Abstract
Spinal cord injury (SCI) results in rapid muscle loss. Exogenous molecular interventions to slow muscle atrophy after SCI have been relatively ineffective and require the search for novel therapeutic targets. Connexin hemichannels (CxHCs) allow nonselective passage of small molecules into and out of the cell. Boldine, a CxHC-inhibiting aporphine found in the boldo tree (Peumus boldus), has shown promising preclinical results in slowing atrophy during sepsis and restoring muscle function in dysferlinopathy. We administered 50 mg/kg/day of boldine to spinal cord transected mice beginning 3 days post-injury. Tissue was collected 7 and 28 days post-SCI and the gastrocnemius was used for multiomics profiling. Boldine did not prevent body or muscle mass loss but attenuated SCI-induced changes in the abundance of the amino acids proline, phenylalanine, leucine and isoleucine, as well as glucose, 7 days post-SCI. SCI resulted in the differential expression of ∼7,700 and ∼2,000 genes at 7 and 28 days, respectively, compared with Sham controls. Pathway enrichment of these genes highlighted ribosome biogenesis at 7 days and translation and oxidative phosphorylation at both timepoints. Boldine altered the expression of ∼150 genes at 7 days and ∼110 genes at 28 days post-SCI. Pathway enrichment of these genes indicated a potential role for boldine in suppressing protein ubiquitination and degradation at the 7-day timepoint. Methylation analyses showed minimal differences between groups. Taken together, boldine is not an efficacious therapy to preserve body and muscle mass after complete SCI, though it attenuated some SCI-induced changes across the metabolome and transcriptome.
NEW & NOTEWORTHY This is the first study to describe the multiome of skeletal muscle paralyzed by a spinal cord injury (SCI) in mice across the acute and subacute timeframe after injury. We show large-scale changes in the metabolome and transcriptome at 7 days post-injury compared with 28 days. Furthermore, we show that the alkaloid boldine was able to prevent SCI-induced changes in muscle glucose and free amino acid levels at 7 days, but not 28 days, after SCI.
Keywords: metabolomics, paralysis, skeletal muscle, spinal cord injury, transcriptomics
INTRODUCTION
Immobilization-related muscle atrophy is a highly coordinated process that occurs during periods of disuse. Sustained unloading increases protein degradation (1), reduces RNA translation efficiency (2), and promotes dietary anabolic resistance (3), all of which may lead to reduced size, function, and health of skeletal muscle. One of the most severe forms of muscle atrophy occurs after spinal cord injury (SCI). Motor-complete SCI [ASIA Impairment Score (AIS) A/B] results in paralysis and rapid loss in whole muscle mass and myofiber size leading to reduced contractile and metabolic function (4). In mice, our group has consistently observed rapid losses in muscle mass 7–14 days post-SCI, with continued losses out to 28 days post-SCI that then become relatively stable up to 84 days after injury (5–8). We have previously used unbiased metabolomics analyses to understand how SCI altered levels of metabolites in gastrocnemius muscle at 7 and 28 days after a complete spinal cord transection and found levels of glucose, pyruvate, and lactate were reduced at 7 but not 28 days (7). This acute disruption of metabolic function after SCI is likely to be related to other large-scale molecular changes within the muscle. However, the effects of acute and subacute SCI on the transcriptome and methylome of paralyzed muscle have not been extensively studied.
A growing number of mechanisms have been reported to drive the initial loss of muscle mass during disuse (9–11). Some of these mechanisms may be driven by connexin hemichannels (CxHCs) (12–17). CxHCs are nonselective pore proteins localized to the cytoplasmic membrane that can bind with CxHCs from adjacent cells to form gap junctions or remain as hemichannels that connect cytoplasmic and extracellular compartments. Gap junctions and CxHCs are essential for muscle differentiation, regeneration, and development (12) but are absent from the sarcolemma in healthy adult skeletal muscle fibers (12, 18). However, sciatic nerve transection (13), dexamethasone administration (14), and endotoxemia/sepsis (19) can cause the reappearance of sarcolemmal CxHCs in fast-twitch muscle fibers. Conditionally knocking out both Cx43 and Cx45 in skeletal muscle reduces muscle atrophy following a sciatic nerve transection (13), and inhibiting their activity improves muscle health (15, 16, 19, 20). Boldine [an alkaloid derived from the Chilean boldo tree (Peumus boldus)] can inhibit CxHC activity without blocking gap junction function (21) and can prevent sepsis-induced increases in cytosolic calcium concentrations and cytokine-induced small molecule dye uptake in isolated muscle fibers (19). Detailed analysis of the effect of sarcolemmal CxHC on the molecular profile of muscle has not been performed and whether sarcolemmal CxHC alters muscle metabolism or function is unknown.
Immunofluorescence studies indicate that a complete thoracic spinal cord transection leads to increased sarcolemmal expression of Cx39, Cx43, and Cx45 in the gastrocnemius of young adult rats at 56 days after surgery (13). However, it is unknown whether inhibition of CxHC activity in the acute and subacute timeframe protects against loss of muscle mass or metabolic perturbations (e.g., low glucose, pyruvate, and lactate) or other molecular abnormalities in skeletal muscle after SCI. Therefore, in this study, we aimed to: 1) describe how SCI affects the metabolome, transcriptome, and methylome of adult mouse gastrocnemius muscle; 2) determine if boldine could prevent losses in body and muscle mass following a complete thoracic SCI; and 3) determine if boldine could alter molecular profiles in a way that could improve muscle health and/or function.
METHODS
Animals
Male C57BL/6NCrl mice aged 4 mo were used for these studies. Mice were purchased from Charles River Laboratories (Wilmington, MA) and kept in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal facility at the James J. Peters Veterans Affairs Medical Center (VAMC) for a minimum of 2 wk before surgery. Mice were kept on a 12:12-h light-dark cycle with ad libitum access to standard chow (LabDiet rodent Diet No. 5001) and water. All mice were randomly selected for a 7 or 28 days post-surgery timepoint, then further randomized for a laminectomy (Sham) or laminectomy + complete T10 spinal cord transection with vehicle (SCIv) or boldine (SCIb) treatment. Thirty-two mice were used for this study, with n = 4 for both 7 and 28 days Sham groups and n = 6 for all 7 and 28 days SCI groups. All studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the James J. Peters VAMC (Protocol No. CAR-16-54).
Laminectomy and Spinal Cord Transection
Detailed methods for the laminectomy and spinal cord transection used by our group have been previously published (5). In brief, mice were weighed and then placed under deep anesthesia using inhalation of 2%–3% continuous-flow isoflurane. Hair along the back of the animal was shaved and the skin was cleaned with 70% ethanol and a 2% betadine solution. A sterile scalpel was then used to make an incision from T7-11 and the spinal column was exposed by blunt dissection and removal of the para-vertebral muscles. The vertebral arch of the T10 vertebral body was removed by cutting the lateral processes with surgical scissors and gently lifting with fine forceps. With the dura exposed, the incision site for Sham mice was closed in layers using sutures for the muscle layer and surgical staples for skin. For the SCI mice, a micro-scalpel was passed through the spinal cord. The transection site was inspected for residual tissue bridges, which were cut by a second pass of the scalpel when present. An inert gel foam was placed in between the ends of the severed spinal cord and the incision site was sutured closed as above, followed by placing the mice in a clean cage. Clean cages included Αlpha-dri+ bedding, standard chow placed on the floor for paralyzed mice, peanut butter, and Bio-Serv fruit crunch treats. All mice were single-housed for the remainder of the study.
Postoperative Care and Boldine Administration
Mice were kept on warming pads heated to 37°C with recirculating water for 24 h post-surgery. They were given a daily injection of ketophen (5 mg/kg) and Baytril (5 mg/kg) for 3 days post-surgery and a total daily volume of 1 mL of lactated Ringer’s solution for 7 days to prevent dehydration. Bladders were expressed two times per day, and mice were carefully monitored to ensure no signs of an incomplete transection (hindlimb movement, spasms, grooming, manifestations of pain, etc.). Mice were familiarized with peanut butter for a week before surgery and boldine was administered in a 1.0 g peanut butter bolus at a dose of 50 mg/kg/day starting at 3 days post-injury. All mice consumed 100% of their daily peanut butter by day 3 post-surgery and continued to do so throughout the remainder of the study.
Body and Tissue Mass
Mice were euthanized at 7 or 28 days post-surgery. Body mass was recorded before mice were placed under deep anesthesia using inhalation of 2%–3% continuous-flow isoflurane. Muscles [soleus, plantaris, gastrocnemius, tibialis anterior (TA) and extensor digitorum longus (EDL), biceps, triceps] were excised, wet-weighed, then flash-frozen in liquid nitrogen. Mice were euthanized using a combination of blood collection via ventricular puncture and cervical dislocation while under deep anesthesia. Although the major hindlimb muscles were collected, only the gastrocnemius was selected for all multiomics experiments as it is large enough after severe/complete SCI (∼60 mg for a 25 g mouse) for one single muscle to be used across all -omics platforms, allowing for better comparison across the different -omics data sets.
Metabolomics and Analysis
Approximately 10 mg of the left gastrocnemius containing equal portions of medial and lateral heads was sent on dry ice to West Coast Metabolomics, a regional NIH Resource Core located at the University of California-Davis, for untargeted primary metabolomics analyses using GC-TOF mass spectroscopy. Complete sample processing, data acquisition, and data processing have been reported in detail (22). Our group has used these parameters to interrogate changes in muscle from female mice at 7 and 28 days post-SCI (7) and others have used these parameters to describe other metabolic changes in mouse skeletal muscle (23). We analyzed peak spectra for each metabolite following normalization to the median, log transformation, and Pareto scaling. All spectra were matched to known metabolites using the BinBase algorithm (24) while unconfirmed metabolites were matched to numerical BinBase IDs with identical spectra and retention times. Four mice from each group were used for these analyses.
DNA Isolation and Reduced Representation Bisulfite Sequencing
Approximately 10 mg of the left gastrocnemius was cut to contain equal amounts of both medial and lateral heads and DNA was isolated after an overnight incubation in proteinase K using the Qiagen Blood and Tissue kit following the manufacturer’s guidelines. DNA was sent to Novogene, LLC for bisulfite conversion, library preparation, and reduced representation bisulfite sequencing (RRBS). DNA quality was checked with 1% agarose gel electrophoresis and quantified using a Qubit fluorometer (Thermo Fisher). Samples were digested with MspI and fragments were repaired, adenylated, and ligated to 5-methylcytosine-modified adapters. DNA fragments were size-selected (40–220 bp) using gel cutting and bisulfite treated using the Zymo Research EZ DNA Methylation Gold Kit. PCR amplification was used to generate the final DNA library. Library quality control was carried out by examining the inset size using the Agilent 2100 Bioanalyzer and the library was quantified using qPCR. Sequencing was then completed using a NovaSeq6000. DNA sequencing was completed on four Sham mice and six mice for both SCIv and SCIb groups at each timepoint.
