Abstract
Objective
Early atrial arrhythmia recurrence following atrial fibrillation (AF) ablation is common. Current guidelines promulgate a 3-month blanking period. We hypothesize that early atrial arrhythmia recurrence during the blanking period may predict longer-term ablation outcomes.
Methods and results
A total of 688 patients with persistent AF undergoing catheter ablation were included in the DECAAF II trial database. The primary endpoint of the study was the first confirmed recurrence of atrial arrhythmia. Recurrence was also monitored during the 90-day blanking period. A total of 287 patients experienced recurrent atrial arrhythmia during the blanking period, while 401 remained in sinus rhythm. Rates of longer-term arrhythmia recurrence were substantially higher among those who developed recurrence during the blanking period compared to those who remained in sinus rhythm throughout the blanking period (68% vs. 32%, P < 0.001). The study cohort was divided into three groups according to the timing of arrhythmia recurrence during the blanking period. Of those who had recurrent arrhythmia during the first month of the blanking period (Group 1), 43.9% experienced longer-term recurrence, compared to 61.6% who recurred during the second month of the blanking period (Group 2), and 93.3% of those who had arrhythmia recurrence during the third month (Group 3, P < 0.001). The risk of recurrent arrhythmia was highest in Group 3 (HR = 10.15), followed by Group 2 (HR = 2.35) and Group 1 (HR = 1.5). Receiver operating characteristic analysis was performed to assess the relationship between the timing of arrhythmia recurrence and the primary outcome (AUC = 0.746, P < 0.001). The optimal blanking period duration was identified as 34 days. Atrial fibrillation burden determined by smartphone electrocardiogram technology over the 18 months follow-up period was significantly higher in Group 3 (29%) compared to Groups 1 (6%) and 2 (7%) and in patients who stayed in sinus rhythm during the blanking period (5%) (P < 0.0001).
Conclusion
Early atrial arrhythmia recurrence during the blanking period, particularly during the third month, is significantly associated with later recurrence. Although a blanking period is warranted, it should be abbreviated.
Keywords: Catheter ablation, Atrial fibrillation, Blanking period, Arrhythmia recurrence, Atrial fibrillation burden
Graphical Abstract
Graphical Abstract.
What’s new?
Early atrial arrhythmia recurrence during the blanking period, particularly during the third month, is significantly associated with later recurrence.
Although a blanking period is warranted, it should be abbreviated, allowing for earlier retreatment of arrhythmia if indicated.
Atrial fibrillation burden assessed by smartphone electrocardiogram technology is significantly higher in patients who have recurrence during the third month of the blanking period compared to their counterparts who had recurrence during the first and second months of the blanking period.
Introduction
Catheter ablation (CA) is a common rhythm-control strategy for the management of atrial fibrillation (AF). Current guidelines recommend a 90-day blanking period following CA during which arrhythmia recurrence is not considered a procedural outcome.1 Early arrhythmia recurrence during the blanking period is common, and the longer-term prognostic significance of these early recurrences remains controversial. Approximately 50% of those who develop early arrhythmia recurrence during this monitoring period do not experience longer-term recurrence.1–3 Several factors account for these transient recurrences, including the inflammation caused by the ablation lesions,4,5 a temporary alteration in autonomic balance6 and immaturity of the ablation scar.7
Although the 90-day blanking period remains standard of care, recent studies have demonstrated that early recurrence during the blanking period provides important prognostic information with respect to long-term CA outcomes and have advocated for a shorter blanking period.8–10
The aims of our study were two-fold: (i) to identify the prognostic significance of arrhythmia recurrence during the blanking period and (ii) to define an alternative blanking period duration that is sensitive and specific for longer-term arrhythmia recurrence.
Methods
Study population
Details of the DECAAF II trial have been published elsewhere.11 In short, the DECAAF II trial was a large investigator-initiated, industry-sponsored, prospective, multicenter (44 sites, three continents), randomized controlled clinical trial in which 815 patients with persistent AF were randomized into two treatment arms comparing magnetic resonance imaging (MRI)-guided fibrosis ablation+pulmonary vein isolation (PVI) vs. PVI alone. To be enrolled in the trial, patients had to have persistent AF (defined as 7 days or more of AF as evidenced by either rhythm strip or documentation on chart review) and must have been undergoing de novo AF ablation. Major exclusion criteria were contraindication to gadolinium and/or MRI and previous AF ablation or valvular cardiac surgery.
Follow-up
All patients received a handheld smartphone electrocardiogram (ECG) device (ECG Check, Cardiac Designs, Spring TX) and were required to record daily ECG strips in addition to sending a strip to the ECG core laboratory if they experienced symptoms during the follow-up period. Ambulatory monitoring and 12-lead ECG data performed as part of clinical care were also included. All ECG strips were transmitted automatically to the ECG core laboratory at the University of Washington and were analysed by trained experts masked from treatment assignment.
