Abstract
Galactosyl transferase knock-out pig lungs fail rapidly in baboons. Based on previously identified lung xenograft injury mechanisms, additional expression of human complement and coagulation pathway regulatory proteins, anti-inflammatory enzymes and self-recognition receptors, and knock-down of the β4Gal xenoantigen were tested in various combinations. Transient life-supporting GalTKO.hCD46 lung function was consistently observed in association with either hEPCR (n = 15), hTBM (n = 4), or hEPCR. hTFPI (n = 11), but the loss of vascular barrier function in the xenograft and systemic inflammation in the recipient typically occurred within 24 h. Co-expression of hEPCR and hTBM (n = 11) and additionally blocking multiple pro-inflammatory innate and adaptive immune mechanisms was more consistently associated with survival >1 day, with one recipient surviving for 31 days. Combining targeted genetic modifications to the lung xenograft with selective innate and adaptive immune suppression enables prolonged initial life-supporting lung function and extends lung xenograft recipient survival, and illustrates residual barriers and candidate treatment strategies that may enable the clinical application of other organ xenografts.
Keywords: animal models: nonhuman primate, basic (laboratory) research/science, genetics, graft survival, immunobiology, lung transplantation/pulmonology, translational research/science, xenoantibody, xenotransplantation
1 |. INTRODUCTION
Substantial progress has recently been reported in the preclinical kidney1 and heart xenograft models.2 In contrast, relatively formidable biologic barriers currently prevent similarly successful preclinical lung xenotransplantation.3,4 Our results in an ex vivo lung perfusion model using human blood reveal that lungs from pigs genetically modified to lack Gal-α1–3Galactose (GalTKO) and to express human CD46 (hCD46) are incompletely protected from rapid lung injury,5,6 with lung failure manifested as a rapid rise in pulmonary vascular resistance (PVR), often accompanied loss of vascular barrier function. Lungs with additional expression of human thrombomodulin (hTBM)7,8 or endothelial protein C receptor (hEPCR)9 are incompletely protected from injury during perfusion with human blood, with modest improvement in the proportion of lungs surviving beyond 4 h with hTBM, and consistent prolongation of graft survival with hEPCR. Similarly, additional knockout of the cytidine monophosphate-Neu5Ac hydroxylase gene (which encodes the enzyme that converts Neu5Ac to Neu5Gc: Neu5GcKO) alone10 or with additional β4 galactosyl transferase gene knockout (β4GalKO: “triple knock-out,” TKO),11 delays lung xenograft injury in the ex vivo perfusion model. Additional genetic modifications to activate anti-inflammatory self-recognition receptors (hCD47,12,13 HLA-E14), modulate dysregulated coagulation (human tissue factor pathway inhibitor: hTFPI,15 hCD3916), and dampen inflammation (hemeoxygenase-1: HO-117) have also been introduced.
Here we evaluated lungs from pigs genetically modified based on known physiologic incompatibilities between pigs and humans, and tested in a baboon in vivo lung xenotransplant model in the context of additional mechanism-directed interventions informed by our ex vivo lung perfusion studies.18–20
2 |. MATERIALS AND METHODS
2.1 |. In vivo methods
Single left lung transplantation was performed using previously described procedures and techniques (detailed in Supplemental Materials and Refs. [3,4]). From a consecutive series of 73 procedures (IBN1–6; ILB1–67), three left lung allograft (IBN6, ILB3, 44) and 53 left lung xenografts are reported here (Tables 1 and 2). IBN experiments have been reported previously.3 GalTKO. hCD46 swine, most with additional genetic modifications, were provided for ILB experiments by Revivicor at 10–20 kg (months). Lung phenotypes were evaluated at the gene (pig genotype) and protein level (confirmed pig phenotype) by Revivicor; transgenes for which protein expression was not detected were considered absent (Table 2).
TABLE 1.
Lung phenotypes by group
| Group | Pig phenotypes | n |
|---|---|---|
| Allogeneic | (NA) | 3 |
| Background | GalTKO | 3 |
| GalTKO.hCD46 | 5 | |
| GalTKO.hCD46.hCD47 | 2 | |
| hEPCR | GalTKO.hCD46.hEPCR | 6 |
| GalTKO.hCD46.hCD55.hEPCR | 6 | |
| GalTKO.hCD46.hEPCR.HO-1 | 2 | |
| GalTKO.hCD46.hEPCR.hCD47 | 1 | |
| hTBM | GalTKO.hCD46.hTBM (1 w hCD47) | 4 |
| hEPCR.hTFPI.hCD47.hCD55 | GalTKO.hCD46.hCD55.hEPCR.hTFPI.hCD47 | 11 |
| hEPCR.hTBM/Others | GalTKO.hCD46.hEPCR.hTBM.HO-1 | 3 |
| GalTKO.hCD46.hEPCR.hTBM.hCD47.HO-1 | 3 | |
| GalTKO.hCD46.hCD55.hEPCR.hTBM (3 w hCD39) | 5 | |
| B4GalKD.hEPCR.hTBM (1 w hCD47) | GalTKO.B4GalKD.hCD46.hEPCR.hTBM.hCD47 | 1 |
| GalTKO.B4GalKD.hCD46.hEPCR.hTBM.HO-1 | 1 |
Note: All experiments were grouped according to the genetic background of the used organ donor animal. To facilitate identification of the experiments, groups are displayed using the same group colors throughout the publication. Phenotypes of pig lungs were confirmed by mRNA and protein expression as described in Methods. GalTKO.hCD46 pig groups were formed according to expression of thromboregulatory genes (hEPCR, hTBM, hTFPI) and carbohydrate gene knock-down (β4GalKD).
TABLE 2.
Experimental outcomes and regimen details
| Exp. ID | Pig genotype | Confirmed pig phenotype | Survival (d) | Initial life-supporting Function | Preformed AB levels |
Onset of thrombocytopenia (<60% BL count) (h) | Onset of neutropenia (<50% BL count) (h) | Onset of anemia (<70% BL count) (h) | Immunosuppression | Adjunctive Treatments |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| anti-Gal IgM (fold of human pool 1:2 median IgM FI) | anti-Gal IgG (fold of human pool 1:2 median IgG FI) | A1AT | Selectin integrin blockade | CVF, αIL8R, TNFα blockade | Liposomes | Kidney pre- perf. AbAds | Splenectomy | |||||||||
| IBN6 | NA | >0.5 | Yes | Not available | Not available | |||||||||||
| ILB3 | NA | >1 | Yes | Not available | Not available | 24 | ||||||||||
| ILB44 | NA | 29 | Yes | 1.6 | Not available | αCD40,MMF | X | X | X | |||||||
| IBN3 | GalTKO | GalTKO | 0.25 | No | 0.9 | 0.1 | ||||||||||
| IBN4 | GalTKO | GalTKO | 0.25 | Yes | 2.5 | 0.6 | 2 | |||||||||
| IBN5 | GalTKO | GalTKO | 0.25 | Yes | 4.2 | 0.6 | 2 | |||||||||
| IBN1 | GalTKO.hCD46 | GalTKO.hCD46 | 0.25 | No | 2.3 | 1.9 | 2 | 2 | ||||||||
| IBN2 | GalTKO.hCD46 | GalTKO.hCD46 | 0.25 | Yes | 2.5 | 0.3 | 2 | |||||||||
| ILB2 | GalTKO.hCD46 | GalTKO.hCD46 | 0.25 | No | 1.5 | 0.5 | 2 | |||||||||
| ILB4 | GalTKO.hCD46 | GalTKO.hCD46 | 0.25 | Partial | 2.9 | 1 | 12 | |||||||||
| ILB5 | GalTKO.hCD46 | GalTKO.hCD46 | 0.25 | Yes | 2.3 | 0.4 | ||||||||||
| ILB18 | GalTKO.hCD46. hTBM.hCD47. hCD39.A20 |
GalTKO.hCD46. hCD47 |
0.5 | No | 0.6 | 0.3 | 2 | |||||||||
| ILB19 | GalTKO.hCD46. hTBM.hCD47. hCD39.A20 |
GalTKO.hCD46. hCD47 |
0.5 | No | 1.8 | 0.2 | 2 | |||||||||
| ILB6 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.25 | No | 2.5 | 0.1 | 12 | |||||||||
| ILB7 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.25 | No | 0.7 | 0.1 | 12 | 2 | ||||||||
| ILB8 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.5 | Yes | 6.4 | 0.1 | 12 | |||||||||
| ILB9 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.25 | No | 1.9 | 0.5 | ||||||||||
| ILB13 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.5 | Yes | 1.1 | 0.2 | 12 | 2 | 12 | |||||||
| ILB14 | GalTKO.hCD46. hEPCR |
GalTKO.hCD46. hEPCR |
0.5 | Yes | 2 | 0 | 2 | 2 | 6 | |||||||
| ILB15 | GalTKO.hCD46. hCD55.hEPCR |
GalTKO.hCD46. hCD55. hEPCR |
0.25 | Yes | 0.5 | 0.1 | 2 | 6 | ||||||||
| ILB22 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hTFPI |
GalTKO.hCD46. hCD55. hEPCR |
0.25 | No | 1.2 | 0.1 | FK506,MMF | |||||||||
| ILB23 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hTFPI |
GalTKO.hCD46. hCD55. hEPCR |
0.25 | Yes | 0.8 | 0.3 | FK506,MMF | |||||||||
| ILB28 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR |
8 | Yes | 0.7 | 0.2 | 24FK506,MMF | |||||||||
| ILB29 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hTFPI |
GalTKO.hCD46. hCD55. hEPCR |
1 | Yes | 3.4 | 0.1 | FK506,MMF | |||||||||
| ILB30 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR |
2 | No | 1 | 0.1 | 2 | 2 | 24 | FK506,MMF | ||||||
| ILB37 | GalTKO.hCD46. hEPCR.hTFPI. hCD47.HO-1 |
GalTKO.hCD46. hEPCR.HO-1 |
2 | Yes | Not available | Not available | 2 | αCD40,MMF | X | |||||||
| ILB38 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hEPCR. hCD47 |