RRBS Data Processing and Data Analysis
Paired-end fastq files generated from RRBS were checked for quality using FastQC (v0.11.9) (25), trimmed using Trim Galore (v0.6.6) and CutAdapt (v3.1) (26), and aligned against the GRCm38 reference genome using Bismark (27), which then generated methylation coverage files. From these coverage files, differentially methylated CpG sites (DMCs) were detected via methylKit [v1.16.1; (28)] in R [v4.03 (29)] and R Studio [v1.3.1093 (30)] across the different groups. To complement methylKit’s logistic regression-based model and provide additional validation, methylSig [v1.7.0, (31)] with its beta-binomial model was also used. HOMER motif enrichment analysis was performed on the promoter-associated (defined as CpG sites occurring in 0–5,000 bp upstream of a transcriptional start site) DMCs, ±10 bp, for each analysis using the “findMotifsGenome.pl” command (32). In addition, to look for possible methylation-based enhancer regulation, all DMCs were compared with EnhancerAtlas 2.0s database using the most relevant available tissue/cell type, “Gastrocnemius_muscle_myoblast.” The R code and list of R packages used can be found in Supplemental File S1 (https://doi.org/10.6084/m9.figshare.20419122).
RNA Isolation and Sequencing
RNA was isolated from ∼20 mg of the gastrocnemius cut to contain equal amounts of both medial and lateral heads of the gastrocnemius using the miRNeasy kit (Qiagen) according to the manufacturer’s recommendations, with minor deviations. Samples were homogenized in 1 mL Qiazol (Qiagen) with 5 µL β-mercaptoethanol using a beadmill homogenizer cooled with liquid nitrogen and then phase separated using 1-bromo-3-chloropropane (BCP). The clear phase was mixed with 100% ethanol and subjected to column isolation and centrifugation using buffer RWT and RPE as recommended by the manufacturer. The columns were then washed with two changes of 80% ethanol at 8,000 g for 60 s at room temperature. RNA was eluted with RNase-free water heated to 37°C, quantified at 260 nm with a Nanodrop (Thermo Fisher), and quality was checked with an Agilent 2100 Bioanalyzer. Samples were then shipped to Novogene, LLC for library preparation and mRNA sequencing with 1 μg of RNA per sample being used as input material for all RNA sample preparations. Sequencing libraries were generated using NEBNext UltraTM RNA Library Prep Kit for Illumina (New England BioLabs) following the manufacturer’s recommendations. Briefly, mRNA was purified from total RNA using polyT, oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). First-strand cDNA was synthesized using random hexamer primer and M-MuLV reverse transcriptase (RNase H minus). Second-strand cDNA synthesis was then performed using DNA polymerase I and RNase H plus. The remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of the 3′ ends of DNA fragments, NEBNext Adaptor with hairpin loop structure was ligated to prepare for hybridization. To select cDNA fragments of 150–200 bp, the library fragments were purified with AMPure XP system (Beckman Coulter). Three microliters of USER Enzyme (New England BioLabs) was then used with size-selected, adaptor-ligated cDNA at 37°C for 15 min, which was followed by another incubation of 5 min at 95°C before PCR. PCR was performed with Phusion High-Fidelity DNA polymerase (New England Biolabs), Universal PCR primers, and Index (X) Primer. Finally, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using PE Cluster Kit cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced using a NovaSeq6000 and paired-end reads (150 bp) were generated. RNA sequencing was completed on four Sham mice and six mice for both SCIv and SCIb groups at each timepoint.
RNAseq Data Processing and Analysis
Paired-end fastq files generated from sequencing were checked for quality using FastQC (v0.11.9) (33), then trimmed of adapters and low-quality reads (PHRED < 20) using Trim Galore (v0.6.6) and CutAdapt (26). Alignment to the mouse genome (GRCm38) and gene counts were then generated using Spliced Transcripts Alignment to a Reference [STAR (34)]. DESeq2 was used for differentially expressed gene (DEG) analyses. DESeq2 first calculates dispersion estimates through maximum likelihood, after which it calculates gene-wise dispersion using the empirical Bayes method (35). Statistical significance was computed via a Wald test, with P values adjusted using Benjamini–Hochberg false discovery rate (FDR). Pathway analyses of DEG sets were completed using Reactome 2016, Gene Ontology: Biological Processes 2021, Wikipathway 2019, and KEGG 2019 using EnrichR (36). Protein–protein interaction (PPI) networks using DEG profiles as inputs were generated using STRING v11 with clustering carried out using Molecular Complex Detection [MCODE (37)] and plotted using Cytoscape (38). Heatmaps were generated using pheatmap (v1.0.12). DESeq2 and visualization analyses were completed using R (v4.0.3) and R studio (v1.3.1093). The R code and list of R packages used can be found in Supplemental File S1 (https://doi.org/10.6084/m9.figshare.20419122).
We used Pathway-Level Information ExtractoR (PLIER) for exploratory RNAseq analyses (33). Read counts were filtered for low expression and normalized using trimmed mean of M values (TMM). Normalized reads were then deconvoluted using PLIER to generate latent variables (LVs) that summarize the overall structure and increase the biological interpretability of the data set. Briefly, PLIER performs a singular value decomposition constrained by prior knowledge from established pathways in the literature and other gene ontology resources. LVs were compared across samples using a mixed-model ANOVA to determine whether there were any molecular patterns associated with boldine, treatment or timepoint. Data were generated using the server-hosted version of PLIER (https://gobie.csb.pitt.edu/PLIER/) with default settings, then visualized using R version 4.1.2.
Statistics
Two-way mixed-model ANOVAs (treatment × timepoint) were used to compare body and muscle mass among groups with P < 0.05 being our statistical threshold for meaningful group differences. MetaboAnalyst 5.0 (39) was used for two-way mixed-model and one-way exploratory ANOVA analyses of metabolomics data, with an FDR < 0.10 as the statistical threshold for meaningful differences. Within the principal component analysis (PCA) of each -omics platform, Spearman’s rank correlation coefficient (rho, ρ) and associated P values were calculated between the top principal components (calculated by Horn’s parallel analysis) and the experimental groups coded as binary variables. Statistical thresholds for DEGs were Ward’s P test with an FDR < 0.10. For DNA methylation, a chi-squared test with overdispersion correction (methylKit) and a beta-binomial approach (methylSig) were both used with an FDR < 0.10 for the principal analysis for group comparisons, with exploratory analyses completed on DMCs with raw P values of <0.01 and |Δ in methylation| >10%. The PLIER statistical threshold was conservatively set by a treatment × timepoint interaction at P = 0.05 divided by the number of LVs detected by the model (0.05/18), making the final PLIER threshold P < 0.003. Tukey’s multiple comparison tests were used for follow-up testing of LVs meeting the interaction effect threshold.
All raw fastq files for RNAseq and RRBS have been deposited to Gene Expression Omnibus under accession number GSE210392. Data files containing unnormalized read counts for RNAseq and Bismark methylation coverage files can be found using the GEO accession number and peak spectra for metabolomics can be found in Supplemental Table S1 (https://doi.org/10.6084/m9.figshare.20260152).
RESULTS
Body Mass
There was no interaction between timepoint and absolute body mass (Fig. 1A; P = 0.901). There was a main effect of treatment on absolute body mass (P < 0.001) with SCI groups being lower compared with Sham, as well as for timepoint (P = 0.019), with 7 days being greater than 28 days. There was no interaction effect for body mass percent change (Fig. 1B; P = 0.187). There was a main effect of treatment (P < 0.001), with the Sham group being greater compared with the SCI groups; there was no main effect of timepoint for percent change in body mass (P = 0.702).
Figure 1.
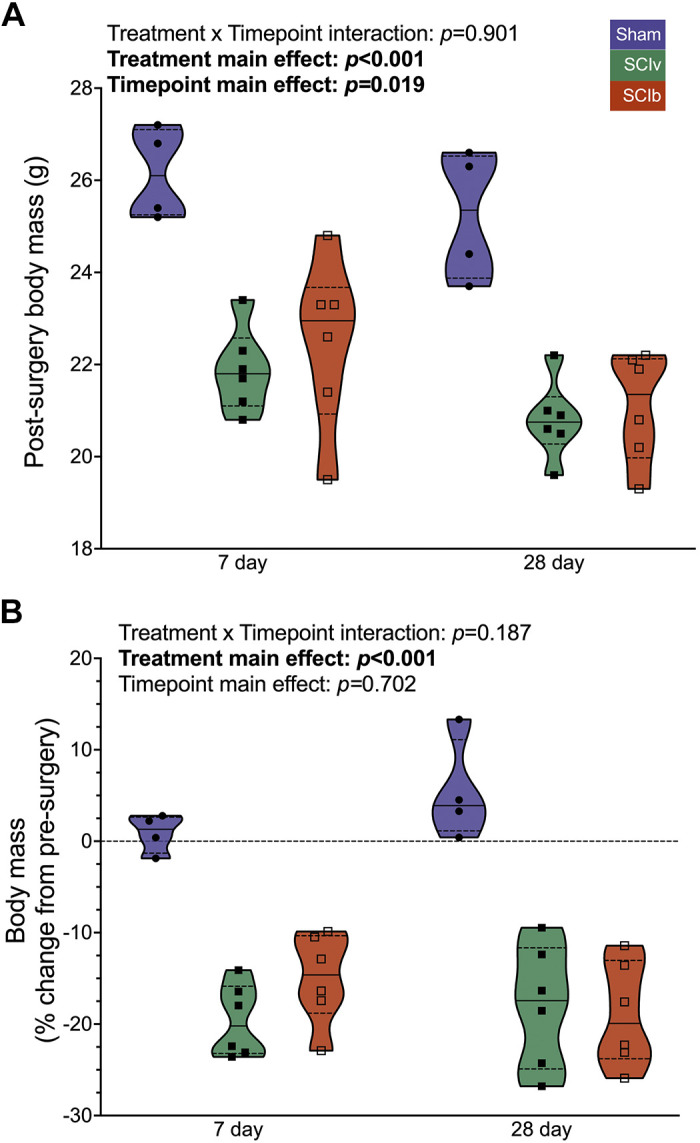
Body mass changes after SCI with and without boldine. Absolute (A) and relative (B) changes in body mass 7 days and 28 days are reduced after a complete SCI, with no effect of boldine. Individual data points are presented in violin plots with 95% confidence interval boundaries. The solid black line represents the median value and hashed lines represent quartiles. SCI, spinal cord injury.