Atrial fibrillation burden
All patients from the DECAAF II mobile ECG database with at least 10 ECG strips submitted for analysis were included. All patients with fewer than 10 single-lead ECG strips submitted during the blanking period (0–90 days) were excluded from the analysis. Atrial Fibrillation burden was defined as the proportion of days on which the submitted ECG strips showed evidence of AF, out of the total number of days on which ECG strips were submitted for each patient during a specified period. If multiple ECG strips were provided on the same day, and all showed sinus rhythm, that day was considered a sinus rhythm day. However, if at least one ECG strip showed evidence of AF on that day, the day was classified as an AF day. Clinical and ambulatory ECGs were not used in the calculation of AF burden for this analysis.
Primary outcome
The primary end point of the study was the first confirmed recurrence of atrial arrhythmia (including AF, atrial flutter or atrial tachycardia) lasting for at least 30 s after the 90-day blanking period, demonstrated by at least two consecutive 1-lead smartphone ECG device tracings, 1 positive reading on a clinical 12-lead ECG tracing, ambulatory monitor or if the patient underwent repeat ablation. The daily smartphone ECGs were intended as the primary method for assessing atrial arrhythmia recurrence, but clinical and ambulatory ECGs served as back-up methods for detecting recurrence in patients who failed to reliably transmit smartphone ECG readings. A core laboratory at the University of Washington adjudicated the ECG findings. The last recurrence within the 3-month blanking period was tracked as a continuous variable. It was subsequently sorted based on whether it happened in the first, second, or third month of the blanking period.
Statistics
All continuous variables are presented as mean ± SD and compared using Student’s t-tests and Mann–Whitney tests, according to the results of normality assumption check through Shapiro–Wilk test. Categorical variables are presented as percentages or frequencies and compared using Chi-square tests. A Cox proportional hazards model was used to assess for independent factors associated with recurrence during the blanking period. The proportional hazard assumption has been checked by introducing an interaction term of log time. Receiver operating characteristic curve analysis was performed to assess the correlation of day-to-recurrence during the blanking period with late recurrence after the blanking period. Kaplan–Meier method was used to determine time to recurrence, and comparison was done using the log rank test. All the statistical analyses were conducted with R 4.2.0 (http://www.R-project.org, The R Foundation) with a two-sided significance level of 0.05 by default.
Results
Baseline characteristics
A total of 688 patients with at least 10 ECG strips were included in this analysis. Two hundred and eighty-seven patients had recurrent atrial arrhythmia during the blanking period, while 401 remained in sinus rhythm. Patients who experienced recurrence during the blanking period tended to be slightly older (62.9 vs. 61.2 years, P = 0.017), had more left atrial fibrosis (19.6 vs. 18%, P = 0.004) and a higher left atrial volume (140.6 vs. 124.2 mL, P < 0.001) at baseline, and were less likely to be taking antiarrhythmic medications (51 vs. 41%, P = 0.008). Baseline characteristics, comorbidities and medication history are shown in Table 1. Table 2 shows the baseline characteristics of patients in whom the last arrhythmia recurrence during the blanking period occurred in the first month (Group 1), second month (Group 2) and third month (Group 3). The breakdown of ECG strip classifications throughout the blanking period and the total follow-up is represented in Supplementary material online, Table S1. The initial randomization effect from the DECAAF II trial was assessed in Supplementary material online, Table S2 of the supplement. Table S3 shows the effect of baseline fibrosis and ablation scar on early recurrence of arrhythmia and late recurrence.
Table 1.