1 | Yes | Not available | Not available | 6 | αCD40,MMF | X | |||||||
| ILB50 | GalTKO.hCD46. hEPCRhTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR.HO-1 |
0.5 | Partial | Not available | Not available | 2 | 12 | αCD40,MMF | X | X | X | ||||
| ILB10 | GalTKO.hCD46. hTBM |
GalTKO.hCD46. hTBM |
0.25 | Yes | 3 | 0.3 | ||||||||||
| ILB11 | GalTKO.hCD46. hTBM |
GalTKO.hCD46. hTBM |
0.25 | No | 1.1 | 0.3 | ||||||||||
| ILB12 | GalTKO.hCD46. hTBM |
GalTKO.hCD46. hTBM |
0.25 | Yes | 1.2 | 0.1 | 12 | 12 | ||||||||
| ILB43 | GalTKO.hCD46. hTBM.hTFPI. hCD47.HO-1 |
GalTKO.hCD46. hTBM. hCD47 |
1 | Yes | Not available | Not available | αCD40,MMF | X | ||||||||
| ILB16 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1 | Yes | 3 | 0.1 | 12 | 24 | ||||||||
| ILB17 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
0.5 | Yes | 0.6 | 0.2 | 12 | |||||||||
| ILB24 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1.25 | Yes | 2.8 | 0.7 | 12 | 2 | FK506,MMF | |||||||
| ILB25 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1.25 | Yes | 1.7 | 0.7 | FK506,MMF | |||||||||
| ILB26 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
0.25 | No | 1.7 | 0.4 | FK506,MMF | |||||||||
| ILB33 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1.5 | Yes | 1.4 | 0.4 | αCD40,MMF | |||||||||
| ILB34 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1 | Partial | Not available | Not available | 12 | 2 | αCD40,MMF | |||||||
| ILB35 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
1 | No | 0.9 | 0.2 | 12 | αCD40,MMF | ||||||||
| ILB36 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
2 | Yes | 1.2 | Not available | 6 | 2 | 24 | αCD40,MMF | ||||||
| ILB41 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
2 | No | 1.8 | Not available | 24 | αCD40,MMF | X | X | ||||||
| ILB42 | GalTKO.hCD46. hCD55.hEPCR. hTFPI.hCD47 |
GalTKO.hCD46. hCD55. hEPCR. hTFPI.hCD47 |
2 | Yes | 1.1 | Not available | 24 | 2 | 48 | αCD40,MMF | X | X | ||||
| ILB49 | GalTKO.hCD46. hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM.HO-1 |
2 | Yes | Not available | Not available | αCD40,MMF | X | X | X | X | |||||
| ILB51 | GalTKO.hCD46. hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM.HO-1 |
0.5 | No | Not available | Not available | 2 | αCD40,MMF | X | X | X | X | ||||
| ILB52 | GalTKO.hCD46. hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM.HO-1 |
7 | Partial | Not available | 0.6 | 48 | αCD40,MMF | X | X | X | |||||
| ILB39 | GalTKO.hCD46. hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM. hCD47.HO-1 |
1.75 | Yes | 0.4 | Not available | 24 | αCD40,MMF | X | X | ||||||
| ILB40 | GalTKO.hCD46. hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM. hCD47.HO-1 |
0.5 | No | 0.9 | Not available | 2 | αCD40,MMF | X | |||||||
| ILB45 | GalTKO.hCD46.hEPCR.hTBM. hCD47.HO-1 |
GalTKO.hCD46. hEPCR. hTBM. hCD47.HO-1 |
9 | Yes | 2.8 | Not available | 12 | 6 | αCD40,MMF | X | X | X | ||||
| ILB20 | GalTKO.hCD46
hCD55.hEPCR. hTBM.hCD39 |
GalTKO.hCD46. hCD55. hEPCR. hTBM. hCD39 |
0.5 | Yes | 3.4 | 0 | 2 | 2 | 12 | |||||||
| ILB21 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hCD39 |
GalTKO.hCD46. hCD55. hEPCR. hTBM. hCD39 |
0.25 | Yes | 0.7 | 0.1 | 6 | 6 | ||||||||
| ILB31 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hCD39 |
GalTKO.hCD46. hCD55. hEPCR. hTBM. hCD39 |
1 | Yes | 1.3 | 0.1 | FK506,MMF | |||||||||
| ILB32 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hCD39 |
GalTKO.hCD46. hCD55. hEPCR.hTBM |
0.5 | No | 1.6 | 0.1 | 6 | 24 | 24 | αCD40,MMF | ||||||
| ILB47 | GalTKO.hCD46. hCD55.hEPCR. hTBM.hCD39 |
GalTKO.hCD46. hCD55. hEPCR.hTBM |
2 | Yes | 1.3 | Not available | 2 | 2 | 24 | αCD40,MMF | X | X | ||||
| ILB63 | GalTKO.β4GalTKD. hCD46.hEPCR. hTBM.hCD47. HO-1 |
GalTKO. β4GalTKD. hCD46. hEPCR. hTBM. hCD47 |
2 | No | 0.9 | 0.5 | 6 | 2 | αCD40,MMF | X | X | X | ||||
| ILB64 | GalTKO.β4GalTKD. hCD46.hEPCR. hTBM.hCD47. HO-1 |
GalTKO. β4GalTKD. hCD46. hEPCR. hTBM.HO-1 |
31 | Yes | 4.4 | 0.4 | 2 | αCD40,MMF | X | X | X | X | ||||
Note: All experiments were grouped according to the genetic background of the used organ donor animal. To facilitate identification of the experiments, groups are displayed using the same group colors throughout the publication. Adjunctive treatments administered to and procedures performed on each experimental subject are detailed, grouped by pig lung phenotype and, within phenotypes, by similarities in other regimen components or additional expressed genes. Intended pig genotype is listed along with confirmed pig phenotype; phenotype was assigned by transgene mRNA expression and, in most instances, by immunohistochemistry of ear clippings and multiple tissues including right lung, and/or by flow cytometry on peripheral blood leukocytes. Experimental identifiers (Exp ID) were assigned sequentially and chronologically in ascending order; the IBN series preceded the ILB series. Survival and function definitions and regimen details are described in the text. Missing experimental numbers are explained in Methods.
2.2 |. Lung function and survival assessment
Before chest closure, life-supporting left graft function was evaluated periodically by transiently obstructing flow to the native right lung using a silastic “vessi-loop” snare around the right pulmonary artery (RPA), and assessing recipient hemodynamics (right heart appearance, systemic arterial blood pressure, and cardiac output, measured via a sterile flow probe on the ascending aorta [PhysioGear 1, Transonic Systems Inc.]), and lung performance (peripheral oxygen saturation). Life support was defined as maintenance of systemic arterial pressure and/or cardiac output >70% of baseline without right heart distention, bradycardia, or desaturation. Partial life support was reported if the proportion of cardiac output transiting the xenograft, measured as the difference between RPA and aortic blood flow with the RPA fully open, was higher than 25%, and if the recipient tolerated 70%–80% of baseline cardiac output transiting the xenograft with the RPA partially occluded. Adjustment of inotropic (dopamine, epinephrine, or dobutamine) and vasopressor (vasopressin, noradrenaline) support was frequently required during lung implant and after graft reperfusion. Anecdotally, circumstances with higher proportion of blood flow transiting the xenograft were associated with a higher inotrope and pressor requirements before life-supporting lung function was tested, and to support stable hemodynamics with the RPA occluded. Failure of the transplanted lung was defined intra-operatively as the absence of full or (after ILB25) partial life-supporting lung function.
While the function of the lung xenograft cannot be ascertained in this model after chest closure, recipient demise due to loss of vascular barrier function and airway flooding from the lung xenograft is used as a graft “survival” surrogate.3,4 After chest closure, lung survival was gauged by radiologic appearance and bronchoscopy, which were performed at 2–3 day intervals or for evaluation of altered recipient clinical condition. The decline in recipient appetite and activity and signs of respiratory distress, including tachypnea, cough, and/or increased work of breathing, reliably heralded progressive opacification of the lung xenograft on x-ray (Figure 2A), new onset of clear or bloody edema fluid emanating primarily from the left (xenograft) airways (Figure 2B), and hemorrhagic consolidation of the lung xenograft at terminal re-exploration. Sample collection and testing were performed as described previously.4,5
FIGURE 2.
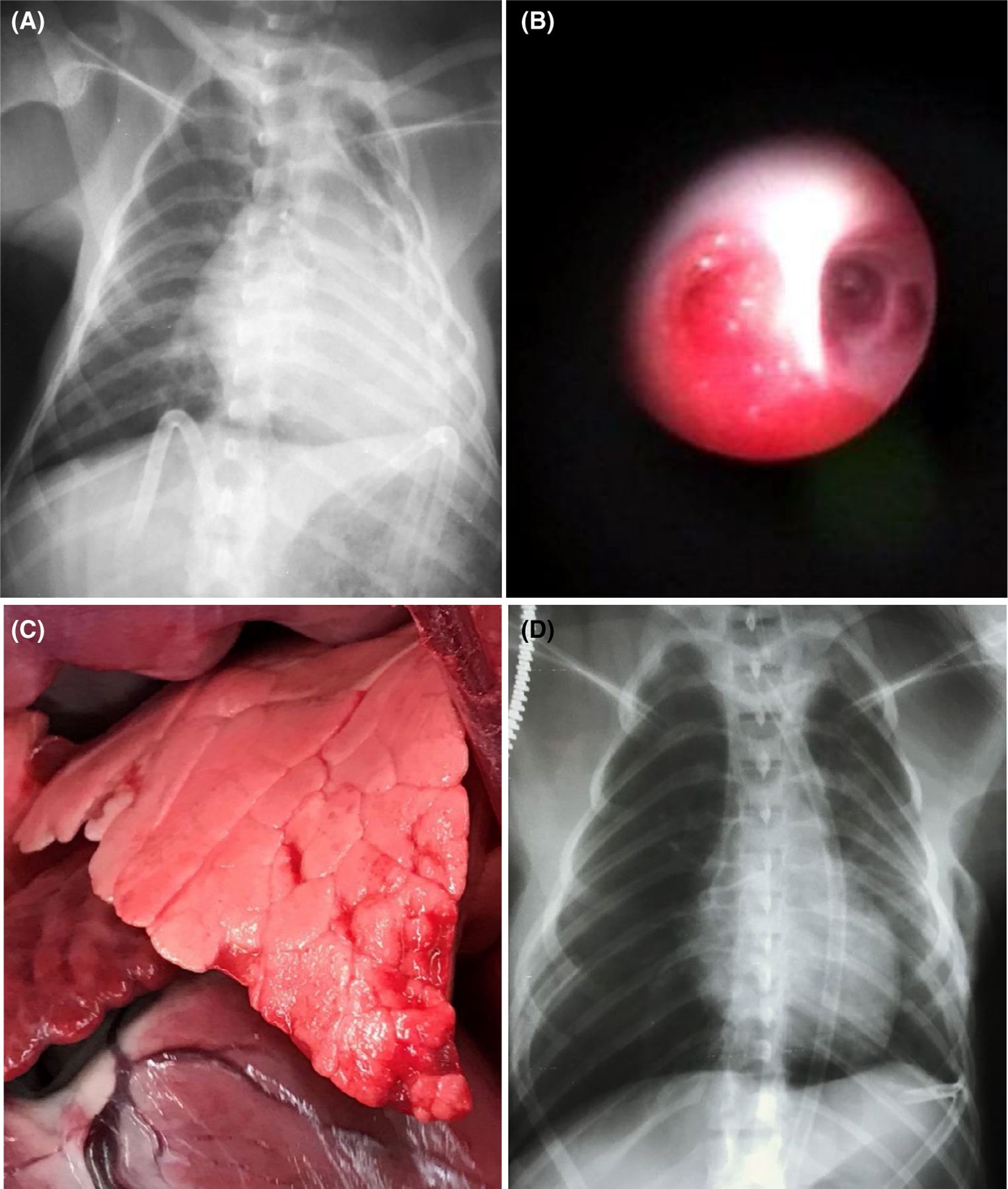
Representative findings after lung xenotransplantation. Chest radiograph illustrating findings associated with interstitial lung edema accumulation (A) in a representative left lung xenograft (ILB18) failing within 12–24 h due to loss of vascular barrier function, manifesting in its late stages as tracheal flooding with edema fluid visualized through a flexible bronchoscope (B). In contrast, the lung parenchyma appears normal (C) in association with life-supporting left lung function in ILB45, which exhibited minimal postoperative interstitial changes in the mid-lung zone (D). Graft opacification after 7 days (not shown) correlated with detection of increasing titers of anti-pig antibody prior to euthanasia for deteriorating clinical condition on day 9
2.2. |. In vitro methods
Baboon blood samples were collected at protocol-driven time points for hematology, cytokine ELISA, and xenoantibody measurements (methods in Supplemental Materials).