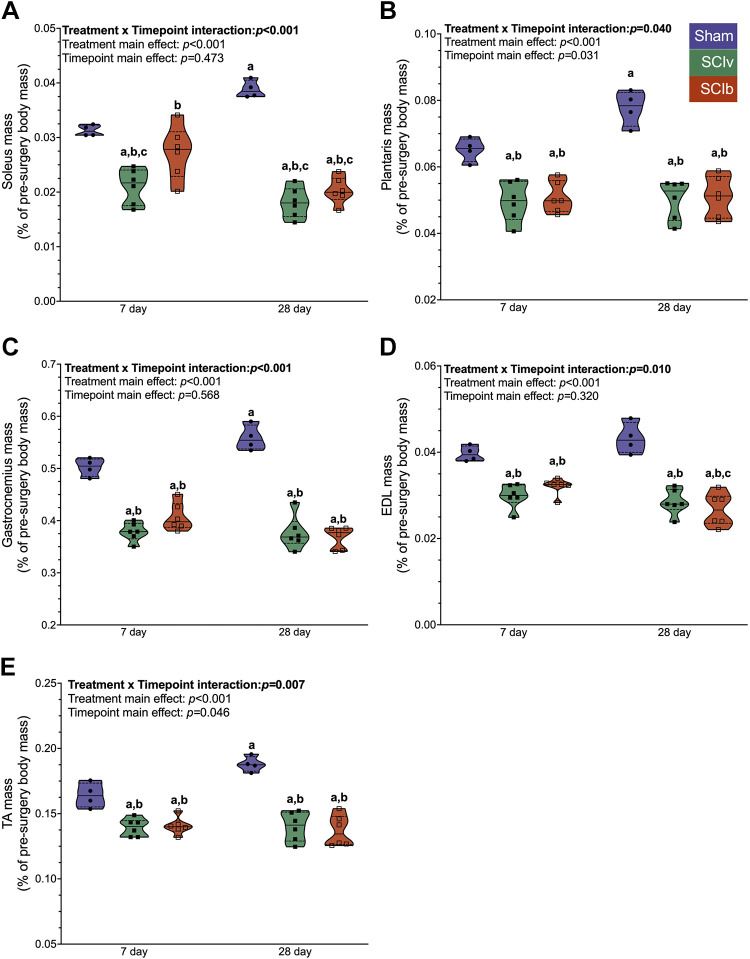
Tissue Mass
There was an interaction effect of timepoint and treatment for the soleus (Fig. 2A; P < 0.001), plantaris (Fig. 2B; P = 0.040), gastrocnemius (Fig. 2C; P < 0.001), EDL (Fig. 2D; P = 0.010), and TA (Fig. 2E; P = 0.007). Post-test multiple comparisons showed that the 28 days Sham group was elevated compared with other groups for these muscles. The 7 days soleus was the only muscle group where SCIb was elevated versus SCIv (P = 0.020). For the biceps, there was no interaction effect (P = 0.631) or treatment effect (P = 0.250). There was a main effect of timepoint (P = 0.001), with the 28 days being greater compared with the 7 days mice (Supplemental File S2, Supplemental Fig. S1A: https://doi.org/10.6084/m9.figshare.20259897). Finally, there was no interaction effect for the triceps (P = 0.637) or main effect for timepoint (P = 0.454). There was a main effect of treatment (P = 0.034), with the Sham group being elevated compared with SCI mice (Supplemental File S2, Supplemental Fig. S1B: https://doi.org/10.6084/m9.figshare.20259897). All individual mouse body masses, body mass changes, and tissue weights can be found in Supplemental Table S2 (https://doi.org/10.6084/m9.figshare.22581958).
Figure 2.
Normalized mass of the major hindlimb muscles post-injury. A: soleus atrophy was blunted with boldine at 7 days but not 28 days post-SCI. SCI led to similar magnitudes of muscle loss with and without boldine for the plantaris (B), gastrocnemius (C), EDL (D), and TA (E). Individual data points are presented in violin plots with 95% confidence interval boundaries. The solid black line represents the median value and hashed lines represent quartiles. Tukey’s multiple comparison test was used for interaction follow-up with a denoting statistical differences vs. 7 days Sham, b vs. 28 days Sham and c vs. 7 days SCIb. EDL, extensor digitorum longus; SCI, spinal cord injury; TA, tibialis anterior.
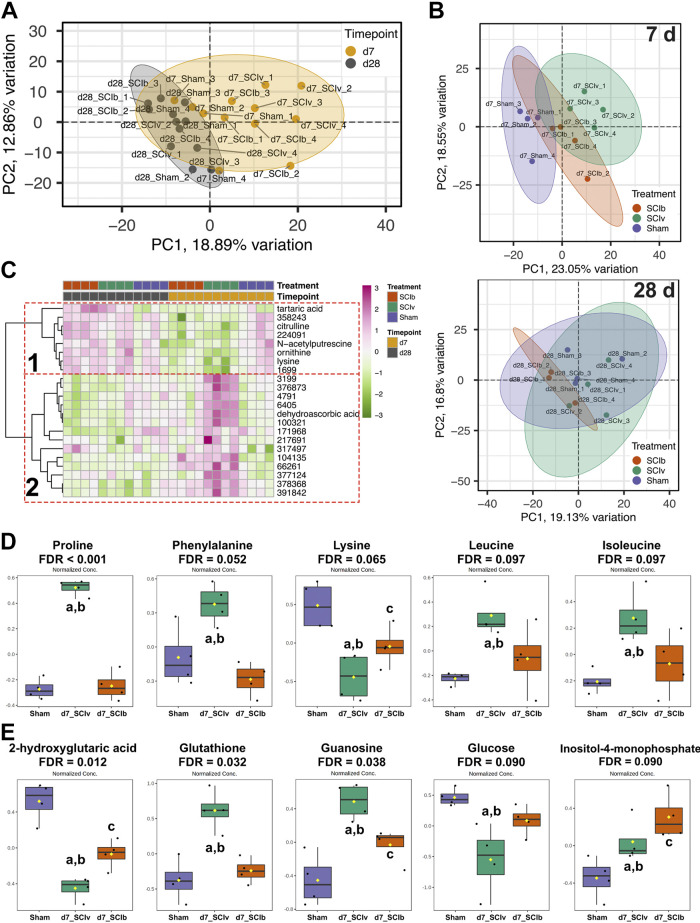
Metabolomics
Five hundred seventy-five metabolites met quality and filtering criteria so that there were no missing data points across surgeries and timepoints (n = 4/group, 24 total). A PCA plot shows clustering by timepoint (Fig. 3A). Additional PCA plots show a clear separation between SCIv and Sham groups, with SCIb being an intermediate cluster at 7 days (Fig. 3B, top panel). At 28 days, there was overlap among all groups (Fig. 3B, bottom panel). Correlation analysis between the top principal components and the experimental groups was performed to better show how much the experimental groups contributed to the variance in the data (Supplemental File S2, Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.20259897). Within the full data set, timepoint was significantly correlated with PC1 (ρ = −0.73, P < 0.001). Additional correlations between the treatment groups were also seen in PC3 through PC6. Within the 7 days timepoint PCA, both “SCIv versus Sham”’ (ρ = 0.87, P = 0.005) and “SCIb versus Sham” (ρ = 0.87, P = 0.005) were correlated with PC1, whereas “SCIb versus SCIv” correlated significantly with PC2 (ρ = −0.76, P = 0.005). At 28 days, there were correlations between PC1 and both “SCIb versus SCIv” (ρ = −0.76, P = 0.027) and “SCIb versus Sham” (ρ = −0.76, P = 0.027), as well as a correlation between “SCIb versus Sham” and PC4 (ρ = 0.76, P = 0.027). Twenty-two metabolites met the criteria for an interaction effect using a mixed-model two-way ANOVA (FDR < 0.10), with six of them being annotated (Supplemental Table S3: https://doi.org/10.6084/m9.figshare.20261475). A hierarchical clustering heatmap of these 22 metabolites shows two clear patterns: 1) reduced abundance of metabolites at 7 days in both SCI groups returning to Sham levels by 28 days and 2) boldine blunted the 7 days SCI response (Fig. 3C). One hundred sixty-two metabolites had an FDR < 0.10 for the main effect of timepoint, and 89 metabolites had an FDR < 0.10 for the main effect of treatment (Supplemental File S3: https://doi.org/10.6084/m9.figshare.20259594). Exploratory one-way ANOVAs at each timepoint resulted in 76 metabolites with FDR < 0.10 at 7 days, while only one unannotated metabolite had an FDR < 0.10 among groups at 28 days. At the 7 days timepoint, Tukey’s multiple comparisons follow-up testing showed boldine either prevented or attenuated SCI-induced changes in amino acids such as proline, phenylalanine, lysine, leucine, and isoleucine (Fig. 3D), and other notable metabolites such as 2-hydroxyglutarate, glucose, glutathione, guanosine, while further elevating levels of inositol-4-monophosphate (Fig. 3E). The full list of differentially regulated metabolites from the one-way ANOVAs is available in Supplemental Table S4 (https://doi.org/10.6084/m9.figshare.20263659).
Figure 3.
Metabolomics profiles of the gastrocnemius 7 and 28 days post-SCI. A: PCA demonstrating timepoint-based clustering of all samples. B: top panel shows unique clustering of groups at 7 days, with the bottom panel showing more overlap. C: fixed-sample clustering heatmap of features with FDR < 0.10 using a mixed-model ANOVA shows a cluster of metabolites with reduced abundance in SCI mice at 7 days (dotted red box 1) and a cluster with boldine-elevated metabolites at 7 days (dotted red box 2). Boldine-associated changes in annotated metabolites 7 days after complete spinal cord transection for key amino acids (D) and other key molecules (E) associated with muscle health. Individual data points are presented in box and whisker plots with 95% confidence interval. The solid black line represents the median value and the edges of the box represent quartiles. Tukey’s multiple comparison test was used for follow-up analyses with adenoting statistical differences vs. Sham, bvs. SCIb and cvs. SCIv. FDR, false discovery rate; SCI, spinal cord injury.
We used MetaboAnalyst to generate correlation coefficients to determine associations of metabolites with glucose, proline, and inositol-4-monophosphate. The top 25 associations for each comparison are shown in Supplemental File S2, Supplemental Fig. S3 (https://doi.org/10.6084/m9.figshare.20259897). For glucose, 152 metabolites had correlations with FDR < 0.10. Most of the highly correlated features were not annotated but five of the top 25 features were sugar species. There were 159 metabolites that were correlated with proline (FDR < 0.10). Like glucose, a majority of highly correlated features were unannotated but there were five amino acids all highly and positively correlated with the proline response. For inositol-4-monophosphate, there were 113 correlated metabolites with FDR < 0.10 but no response of similar species. All statistics generated for these analyses are provided in Supplemental File S3 (https://doi.org/10.6084/m9.figshare.20259594).
