs are characterized by an LBBB pattern, inferior axis, and early precordial transition (R/s = 1 by V2 or V3). Fascicular PVCs are characterized by a typical RBBB and QRS duration less than 130 milliseconds (anterior = inferior axis, posterior = superior axis). In contrast, patterns concerning for underlying myocardial disease in athletes include an atypical RBBB with QRS 130 milliseconds or greater (suggestive of mitral valve annulus, papillary muscles, or left ventricular sites of origin; Fig. 7) or an LBBB pattern with superior or intermediate axis (right ventricular free wall, interventricular septum).102
Fig. 6.
RVOT PVCs. Example of ECG with RVOT PVCs. Pertinent features include left bundle branch block pattern, inferior axis, and late precordial transition. RVOTs are broadly considered more likely to have a benign course.
Fig. 7..
Papillary muscle PVCs. Example of ECG with PVCs originating from papillary muscle. Note the atypical right bundle branch block pattern with wide (≥130 milliseconds) QRS. This PVC morphology is more often associated with myocardial disease and increased risk for malignant clinical course.
The prevalence of PVCs in young competitive athletes versus sedentary controls has been evaluated in many studies with mixed results.103-107 Most studies were in small populations and have shown similar overall burden of PVCs in athletes as in control populations.103,104,106 However, in a recent study by Zorzi and colleagues assessing the burden of PVCs and arrhythmias in athletes (n = 288) versus controls (n = 144), the presence of 1 or greater PVC on 24-hour 12-lead ECG monitoring was higher in the athlete cohort (59% vs 40%, P < .0001).107 Although athletes may have a higher prevalence of PVCs depending on the study, studies have consistently shown that frequent or complex ventricular arrhythmias (couplets, triplets, or NSVT) seem rare in young competitive athletes (6%–13%).103-108
Given these collective findings, a limitation in the current International Criteria is that recommendations for additional testing are based on the quantity of PVCs on a 12-lead ECG without consideration of the PVC morphology. An athlete could have 1 PVC from a concerning origin (eg, LBBB with superior or intermediate axis) and not undergo further evaluation for structural heart disease, whereas another athlete with 2 PVCs of outflow tract or fascicular origin may undergo an extensive workup when underlying disease is unlikely. Future iterations of ECG screening criteria should consider the addition of PVC morphology in some form, and research should assess the combined diagnostic effect of PVC morphology and PVC burden on the surface ECG in the PPCS setting.
ST-Segment Depressions
Current International Criteria recommendations consider an ECG abnormal if there are ST-segment depressions 0.5 mm or greater in 2 or more contiguous leads.11 ST-depressions are frequently a marker of underlying myocardial disease and can be found in conditions leading to SCD in young competitive athletes such as hypertrophic cardiomyopathy.109,110 However, the current guidelines do not specifically comment on ST-segment depression morphology (eg, upsloping, horizontal, or downsloping) as a component of this assessment, likely because prior studies in athletes have focused on the presence or absence of ST-segment depressions and have not reported the morphology of the ST changes in detail. Given that ST-segment depressions are generally considered abnormal and possibly associated with pathologic condition in the general population when they are horizontal or downsloping, research is needed to determine if upsloping ST-segment depressions among athletes truly warrants more evaluation or if it could be considered a normal or borderline finding.
Borderline Findings
The International Criteria currently includes a section of “borderline” ECG abnormalities (left atrial enlargement, right atrial enlargement, left axis deviation, right axis deviation, complete RBBB) where 2 or more abnormalities in this category are needed to warrant further testing.11 The borderline category was created to account for the findings of the seminal study by Gati and colleagues, which demonstrated that athletes with isolated axis deviation or atrial enlargement (n = 579) did not have any major structural or functional abnormalities on TTE.38 Complete RBBB patterns are also included in the borderline category on the basis of a study of 510 US athletes, which found 2.5% (n = 13) to have a complete RBBB, all of whom were free of pathologic structural heart disease.111 The authors of this study also subsequently assessed the association of RBBB with pathologic condition from previous athlete studies and found no reported cardiac pathologic condition among asymptomatic athletes with complete RBBB.112
Although this group of borderline findings has been a major driver in reducing false-positive interpretations, considerable uncertainty remains regarding whether specific combinations of borderline ECG abnormalities are associated with high-risk conditions or whether certain combinations may actually be considered normal findings. As such, additional research adding granularity to athletes with 2 or more borderline ECG findings would be valuable.