3 |. RESULTS
3.1 |. Xenograft recipient survival associated with pig lung genetics and adjunctive treatments
Survival of ILB44 to 29 days (Figure 1A, Table 2) demonstrated that the donor pretreatment and anti-inflammatory and immunosuppressive drug regimen used for the more recent lung xenograft experiments (Figure S1) were not associated with unexpected early lung allograft injury. Lung allografts consistently (3/3) exhibited life-supporting lung function (Figure 1B). At euthanasia for failure to thrive, necropsy of ILB44 failed to confirm suspected viral or bacterial pneumonia, and excluded septicemia.
FIGURE 1.
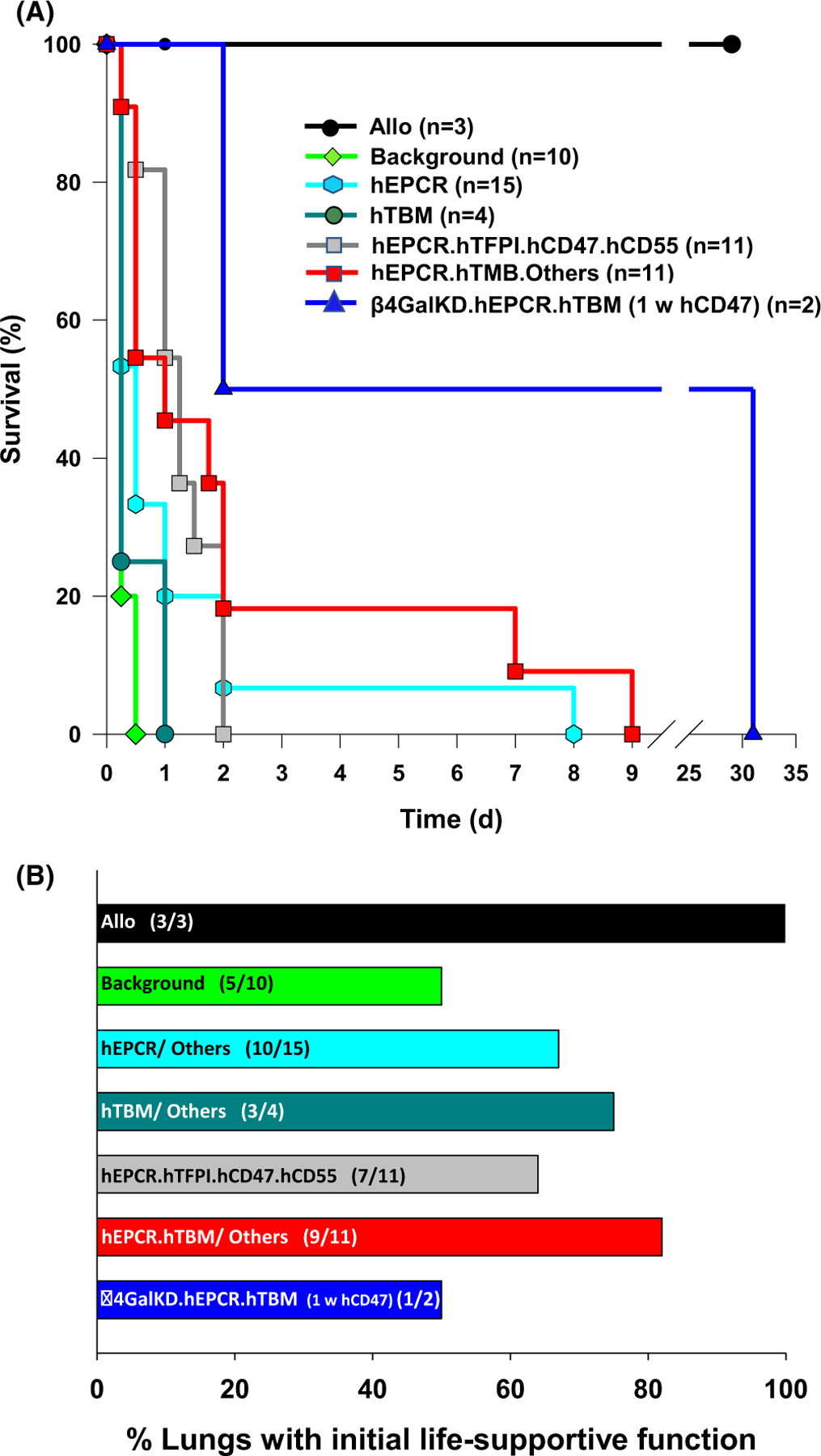
Lung xenograft recipient clinical outcomes. Survival of GalTKO.hCD46 lung xenograft recipients (A), grouped by coagulation pathway regulatory gene expression phenotype, categorized as detailed in Table 1 and Table S1. Survival to 24 h and beyond was observed more frequently with expression of hEPCR with either hTFPI or hTMB relative to hTMB or hEPCR alone. Occasional survivors beyond 2 days were associated with a variety of treatments designed to modulate inflammation as described in the text and Table S2. The incidence (B) and duration (not illustrated) of life-supporting lung function was increased with lungs expressing one or more thromboregulatory genes
GalTKO and GalTKO.hCD46 lungs exhibited life-supporting function for 90–240 mins in five of eight experiments (Figure 1B); two GalTKO.hCD46.hCD47 grafts (ILB18, 19) failed to support life due to high PVR and developed tracheal flooding with edema fluid (TE) within 12 h (Figure 2A,B; Table 2). Lung failure was associated with tracheal flooding from the xenograft in both life-supporting GalTKO lung xenografts, whereas GalTKO.hCD46 lung xenografts failed due to progressive rise in PVR with less prominent TE. All life-supporting “background genetics” lung xenograft recipients required high-dose inotropic support, and most developed moderate to severe metabolic acidosis, with or without native lung edema, consistent with profound systemic inflammation and secondary native lung injury.
Of 15 lungs expressing hEPCR on the GalTKO.hCD46 background, initial life-supporting lung xenograft function was frequently observed in association with hEPCR alone (3/6) or with additional expression of hCD55 (4/6), HO-1 (2/2), or hCD47 (1/1). However, expression of hEPCR alone or with hCD47 or HO-1 did not consistently prevent rapid (within 12–18 h) appearance of interstitial edema and TE. In two instances where the lung expressed hCD55 (ILB28, 30) appearance of TE was delayed beyond 24 h. In one case (ILB30), graft thrombosis occurred around the postoperative day (POD) 2 in a graft that never exhibited life-supporting lung function. In the other (ILB28), gradual opacification of the graft occurred by POD 8 in association with detection of rising anti-non-Gal antibody titers in the context of immunosuppression with FK 506, MMF, and steroids. The appearance of TE was delayed beyond 24 h in one of two GalTKO.hCD46 lungs with hEPCR and HO-1 expression (ILB37).
Expression of hTBM was associated with initially life-supporting GalTKO.hCD46 lung xenograft function in three of four experiments. TE occurred within 8 h except in association with lung from a donor that also expressed hCD47 and was treated with clodronate liposomes, where airway flooding occurred at about 24 h.
Co-expression of hEPCR with hTFPI, hCD55, and hCD47 was associated with initially life-supporting GalTKO.hCD46 lung function in 7 of 11 experiments. Systemic inflammation and progressive acidosis in the recipient, usually accompanied by TE from the xenograft, consistently occurred within 48 h despite conversion from FK506 to costimulation-based immunosuppression (IS, 6/11) and adding candidate anti-inflammatory treatments, including: αIL6R (9/11), A1AT (2/11), and liposomal clodronate donor pretreatment (2/11) (detailed in Table 2).
Co-expression of hEPCR and hTBM with HO-1 and full (ILB49, 51) or partial (ILB52) donor clodronate liposome pretreatment yielded hemorrhagic graft consolidation and recipient hemodynamic instability within 24 h in ILB51. In ILB49, with additional donor kidney pre-perfusion, TE remained absent at 48 h, but the recipient exhibited persistent high-dose inotrope and pressor requirement and failure to wean from mechanical ventilation, necessitating euthanasia. In ILB52, without renal pre-perfusion, the hEPCR.hTBM.HO-1 graft was partially life-supporting initially, with preserved graft aeration by CXR on POD 4. This recipient developed a fever on day 6 and was euthanized on POD 7 due to increasing respiratory distress, fever >39℃, and hypotension. Pleural space bacterial and fungal infection found at pre-mortem chest exploration, presumably reflecting a perioperative break in sterile technique with polymicrobial chest contamination. Two of three GalTKO.hCD46.hEPCR.hTBM.HO-1 lungs with additional expression of hCD47, donor clodronate liposome treatment, and recipient treatment with A1AT were initially life-supporting; ILB39 developed TE at 40 h, and ILB45, with additional selectin blockade, exhibited normal gross appearance after implantation (Figure 2C) and minimal dependent edema on postoperative x-ray (Figure 2D), before gradually developing graft opacification and TE by POD 9. Four of five GalTKO.hCD46.hCD55.hEPCR. hTBM lungs, three of which also expressed hCD39, were initially life-supporting. One without hCD39 (ILB32) succumbed at 12 h with TE attributed to pulmonary vein thrombosis; the three with hCD39 (ILB20, 21, and 31) exhibited TE associated with escalating recipient acidosis and pressor requirement between 12 and 18 h. TE was delayed to 44 h in ILB47 in association with additional A1AT, selectin and integrin blockade, and costimulation-based IS.