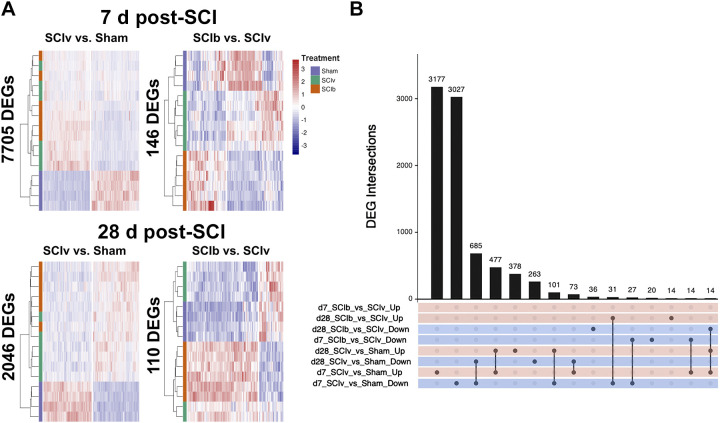
Differential mRNA Expression
PCA and principal component correlation analysis of our mRNA transcriptomic profiles highlighted significant variability by both timepoint, and by treatment group within each timepoint (Supplemental File S2, Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.20259897). Therefore, we investigated these profiles between treatments at 7 and 28 days post-SCI separately to simplify and increase the power for the analyses. Our primary comparisons were “SCIv versus Sham,” and “SCIb versus SCIv.” Figure 4A shows the hierarchical clustering heatmaps of DEGs across timepoints and comparisons with all three treatment groups included for relative comparison. Acute SCI resulted in the largest change in the transcriptome with 7,705 genes (3,787 upregulated and 3,918 downregulated) in the “SCIv versus Sham” comparison. “SCIb versus SCIv” led to 146 DEGs (59 upregulated and 87 downregulated) at 7 days, 105 of which were shared with “SCIv versus Sham” and 57 inversely regulated. The “SCIv versus Sham” comparison resulted in 2,046 DEGs (1,002 upregulated and 1,044 downregulated) at 28 days post-SCI, with “SCIb versus SCIv” having 110 DEGs (82 upregulated and 28 downregulated); 34 of these DEGs were shared between the comparisons. An UpSet plot of the top 15 unique and shared sets of DEGs across all comparisons is presented in Fig. 4B. The “SCIv versus Sham” comparison at 7 days had ∼3,000 unique up- and downregulated genes. There were DEGs shared by the “SCIv versus Sham” comparisons at both 7 and 28 days that are changed in the same direction (685 downregulated and 477 upregulated), with additional sets of up- and downregulated genes specific to SCI at the 28 days timepoint. Other intersections of note show 36 genes unique to upregulation in the “SCIb versus SCIv” comparison at 28 days, as well as 31 genes shared in the 7 days “SCIb versus SCIv” upregulated comparison that were downregulated in the 7 days “SCIv versus Sham” comparison. The full list of differentially regulated genes in each analysis is available in Supplemental File S4 (https://doi.org/10.6084/m9.figshare.20261460).
Figure 4.
Transcriptomic signatures of complete SCI at acute and subacute timeframes after injury. A: hierarchical clustering heatmaps across the main comparisons and timepoints. B: UpSetR plot showing the top 15 unique and overlapping DEG sets across all comparisons. DEG, differentially expressed gene; SCI, spinal cord injury.
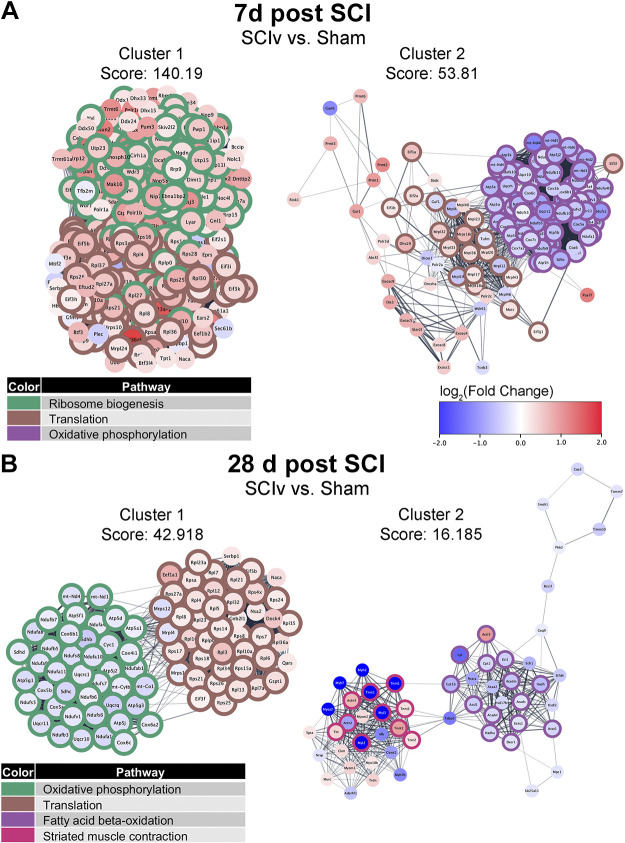
Pathway Analyses, Network Clustering, and PLIER
EnrichR was used to perform pathway analyses on the DEGs from each comparison. The top five nonredundant pathways and ontologies for both upregulated and downregulated gene sets are shown in Table 1, while the full pathway analysis results are available in Supplemental File S5 (https://doi.org/10.6084/m9.figshare.20419386). PPI networks were generated for each analysis with the goal of identifying clusters of highly related genes and their associations with specific pathways. MCODE analysis within the PPI networks identified 84 clusters from the 7 days “SCIv versus Sham” comparison (Supplemental File S6: https://doi.org/10.6084/m9.figshare.20422227). Cluster 1 contained primarily upregulated genes associated with both “Ribosome biogenesis” and “Translation,” whereas cluster 2 demonstrated downregulation of genes associated with “Oxidative phosphorylation” and mixed regulation of additional “Translation” associated genes (Fig. 5A). Clusters 3 to 5 can be seen in Supplemental File S2, Supplemental Fig. S5A (https://doi.org/10.6084/m9.figshare.20259897). They highlighted pathways related to increased transcriptional activity, inflammatory responses, and protein metabolism (Supplemental File S7: https://doi.org/10.6084/m9.figshare.21899016). Three MCODE clusters were seen for the “SCIb versus SCIv” comparison and are shown in Supplemental File S2, Supplemental Fig. S6A (https://doi.org/10.6084/m9.figshare.20259897). Each of these clusters had only three to four genes per comparison. Cluster 1 of the 7 days “SCIb versus SCIv” comparison was annotated to “Ubiquitin-mediated proteolysis”, cluster 2 to “Starch and sucrose metabolism,” and cluster 3 to “N-glycan trimming in the ER and calnexin/calreticulin pathway.”
Table 1.
Selected top pathways enriched after spinal cord injury
| Overlap | FDR | Pathway Analysis Tool | |
|---|---|---|---|
| 7 days post-spinal cord injury | |||
| SCIv vs. Sham upregulated | |||
| Ribosome biogenesis | 154/192 | 2.08E-72 | GO_Biological_Process_2021 (GO: 0042254) |
| rRNA processing | 142/173 | 2.21E-69 | GO_Biological_Process_2021 (GO: 0006364) |
| rRNA metabolic process | 129/162 | 6.06E-60 | GO_Biological_Process_2021 (GO: 0016072) |
| Translation | 119/151 | 8.82E-55 | Reactome_2016 |
| ncRNA processing | 138/201 | 1.06E-50 | GO_Biological_Process_2021 (GO:0034470) |
| SCIv vs. sham downregulated | |||
| The citric acid (TCA) cycle and respiratory electron transport | 114/153 | 7.33E-46 | Reactome_2016 |
| Thermogenesis | 119/231 | 3.62E-25 | Reactome_2016 |
| Oxidative phosphorylation | 76/134 | 1.39E-19 | KEGG_2016 |
| Complex I biogenesis | 41/49 | 2.30E-19 | Reactome_2016 |
| Muscle contraction | 72/129 | 5.50E-17 | GO_Biological_Process_2021 (GO: 0006936) |
| SCIb vs. SCIv upregulated | |||
| Riboflavin metabolism | 2/8 | 1.59E-02 | KEGG_2019_Mouse |
| Contact inhibition | 2/6 | 3.15E-02 | GO_Biological_Process_2021 (GO: 0060242) |
| Extracellular matrix organization | 6/300 | 4.13E-02 | GO_Biological_Process_2021 (GO: 0030198) |
| Supramolecular fiber organization | 6/351 | 4.71E-02 | GO_Biological_Process_2021 (GO: 0097435) |
| Negative regulation of cell adhesion | 2/36 | 8.46E-02 | GO_Biological_Process_2021 ( GO:0007162) |
| SCIb vs. SCI downregulated | |||
| Antigen processing: ubiquitin and proteosome degradation | 9/260 | 8.65E-04 | Reactome_2016 |
| Protein K48-linked ubiquitination | 5/57 | 3.23E-03 | GO_Biological_Process_2021 (GO: 0070936) |
| Adaptive immune system | 11/762 | 4.55E-02 | Reactome_2016 |
| Membrane binding and targeting of GAG proteins | 2/11 | 4.93E-02 | Reactome_2016 |
| Neuron projection fasciculation | 2/10 | 5.92E-02 | GO_Biological_Process_2021 (GO: 0106030) |
| 28 days post-spinal cord injury | |||
| SCIv vs. sham upregulated | |||
| Translation | 47/151 | 6.16E-22 | Reactome_2016 |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 38/107 | 2.67E-20 | Reactome_2016 |
| Selenoamino acid metabolism | 36/111 | 3.74E-18 | Reactome_2016 |
| Nonsense-mediated decay | 35/106 | 4.79E-18 | Reactome_2016 |
| Protein targeting to ER | 35/103 | 2.99E-17 | GO_Biological_Process_2021 (GO: 0045047) |
| SCIv vs. sham downregulated | |||
| The citric acid (TCA) cycle and respiratory electron transport | 55/153 | 8.85E-29 | Reactome_2016 |
| Oxidative phosphorylation | 43/134 | 2.20E-20 | KEGG_2019_Mouse |
| Thermogenesis | 55/231 | 1.11E-19 | KEGG_2019_Mouse |
| Cardiac muscle contraction | 26/78 | 3.44E-13 | KEGG_2019_Mouse |
| Mitochondrial fatty acid beta-oxidation | 12/17 | 4.42E-10 | Reactome_2016 |
| SCIb vs. SCIv upregulated | |||
| Antigen processing: ubiquitin and proteosome degradation | 7/260 | 2.84E-02 | GO_Biological_Process_2021 (GO: 0042254) |
| Positive regulation of autophagosome maturation | 2/6 | 5.42E-02 | GO_Biological_Process_2021 (GO: 1901098) |
| Regulation of histone H3K9 acetylation | 2/11 | 5.42E-02 | GO_Biological_Process_2021 (GO: 2006215) |
| Negative regulation of gene silencing by miRNA | 2/12 | 5.42E-02 | GO_Biological_Process_2021 (GO: 0060965) |
| Regulation of nucleocytoplasmic transports | 2/13 | 5.42E-02 | GO_Biological_Process_2021 ( GO:0046822) |
| SCIb vs. SCI downregulated | |||
| Keratan sulfate degradation | 2/12 | 5.63E-03 | KEGG_2019_Mouse |
| Scavenging by Class A receptors | 2/19 | 1.16E-02 | Reactome_2016 |
| Golgi-to-ER retrograde transport | 3/110 | 1.31E-02 | Reactome_2016 |
| Relaxin signaling pathway | 3/131 | 2.31E-02 | KEGG_2019_Mouse |
| PI3K-Akt signaling pathway | 4/347 | 2.82E-02 | KEGG_2019_Mouse |
FDR, false discovery rate; SCI, spinal cord injury.