Beat-to-Beat Variation
Although the International Criteria provide a framework for normal and abnormal ECG findings in athletes, a frequently encountered issue not covered in the text is how to handle beat-to-beat variation. When interpreting an athlete’s ECG, it is very common that there may be 2 to 3 beats available for each lead that is not included in the rhythm strip. Interpretation of abnormal findings can be difficult if an abnormality is visualized in a subset of the beats available in any specific lead (eg, TWI meets criteria in 1/3 or 2/3 beats). Guidance on how to interpret beat-to-beat variation would be valuable in future iterations of ECG interpretation guidelines. For instance, greater than 50% of the beats in a given lead might be required to define an abnormality as “present.” Although published research on this topic is lacking, it seems likely that the proportion of beats with a given finding will have implications for test sensitivity and specificity. For example, if TWI is only required in 1/3 beats to be considered abnormal as opposed to 2/3 or 3/3 beats, this definition may be more sensitive but likely less specific.
SUMMARY
Criteria for the evaluation of the athlete’s ECG have evolved considerably during the past 20 years because the scientific understanding of physiologic versus pathologic findings has expanded. With ongoing refinement, metrics of test performance have markedly improved. Nevertheless, important challenges and pitfalls to the application and interpretation of these criteria remain with several important areas of future research identified to fill existing knowledge gaps. Ongoing efforts are required to further refine ECG interpretation standards in athletes.
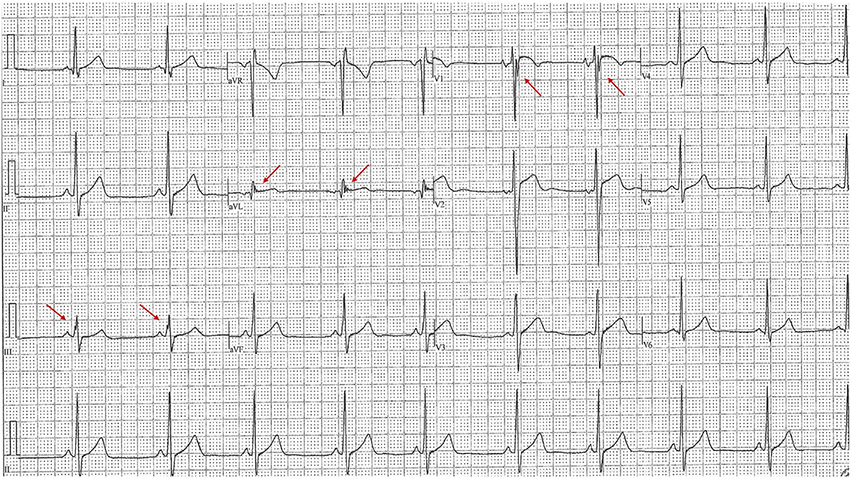
Fig. 2.
Common Pitfalls—Black athlete repolarization pattern. Example of an ECG with a Black athlete repolarization pattern (J point elevation with convex ST elevation and TWIs confined to V1–V4—denoted here with blue circles). This ECG is considered normal per the International Criteria. TWIs extending into V5 are always considered abnormal.
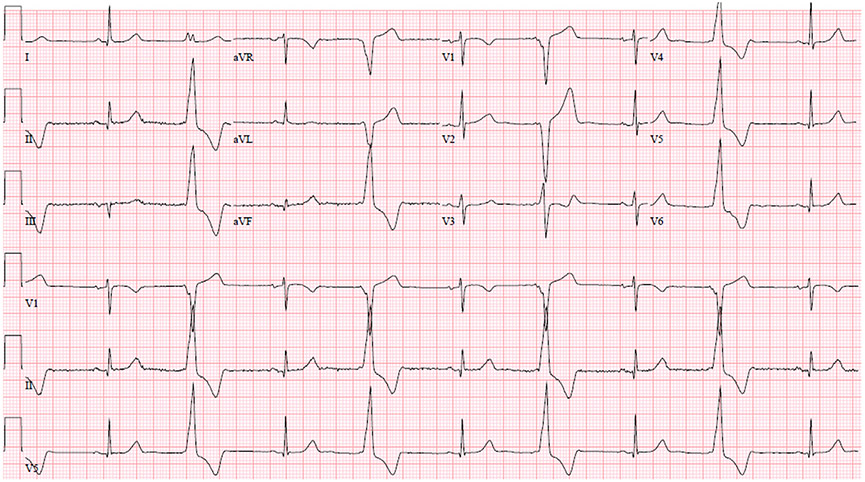
Fig. 3.