One GalTKO.hCD46.hEPCR.hTBM.hCD47 lung (ILB63) with reduced expression of the β4Gal carbohydrate antigen (β4GalKD) and without donor kidney pre-perfusion was not life-supporting, and the recipient was euthanized for failure to thrive on POD 2, with native lung interstitial edema and xenograft hemorrhage and TE at necropsy. ILB64 with the addition of donor kidney pre-perfusion was initially life-supporting. Partial graft consolidation progressed by chest x-ray between POD 1 (Figure 3A) and POD 3 (Figure 3B) without TE or recipient respiratory distress before improving progressively between POD 14 (Figure 3C) and 28 (Figure 3D); healthy xenograft bronchial mucosa with minimal tracheal edema was observed on bronchoscopy on POD 21 and 28. The recipient remained clinically well (active, eating, afebrile) until developing progressive dyspnea and cough on POD 30–31. At chest exploration on POD 31 the graft was shrunken and consolidated with hemorrhagic bronchial mucosa. Cultures of lung xenograft and blood grew Enterococcus and Escherichia coli, consistent with polymicrobial pneumonia and septicemia. Organizing thrombi were observed by light microscopy in the small arterial and venous vessels of the xenograft despite widely patent vascular anastomoses. Epithelial, endothelial and mesenchymal cell nuclei were pyknotic or absent throughout the consolidated, macrophage-filled lung parenchyma.
FIGURE 3.
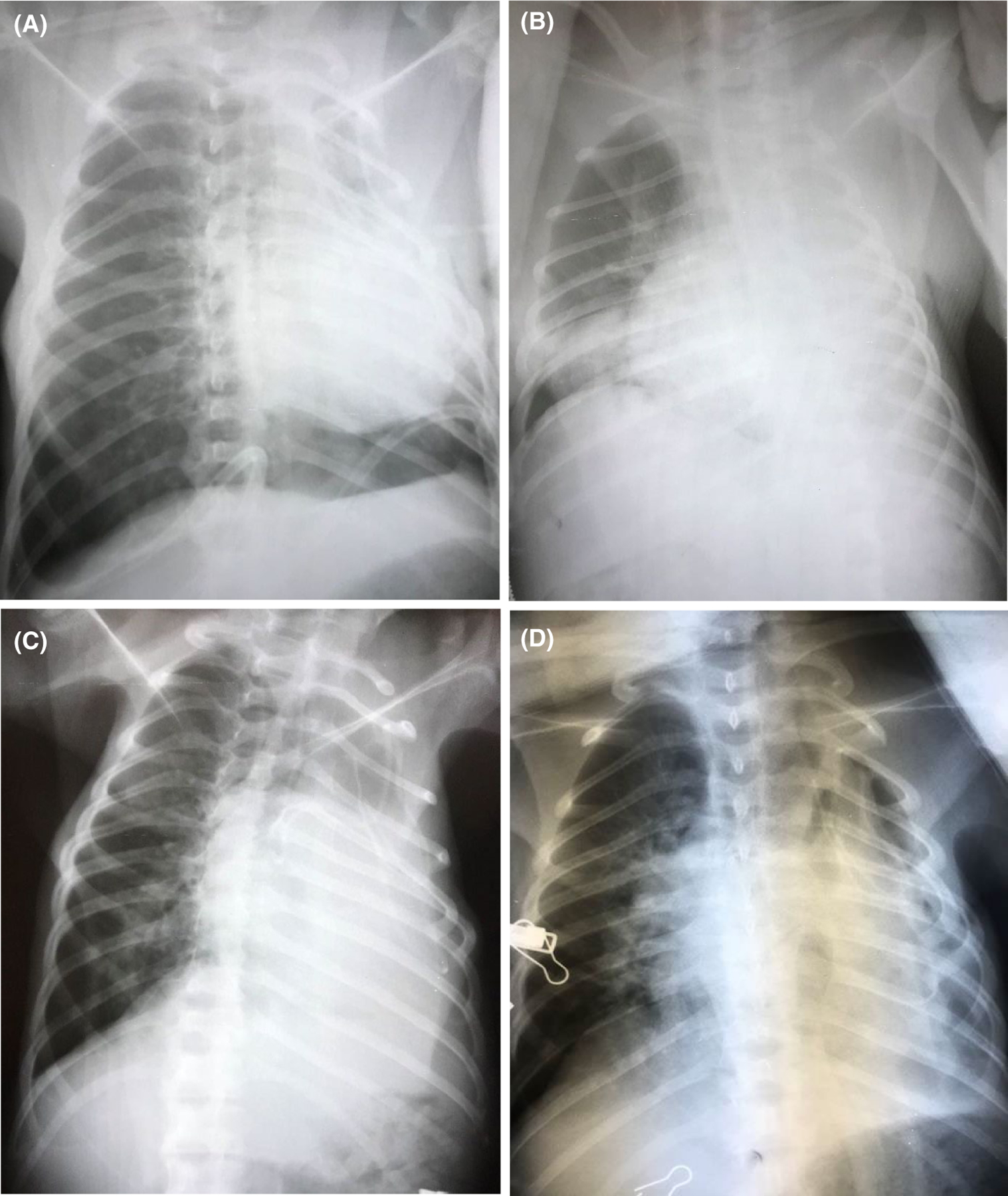
ILB64 chest x-rays. In the recipient with 31-day survival, partial graft consolidation progressed between day 1 (Figure 3A) and day 3 (Figure 3B) without TE on bronchoscopy or recipient respiratory distress. Left upper lung zone aeration improved by day 14 (Figure 3C), with additional partial re-expansion of the left lower lung field by day 28 (Figure 3D)
3.2 |. Association of regimen components with lung and recipient outcomes
Within lung phenotypes grouped according to thromboregulatory gene expression (hEPCR, hEPCR.hTFPI, hEPCR.hTBM), elaboration of IL-6, but not IL-8, TNFα, or IFNγ, was significantly reduced at 12 h in association with the expression of hEPCR.hTFPI (2557 ± 1517 vs. EPCR 4810 ± 1781; p = .054) or hEPCR.hTBM (2166 ± 983; p = .018 vs. EPCR) relative to hEPCR alone (Figure 4A-C). A1AT treatment was associated with reduced elaboration of IL6 (1344 ± 425 vs. 3673 ± 891 at 4 h; p = .0007), IL8 (670 ± 193 vs. 1615 ± 342 at 4 h; p = .007), and IFNγ (1.5 ± 0.51 vs. 8.1 ± 2.4 at 12 h; p = .009), but not TNFα (18.9 ± 12.9 vs. 46.7 ± 22.6 at 12 h; p = .27) (Figure S2). A1AT was also associated with longer recipient survival, including the two longest survivors in the hEPCR.hTBM group (ILB45, 52), as was additional cytokine inhibition (soluble TNFα-R, αIL-8R) and complement depletion using cobra venom factor (ILB63, 64). Platelet activation, reflected by elaboration of β-thromboglobulin (βTG), was delayed and reduced in association with expression of hTBM alone or with hEPCR (Figure 4E) and tended to be lower when A1AT was used with hEPCR.hTFPI or hEPCR.hTBM lungs (Table S1, βTG tab).
FIGURE 4.