Baseline characteristics
| No Recurrence in BP | Recurrence in BP | P-value | |
|---|---|---|---|
| n | 401 | 287 | |
| PVI + MRI (%) | 202 (50.4) | 138 (48) | ns |
| Age (y) | 61.2 | 62.9 | 0.017 |
| Sex (Female %) | 86 (21) | 54 (18.8) | ns |
| Baseline Fibrosis (%) | 18.0 | 19.6 | 0.004 |
| AAD (%) | 206 (51.4) | 118 (41.1) | 0.008 |
| Beta Blockers (%) | 306 (76.3) | 205 (71.4) | ns |
| CCB (%) | 86 (21.4) | 67 (23.3) | ns |
| ACEi (%) | 113 (28.1) | 79 (27.5) | ns |
| ARB (%) | 107 (26.7) | 77 (26.8) | ns |
| Statin (%) | 128 (31.9) | 103 (35.9) | ns |
| Anticoagulation (%) | 389 (97) | 272 (94.8) | ns |
| CHF (%) | 75 (18.7) | 53 (18.4) | ns |
| HTN (%) | 234 (58.35) | 176 (61.3) | ns |
| DM (%) | 47 (11.7) | 26 (9) | ns |
| Stroke (%) | 27 (6.7) | 24 (8.4) | ns |
| VascularDx (%) | 46 (11.5) | 24 (8.4) | ns |
| Tobacco (%) | 153 (38.1) | 117 (41) | ns |
| CAD (%) | 50 (12.5) | 30 (10.4) | ns |
| CABG (%) | 4 (1) | 5 (1.7) | ns |
| Hyperlipidemia (%) | 139 (34.7) | 97 (33.8) | ns |
| Recurrence at 18-month (%) | 130 (32.4) | 196 (68.2) | 0.0001 |
| Time to recurrence (days) | 344 | 180 | 0.0001 |
| LA Volume (mL) | 124.2 | 140.6 | 0.0001 |
| Smartphone ECG recordings during the blanking period | 75 | 78 | ns |
MRI, magnetic resonance imaging; PVI, pulmonary vein isolation.
Table 2.
Baseline characteristics of the different groups. (*: no significant difference)
| No Recurrence in BP | Recurrence in 1st mo (Group 1) | Recurrence in 2nd mo (Group 2) | Recurrence in 3rd mo (Group 3) | P-value | |
|---|---|---|---|---|---|
| n | 401 | 107 | 60 | 120 | |
| PVI + MRI (%) | 202 (50.4) | 52 (48.5) | 24 (40) | 62 (51.7) | ns |
| Age (y) | 61.2 | 62.2 | 63.0 | 63.5 | ns |
| Sex (Female %) | 86 (21) | 14 (13) | 13 (21) | 27 (22.5) | ns |
| Baseline Fibrosis (%) | 18.0 | 19.3 | 19.7 | 19.8 | ns |
| AAD (%) | 206 (51.4) | 42 (39.25) | 27 (45) | 49 (40.8) | ns |
| Beta Blockers (%) | 306 (76.3) | 78 (72.9) | 39 (65) | 88 (73.3) | ns |
| CCB (%) | 86 (21.4) | 30 (28) | 14 (23.3) | 23 (19.2) | ns |
| ACEi (%) | 113 (28.1) | 30 (28) | 16 (26.7) | 33 (27.5) | ns |
| ARB (%) | 107 (26.7) | 31 (29) | 11 (18.3) | 35 (29.2) | ns |
| Statin (%) | 128 (31.9) | 37 (34.6) | 18 (30) | 48 (40) | ns |
| Anticoagulation (%) | 389 (97) | 104 (97.2) | 57 (95) | 111 (92.5) | ns |
| CHF (%) | 75 (18.7) | 19 (17.8) | 11 (18.3) | 23 (19.2) | ns |
| HTN (%) | 234 (58.35) | 74 (69.15) | 34 (56.7) | 68 (56.7) | ns |
| DM (%) | 47 (11.7) | 10 (9.3) | 3 (5) | 13 (10.83) | ns |
| Stroke (%) | 27 (6.7) | 7 (6.5) | 5 (8.3) | 12 (10) | ns |
| VascularDx (%) | 46 (11.5) | 6 (5.6) | 5 (8.3) | 13 (10.8) | ns |
| Tobacco (%) | 153 (38.1) | 37 (34.6) | 26 (43.4) | 54 (45) | ns |
| CAD (%) | 50 (12.5) | 9 (8.4) | 7 (11.7) | 14 (11.7) | ns |
| CABG (%) | 4 (1) | 0 (0) | 2 (3.3) | 3 (2.5) | ns |
| Hyperlipidemia (%) | 139 (34.7) | 35 (32.7) | 20 (33.3) | 42 (35) | ns |
| Recurrence at 18 months (%) | 130 (32.4) | 47 (44) | 37 (61.7) | 112 (93.3) | 0.0001 |
| Time to recurrence (days) | 344 | 285* | 234* | 58 | 0.0001 |
| LA Volume (mL) | 124.2 | 139.7* | 140.1* | 141.7* | 0.001 |
| Smartphone ECG recordings per month during the blanking period | 25.1 | 24.7 | 25.3 | 26.9 | ns |
| Smartphone ECG recordings per month after the blanking period | 21 | 21.5 | 21 | 19.8 | ns |
ECG, electrocardiogram; PVI, pulmonary vein isolation; MRI, magnetic resonance imaging.