Common Pitfalls—Nonpathologic Q waves. Example of an ECG with Q waves greater than 3 mm in the lateral leads (blue arrows). This ECG is normal per the International Criteria, given the International Criteria requires Q/R ratio of 0.25 or greater or q of 40 milliseconds or greater in 2 or more leads (excluding III and aVR).
CLINICS CARE POINTS.
The International Criteria for ECG interpretation in athletes have improved specificity of ECG interpretation in this population. These can be used as a reference at the point of care by clinicians evaluating athlete ECGs.
Common pitfalls in athlete ECG interpretation that the clinician should be aware of include identification of pathologic inferior T-wave inversions and pathologic Q waves, recognition of the black athlete repolarization pattern, and correct application of the criteria’s ‘borderline’ finding category.
Normative ECG data specific to the sport and population in question are required, and caution should be exercised applying data from different populations and athletic contexts.
Other areas requiring additional research include the significance of low QRS voltage and QRS fragmentation, greater granularity regarding the implications of specific PVC morphologies, and more specific guidance regarding beat-to-beat variation in ECG findings.
KEY POINTS.
The International Criteria for electrocardiogram (ECG) interpretation is the current standard of care for preparticipation ECG screening in young competitive athletes.
Common pitfalls using the International Criteria include incorrect interpretation of inferior T-wave inversions, black athlete repolarization patterns, pathologic Q waves, and borderline ECG criteria.
Future directions to consider for new ECG screening criteria include expanding age/sex/geographic origin-specific ECG changes, addressing low QRS voltage criteria and QRS fragmentation, defining nuanced preventricular contraction (PVC) and ST-segment depression morphology, and providing interpretation guidance when beat-to-beat variation is present.
Footnotes
DISCLOSURE
The authors report no disclosures.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Ljungqvist A, Jenoure P, Engebretsen L, et al. The international Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes March 2009. Br J Sports Med 2009;43(9):631–43. [DOI] [PubMed] [Google Scholar]
- 2.Mont L, Pelliccia A, Sharma S, et al. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Ep Europace; 2016; 19(1):139–63. [DOI] [PubMed] [Google Scholar]
- 3.Löllgen HBM, Cummiskey J, Bachl N, et al. The pre-participation examination in sports: EFSMA statement on ECG for pre-participation examination. Dtsch Z Sportmed 2015;66:151–5. [Google Scholar]
- 4.Dvorak J, Kramer EB, Schmied CM, et al. The FIFA medical emergency bag and FIFA 11 steps to prevent sudden cardiac death: setting a global standard and promoting consistent football field emergency care. Br J Sports Med 2013;47(18):1199–202. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Levine BD, Washington RL, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66(21):2356–61. [DOI] [PubMed] [Google Scholar]
- 6.Drezner JA, O’connor FG, Harmon KG, et al. AMSSM position statement on cardiovascular pre-participation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med 2017;51(3):153–67. [DOI] [PubMed] [Google Scholar]
- 7.Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 2009;31(2):243–59. [DOI] [PubMed] [Google Scholar]
- 8.Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the ‘Seattle criteria. Br J Sports Med 2013;47(3):122–4. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation 2014;129(16):1637–49. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol: consensus statement of the study group of sport cardiology of the working group of cardiac Rehabilitation and exercise Physiology and the working group of myocardial and Pericardial diseases of the European society of cardiology. Eur Heart J 2005;26(5):516–24. [DOI] [PubMed] [Google Scholar]
- 11.Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017;51(9):704–31. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Biffi A, Basso C, et al. 12-lead ECG in the athlete: physiological versus pathological abnormalities. Br J Sports Med 2009;43(9):669–76. [DOI] [PubMed] [Google Scholar]