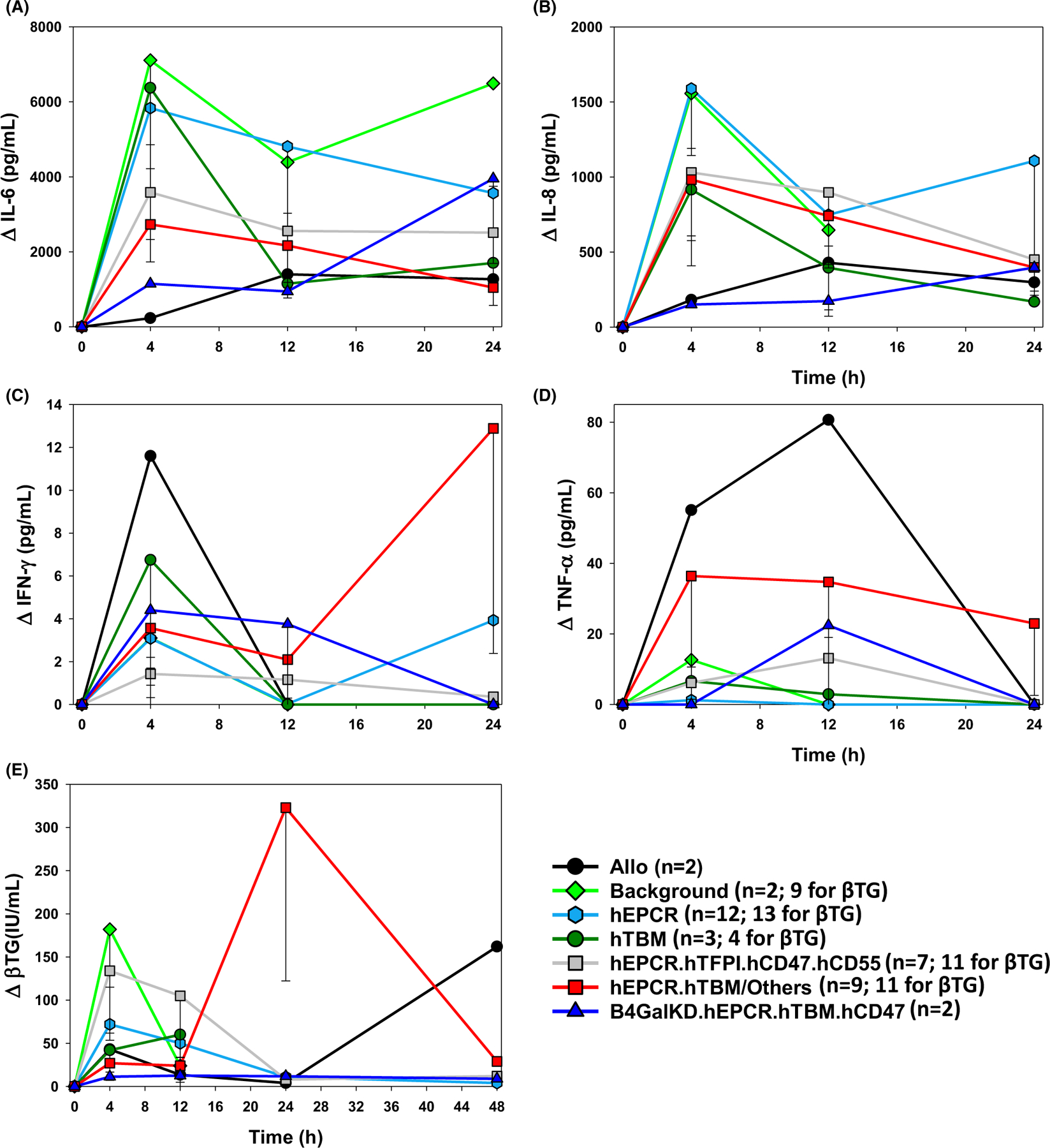
Cytokine and βTG elaboration by lung xenograft phenotype. Expressions of IL-6, IL-8, TNFα, IFNγ, and βTG were measured by Luminex at intervals over the first 12–48 h in an arbitrarily selected subset of experiments and expressed as mean with standard error of the mean. Prolific elaboration of IL-6 (A) during the first 12 hours after GalTKO.hCD46 lung xenotransplantion was attenuated in association with the expression of hEPCR with hTFPI or hTBM and with β4GalKD.hEPCR.hTBM, but not with hTBM or hEPCR alone, whereas IL-8 was lower only with allografts and with β4GalKD.hEPCR.hTBM xenografts (B). TNFα and IFNγ were highest in 2 lung allografts and did not vary statistically significantly in association with the combinations of thromboregulatory genes and drug treatments tested here (B,C). βTG elaboration 4 h after transplant was lower, and similar to lung allografts, in association with hTBM expression alone (42 ± 17 IU/ml; n too small for p-value calculation) or with hEPCR (27 ± 10 IU/ml; p = .035) relative to high levels with GalTKO.hCD46 background genetics (182 ± 67 IU/ml), while βTG elevations were intermediate with hEPCR (72 ± 19 IU/ml; p = .08). Of note, except for TNFα, pro-inflammatory cytokine and βTG elaboration were generally lowest in association with β4GalKD.hEPCR.hTBM lung xenografts, including ILB64 (31 d)
E- and P-selectin and Mac-1/β2 integrin blockade (ILB45, 47, 49, 51, 52) did not consistently attenuate platelet, neutrophil, or monocyte sequestration or anemia (data not illustrated; Table S1). Kidney pre-perfusion (ILB49, 50, 51, 64) and donor clodronate liposome pretreatment (ILB41–45, 49–52) were associated with exceptional survivals beyond POD 2, but were neither necessary to reproducibly enable initial life-supporting lung performance (prevent early rise in PVR) nor sufficient to consistently prevent loss of xenograft barrier function within 24 h.
Conventional calcineurin inhibitor-based immunosuppression (ILB22–31) failed to prevent elaboration of anti-donor antibody (ILB28) (Figure 5) despite pre-operative B- and T-cell depletion. In contrast to findings in the kidney1 and heart2,21 models, anti-donor antibody elaboration was detected within 14 days despite αCD40 treatment, alone or with additional ATG, αCD20, and splenectomy. Although bronchial mucosal viability was preserved beyond POD 28 in ILB64, elaboration of anti-non-Gal antibodies (IgM and IgG) by POD 14, rising further by POD 31, implicates elicited antibody in graft demise.
FIGURE 5.
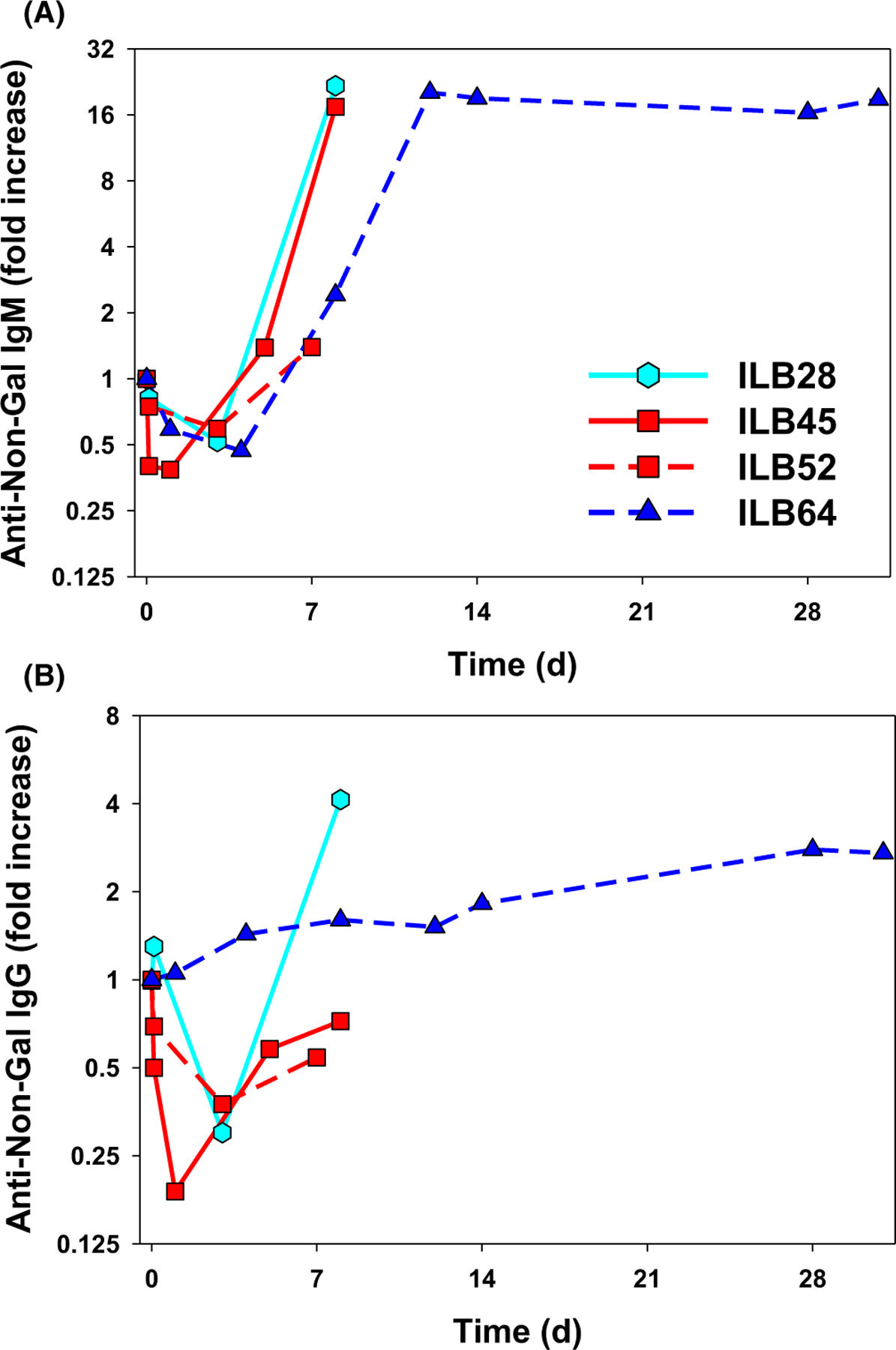
Anti-non-Gal antibody titers after lung xenotransplantation. Levels of baboon-anti-pig IgM (A) and IgG (B) antibody were measured by flow cytometry in the serum or plasma of four baboons that exhibited graft survival ≥7 days using a GalTKO endothelial cell line, expressed as a fold increase in MFI relative to pretransplant serum.1 Initial decline in IgM and IgG titers presumably reflected adsorption of preformed non-Gal anti-pig antibody to the lung xenograft. In association with calcineurin-based IS (ILB28), increased IgM and IgG were detected on POD 7 around the time of euthanasia for graft failure on POD 8. In three animals treated with αCD40, IgM rise was appreciated by day 7–8, but IgG elaboration appeared to be attenuated, consistent with prevention of class-switching that would be expected in association with effective blockade of the CD40/CD154 costimulatory pathway
Perfusion of the donor kidneys was associated with small (<40%) and inconsistent decline in anti-donor antibody titer, and, while associated with 31-day recipient survival in ILB 64, did not consistently prevent early graft demise.
3.3 |. Hematologic observations by lung xenograft phenotype and treatment regimen
In most lung xenograft recipients, but not allograft recipients, thrombocytopenia was moderate (40%–60% decline from immediately before graft revascularization, “time 0”) to severe (>60% decline) within 2–12 h after graft revascularization (Figure 6A, Table 2, Figure S3). In association with hEPCR, hTBM, or hEPCR.hTFPI expression, thrombocytopenia was delayed in onset from <2 to 6–12 h relative to GalTKO or GalTKO.hCD46 alone, and tended to be attenuated in severity (mild to moderate) and transient. Addition of E- and P-selectin and Mac-1/β2 integrin blockade (after ILB44) and IL-8 receptor inhibition (ILB63, 64) did not consistently prevent thrombocytopenia relative to GPIb blockade and expression of human coagulation pathway regulatory molecules alone, although the number of observations with full treatment was limited. Severe thrombocytopenia during the first 12 h was sometimes associated with failure to support life (31% nadir, from 239 to 73 K in ILB32; 11% nadir, from 245 to 26 K at 2 h in ILB40) and/or rapid loss of barrier function (9% nadir, from 256 to 22 K at 2 h ILB38), whereas less severe initial decline (70% nadir, from 294 to 220 K at 12 h in ILB52, 7-day survival) or recovery by 24 h (30% nadir at 4 h rebounding to 79% at 24 h in ILB 45, 9-day survival; 66% nadir at 12 h in ILB64, 31-day survival) was associated with delay in loss of graft vascular barrier function and longer duration of recipient survival in some instances.
FIGURE 6.