Arrhythmia recurrence
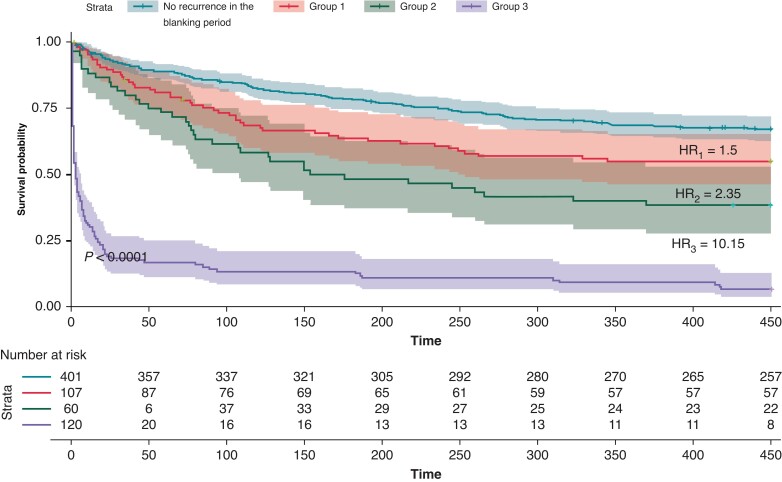
At the end of the 18-month follow-up period, the recurrence rate was significantly higher among patients who had recurrent arrhythmia during the blanking period compared to those who remained in sinus rhythm (68% vs. 32%, P < 0.001). Longer-term arrhythmia recurrence rates were significantly higher in those in Group 3 (93.3%), compared to those in Groups 2 (61.6%) and 1 (43.9%, P < 0.001).
Time-to-primary-outcome was significantly shorter in Group 3 (58 days) than in Groups 1 (285 days) and 2 (234 days, P < 0.001). Survival analysis was performed to evaluate the time to recurrence in the different groups. The risk of arrhythmia recurrence was highest in Group 3 [HR3 = 10.15, 95% (CI 7.8–13.21), P < 0.001], followed by group 2 [HR2 = 2.35, 95% CI (1.63–3.39), P < 0.001] and group 1 [HR1 = 1.5, 95% CI (1.07–2.09), P = 0.018] (Figure 1).
Figure 1.
Primary endpoint of atrial arrhythmia recurrence in the different groups.
Optimal blanking period duration
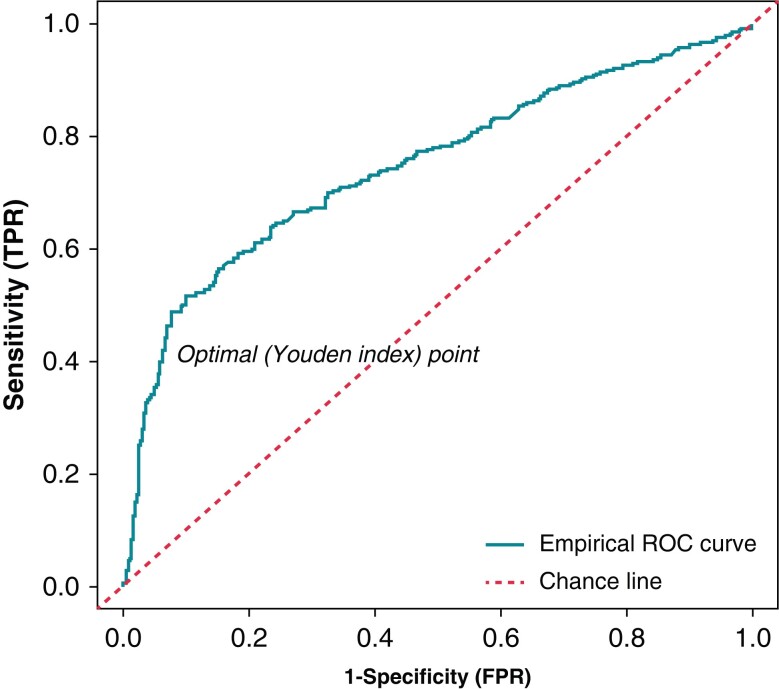
Receiver operating characteristic curve analysis was performed to assess the association between the timing of arrhythmia recurrence during the blanking period and the primary outcome (AUC = 0.746, P < 0.001). The most discriminatory value was 34 days with a Youden index of 0.41 (Figure 2).
Figure 2.
Receiver operator characteristics curve to assess correlation of day of recurrence during the blanking period and atrial arrhythmia recurrence.