- 13.Corrado D, Basso C, Schiavon M, et al. Screening for hypertrophic cardiomyopathy in young athletes. New Engl J Med 1998;339(6):364–9. [DOI] [PubMed] [Google Scholar]
- 14.Baggish AL, Hutter AM, Wang F, et al. Cardiovascular screening in college athletes with and without electrocardiography: a cross-sectional study. Ann Intern Med 2010;152(5):269–75. [DOI] [PubMed] [Google Scholar]
- 15.Magalski A, McCoy M, Zabel M, et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med 2011;124(6):511–8. [DOI] [PubMed] [Google Scholar]
- 16.Le V-V, Wheeler MT, Mandic S, et al. Addition of the electrocardiogram to the preparticipation examination of college athletes. Clin J Sport Med 2010;20(2):98–105. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra R, West JJ, Dent J, et al. Cost and yield of adding electrocardiography to history and physical in screening Division I intercollegiate athletes: a 5-year experience. Heart Rhythm 2011;8(5):721–7. [DOI] [PubMed] [Google Scholar]
- 18.Alattar A, Ghani S, Mahdy N, et al. Pre-participation musculoskeletal and cardiac screening of male athletes in the United Arab Emirates. Transl Med Unisa 2014;9:43. [PMC free article] [PubMed] [Google Scholar]
- 19.Brosnan M, La Gerche A, Kalman J, et al. The Seattle Criteria increase the specificity of preparticipation ECG screening among elite athletes. Br J Sports Med 2014;48(15):1144–50. [DOI] [PubMed] [Google Scholar]
- 20.Chandra N, Bastiaenen R, Papadakis M, et al. Prevalence of electrocardiographic anomalies in young individuals: relevance to a nationwide cardiac screening program. J Am Coll Cardiol 2014;63(19):2028–34. [DOI] [PubMed] [Google Scholar]
- 21.Deligiannis AP, Kouidi EJ, Koutlianos NA, et al. Eighteen years’ experience applying old and current strategies in the pre-participation cardiovascular screening of athletes. Hellenic J Cardiol 2014;55(1):32. [PubMed] [Google Scholar]
- 22.Dhutia H, Malhotra A, Finocchiaro G, et al. Impact of the international recommendations for electrocardiographic interpretation on cardiovascular screening in young athletes. J Am Coll Cardiol 2017;70(6):805–7. [DOI] [PubMed] [Google Scholar]
- 23.Dunn TP, Pickham D, Aggarwal S, et al. Limitations of current AHA guidelines and proposal of new guidelines for the preparticipation examination of athletes. Clin J Sport Med 2015;25(6):472–7. [DOI] [PubMed] [Google Scholar]
- 24.Fudge J, Harmon KG, Owens DS, et al. Cardiovascular screening in adolescents and young adults: a prospective study comparing the Pre-participation Physical Evaluation Monograph 4th Edition and ECG. Br J Sports Med 2014;48(15):1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra A, Dhutia H, Yeo T-J, et al. Accuracy of the 2017 international recommendations for clinicians who interpret adolescent athletes’ ECGs: a cohort study of 11 168 British white and black soccer players. Br J Sports Med 2019;54(12):739–45. [DOI] [PubMed] [Google Scholar]
- 26.McClean G, Riding NR, Pieles G, et al. Diagnostic accuracy and Bayesian analysis of new international ECG recommendations in paediatric athletes. Heart 2019;105(2):152–9. [DOI] [PubMed] [Google Scholar]
- 27.Menafoglio A, Di Valentino M, Segatto J-M, et al. Costs and yield of a 15-month preparticipation cardiovascular examination with ECG in 1070 young athletes in Switzerland: implications for routine ECG screening. Br J Sports Med 2014;48(15):1157–61. [DOI] [PubMed] [Google Scholar]
- 28.Pickham D, Zarafshar S, Sani D, et al. Comparison of three ECG criteria for athlete pre-participation screening. J Electrocardiol 2014;47(6):769–74. [DOI] [PubMed] [Google Scholar]
- 29.Price DE, McWilliams A, Asif IM, et al. Electrocardiography-inclusive screening strategies for detection of cardiovascular abnormalities in high school athletes. Heart Rhythm 2014;11(3):442–9. [DOI] [PubMed] [Google Scholar]
- 30.Riding NR, Sheikh N, Adamuz C, et al. Comparison of three current sets of electrocardiographic interpretation criteria for use in screening athletes. Heart 2015;101(5):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snoek JA, Jongman JK, Brandon T, et al. Performance of the Lausanne questionnaire and the 2010 European Society of Cardiology criteria for ECG interpretation in athletes. Eur J Prev Cardiol 2015;22(3):397–405. [DOI] [PubMed] [Google Scholar]