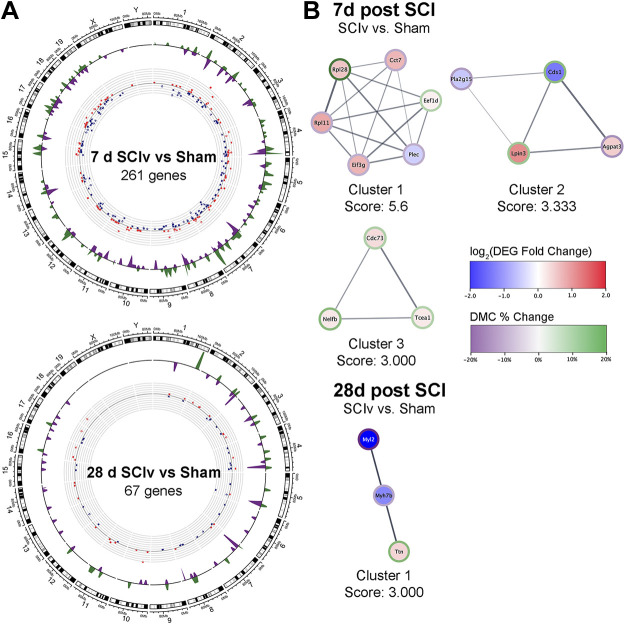
Figure 5.
The top two MCODE clusters identified within STRING association networks from DEGs for “SCIv vs. Sham” analyses at 7 days (A) and 28 days (B) post-injury. The colored borders identify selected top enriched pathways within that cluster. DEG, differentially expressed gene; SCI, spinal cord injury.
Forty clusters were identified in the “SCIv versus Sham” comparison at 28 days, with the top clusters shown in Fig. 5B. Cluster 1 showed maintained alterations in pathways annotated to “Oxidative phosphorylation” and “Translation.” Cluster 2 was composed of downregulated genes enriched for “Fatty acid oxidation” and “Striated muscle contraction.” Clusters 3 to 5 can be seen in Supplemental File S2, Supplemental Fig. S5B (https://doi.org/10.6084/m9.figshare.20259897) and were composed of DEGs that annotated to pathways related to cell cycle, SCI, translation, and collagen formation (Supplemental File S7: https://doi.org/10.6084/m9.figshare.21899016). Of the four clusters identified for “SCIb versus SCIv” at 28 days, cluster 2 was annotated to “Focal Adhesion,” whereas cluster 4 was enriched by upregulated genes relating to “Gene Silencing by RNA” (Supplemental File S2, Supplemental Fig. S6B: https://doi.org/10.6084/m9.figshare.20259897). The list of MCODE clusters and the full pathway analyses for the top clusters can be found in Supplemental Files S6 and S7, respectively.
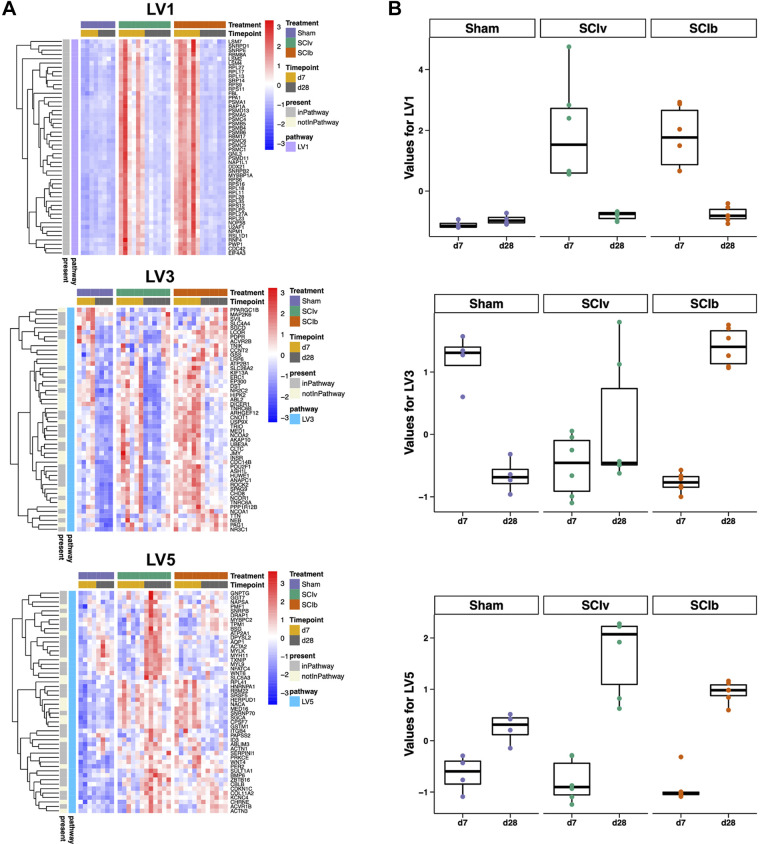
PLIER constructed 18 LVs following deconvolution (Table 2). Three LVs had an interaction effect for timepoint and treatment (LV1, 3, and 5), four had a main effect of timepoint (LV1, 2, 5, and 7) and three had a main effect of treatment (LV1, 2 and 3). The LVs constructed by PLIER contained genes associated with pathways identified in the MCODE clustering such as alterations in translation and mitochondrial ribosomes (LV1, 2, and 5). Heatmaps of the top-loaded genes for these constructed LVs and box plots of the individual sample scores highlighting the interaction effects are shown in Fig. 6, A and B, respectively. LV1, annotated to “MIPS Nop56p-associated pre-rRNA Complex” and LV5, annotated to “MIPS Spliceosome,” demonstrated clear SCI-induced timepoint effects without any effect of boldine. LV3 annotated to “PID Androgen Receptor Transcription Factor Pathway” and showed a potent effect of the Sham surgery at 7 and 28 days. In addition, it showed a unique boldine effect as group loading values were elevated in the SCIb mice compared with SCIv at 28 days. LV2, annotated to “MIPS 55S Ribosome Mitochondrial” and LV7, annotated to “Reactome Antigen Processing Ubiquitination and Proteasomal Degradation,” are presented in Supplemental File S2, Supplemental Fig. S7 (https://doi.org/10.6084/m9.figshare.20259897). LV2 had strong SCI-induced downregulation at 7 days, with all groups having a positive response by 28 days. LV7 had SCI-induced upregulation at 7 days, with all treatment groups having similar values by 28 days. All multiple comparison follow-up test P values can be found in Supplemental Table S5 (https://doi.org/10.6084/m9.figshare.20263668).
Table 2.
Pathway level information ExtractoR results
| LVs Built by PLIER | Interaction Effect | Timepoint Main Effect | Treatment Main Effect | Annotation (If Present) |
|---|---|---|---|---|
| LV1 | 0.002 | 0.000 | 0.001 | MIPS_NOP56P_ASSOCIATED_PRE_RRNA_COMPLEX |
| LV2 | 0.073 | 0.000 | 0.000 | MIPS_55S_RIBOSOME_MITOCHONDRIAL |
| LV3 | 0.000 | 0.005 | 0.083 | PID_AR_TF_PATHWAY |
| LV4 | 0.081 | 0.317 | 0.221 | N/A |
| LV5 | 0.002 | 0.000 | 0.008 | MIPS_SPLICEOSOME |
| LV6 | 0.039 | 0.083 | 0.022 | N/A |
| LV7 | 0.224 | 0.000 | 0.098 | REACTOME_ANTIGEN_PROCESSING_UBIQUITINATION_PROTEASOME_DEGRADATION |
| LV8 | 0.811 | 0.383 | 0.501 | REACTOME_GPCR_DOWNSTREAM_SIGNALING |
| LV9 | 0.121 | 0.873 | 0.013 | KEGG_AXON_GUIDANCE |
| LV10 | 0.363 | 0.116 | 0.220 | KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION |
| LV11 | 0.847 | 0.249 | 0.274 | N/A |
| LV12 | 0.835 | 0.623 | 0.131 | REACTOME_GPCR_DOWNSTREAM_SIGNALING |
| LV13 | 0.148 | 0.369 | 0.139 | KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION |
| LV14 | 0.083 | 0.399 | 0.728 | N/A |
| LV15 | 0.270 | 0.936 | 0.288 | REACTOME_GPCR_DOWNSTREAM_SIGNALING |
| LV16 | 0.618 | 0.556 | 0.289 | N/A |
| LV17 | 0.778 | 0.807 | 0.280 | N/A |
| LV18 | 0.364 | 0.446 | 0.425 | PID_PLK1_PATHWAY |
LV, latent variable; PLIER, Pathway-Level Information ExtractoR. Significant values in bold.
Figure 6.
Pathway-Level Information ExtractoR (PLIER) highlights unique molecular circuits after SCI. A: heatmap of the top loaded genes from constructed LVs with timepoint × treatment interaction effects. B: box plots showing the overall loading score of each animal for the respective LV. LV, latent variable; SCI, spinal cord injury.