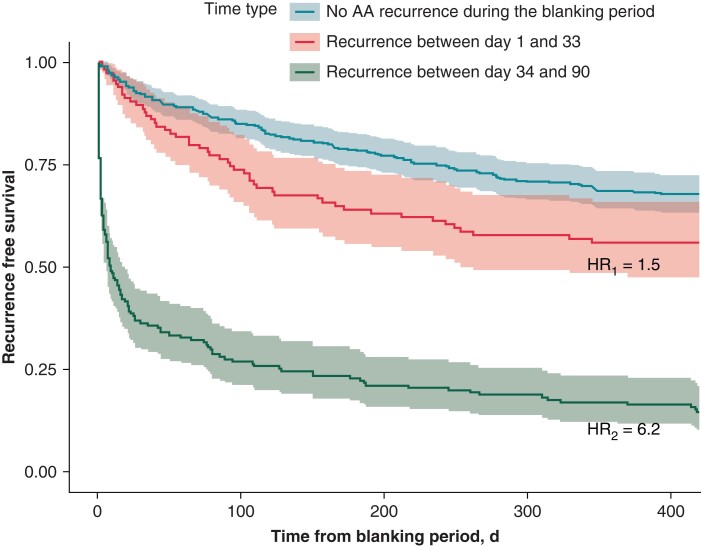
The risk of longer-term recurrence was significantly higher in patients who had arrhythmia recurrence after 34 days [HR = 6.18, 95% CI (4.9–7.9), P < 0.001] (Figure 3). The positive predictive value (PPV) of late recurrence if early recurrence occurred between 34 and 90 days was 85%. The PPV of late recurrence if early recurrence occurred between 45 and 90 days was 90%, and the PPV of late recurrence if early recurrence occurred between 60 and 90 days was 95%. The NPV of staying in SR during the blanking period was 65.2%.
Figure 3.
Primary endpoint of atrial arrhythmia recurrence in patients who had early recurrence between 1–33 days and 34–90 days.
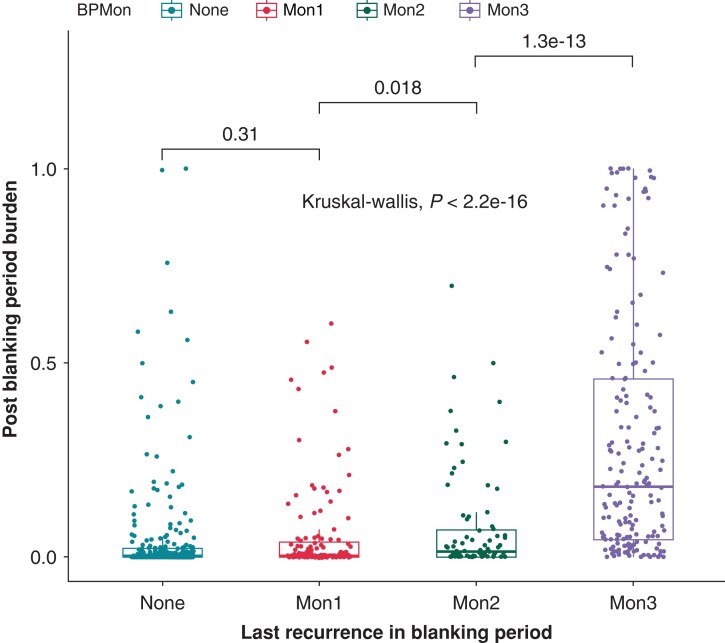
Atrial fibrillation burden
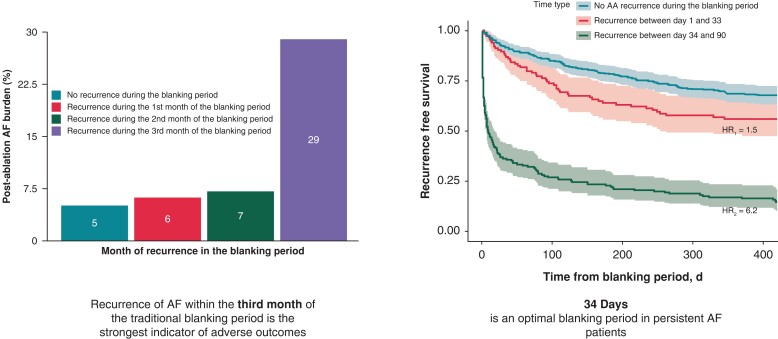
Patients who had recurrence during the third month (29%) of the blanking period had a significantly higher AF burden over the 18-month follow-up period compared to patients who had recurrence during the first (6%) and second month (7%) of the blanking period and compared to patients who did not have recurrence in the blanking period (5%) (P < 0.0001). Additionally, AF burden was significantly higher in in Group 2 (7%) compared to Group 1 (6%) (P = 0.02) (Figure 4).
Figure 4.
Post-ablation AF burden comparison in the different groups.