- 32.Wasfy MM, DeLuca J, Wang F, et al. ECG findings in competitive rowers: normative data and the prevalence of abnormalities using contemporary screening recommendations. Br J Sports Med 2015;49(3):200–6. [DOI] [PubMed] [Google Scholar]
- 33.Weiner RB, Hutter AM, Wang F, et al. Performance of the 2010 European Society of Cardiology criteria for ECG interpretation in athletes. Heart 2011;97(19):1573–7. [DOI] [PubMed] [Google Scholar]
- 34.Wilson MG, Chatard J, Carré F, et al. Prevalence of electrocardiographic abnormalities in West-Asian and African male athletes. Br J Sports Med 2012;46(5):341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J 2011;32(18):2304–13. [DOI] [PubMed] [Google Scholar]
- 36.Drezner JA, Owens DS, Prutkin JM, et al. Electrocardiographic screening in national collegiate athletic association athletes. Am J Cardiol 2016;118(5):754–9. [DOI] [PubMed] [Google Scholar]
- 37.Williams EA, Pelto HF, Toresdahl BG, et al. Performance of the American heart association (AHA) 14-point evaluation versus electrocardiography for the cardiovascular screening of high school athletes: a prospective study. J Am Heart Assoc 2019;8(14):e012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gati S, Sheikh N, Ghani S, et al. Should axis deviation or atrial enlargement be categorised as abnormal in young athletes? The athlete’s electrocardiogram: time for re-appraisal of markers of pathology. Eur Heart J 2013;34(47):3641–8. [DOI] [PubMed] [Google Scholar]
- 39.Fuller C, Scott C, Hug-English C, et al. Five-year experience with screening electrocardiograms in national collegiate athletic association Division I athletes. Clin J Sport Med 2016;26(5):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drezner JA. 18 highlights from the International Criteria for ECG interpretation in athletes. In: BMJ publishing group Ltd and British association of sport and exercise medicine. 2019. [DOI] [PubMed] [Google Scholar]
- 41.Hyde N, Prutkin JM, Drezner JA. Electrocardiogram interpretation in NCAA athletes: comparison of the ‘Seattle’and ‘International’criteria. J Electrocardiol 2019;56:81–4. [DOI] [PubMed] [Google Scholar]
- 42.Conway JJ, Krystofiak J, Quirolgico K, et al. Evaluation of a preparticipation cardiovascular screening program among 1,686 national collegiate athletic association Division I athletes: comparison of the Seattle, refined, and international electrocardiogram screening criteria. Clin J Sport Med 2020;32(3):306–12. [DOI] [PubMed] [Google Scholar]
- 43.Halasz G, Cattaneo M, Piepoli M, et al. Pediatric athletes’ ECG and diagnostic performance of contemporary ECG interpretation criteria. Int J Cardiol 2021;335:40–6. [DOI] [PubMed] [Google Scholar]
- 44.Churchill TW, Petek BJ, Wasfy MM, et al. Cardiac structure and function in elite female and male soccer players. JAMA Cardiol 2021;6(3):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petek BJ, Drezner JA, Prutkin JM, et al. Electrocardiogram interpretation in college athletes: local institution versus sports cardiology center interpretation. J Electrocardiol 2020;62:49–56. [DOI] [PubMed] [Google Scholar]
- 46.Waase MP, Mutharasan RK, Whang W, et al. Electrocardiographic findings in national Basketball association athletes. JAMA Cardiol 2018;3(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss M, Rao P, Johnson D, et al. Physician adherence to ‘Seattle’and ‘International’ECG criteria in adolescent athletes: an analysis of compliance by specialty, experience, and practice environment. J Electrocardiol 2020;60:98–101. [DOI] [PubMed] [Google Scholar]
- 48.Rambarat CA, Reifsteck F, Clugston JR, et al. Pre-participation cardiac evaluation findings in a cohort of collegiate female athletes. Am J Cardiol 2021;140:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas JA, Perez-Alday EA, Junell A, et al. Vector-cardiogram in athletes: the Sun valley Ski study. Ann Noninvasive Electrocardiol 2019;24(3):e12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown B, Millar L, Somauroo J, et al. Left ventricular remodeling in elite and sub-elite road cyclists. Scand J Med Sci Sports 2020;30(7):1132–9. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra A, Oxborough D, Rao P, et al. Defining the normal Spectrum of electrocardiographic and left ventricular Adaptations in mixed-race male adolescent soccer players. Circulation 2021;143(1):94–6. [DOI] [PubMed] [Google Scholar]