DNA Methylation
The RRBS analysis strategy was identical to that used for RNAseq, with analyses separated by timepoint and key comparisons being “SCIv versus Sham” and “SCIb versus SCIv.” PCA and principal component correlation analysis of our data showed only moderate separation either by timepoint or by treatment group within each timepoint, even when filtered to the top 2,000 most variable CpG sites (Supplemental File S2, Supplemental Fig. S8). Using methylKit, an FDR threshold < 0.10 resulted in < 10 DMCs in each comparison at both timepoints (Supplemental Table S6: https://doi.org/10.6084/m9.figshare.20263674). We thus expanded our approach using DMCs with a raw P value < 0.01 and |Δ methylation change| > 10%. The 7 days “SCIv versus Sham” comparison resulted in 2,991 DMCs (1,614 hypermethylated, 1,377 hypomethylated) across 1,781 annotated genes, with the “SCIb versus SCIv” comparison resulted in 2,217 DMCs (1,199 hypermethylated, 1,018 hypomethylated) across 1,365 genes. Similar numbers of DMCs were observed at 28 days, with SCI resulting in 2,645 DMCs (872 hypermethylated, 1,773 hypomethylated) across 1,560 genes compared with Sham mice, and boldine treatment leading to 2,459 DMCs (1,742 hypermethylated, 717 hypomethylated) across 1,419 genes compared with vehicle after SCI. DMC intersections among comparisons are shown in an UpSet plot in Supplemental File S2 (Supplemental Fig. S9A: https://doi.org/10.6084/m9.figshare.20259897), highlighting that each comparison had a mostly unique set of DMCs. The complete list of all significant methylKit DMCs is provided in Supplemental File S8 (https://doi.org/10.6084/m9.figshare.21899394).
Promoter-associated CpG sites, split by hyper or hypomethylation, were run through HOMER motif enrichment analysis to identify differences in methylation of key transcription factor binding motifs. The results are presented in Supplemental Table S7 (https://doi.org/10.6084/m9.figshare.21899421). The 7 days “SCIv versus Sham” comparison identified hypermethylation of the motif for PRDM1. The “SCIb versus SCIv” comparison resulted in no hypermethylation that met FDR criteria but was associated with hypomethylation of motifs for ELK1 and ELK4, ETV1 and ETV4, FLI1, SPDEF, and EFL1 (FDR < 0.10). The “SCIv versus Sham” comparison at 28 days resulted in no motif meeting hyper- or hypomethylation FDR criteria. There was no hypermethylation of motifs associated with the “SCIb versus SCIv” comparison at 28 days but hypomethylation of ELF1. All significant CpG sites were also analyzed for possible enhancer annotation using EnhancerAtlas 2.0, but no overlaps were identified in the available gastrocnemius data set.
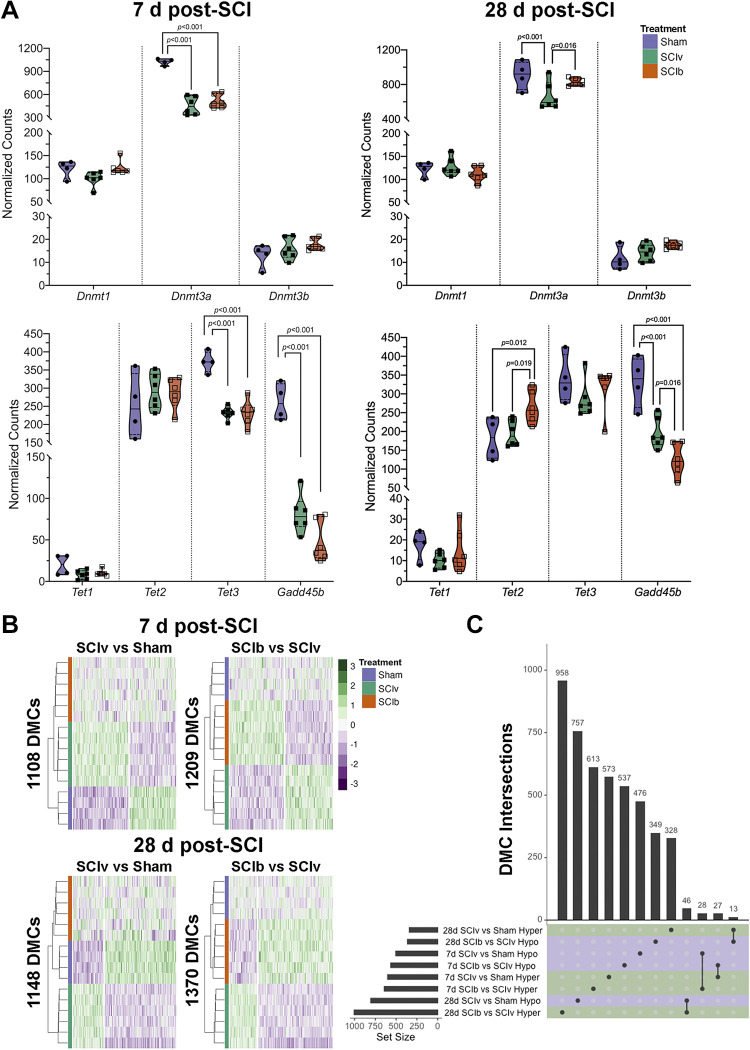
Normalized read counts from our transcriptomics data show the major DNA methyltransferase in skeletal muscle, Dnmt3a, was reduced by SCI with no effect of boldine (Fig. 7A, top left panel). Normalized read counts of genes associated with DNA demethylation show Tet3 and Gadd45b reduced following SCI, with no change in Tet2 and minimal overall expression of Tet1 (Fig. 7A, bottom left lower panel). Expression of Dnmt3a was preserved with boldine treatment at 28 days using normalized read counts, with boldine leading to increased expression of Tet2 and further reductions in Gadd45b (Fig. 7A, top and bottom right panels, respectively).
Figure 7.
DNA methylation of the gastrocnemius post-complete SCI. A: gene expression using normalized read counts of factors associated with methylation and demethylation. Dmt3a, Tet3, and Gadd45b are greatly downregulated at 7 days, with boldine being associated with improved levels of Dmt3a and reduced expression of Gadd45b at 28 days. B: hierarchical clustering heatmaps of the hyper- and hypomethylated consensus DMCs for each comparison. C: UpSet plot showing that each comparison has a mostly unique set of DMCs, with the shared DMCs for “SCIb vs. SCIv” and “SCIv vs. Sham” comparisons at both 7 and 28 days being inverse to each. DMCs, differentially methylated CpG sites; SCI, spinal cord injury.
Confirmation of DNA Methylation Profile
The minimal amount of DMCs that met our FDR threshold using methyKit led us to cross-validate these findings using methylSig. methylSig identified no DMCs that met our FDR criteria. We did observe a larger number of DMCs using matching cutoffs from our exploratory analyses (raw P value < 0.01 and |Δ in methylation| >10%; Supplemental File S9: https://doi.org/10.6084/m9.figshare.21899451), with similar patterns in the proportion of CpGs uniquely regulated or inversely regulated between the groups (Supplemental File S2, Supplemental Fig. S9B: https://doi.org/10.6084/m9.figshare.20259897). These results showed only moderate overlap with our initial analysis (Supplemental File S2, Supplemental Fig. S10), leading us to repeat the downstream HOMER and EnhancerAtlas 2.0 analyses with the methylSig results. Despite the modest overlap between identified DMCs between analyses, we saw similar results in HOMER promoter motif enrichment (Supplemental Table S8: https://doi.org/10.6084/m9.figshare.21899430), with the biggest difference being that both ETV4 and ELK4 met FDR criteria in the “SCIv versus Sham” comparison at 28 days. Like the methylKit results, no enhancers were identified to overlap with the significant DMCs in this analysis. To offset the likelihood of false positives in our data due to using a lower statistical threshold, we decided to proceed with further analyses using only consensus DMCs meeting our thresholds via both tools (Supplemental File S10: https://doi.org/10.6084/m9.figshare.21899457). Heatmaps showing consensus DMC profiles from each comparison are shown in Fig. 7B. DMC intersections are also shown in Fig. 7C and highlight a small selection of 55 DMCs at 7 days and 59 DMCs at 28 days that were inversely methylated.
DEG and DMC Overlap
Using the newly defined consensus DMCs, we examined the overlap of DEGs and DMCs for each comparison (Supplemental File S11: https://doi.org/10.6084/m9.figshare.21899460). The 7 days “SCIv versus Sham” had 283 DMCs that overlapped with 261 DEGs (Fig. 8A, top panel), with these numbers reduced to 79 DMCs overlapping 67 DEGs at 28 days (Fig. 8A, bottom panel). The “SCIb versus SCIv” comparison resulted in nine DMCs overlapping seven DEGs at 7 days and three DMCs/DEGs at 28 days. EnrichR was used to perform pathway analyses on the DEGs from the “SCIv versus Sham” analyses at both the 7 days and 28 days timepoints. The gene lists from “SCIb versus SCIv” analyses at both timepoints were deemed too small to provide biologically relevant pathway analyses and were excluded. The top non-redundant pathways and ontologies were related to cell proliferation and differentiation, axon guidance, and cell junction organization for the 7 day “SCIv vs. Sham” comparison, and principally skeletal muscle sarcomere and contractile machinery assembly for the 28 day “SCIv vs. Sham” comparison. Full pathway analysis results are available in Supplemental File S12 (https://doi.org/10.6084/m9.figshare.21899466). As before, PPI networks were generated for each analysis with the goal of identifying clusters of highly related genes and their associations with specific pathways. Neither “SCIb versus SCIv” analysis showed any connections between their small gene sets. MCODE analysis within the “SCIv versus Sham” PPI networks identified three clusters at the 7 days timepoint and one cluster at the 28 days timepoint (Fig. 8B). Within the 7 days timepoint, cluster 1 showed enrichment for “Translation” and “Metabolism of proteins”; cluster 2 was entirely composed of genes related to “Glycerophospholipid metabolism”; and cluster 3 genes were related to “Formation of RNA Pol II elongation complex.” At the 28 days timepoint, cluster 1 annotated to “Striated Muscle Contraction” and “Cardiac myofibril assembly.” The data for the MCODE clusters and the pathway analysis for each can be found in Supplemental Files S6 and S7, respectively.
Figure 8.
DEG/DMC overlaps. A: Circos plots demonstrating the overlap and magnitude of change in DEGs and DMCs across the “SCIv and Sham” comparisons at 7 days and 28 days post-SCI. Red dots = upregulated genes, blue dots = downregulated genes; green waves = hypermethylated DMCs, purple waves = hypomethylated DMCs. B: all identified MCODE clusters from STRING association networks using genes with associated methylation changes for “SCIv vs. Sham” analyses at 7 days and 28 days post-SCI. DEG, differentially expressed gene; DMCs, differentially methylated CpG sites; SCI, spinal cord injury.