Discussion
The main findings of our study are: (i) early arrhythmia recurrence during the blanking period is prognostic of longer-term ablation outcomes; (ii) arrhythmia recurrence during the third month of the blanking period has the highest predictive value for subsequent recurrence; and (iii) the currently recommended 90-day blanking period should be abbreviated. Our study suggests an optimal blanking period duration of 34 days.
The blanking period is currently considered a monitoring period during which arrhythmia recurrence is not considered a procedural outcome, as several factors such as inflammation and lesion immaturity contribute to increased arrhythmogenicity during this timeframe.1–3 However, several studies have shown that arrhythmia recurrence during the blanking period may predict longer-term procedural outcomes.8,9,12 In our population, 42% of patients had a recurrence within the first 3 months. Our data show a clear gradient of arrhythmia recurrence risk depending on the timing of early recurrence during the blanking period, as the hazard ratio increases from 1.5 in the first month to 2.35 in the second and 10.15 in the third month. In a post hoc analysis of the ADVICE study, Willems et al. show a similar gradient with a hazard ratio increasing from 1 at 1 month to 4 at 2 months and 9 at 3 months.8 Alipour et al. report similar results in their cohort, with odds ratios ranging from 4.35 at month 1 to 9.06 at month 2 and 20.8 at month 3 in a multivariable model.9 Furthermore, in the EAST-AF study population, Onishi et al. show that arrhythmia-free survival is significantly reduced in patients who experience a recurrence in the ‘late phase’ of the blanking period, defined as 30–90 days, compared with patients who experience a recurrence during the first month of the blanking period (17.1% vs. 38%, P < 0.001).12
Several pathophysiological processes are associated with early recurrence, such as the inflammatory process resulting from the energy delivered during ablation lesions,4,5 a transient imbalance in autonomic nervous system activation6 and immaturity of the ablation scar.7 Early recurrences occur most frequently in the first month of the blanking period, especially in the first 2 weeks after the ablation procedure when the transitory processes are at their peak.12–14 In the later stages of the blanking period, recurrence is usually due to pulmonary vein reconnection. Das et al. reported that pulmonary vein reconnection causes early recurrence during the second and third months of the blanking period.15 Nevertheless, recurrence during the first month of the blanking period significantly increased the risk of subsequent arrhythmia recurrence in our population, albeit weakly (HR = 1.5). Willems et al. also reported a significant hazard ratio of 1.84 during the first month,8 and Xue et al. even reported significant predictive power of recurrences occurring during the first 7 days after the ablation procedure.16 This can be explained by a minority of patients experiencing acute pulmonary vein reconnection after ablation, implying early failure of the procedure. In our population, 93.3% of patients who experienced an early recurrence in the third month of the blanking period had another recurrence after an average of 58 days. This is consistent with a study by Willems et al. who found a recurrence rate of 92.2% in the same population.8 It is highly suggestive that recurrences in the third month of the blanking period are mostly due to pulmonary vein reconnection. It is even debatable whether recurrences in the third month should be considered as early recurrences and directly considered as procedure-related outcomes, similar to any recurrence after the 90-day blanking period. In practice, a physician-based survey carried out by the European Heart Rhythm Association (EHRA), which gathered responses from 436 cardiologists, indicated that nearly 28% of the participants considered recurrences in the third month of the blanking period as an independent predictor of late recurrences.17 Another significant contribution of our study is the calculation of AF burden using single-lead ECG strips. Our findings indicate that while ERAFs during the first and second months of the blanking period are statistically correlated with later recurrences, they do not signify a significantly worse outcome compared to recurrences in the third month. These later recurrences are associated with the highest AF burden post-blanking period.
Our data show that the optimal length of the blanking period should be 34 days, because 34 days has the highest discriminatory ability with a Youden index of 0.41. Willem et al. suggest an optimal blanking period of 50 days with a Youden index of 0.58.8 Similarly, Alipour et al. propose an optimal blanking period of 23 days with a Youden index of 0.3.9 A recent meta-analysis conducted by Saglietto et al. suggested a 4-week optimal blanking period post-CA, corroborating our findings.18 The difference in our results is due to the fact that our population exclusively represents patients with persistent AF, and we used smartphone ECG monitoring of arrhythmias, which provides a more comprehensive picture of the arrhythmia profile during the follow-up period and more accurate results than the regular monitoring methods used in previous studies. However, even though 34 days is statistically the optimal cut-off point, implementation in practice is challenging. Even though patients who had an early recurrence after 34 days had a hazard ratio of recurrence of 6.19 compared with patients who had an early recurrence before 34 days, 15% of patients from the former group did not present with later recurrence, which presents a challenge for early management in the clinic. Nevertheless, early recurrence after 60 days of the blanking period has a positive predictive value of 95% for later recurrence. Considering the physiological process of scar formation, it is also very likely that recurrence in the third month of the blanking period should be considered an indicator of treatment failure and not disregarded as a transient event.