- 52.Morrison B, Mohammad A, Oxborough D, et al. The 12-lead electrocardiogram of the elite female footballer as defined by different interpretation criteria across the competitive season. Eur J Sport Sci 2021;1–24 (just-accepted). [DOI] [PubMed] [Google Scholar]
- 53.Panhuyzen-Goedkoop NM, Wellens HJ, Verbeek AL, et al. ECG criteria for the detection of high-risk cardiovascular conditions in master athletes. Eur J Prev Cardiol 2020;27(14):1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karagjozova I, Petrovska S, Nikolic S, et al. Frequency of electrocardiographic changes in trained athletes in the Republic of Macedonia. Open access Macedonian J Med Sci 2017;5(6):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakubiak AA, Konopka M, Bursa D, et al. Benefits and limitations of electrocardiographic and echocardiographic screening in top level endurance athletes. Biol Sport 2021;38(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zorzi A, Calore C, Vio R, et al. Accuracy of the ECG for differential diagnosis between hypertrophic cardiomyopathy and athlete’s heart: comparison between the European Society of Cardiology (2010) and International (2017) criteria. Br J Sports Med 2018;52(10):667–73. [DOI] [PubMed] [Google Scholar]
- 57.Calò L, Martino A, Tranchita E, et al. Electrocardiographic and echocardiographic evaluation of a large cohort of peri-pubertal soccer players during pre-participation screening. Eur J Prev Cardiol 2019;26(13):1444–55. [DOI] [PubMed] [Google Scholar]
- 58.Vessella T, Zorzi A, Merlo L, et al. The Italian pre-participation evaluation programme: diagnostic yield, rate of disqualification and cost analysis. Br J Sports Med 2020;54(4):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beale AL, Julliard MV, Maziarski P, et al. Electrocardiographic findings in elite professional cyclists: the 2017 international recommendations in practice. J Sci Med Sport 2019;22(4):380–4. [DOI] [PubMed] [Google Scholar]
- 60.Schneiter S, Trachsel LD, Perrin T, et al. Inter-observer agreement in athletes ECG interpretation using the recent international recommendations for ECG interpretation in athletes among observers with different levels of expertise. PLOS ONE 2018;13(11):e0206072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen X, Huang Y-m, Shen T-H, et al. Prevalence of abnormal and borderline electrocardiogram changes in 13, 079 Chinese amateur marathon runners. BMC Sports Sci Med Rehabil 2021;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aziz MA, Hanifah RA. Characteristics OF RESTING ECG among SABAH professional male FOOTBALLERS. Malaysian J Move Health Exerc 2021;10(1). [Google Scholar]
- 63.Lim ZL, Mokhtar AH, Jaffar MR. Pre-participation evaluation of Malaysian university athletes–the importance of cardiovascular screening. Malaysian J Move Health Exerc 2017;6(2). [Google Scholar]
- 64.Ariffin F, Khir RN, Mohamed-Yassin M-S, et al. Identifying electrocardiogram pattern changes and Their association with echocardiography among Malaysian Footballers Attending pre-participation evaluation. J Clin Health Sci 2020;5(1):49–59. [Google Scholar]
- 65.Chatard J-C, Espinosa F, Donnadieu R, et al. Pre-participation cardiovascular evaluation in Pacific Island athletes. Int J Cardiol 2019;278:273–9. [DOI] [PubMed] [Google Scholar]
- 66.Johnson C, Forsythe L, Somauroo J, et al. Cardiac structure and function in elite native Plawaiian and Pacific Islander Rugby football League athletes: an exploratory study. Int J Cardiovasc Imaging 2018;34(5):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medrano Plana Y, Castillo Marcillo ÁR, Lugo Morales AM, et al. Alteraciones electrocardiográficas en jóvenes atletas de alto rendimiento. CorSalud 2019;11(4):296–301. [Google Scholar]