DISCUSSION
Our data support the following primary conclusions: 1) SCI leads to large-scale alterations in the metabolome and transcriptome of paralyzed muscle at 7 days, with only the transcriptome remaining altered at 28 days; 2) boldine treatment did not prevent losses in body mass and hindlimb muscle mass; 3) boldine blunted changes in abundance of glucose and amino acids 7 days post-SCI; and 4) boldine altered expression of a select group of mRNAs at 7 days post-SCI. These molecular profiles provide an attractive starting point for future studies to determine the biological significance of CxHCs on skeletal muscle health and function using other SCI or atrophy models.
The finding that boldine did not preserve body or tissue mass after SCI suggests that CxHCs are not a primary driver of muscle atrophy after SCI. Interpretation is somewhat complicated for our study as our gastrocnemius transcriptomics data shows downregulation of Gja1 and Gjc1, the genes that encode Cx43 and Cx45, respectively, at 7 days post-SCI. It remains possible that mRNA levels are not indicative of sarcolemmal protein levels as translation of these transcripts is under post-transcriptional control by miRNAs (40). The delayed reduction in muscle loss in the soleus is intriguing as it has the highest type 1 percentage of the principal hindlimb muscles in C57BL6 mice (35%–40%), and CxHCs seem to reappear de novo only in denervated fast twitch fibers (13). We selected the gastrocnemius for our analysis of the transcriptome, metabolome, and DNA methylome because prior work examining the roles of CxHCs in muscle atrophy and other muscle disorders has found that these membrane pores function in fast but not slow twitch fibers. In addition, the soleus’ size post-SCI does not allow one to use samples from a single muscle for multiple different -omic platforms. Future studies focusing on how boldine affects type I fibers during atrophy would be greatly informative.
Our RNAseq data is suggestive of neuromuscular junction (NMJ) remodeling as Musk, Ncam1, and Scn5a, common markers of denervation, are upregulated in the SCIv mice compared to Sham at 7 days, with Musk and Ncam1 remaining elevated at 28 days. However, there is very little known regarding the effect of SCI on NMJs. Several factors could contribute to NMJ remodeling after SCI and include denervation and motor unit loss in the acute and subacute timespans post-injury. In one study, there was an approximately 30% loss in quadriceps motor neuron counts 6 mo following an L2 contusion SCI in rats (41) and motor neuron death was highest at the level just below the epicenter of injury (42). Further study is needed to understand if motor neuron death occurs in lumbar spinal segments containing motor neurons innervating hindlimb muscles over the time frame studied in the current investigation.
For metabolomics, the 7 days timepoint was the most affected by complete SCI, similar to our previous report in female mice (7). We demonstrated SCI-induced changes in amino acids, which may be expected post-SCI due to protein degradation and anaplerotic potential (43). Our 7-day transcriptomics data shows boldine downregulated Ubb, which encodes ubiquitin, as well as genes associated with protein ubiquitination, including Vps37a, Cul3, Asb11/14, Ube2e1, and multiple E3 ligases (Arih2, Amfr, Nedd4l, Herc3, and Wwp1). So, although boldine possibly reduced protein breakdown through reduced ubiquitination, it was insufficient to prevent atrophy as measured by muscle weights. Another possible function of boldine is protection against extracellular matrix (ECM) turnover. Proline is a major component of collagen and the most abundant ECM protein in skeletal muscle (44). Boldine completely prevented SCI-induced proline increases at 7 days post-injury. Matrix metalloproteinases are the predominant enzymes for protein breakdown in the ECM (45); boldine reversed SCI-related increased expression of Adam9, a widely expressed metalloprotease upregulated in pathological conditions (46). Boldine also upregulated peptidase inhibitor 16 (Pi16), a predicted negative regulator of peptidase activity in the ECM (47). Genes associated with the extracellular matrix, Postn, Col12a1, and Col14a1 were upregulated in the SCIb mice compared with SCIv. Furthermore, Pxdn, which encodes peroxidasin, a protein involved in extracellular matrix formation, was upregulated. Thus, a potential mechanism of action for boldine may be inhibiting the breakdown of proline-rich ECM proteins like collagen through the upregulation of PI16. Notably, 4-hydroxyproline, an amino acid formed through collagen post-translational modification, was not detected in our metabolomics data set, and, as such, it remains unclear if boldine reduced collagen turnover and ECM remodeling.
The SCIv mice had reduced glucose levels similar to our previous report that also noted reduced lactate and pyruvate (7); boldine prevented this SCI-induced reduction. At the mRNA level, SCI resulted in elevated Ide expression, which encodes insulin-degrading enzyme (IDE). Boldine limited this elevation, suggesting the potential for a longer local duration of insulin action. Finally, the levels of inositol-4-monophosphate were elevated in the SCIb mice compared with both SCIv and Sham mice. Cellular levels of inositol and related metabolites are usually inversely regulated by glucose levels (as seen in the SCIv and Sham mice), but they can act as insulin mimetics once in the cell via second messenger systems (48). Although speculative, our data hints at boldine being able to transiently preserve glucose and amino acid levels through insulin signaling pathways. A systematic study of boldine’s role in regulating intramuscular glucose would be an important future direction.
Acute SCI led to major disruptions in gene expression between SCIv mice and Sham controls. The molecules involved in muscle atrophy post-injury share pathways common to other models of disuse atrophy. Peripheral nerve crush results in ∼7,000 DEGs in muscle 3 days after injury, with gene set enrichment and pathways analyses associated with the TCA cycle, protein translation, and protein synthesis (49). mRNA profiles from mice that were fasted, casted, or denervated from a sciatic nerve transection led to upregulation in genes associated with ribosomal processing and protein catabolism observed alongside the downregulation of genes associated with metabolism and the extracellular matrix (50). These are shared in our study, highlighting that SCI-induced atrophy follows the general atrophy program at the transcriptomic level.
PLIER found SCI-induced changes in translation and mitochondrial function (LVs 1, 2, 5, and 7). However, the main objective with the use of PLIER was to find LVs altered in SCIb mice compared with SCIv mice. LV3 was upregulated in the 28 days SCIb group compared with 28 days SCIv and annotated to the GSEA “PID Androgen Receptor Transcription Factor Pathway.” Boldine has not been linked to these cell processes in any tissue to our knowledge. SCI is well known to lead to hypogonadism, as shown by reduced testosterone levels in male rats (51), whereas those with SCI have four- to fivefold higher prevalence compared with the able-bodied (52, 53). Further study is needed to determine how boldine may regulate androgen signaling. In addition, LV3 provides interesting information regarding the laminectomy control surgeries, as there were clear time-related group differences between the 7 and 28 days Sham mice. This demonstrates the importance of having sham surgical controls even for analyses of tissues peripheral to a surgical site.
Data focused on the methylome of acute and subacute SCI do not exist to our knowledge, though methyl array data do exist for individuals with chronic SCI (54, 55). A limited number of DNA methylation changes met our FDR thresholds across comparisons and timepoints, even with the use of two different analysis tools that use distinct statistical approaches. RRBS has been used to show changes in mouse gastrocnemius in aging and exercise studies with similar group sizes using more conservative FDR criteria than our report (56, 57). However, although a complete spinal cord transection is a uniform model of paralysis, variability among mice and the small sample size may have prevented the detection of biologically significant changes methylation in DNA methylation.
We used less conservative statistical thresholds for the methylome for exploratory analyses, as the reduced read counts we observed for Dmnt3a, Tet3, and Gadd45b across time suggested the potential for differences in methylation profiles. Although the identified DMCs were largely unique to each comparison, boldine was able to revert the methylation state of 55 DMCs at 7 days post-SCI and 59 DMCs at 28 days. We then investigated whether changes in promoter-associated DMCs were enriched for transcription factor motifs using HOMER. PRDM15 was detected in the 7 days “SCIv versus Sham” comparison and though it has not been described in skeletal muscle, it has been shown to regulate the PI3K signaling cascade in cancer cells to improve metabolic function (58). Several hypomethylated motifs related to erythroblast transformation specific (ETS) family members met FDR criteria in the 7 days “SCIb versus SCIv” and 28 days “SCIv versus Sham” comparisons. Due to the range of overlap of these factors, it is difficult to highlight a key biological process that would be related to boldine administration. Of course, methylation can regulate gene transcription beyond the promoter, and while genome methylation patterns are difficult to identify or interpret, we did attempt to identify the overlap of our DMCs with known enhancers using the EnhancerAtlas 2.0 database. Unfortunately, we were unable to find any annotation of our DMCs among the ∼1,000 gastrocnemius enhancers identified in that data set.
We found a consistent pattern of mRNA changes that are associated with the changing methylation state of their gene in the “SCIv versus Sham” comparisons at both timepoints. This is not seen in the “SCIb versus SCIv” comparisons, likely due to the small number of DEGs. The moderate numbers of DEGs that overlapped with DMCs provided a bit more insight into the potential role of the changing DNA methylation in SCI through highlighting altered pathways and providing potential gene targets for future studies. These data must be interpreted carefully since we did not probe chromatin accessibility or alter the DNA methylation at any identified CpG sites, we cannot infer transcriptional regulation from the overlap alone.
Our study has several limitations: 1) This study only investigated male mice. In humans, males make up ∼80% of people with SCI (59), which is even further skewed (∼95%) within the Veteran population (60). Although portions of our metabolomics data support our previous investigation in female mice (8), a follow-up study repeating these approaches using female mice would be a great complement to our report; 2) We used a complete spinal cord transection as it is a reproducible and consistent model of paralysis. However, this is not a direct clinical analog as anatomically complete transections are rare in humans. Studies investigating these parameters in various models of contusion SCI would be a great benefit to the field; 3) The unexpectedly low number of FDRs for methylomics hindered rigorous biological interrogation. Our two-parameter exploratory analyses using raw P values and a ±10% methylation change likely capture legitimate physiological changes, but increase the chance for false positives. Further investigations using high-throughput arrays, other methylation library preparations (e.g., enzymatic CpG detection), or larger sample sizes may help increase the rigor for these types of analyses; 4) Boldine may target cellular processes not directly related to CxHCs, such as pannexins, which allow small molecules such as calcium into the cell (12, 17). However, muscle-restricted knockout of pannexins does not prevent atrophy after denervation suggesting that if pannexins participate, their role is dependent on CxHCs (13); 5) We solely focused on skeletal muscle in this report. Our intervention was systemic so there is a chance any changes we have observed may be coming from temporally preserved function of upstream factors such as the motor neuron or even the spinal cord itself; and 6) Changes in single DMCs do not necessarily account for changes in mRNA expression. Chromatin profiling and accessibility using ATACseq would be a very useful complementary approach, as accessibility changes surrounding a DMC (or group of nearby DMCs) would imply biological significance.