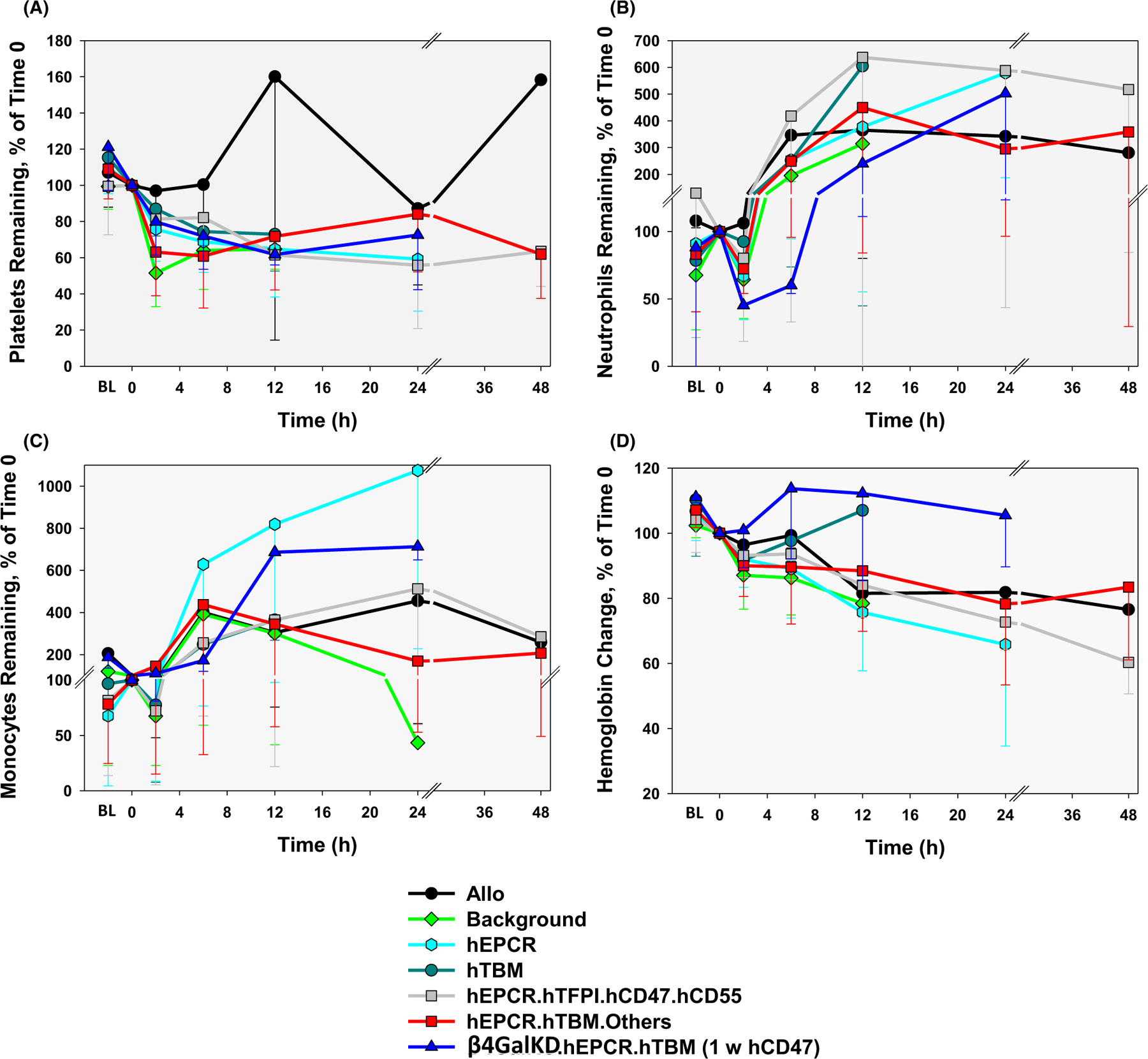
CBC data by lung phenotype after lung xenotransplantation. Platelet (A), neutrophil (B), and monocyte (C) counts as well as hemoglobin levels (D) during intervals after lung transplantation (nadir within the first 4 h and between 4 and 8 h; and peak at 12, 24, and 48 h) are expressed as a percent of pre-transplant (t = 0). Absolute counts and calculations are shown in Table S2
Mild to moderate leukopenia before transplant became prevalent after the introduction of preoperative recipient treatment with ATG (with ILB22) (Table S1). Increased leukopenia was consistently observed within 2–6 h after graft perfusion. Despite administration of high-dose steroids before revascularization, neutropenia was moderate (below baseline) to severe (<50% baseline) in the majority of xeno lung recipients within 2–6 h after graft perfusion, but not in allograft recipients (Figure 6B, Table 2, Figure S4). Transient neutropenia was not consistently inhibited in association with any of the pig genetics tested, or in association with the addition of selectin and integrin blockade (ILB45, 47, 49, 51, 52, 63, 64) or IL-8 receptor inhibition (ILB63, 64). Neutropenia typically resolved within 24 h, but often recurred around the time of lung xenograft demise. Monocyte counts tended to be markedly decreased in the first 2 h in most groups, but rose significantly above pre-transplant levels within 12–24 h in the majority of recipients (Figure 6C, Figure S5).
A mild (20%–30%) or moderate (30%–40%) decline in hemoglobin was frequently observed within 2–6 h, and was not accounted for by crystalloid administration since it was not observed in allograft recipients (Figure 6D, Table 2, Figure S6). In some xenograft recipients, anemia became severe (>40% decline) around the time of graft failure due to loss of barrier function, in the setting of hemorrhage into the lung xenograft (ILB41, 42). In other recipients, including animals with preserved life-supporting lung function such as in ILB45, where hemoglobin fell from 12.5 before graft reperfusion to 7.5 g/dl at 6 h, the etiology of moderate or severe anemia was unclear. Anemia responded appropriately to peri-operative blood transfusion but tended to recur within 24–48 h in longer-surviving recipients despite routine high-dose erythropoietin administration. Gastrointestinal and surgical site blood loss were consistently excluded at necropsy. Recipient splenectomy (ILB63, 64) was associated with avoidance of the moderate or severe anemia consistently observed within the first 12 h in all other groups.
3.4 |. Histologic features of lung xenograft failure
Compared to normal baboon (Figure 7A) or pig lung (Figure 7B) before left lung xenotransplantation, alveolar septal cellularity and capillaries engorged by red blood cells were consistent findings in failing lung xenografts (Figure 7C–E) and to a lesser degree in native right lung at experimental termination, but absent at necropsy in electively terminated allografts (histology not shown). Lung xenograft parenchyma was typically histologically normal in experiments where lung failure was ascribed to elevated pulmonary vascular resistance (Figure 7C) or when the recipient died with a functioning xenograft. Subpleural and septal edema, often with erythrocytes crowding the subpleural space and filling interstitial lymphatics, became prominent by 6–12 h in longer-surviving grafts. When loss of barrier function and tracheal flooding was the primary mode of graft failure, proteinaceous pulmonary edema and alveolar hemorrhage (Figure 7D) involved varying proportions of the lung parenchyma, predominating in but not confined to dependent areas. Endothelial activation with prominent endothelial cell nuclei and adherent leukocytes (Figure 7E) were commonly identified, primarily in pulmonary arterial vessels of all sizes. Both white and red intravascular thrombi were occasionally identified (Figure 7F), but were not prominent, particularly in lung phenotypes expressing hTBM and hEPCR, corroborating prior results.5,8,9
FIGURE 7.
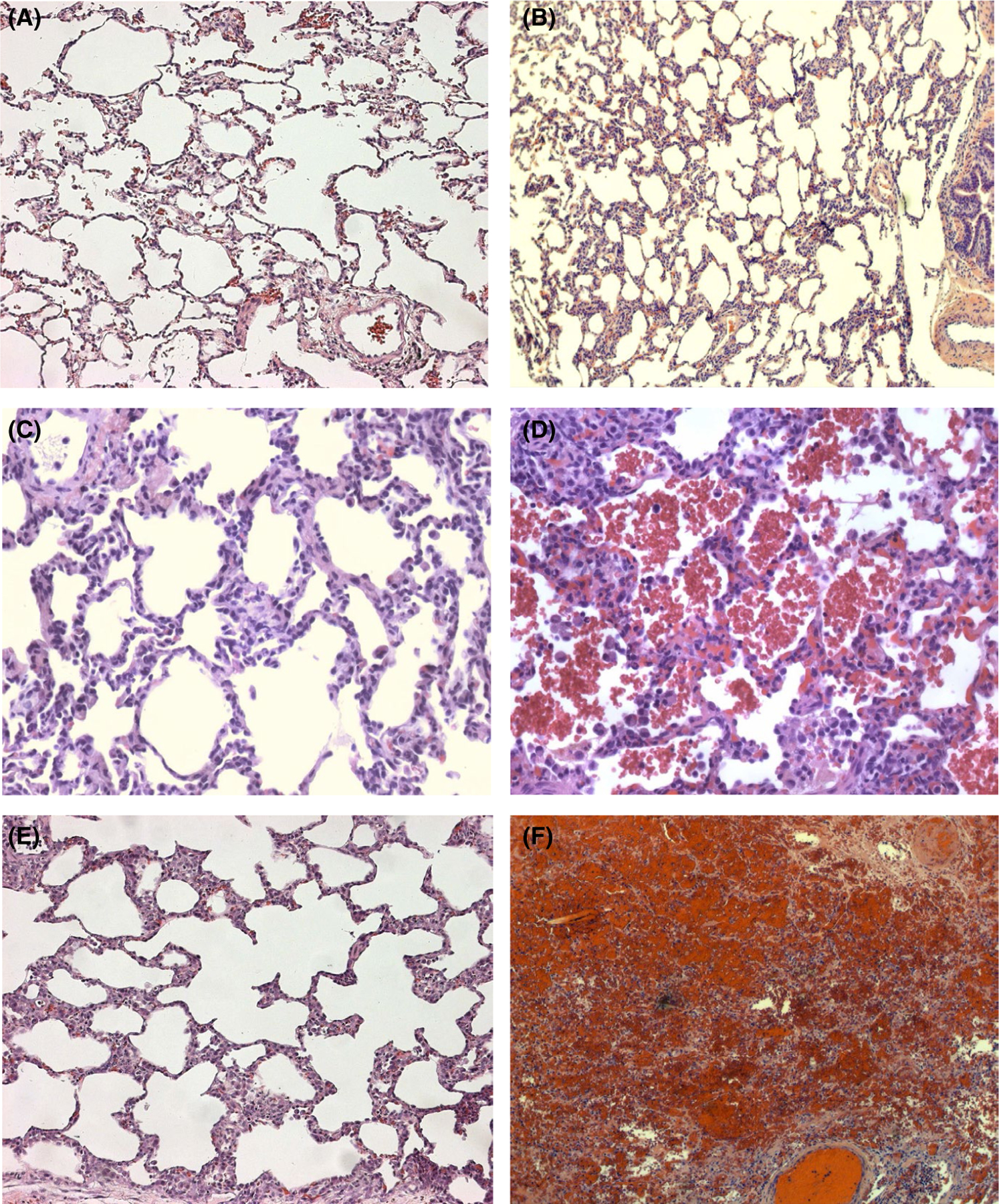
Representative lung histology. Lung samples obtained within hours after implantation (A, B) or at xenograft explant and recipient euthanasia (C–F) were fixed, processed, and stained as described in in vitro methods.5 Alveolar septal cellularity and capillaries engorged by red blood cells were consistent findings in failing lung xenografts (Figure C–E) and to a lesser degree in the native right lung at experimental termination, but absent in normal baboon (Figure A) or pig lung (Figure B). When experiments were terminated due to high pulmonary vascular resistance in the transplant, lung xenograft architecture was otherwise minimally perturbed (Figure C), whereas proteinaceous pulmonary edema and alveolar hemorrhage (Figure D) became prominent when tracheal flooding due to loss of barrier function was observed. Lung endothelium activation with prominent endothelial cell nuclei and adherent leukocytes (Figure E) was prominent in pig pulmonary arterial and arteriolar vessels; both white (presumed platelet-rich fibrin aggregates) and red thrombi (clots with entrapped erythrocytes) were identified more commonly in lungs lacking thromboregulatory gene expression (Figure F)
4 |. DISCUSSION
Non-human primates consistently survive for over a year with kidneys of various relatively simple genotypes,1 and for over six months with life-supporting GalTKO.hCD46.hTBM heart xenografts,2 raising hopes for early clinical translation for those organs. In stark contrast, our experience demonstrates that inflammation in the graft and in the recipient is particularly prominent for lung xenografts3–6,18–20 relative to other pig organs.1,2,22–26 This inflammation is consequential, manifesting early after transplant as parenchymal hemorrhage, loss of graft barrier function, and systemic inflammation. The initial inflammation is also associated with the elaboration of anti-pig antibody in the context of a costimulation-based IS regimen that is consistently effective for the heart2 and kidney xenografts.1,27 Encouragingly, several different combinations of anti-inflammatory pharmacologic interventions and donor and recipient treatment regimens were sufficient to demonstrate the feasibility of at least transiently life-supporting lung xenograft function, and recipient survival beyond 48 h in association with a variety of multi-gene lung phenotypes.