- 68.Ramognino F, Ferraro F, BLUMBERG ES, et al. Hallazgos electrocardiográficos anormales en deportistas amateur: comparación de los criterios de Seattle 2013 y 2017. Revista Argentina de Cardiología 2019;87(2):146–51. [Google Scholar]
- 69.Pambo P, Adu-Adadey M, Ankrah PT, et al. Electrocardiographic and echocardiographic findings in Ghanaian female soccer players. Clin J Sport Med 2020;31(6):e367–72. [DOI] [PubMed] [Google Scholar]
- 70.Gassina L-G, Jerson MN, Guessogo WR, et al. Electrocardiographic CHARACTERISTICS OF athletes OF MOUNT Cameroon ASCENT: prevention OF sudden death. Eur J Phys Education Sport Sci 2019;. https://oapub.org/edu/index.php/ejep/article/view/2243. Accessed 6 September 2022. [Google Scholar]
- 71.Sokunbi OJ, Okoromah CA, Ekure EN, et al. Electrocardiographic pattern of apparently healthy African adolescent athletes in Nigeria. BMC Pediatr 2021;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riding NR, Sharma S, McClean G, et al. Impact of geographical origin upon the electrical and structural manifestations of the black athlete’s heart. Eur Heart J 2019;40(1):50–8. [DOI] [PubMed] [Google Scholar]
- 73.Pambo P, Adu-Adadey M, Agbodzakey H, et al. Electrocardiographic and echocardiographic findings in elite Ghanaian male soccer players. Clin J Sport Med 2019;31(6):e373–9. [DOI] [PubMed] [Google Scholar]
- 74.Brosnan M, La Gerche A, Kumar S, et al. Modest agreement in ECG interpretation limits the application of ECG screening in young athletes. Heart Rhythm 2015;12(1):130–6. [DOI] [PubMed] [Google Scholar]
- 75.Drezner JA, Asif IM, Owens DS, et al. Accuracy of ECG interpretation in competitive athletes: the impact of using standardised ECG criteria. Br J Sports Med 2012;46(5):335–40. [DOI] [PubMed] [Google Scholar]
- 76.Hill AC, Miyake CY, Grady S, et al. Accuracy of interpretation of preparticipation screening electrocardiograms. J Pediatr 2011;159(5):783–8. [DOI] [PubMed] [Google Scholar]
- 77.Dhutia H, Malhotra A, Yeo TJ, et al. Inter-rater reliability and downstream financial implications of electrocardiography screening in young athletes. Circ Cardiovasc Qual Outcomes 2017;10(8):e003306. [DOI] [PubMed] [Google Scholar]
- 78.Calore C, Zorzi A, Sheikh N, et al. Electrocardiographic anterior T-wave inversion in athletes of different ethnicities: differential diagnosis between athlete’s heart and cardiomyopathy. Eur Heart J 2015;37(32):2515–27. [DOI] [PubMed] [Google Scholar]
- 79.McClean G, Riding NR, Pieles G, et al. Prevalence and significance of T-wave inversion in Arab and Black paediatric athletes: should anterior T-wave inversion interpretation be governed by biological or chronological age? Eur J Prev Cardiol 2019;26(6):641–52. [DOI] [PubMed] [Google Scholar]
- 80.Petek BJ, Wasfy MM. Cardiac Adaption to exercise training: the female athlete. Curr Treat Options Cardiovasc Med 2018;20(8):68. [DOI] [PubMed] [Google Scholar]
- 81.Finocchiaro G, Dhutia H, D’Silva A, et al. Effect of sex and sporting discipline on LV Adaptation to exercise. JACC: Cardiovasc Imaging 2017;10(9):965–72. [DOI] [PubMed] [Google Scholar]
- 82.Malhotra A, Dhutia H, Gati S, et al. Anterior T-wave inversion in young white athletes and Nonathletes. Prevalence and Significance 2017;69(1):1–9. [DOI] [PubMed] [Google Scholar]
- 83.D’Ascenzi F, Biella F, Lemme E, et al. Female athlete’s heart: sex effects on electrical and structural remodeling. Circ Cardiovasc Imaging 2020;13(12):e011587. [DOI] [PubMed] [Google Scholar]
- 84.Madias JE. Low QRS voltage and its causes. J Electrocardiol 2008;41(6):498–500. [DOI] [PubMed] [Google Scholar]
- 85.Corrado D, van Tintelen PJ, McKenna WJ, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J 2019;41(14):1414–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34(33):2636–48. [DOI] [PubMed] [Google Scholar]
- 87.Finocchiaro G, Papadakis M, Dhutia H, et al. Electrocardiographic differentiation between ‘benign T-wave inversion’ and arrhythmogenic right ventricular cardiomyopathy. EP Europace 2018;21(2):332–8. [DOI] [PubMed] [Google Scholar]
- 88.Brosnan MJ, Te Riele A, Bosman LP, et al. Electrocardiographic features differentiating arrhythmogenic right ventricular cardiomyopathy from an athlete’s heart. JACC Clin Electrophysiol 2018;4(12):1613–25. [DOI] [PubMed] [Google Scholar]