If a shortened blanking period is adopted, this could have implications for the treatment of recurrences, which would potentially include early retreatment. There are data suggesting that early treatment of recurrence is beneficial. Malasana et al. showed that cardioversion during the blanking period is beneficial.19 Lellouche et al. showed that in patients who develop an early recurrence of arrhythmia, repeat ablation in the first month is associated with lower recurrence rates than in patients without redo ablation at the end of an 11-month follow-up period (51% vs. 91%, P < 0.001).20 However, the disadvantage of early redo ablation is the additional procedures, hospitalization and higher healthcare utilization, as seen in Lellouche's study, in which patients with early repeat ablation had a higher total number of procedures throughout the follow-up period (2.5 vs. 2.2, P = 0.02). Pokushalov et al. confirmed these findings in a prospective randomized trial showing that early redo ablation for triggered AF recurrence during the blanking period was superior to 6 weeks of transient AAD treatment in reducing subsequent recurrences during the 12-month follow-up period.21 However, Pokushalov et al. showed that the strategy of early reablation did not result in a significant increase in overall recurrences (1.79 vs. 1.82, P = 0.28).21 Early AAD therapy has also been studied. In the 5A trial, Roux et al. reported that AAD therapy in the first 6 weeks after ablation led to a reduction in arrhythmia recurrences during the same period (19% vs. 42%, P = 0.005).22 However, in a follow-up study, the recurrence rate at 6 months was similar between the two groups (72% vs. 68%, P = 0.84).23 A post hoc analysis of the EAST-AF trial confirmed these findings, as AAD therapy during the 90-day blanking period did not significantly affect event-free rates at 1-year follow-up (69.5% vs. 67.8%, P = 0.38).24 According to the EHRA survey, a majority of electrophysiologists tend to favour the use of cardioversion and antiarrhythmic drugs over early re-ablation during the blanking period. However, 20% are inclined towards early re-ablation if the patient experiences multiple early recurrences.17
Limitations
Although this study is a post-hoc analysis of the DECAAF II randomized controlled trial, the analysis is still retrospective, which introduces bias. Follow-up was performed using smartphone ECG recordings. Although this method allows dynamic assessment, it is not as accurate as continuous monitoring with implantable monitors, despite the obvious advantages of this technology. Nevertheless, the ECG recording rate was 0.84/patient/day in our population, which should allow accurate assessment of the arrhythmia profile. In addition, patients did not submit the same number of strips for analysis, which constitutes a limitation to the uniform calculation of AF burden and to this study. Finally, we excluded participants who provided fewer than 10 ECG recordings to ensure a reliable monitoring methodology throughout the blanking period, which aimed to guarantee the accuracy of the results. However, this exclusion criterion may have inadvertently introduced selection bias into the analysis.
Conclusion
Early atrial arrhythmia recurrence during the blanking period after catheter ablation for AF is predictive of later recurrences. Recurrence in the third month of the blanking period is the strongest predictor of long-term ablation outcome. A blanking period is warranted but should be shortened to less than 90 days.
Supplementary Material
Contributor Information
Charbel Noujaim, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Chanho Lim, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Mario Mekhael, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Han Feng, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Nour Chouman, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Hadi Younes, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Ala Assaf, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Botao Shan, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Ghaith Shamaileh, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Aneesh Dhore-Patil, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Daniel Nelson, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Brennan Lanier, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Noor Makan, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Nassir Marrouche, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Eoin Donnellan, Tulane Research Innovation for Arrhythmia Discovery, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70130, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
The DECAAF II trial was funded by Medtronic, GE, Siemens, Boston Scientific, Biosense Webster and Abbott.