GalTKO.hCD46 lungs, even with additional expression of up to five additional genetic modifications, are incompletely protected from loss of vascular barrier function in baboons, unlike GalTKO. hCD46 hearts or kidneys. Expression of hEPCR or hTBM alone on the GalTKO.hCD46 background increased the incidence of initially life-supporting lung function and delayed vascular injury, which in this model is primarily manifest as PVR elevation, or loss of vascular barrier function, which presents as alveolar flooding and TE. Improved hemodynamic performance of GalTKO.hCD46 lung xenografts that expressed hEPCR along with hTBM or hTFPI was consistently associated with reduced IL6 and βTG elaboration relative to hEPCR alone. However, severe systemic inflammation in the recipient, manifesting as inotrope and pressor requirements and native lung injury, remained prominent.
Guided by data from our ex vivo perfusion model, we explored a variety of anti-inflammatory drugs (glucocorticoid, complement inhibition, antihistamine, thromboxane synthase inhibition, A1AT),18–20 cytokine inhibition (TNF, IL-6, IL-8),22–25 selectin (E and P), and integrin (CD11b/18, GPIb) receptor blockers,28,29 and treatment strategies (antibody adsorption, complement depletion, splenectomy, and donor lung macrophage depletion).20,30–35 Various combinations of these additional anti-inflammatory treatments were associated with delayed loss of lung xenograft vascular barrier function, from 6–12 to 24 to >48 h, particularly in the context of genetics including both hEPCR and hTBM or hTFPI. With A1AT, or with β4GalKD and a pilot mechanism-directed anti-inflammatory drug combination (ILB64), three (of 13) recipients of hEPCR.hTBM-expressing lungs survived to 7, 9, and 31 days. A1AT was adopted empirically based on its pleotropic anti-inflammatory effects. Within lung phenotypes grouped according to thromboregulatory gene expression, A1AT was associated with a trend toward attenuated cytokine elaboration and longer recipient survival, including two of the three longest survivors in the hEPCR.hTBM group (7 and 9 days). However, A1AT was not essential to prolonged survival (ILB28, 8 days; ILB64, 31 days): donor lung macrophage depletion,20,35 anti-pig antibody adsorption,30,33–37 cytokine inhibition,20,22–27 and combined integrin and selectin blockade18,28,29 were also associated with longer recipient survivals, but were only adopted late in the series. To date, none of the individual interventions or the various combinations of regimens evaluated consistently prevent loss of lung xenograft vascular barrier function, at least in association with the thromboregulatory and anti-inflammatory gene modifications with which they were combined here. While the independent influence of each of these treatment strategies has not yet been directly evaluated, we conclude that targeting inflammation is the key to successful lung xenografting.
Coagulation cascade activation and dysfunctional thromboregulatory mechanisms are closely linked to failure of kidney and heart xenografts.21,22,34–43 Co-expression of hEPCR with hTBM was associated with reduced βTG elaboration, consistent lung survival beyond 12 h, and a high incidence of initial life-supporting lung function. On human endothelium, hEPCR and hTBM function synergistically to enhance production of activated Protein C (aPC) and more efficiently neutralize thrombin’s multiple procoagulant effects,42–44 while aPC binding to EPCR mediates endothelial cytoprotection.44–47 We hypothesize that the hEPCR.hTBM gene combination delays lung xenograft vascular barrier failure by modulating the coagulation cascade dysregulation typically seen in GalTKO.hCPRP-transgenic pig-to-baboon organ xenografts,27,36–43,48,49 including lung.4,6,43,50,51 Failure to attenuate sequestration of platelets, neutrophils, and other formed blood elements and significant residual elaboration of proinflammatory cytokines demonstrate the importance of additional adhesive, pro-coagulant, and proinflammatory mechanisms to lung xenograft behavior that escape control by the multiple donor genetic modifications and anti-inflammatory treatments tested here. We postulate that identifying and effectively targeting the mechanisms driving these phenomena will significantly simplify the path to clinical lung xenotransplantation.
β4 knock-down lung recipient survival to 31 days after donor kidney pig pre-adsorption (ILB64), but failure within 2 days without this maneuver (ILB63), provisionally implicates antibody directed at non-Gal epitopes in the particular vulnerability of the GalTKO.hCD46. hEPCR.hTBM.hCD47 lung to rapid loss of barrier function. Elicited anti-non-Gal antibody elaboration was consistently observed in association with lung xenograft demise, and was not prevented by either conventional (ILB 28) or αCD40-based (ILB45, 64) immunosuppression. These observations support our working hypothesis that anti-pig antibody is one pivotal proinflammatory mechanism driving lung xenograft injury.4,50
Thrombocytopenia is consistently observed in the first hours after lung xenotransplantation with every combination of pig genetics and pharmacologic strategies we have deployed. During ex vivo pig lung perfusion with human blood, lungs from pigs with humanized von Willebrand’s factor exhibit delayed platelet sequestration (51; unpublished observations). Neutrophil and monocyte sequestration are also prominent within hours in both ex and in vivo models. The extent to which these phenomena are mediated by canonical selectin- and integrin-mediated adhesive and cell trafficking mechanisms, their contribution to loss of vascular barrier function, and whether they can be prevented using clinically relevant treatment regimens, are another focus of our current studies.
Expression of hCD47 has been advanced as a strategy to extended duration of mixed hematopoetic chimerism in the MGH xenotolerance model by preventing detection of hCD47+ pigs cells as “non-self” by baboon recipient reticuloendothelial cells.12,13,52,53 Our experience to date provides examples where hCD47 was not necessary to achieve prolonged recipient survival (ILB28, 8 days; ILB52, 7 days; ILB64, 31 days). Nor was hCD47 co-expression sufficient to assure GalTKO.hCD46 lung survival of one day, by itself (ILB18, 19), or with either hEPCR (ILB38) or hTBM (LB43), albeit in singular examples; or beyond two days in the context of hCD55, hTFPI, and HO-1 (n = 11). This experience is in contrast to the report of Watanabe et al.,53 which suggested that hCD47 expression in endothelium was necessary to permit lung xenograft recipient survival for up to 14 days. We hypothesize that by lowering PVR, anti-inflammatory histamine and thromboxane antagonists augment blood flow through the xenograft, amplifying host-versus-graft injury mechanisms such as those driven by: (1) anti-non-Gal antibody and complement,5,6,10,11,30–32 (2) coagulation pathway dysregulation,8,9,22,33,38–43 and (3) adhesive interactions between baboon platelets and leukocytes and pig endothelium.14,18,28,33,54 Increased trans-xenograft blood flow amplifies systemic inflammation, presumably consequent to release of pig cells and cytokines into the recipient, manifesting in transient inotrope and pressor requirements and variable degrees of native lung injury. We predict that hCD47 is likely to demonstrate an advantage to down-modulate host innate immune responses, as predicted,12,13 once other mechanisms of lung xenograft injury are better controlled. Meanwhile, ongoing work by us and others will evaluate Watanabe and Yamada’s hypothesis that anatomic location of hCD47 may significantly influence transgene efficacy, as also seems probable for the many other genes expressed in genetically engineered pigs.
Moderate or severe anemia was frequently observed in the absence of surgical site bleeding or gastrointestinal blood loss. Anemia tended to be less prevalent and less severe in association with lungs that expressed hEPCR alone or with hTBM. Our working model posits that recipient erythrocytes are damaged by adhesive interactions with damaged endothelium (thrombotic microangiopathy21,38–42,48) or activated macrophages20,35 during transit through the lung xenograft, with subsequent sequestration by the recipient’s reticulendothelial system, or in the lung xenograft via sialoadhesin55 or other mechanisms.54,56 Of note, anemia did not occur within the first day in the two baboons (ILB63, 64) that were treated with selectin and integrin blockade and splenectomized prior to hEPCR.hTBM-lung xenotransplantation.