- 89.Mango F, Caselli S, Luchetti A, et al. Low QRS voltages in Olympic athletes: prevalence and clinical correlates. Eur J Prev Cardiol 2020;27(14):1542–8. [DOI] [PubMed] [Google Scholar]
- 90.Zorzi A, Bettella N, Tatangelo M, et al. Prevalence and clinical significance of isolated low QRS voltages in young athletes. Europace 2022;euab330. 10.1093/europace/euab330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial Inflammation: expert recommendations. J Am Coll Cardiol 2018;72(24):3158–76. [DOI] [PubMed] [Google Scholar]
- 92.Pietrasik G, Zaręba W. QRS fragmentation: diagnostic and prognostic significance. Cardiol J 2012;19(2):114–21. [DOI] [PubMed] [Google Scholar]
- 93.Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG. Circ Arrhythmia Electrophysiol 2008;1(4):258–68. [DOI] [PubMed] [Google Scholar]
- 94.Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart rhythm 2010;7(1):74–80. [DOI] [PubMed] [Google Scholar]
- 95.Peters S, Trümmel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia–cardiomyopathy. Heart Rhythm 2008;5(10):1417–21. [DOI] [PubMed] [Google Scholar]
- 96.Homsi M, Alsayed L, Safadi B, et al. Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol 2009;14(4):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008;118(17):1697–704. [DOI] [PubMed] [Google Scholar]
- 98.Corrado D, Perazzolo Marra M, Zorzi A, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–14. [DOI] [PubMed] [Google Scholar]
- 99.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J 2010;31(7):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ollitrault P, Pellissier A, Champ-Rigot L, et al. Prevalence and significance of fragmented QRS complex in lead V1 on the surface electrocardiogram of healthy athletes. EP Europace 2020;22(4):649–56. [DOI] [PubMed] [Google Scholar]
- 101.Zorzi A, Vio R, Bettella N, et al. Criteria for interpretation of the athlete’s ECG: a critical appraisal. Pacing Clin Electrophysiol 2020;43(8):882–90. [DOI] [PubMed] [Google Scholar]
- 102.Corrado D, Drezner JA, D’Ascenzi F, et al. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54(19):1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palatini P, Maraglino G, Sperti G, et al. Prevalence and possible mechanisms of ventricular arrhythmias in athletes. Am Heart J 1985;110(3):560–7. [DOI] [PubMed] [Google Scholar]
- 104.Talan DA, Bauernfeind RA, Ashley WW, et al. Twenty-four hour continuous ECG recordings in long-distance runners. Chest 1982;82(1):19–24. [DOI] [PubMed] [Google Scholar]
- 105.Viitasalo M, Kala R, Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Heart 1982;47(3):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Viitasalo M, Kala R, Eisalo A. Ambulatory electrocardiographic findings in young athletes between 14 and 16 years of age. Eur Heart J 1984;5(1):2–6. [DOI] [PubMed] [Google Scholar]
- 107.Zorzi A, De Lazzari M, Mastella G, et al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc 2018;7(12):e009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pilcher GF, Cook AJ, Johnston BL, et al. Twenty-four-hour continuous electrocardiography during exercise and free activity in 80 apparently healthy runners. Am J Cardiol 1983;52(7):859–61. [DOI] [PubMed] [Google Scholar]
- 109.Haghjoo M, Mohammadzadeh S, Taherpour M, et al. ST-segment depression as a risk factor in hypertrophic cardiomyopathy. EP Europace 2009;11(5):643–9. [DOI] [PubMed] [Google Scholar]
- 110.Maron BJ, Wolfson JK, Ciró E, et al. Relation of electrocardiographic abnormalities and patterns of left ventricular hypertrophy identified by 2-dimensional echocardiography in patients with hypertrophic cardiomyopathy. Am J Cardiol 1983;51(1):189–94. [DOI] [PubMed] [Google Scholar]
- 111.Kim JH, Noseworthy PA, McCarty D, et al. Significance of electrocardiographic right bundle branch block in trained athletes. Am J Cardiol 2011;107(7):1083–9. [DOI] [PubMed] [Google Scholar]
- 112.Kim JH, Baggish AL. Electrocardiographic right and left bundle branch block patterns in athletes: prevalence, pathology, and clinical significance. J Electrocardiol 2015;48(3):380–4. [DOI] [PubMed] [Google Scholar]