Data availability
Data will be made available upon reasonable request.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrade JG, Macle L, Khairy P, Khaykin Y, Mantovan R, De Martino Get al. . Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol 2012;23:1295–301. [DOI] [PubMed] [Google Scholar]
- 3. Joshi S, Choi AD, Kamath GS, Raiszadeh F, Marrero D, Badheka Aet al. . Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol 2009;20:1089–94. [DOI] [PubMed] [Google Scholar]
- 4. Lim HS, Schultz C, Dang J, Alasady M, Lau DH, Brooks AGet al. . Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:83–9. [DOI] [PubMed] [Google Scholar]
- 5. Liang JJ, Dixit S, Santangeli P. Mechanisms and clinical significance of early recurrences of atrial arrhythmias after catheter ablation for atrial fibrillation. World J Cardiol 2016;8:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masuda M, Yamada T, Mizuno H, Minamiguchi H, Konishi S, Ohtani Tet al. . Impact of atrial fibrillation ablation on cardiac sympathetic nervous system in patients with and without heart failure. Int J Cardiol 2015;199:65–70. [DOI] [PubMed] [Google Scholar]
- 7. Richardson WJ, Clarke SA, Quinn TA, Holmes JW. Physiological implications of myocardial scar structure. Compr Physiol 2015;5:1877–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willems S, Khairy P, Andrade JG, Hoffmann BA, Levesque S, Verma Aet al. . Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation. Circulation: Arrhythmia and Electrophysiology 2016;9:e003909. [DOI] [PubMed] [Google Scholar]
- 9. Alipour P, Azizi Z, Pirbaglou M, Ritvo P, Pantano A, Verma Aet al. . Defining blanking period post-pulmonary vein antrum isolation. JACC Clin Electrophysiol 2017;3:568–76. [DOI] [PubMed] [Google Scholar]
- 10. Riahi S, Larsen JM. The 3-month post-atrial fibrillation ablation blanking period: time to redefine? Europace 2020;22:1759–60. [DOI] [PubMed] [Google Scholar]
- 11. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. . Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onishi N, Kaitani K, Nakagawa Y, Inoue K, Kobori A, Nakazawa Yet al. . The association between late-phase early recurrence within the blanking period after atrial fibrillation catheter ablation and long-term recurrence: insights from a large-scale multicenter study. Int J Cardiol 2021;341:39–45. [DOI] [PubMed] [Google Scholar]
- 13. Mugnai G, de Asmundis C, Hünük B, Ströker E, Velagic V, Moran Det al. . Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: predictive role of atrial arrhythmias occurring in the blanking period on the incidence of late recurrences. Heart Rhythm 2016;13:845–51. [DOI] [PubMed] [Google Scholar]
- 14. Zink MD, Chua W, Zeemering S, di Biase L, Antoni BL, David Cet al. . Predictors of recurrence of atrial fibrillation within the first 3 months after ablation. Europace 2020;22:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das M, Wynn GJ, Morgan M, Lodge B, Waktare JEP, Todd DMet al. . Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhyth Electrophysiol 2015;8:846–52. [DOI] [PubMed] [Google Scholar]
- 16. Xue Y, Wang X, Thapa S, Wang L, Wang J, Xu Zet al. . Very early recurrence predicts long-term outcome in patients after atrial fibrillation catheter ablation: a prospective study. BMC Cardiovasc Disord 2017;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bordignon S, Barra S, Providencia R, de Asmundis C, Marijon E, Farkowski MMet al. . The blanking period after atrial fibrillation ablation: an European heart rhythm association survey on contemporary definition and management. Europace 2022;24:1684–90. [DOI] [PubMed] [Google Scholar]
- 18. Saglietto A, Ballatore A, Xhakupi H, Rubat Baleuri F, Magnano M, Gaita Fet al. . Evidence-based insights on ideal blanking period duration following atrial fibrillation catheter ablation. Europace 2022;24:1899–908. [DOI] [PubMed] [Google Scholar]
- 19. Malasana G, Day JD, Weiss JP, Crandall BG, Bair TL, May HTet al. . A strategy of rapid cardioversion minimizes the significance of early recurrent atrial tachyarrhythmias after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:761–6. [DOI] [PubMed] [Google Scholar]
- 20. Lellouche N, Jaïs P, Nault I, Wright M, Bevilacqua M, Knecht Set al. . Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J Cardiovasc Electrophysiol 2008;19:599–605. [DOI] [PubMed] [Google Scholar]
- 21. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Turov A, Shirokova Net al. . Use of an implantable monitor to detect arrhythmia recurrences and select patients for early repeat catheter ablation for atrial fibrillation. Circ Arrhyth Electrophysiol 2011;4:823–31. [DOI] [PubMed] [Google Scholar]
- 22. Roux J-F, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FEet al. . Antiarrhythmics after ablation of atrial fibrillation (5A study). Circulation 2009;120:1036–40. [DOI] [PubMed] [Google Scholar]
- 23. Leong-Sit P, Roux J-F, Zado E, Callans DJ, Garcia F, Lin Det al. . Antiarrhythmics after ablation of atrial fibrillation (5A study). Circ Arrhyth Electrophysiol 2011;4:11–4. [DOI] [PubMed] [Google Scholar]
- 24. Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi Tet al. . Efficacy of antiarrhythmic drugs short-term use after catheter ablation for atrial fibrillation (EAST-AF) trial. Eur Heart J 2015;37:610–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.