While small experimental groups using different donor genotypes and various treatment regimens significantly limit this study’s utility to discern each variable’s relative contribution, our results demonstrate for the first time that initial life-supporting function and recipient survival for up to 1 month are achievable for lung xenografts. Although the minimum gene set and drug treatment regimen necessary to consistently achieve long-term survival and to prevent anti-pig antibody elaboration remain to be defined, targeting thrombodysregulation and inflammation have yielded significant progress, and established a platform for further mechanistic insights and future advances toward clinical application.
Supplementary Material
ACKNOWLEDGMENTS
Supported by U01 AI66335 and U19 AI090959, by unrestricted educational gifts from United Therapeutics, and by sponsored research agreements from Lung Bioengineering PBC. All listed authors contributed to the design and conduct of the experiments and participated in authorship of the manuscript. CP and DA designed, produced, and provided the genetically modified pigs used in this study, and supervised their genotyping and phenotyping by Revivicor technical staff. LB, CTL, DGH, MRC, NAO, AC, and DP participated in the surgical procedures under supervision of RNP. In vitro assays were performed and data initially interpreted by ZH, ER, DP, and lab technical staff under the direction of AMA. RNP, assisted by AMA, and LB, led the data analysis and writing of the manuscript. All authors have approved the final submission. DA (CEO, Chief Scientific Officer) and CP are employees of Revivicor, a subsidiary of Lung PBC and United Therapeutics; RNP has served as an unpaid scientific advisor to Revivicor. The authors wish to acknowledge invaluable surgical (especially Mitsunori Higuchi), anesthetic (especially Steven Shipley, Elana Rybak, and Ivan Tatarov), and technical support from the many colleagues and staff at University of Maryland School of Medicine who worked with us on this project over 15 years.
Funding information
United Therapeutics Corporation; National Institute of Allergy and Infectious Diseases, Grant/Award Number: U01 AI66335 and U19 AI090959
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Kim SC, Mathews DV, Breeden CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant 2019;19(8):2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018;564(7736):430–433. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen BN, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg 2007;133(5):1354–1363. [DOI] [PubMed] [Google Scholar]
- 4.Burdorf L, Azimzadeh AM, Pierson RN 3rd. Progress and challenges in lung xenotransplantation: an update. Curr Opin Organ Transplant 2018;23(6):621–627. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen BN, Azimzadeh AM, Schroeder C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplant 2011;18(2):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdorf L, Stoddard T, Zhang T, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant 2014;14(5):1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuensch A, Baehr A, Bongoni AK, et al. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplant 2014;97(2):138–147. [DOI] [PubMed] [Google Scholar]
- 8.Harris DG, Gao Z, Sievert EP, et al. Transgenic human thrombomodulin expression reduces xenogeneic thrombosis: a promising means of reducing pig lung xenograft thrombotic injury. J Heart Lung Transplant 2014;33(4):S108. [Google Scholar]
- 9.Burdorf L, Rybak E, Zhang T, et al. Human EPCR expression in GalTKO.hCD46 lungs extends survival time and lowers PVR in a xenogenic lung perfusion model. J Heart Lung Transplant 2013;32(4):137.23260714 [Google Scholar]
- 10.Burdorf L, Ali F, Ramsoondar J, et al. N-Glycolyl-neuraminic acid (Neu5GC) knock-out in GalTKO.HCD46 pig lungs improves pulmonary function in a xenogeneic pig-to-human lung perfusion model. Xenotransplant 2015;22(S84):552. [Google Scholar]
- 11.Burdorf L, Cerel B, Abady Z, et al. β4Gal is an antigen relevant to injury of pig lung xenografts in baboons. Xenotransplant 2019;26:e12553.19. [Google Scholar]
- 12.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA 2007;104:5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Wang H, Ide K, et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPα capable of binding to human CD47. Cell Transplant 2011;20(11–12):1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird CT, Burdorf L, French BM, et al. Transgenic expression of human leukocyte antigen-E attenuates GalKO.hCD46 porcine lung xenograft injury. Xenotransplant 2017;24(2):e12294. doi: 10.1111/xen.12294 [DOI] [PubMed] [Google Scholar]
- 15.Jung SH, Hwang JH, Kim SE, et al. The potentiating effect of hTFPI in the presence of hCD47 reduces the cytotoxicity of human macrophages. Xenotransplant 2017;24(3):e12301. doi: 10.1111/xen.12301 [DOI] [PubMed] [Google Scholar]
- 16.Imai M, Takigami K, Guckelberger O, et al. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplant 2000;70(6):864–870. [DOI] [PubMed] [Google Scholar]
- 17.Shen Z, Teng X, Qian X, et al. Immunoregulation effect by overexpression of heme oxygenase-1 on cardiac xenotransplantation. Transplant Proc 2011;43(5):1994–1997. [DOI] [PubMed] [Google Scholar]
- 18.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplant 2016;23(3):222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdorf L, Harris D, Dahi S, et al. Thromboxane and histamine mediate PVR elevation during xenogeneic pig lung perfusion with human blood. Xenotransplant 2019;26(2):e12458. doi: 10.1111/xen.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol 2001;90(6):2257–2268. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol 2005;16(9):2732–2745. [DOI] [PubMed] [Google Scholar]
- 22.Ezzelarab MB, Ekser B, Azimzadeh AM, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplant 2015;22(1):32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase H, Ekser B, Zhou H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplant 2015;22(5):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwase H, Liu H, Li T, et al. Therapeutic regulation of systemic inflammation in xenograft recipients. Xenotransplant 2017;24(2). doi: 10.1111/xen.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao H, Zhang Q, Chen J, et al. Porcine IL-6, IL-1β, and TNF-α regulate the expression of pro-inflammatory-related genes and tissue factor in human umbilical vein endothelial cells. Xenotransplant 2018;25(5):e12408. doi: 10.1111/xen.12408 [DOI] [PubMed] [Google Scholar]
- 26.Zhang G, Iwase H, Wang L, et al. Is interleukin-6 receptor blockade (tocilizumab) beneficial or detrimental to pig-to-baboon organ xenotransplantation? Am J Transplant 2020;20(4):999–1013. [DOI] [PubMed] [Google Scholar]
- 27.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplant 2017;24(2):e12293. doi: 10.1111/xen.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird CT, Hassanein W, O’Neill NA, et al. P- and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplant 2018;25(2):e12381. doi: 10.1111/xen.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezzelarab MB, Liu YW, Lin CC, et al. Role of P-selectin and P-selectin glycoprotein ligand-1 interaction in the induction of tissue factor expression on human platelets after incubation with porcine aortic endothelial cells. Xenotransplant 2014;21(1):16–24. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer S, Zorn GL 3rd, Kelishadi S, et al. Role of anti-Gal alpha-13Gal and anti-platelet antibodies in hyperacute rejection of pig lung by human blood. Ann Thorac Surg 2001;72(5):1681–1689. [DOI] [PubMed] [Google Scholar]
- 31.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplant 2015;22(4):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azimzadeh AM, Zorn GL 3rd, Blair KS, et al. Hyperacute lung rejection in the pig-to-human model. 2. Synergy between soluble and membrane complement inhibition. Xenotransplant 2003;10(2):120–131. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer S, Zorn GL 3rd, Blair KS, et al. Hyperacute lung rejection in the pig-to-human model. 4: evidence for complement and antibody independent mechanisms. Transplant 2005;79(6):662–671. [DOI] [PubMed] [Google Scholar]
- 34.Wu G, Pfeiffer S, Schröder C, et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplant 2007;14(1):34–47. [DOI] [PubMed] [Google Scholar]
- 35.Gaca JC, Palestrant D, Lukes DJ, et al. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res 2003;112(1):19–25. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima N, Bouchart F, Gundry SR, et al. The role of anti-pig antibody in pig-to-baboon cardiac xenotransplant rejection. Transplant 1994;57(6):923–928. [DOI] [PubMed] [Google Scholar]
- 37.Sablinski T, Gianello PR, Bailin M, et al. Pig to monkey bone marrow and kidney xenotransplantation. Surgery 1997;121(4):381–391. [DOI] [PubMed] [Google Scholar]
- 38.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplant 2000;69(12):2504–2515. [DOI] [PubMed] [Google Scholar]
- 39.Galbusera M, Buelli S, Gastoldi S, et al. Activation of porcine endothelium in response to xenogeneic serum causes thrombosis independently of platelet activation. Xenotransplant 2005;12(2):110–120. [DOI] [PubMed] [Google Scholar]
- 40.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol 2009;21(2):75–80. doi: 10.1016/j.trim.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 2014;14(2):488–489. doi: 10.1111/ajt.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg 2015;23(Pt B):296–300. doi: 10.1016/j.ijsu.2015.07.682 [DOI] [PubMed] [Google Scholar]
- 43.Pierson RN III, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplant 2009;16(5):263–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brummel-Ziedins K, Mann KG. Molecular basis of blood coagulation. In: Hoffman R, Benz EJ, Silberstein LE, et al. , eds. Hematology 7th ed. Amsterdam, the Netherlands: Elsevier; 2018:1885–1905.e8. [Google Scholar]
- 45.Bae JS, Yang L, Manithody C, et al. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood 2007;110(12):3909–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost 2006;32(Suppl 1):49–60. [DOI] [PubMed] [Google Scholar]
- 47.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood 2007;109(8):3161–3172. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol 2008;172(6):1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Cooper DKC, Burdorf L, et al. Overcoming coagulation dysregulation in pig solid organ transplantation in nonhuman primates: recent progress. Transplant 2018;102(7):1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen BH, Zwets E, Schroeder C, et al. Beyond antibody-mediated rejection: hyperacute lung rejection as a paradigm for dysregulated inflammation. Curr Drug Targets Cardiovasc Haematol Disord 2005;5(3):255–269. [DOI] [PubMed] [Google Scholar]
- 51.Connolly MR, Burdorf L, Abady Z, et al. Humanized von Willebrand factor reduces platelet sequestration in pig xenogeneic lung perfusion and pig-to-primate lung transplantation. Transplant. Under review for publication
- 52.Tena A, Kurtz J, Leonard DA, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant 2014;14(12):2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplant 2018;25(5):e12391. doi: 10.1111/xen.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chihara RK, Paris LL, Reyes LM, et al. Primary porcine kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplant 2011;92(7):739–744. [DOI] [PubMed] [Google Scholar]
- 55.Brock LG, Delputte PL, Waldman JP, et al. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant 2012;12(12):3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bongoni AK, Kiermeir D, Denoyelle J, et al. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplant 2015;99(4):693–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