In summary, SCI resulted in a severe alteration in the multiome with the greatest number of changes observed during the acute timeframe. Boldine was ineffective in preserving body and muscle mass after injury though it was able to alter the metabolome at 7 days with provocative findings related to amino acids, energy substrates, and multiple unannotated metabolites. Boldine had a unique transcriptomic signature related to the molecular regulation of glucose metabolism and the ECM. Analyses of the methylome highlighted unique DMCs across comparisons. Our data provide a foundation for a better understanding of the changes that occur in paralyzed muscle in the acute and subacute timeframe after a complete SCI and provide a deeper understanding of the effects of boldine on skeletal muscle in SCI.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20260152.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.22581958.
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.20261475.
Supplemental Table S4: https://doi.org/10.6084/m9.figshare.20263659.
Supplemental Table S5: https://doi.org/10.6084/m9.figshare.20263668.
Supplemental Table S6: https://doi.org/10.6084/m9.figshare.20263674.
Supplemental Table S7: https://doi.org/10.6084/m9.figshare.21899421.
Supplemental Table S8: https://doi.org/10.6084/m9.figshare.21899430.
Supplemental File S1: https://doi.org/10.6084/m9.figshare.20419122.
Supplemental File S2: https://doi.org/10.6084/m9.figshare.20259897.
Supplemental File S3: https://doi.org/10.6084/m9.figshare.20259594.
Supplemental File S4: https://doi.org/10.6084/m9.figshare.20261460.
Supplemental File S5: https://doi.org/10.6084/m9.figshare.20419386.
Supplemental File S6: https://doi.org/10.6084/m9.figshare.20422227.
Supplemental File S7: https://doi.org/10.6084/m9.figshare.21899016.
Supplemental File S8: https://doi.org/10.6084/m9.figshare.21899394.
Supplemental File S9: https://doi.org/10.6084/m9.figshare.21899451.
Supplemental File S10: https://doi.org/10.6084/m9.figshare.21899457.
Supplemental File S11: https://doi.org/10.6084/m9.figshare.21899460.
Supplemental File S12: https://doi.org/10.6084/m9.figshare.21899466.
GRANTS
This study was funded by a Department of Veterans Affairs Office of Research and Development RR&D Service CDA-2 grant (1IK2RX002781 to Z.A.G.) and Center grant (5I50RX002020; PI, William Bauman). L.A.P. is supported by a National Institutes of Health F31 Award (F31HL154571). Metabolomics services were completed by West Coast Metabolomics (UC2ES030158; PI, Oliver Fiehn).
DISCLAIMERS
The contents of this manuscript do not represent the views of the US Government or the Department of Veterans Affairs.
DISCLOSURES
Z.A.G., C.A.T., and C.P.C. are co-inventors of a submitted patent application for the use of boldine to treat neurological injuries. This study falls within the claims of the patent. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
L.A.P., C.A.T., C.P.C., A.R.W., and Z.A.G. conceived and designed research; C.A.T., L.H., and Z.A.G. performed experiments; L.A.P., L.H., K.M.L., C.P.C., A.R.W., and Z.A.G. analyzed data; L.A.P., C.A.T., L.H., K.M.L., C.P.C., A.R.W., and Z.A.G. interpreted results of experiments; L.A.P., A.R.W., and Z.A.G. prepared figures; L.A.P., A.R.W., and Z.A.G. drafted manuscript; L.A.P., C.A.T., L.H., K.M.L., C.P.C., A.R.W., and Z.A.G. edited and revised manuscript; L.A.P., C.A.T., L.H., K.M.L., C.P.C., A.R.W., and Z.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Preprint is available at https://doi.org/10.1101/2022.08.17.503230.
REFERENCES
- 1. Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin K-H, Wilson GM, Blanco R, Steinert ND, Zhu WG, Coon JJ, Hornberger TA. A deep analysis of the proteomic and phosphoproteomic alterations that occur in skeletal muscle after the onset of immobilization. J Physiol 599: 2887–2906, 2021. doi: 10.1113/JP281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wall BT, Dirks ML, Snijders T, van Dijk J-W, Fritsch M, Verdijk LB, van Loon LJC. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am J Physiol Endocrinol Physiol 310: E137–E147, 2016. doi: 10.1152/ajpendo.00227.2015. [DOI] [PubMed] [Google Scholar]
- 4. Biering-Sørensen B, Kristensen IB, Kjaer M, Biering-Sørensen F. Muscle after spinal cord injury. Muscle Nerve 40: 499–519, 2009. doi: 10.1002/mus.21391. [DOI] [PubMed] [Google Scholar]
- 5. Graham ZA, Harlow L, Peng Y, Saez J, Bauman WA, Weiping Q, Cardozo C. A soluble activin receptor IIB fails to prevent muscle atrophy in a mouse model of spinal cord injury. J Neurotrauma 33: 1128–1135, 2016. doi: 10.1089/neu.2015.4058. [DOI] [PubMed] [Google Scholar]
- 6. Graham ZA, Liu X-H, Harlow L, Pan J, Azulai D, Tawfeek HA, Wnek RD, Mattingly AJ, Bauman WA, Yarrow JF, Cardozo CP. Effects of a high-fat diet on tissue mass, bone, and glucose tolerance after chronic complete spinal cord transection in male mice. Neurotrauma Rep 1: 17–31, 2020. doi: 10.1089/neur.2020.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham ZA, Siedlik JA, Harlow L, Sahbani K, Bauman WA, Tawfeek HA, Cardozo CP. Key glycolytic metabolites in paralyzed skeletal muscle are altered seven days after spinal cord injury in mice. J Neurotrauma 36: 2722–2731, 2019. doi: 10.1089/neu.2018.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham ZA, DeBerry JJ, Cardozo CP, Bamman MM. SS‐31 does not prevent or reduce muscle atrophy 7 days after a 65 kdyne contusion spinal cord injury in young male mice. Physiol Rep 10: e15266, 2022. doi: 10.14814/phy2.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Physiol 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49: 59–68, 2014. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 12: 330, 2021. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sáez JC, Cisterna BA, Vargas A, Cardozo CP. Regulation of pannexin and connexin channels and their functional role in skeletal muscles. Cell Mol Life Sci 72: 2929–2935, 2015. doi: 10.1007/s00018-015-1968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C, Willecke K, Latorre R, Sáez JC. De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc Natl Acad Sci USA 110: 16229–16234, 2013. doi: 10.1073/pnas.1312331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cea LA, Balboa E, Puebla C, Vargas AA, Cisterna BA, Escamilla R, Regueira T, Sáez JC. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim Biophys Acta 1862: 1891–1899, 2016. doi: 10.1016/j.bbadis.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15. Cea LA, Fernández G, Arias-Bravo G, Castillo-Ruiz M, Escamilla R, Brañes MC, Sáez JC. Blockade of hemichannels normalizes the differentiation fate of myoblasts and features of skeletal muscles from dysferlin-deficient mice. Int J Mol Sci 21: 6025, 2020. doi: 10.3390/ijms21176025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cisterna BA, Vargas AA, Puebla C, Fernández P, Escamilla R, Lagos CF, Matus MF, Vilos C, Cea LA, Barnafi E, Gaete H, Escobar DF, Cardozo CP, Sáez JC. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun 11: 1073, 2020. doi: 10.1038/s41467-019-14063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plotkin LI, Davis HM, Cisterna BA, Sáez JC. Connexins and pannexins in bone and skeletal muscle. Curr Osteoporos Rep 15: 326–334, 2017. doi: 10.1007/s11914-017-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Araya R, Eckardt D, Maxeiner S, Kruger O, Theis M, Willecke K, Sáez JC. Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J Cell Sci 118: 27–37, 2005. doi: 10.1242/jcs.01553. [DOI] [PubMed] [Google Scholar]
- 19. Cea LA, Balboa E, Vargas AA, Puebla C, Brañes MC, Escamilla R, Regueira T, Sáez JC. De novo expression of functional connexins 43 and 45 hemichannels increases sarcolemmal permeability of skeletal myofibers during endotoxemia. Biochim Biophys Acta Mol Basis Dis 1865: 2765–2773, 2019. doi: 10.1016/j.bbadis.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 20. Cisterna BA, Vargas AA, Puebla C, Sáez JC. Connexin hemichannels explain the ionic imbalance and lead to atrophy in denervated skeletal muscles. Biochim Biophys Acta 1862: 2168–2176, 2016. doi: 10.1016/j.bbadis.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 21. Hernández-Salinas R, Vielma AZ, Arismendi MN, Boric MP, Sáez JC, Velarde V. Boldine prevents renal alterations in diabetic rats. J Diabetes Res 2013: 593672, 2013. doi: 10.1155/2013/593672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PloS One 5: e15234, 2010. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aguer C, Piccolo BD, Fiehn O, Adams SH, Harper ME. A novel amino acid and metabolomics signature in mice overexpressing muscle uncoupling protein 3. FASEB J 31: 814–827, 2017. doi: 10.1096/fj.201600914R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skogerson K, Wohlgemuth G, Barupal DK, Fiehn O. The volatile compound BinBase mass spectral database. BMC Bioinformatics 12: 321, 2011. doi: 10.1186/1471-2105-12-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data (Online). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [2015]
- 26. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 10, 2011. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 27. Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27: 1571–1572, 2011. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13: R87, 2012. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (Online). https://www.R-project.org/ [2020]
- 30.R Studio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. (Online). http://www.rstudio.com/ [2020]
- 31. Park Y, Figueroa ME, Rozek LS, Sartor MA. MethylSig: a whole genome DNA methylation analysis pipeline. Bioinformatics 30: 2414–2422, 2014. doi: 10.1093/bioinformatics/btu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mao W, Zaslavsky E, Hartmann BM, Sealfon SC, Chikina M. Pathway-level information extractor (PLIER) for gene expression data. Nat Methods 16: 607–610, 2019. doi: 10.1038/s41592-019-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM, Jeon M, Ma’ayan A. Gene set knowledge discovery with enrichr. Curr Protoc 1: e90, 2021. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bader GD, Hogue CWV. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 4: 2, 2003. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47: D607–D613, 2019. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46: W486–W494, 2018. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cea LA, Riquelme MA, Cisterna BA, Puebla C, Vega JL, Rovegno M, Sáez JC. Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy. J Membr Biol 245: 423–436, 2012. doi: 10.1007/s00232-012-9485-8. [DOI] [PubMed] [Google Scholar]
- 41. Collazos-Castro JE, Soto VM, Gutiérrez-Dávila M, Nieto-Sampedro M. Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J Neurotrauma 22: 544–558, 2005. doi: 10.1089/neu.2005.22.544. [DOI] [PubMed] [Google Scholar]
- 42. Grumbles RM, Thomas CK. Motoneuron death after human spinal cord injury. J Neurotrauma 34: 581–590, 2017. doi: 10.1089/neu.2015.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ilaiwy A, Quintana MT, Bain JR, Muehlbauer MJ, Brown DI, Stansfield WE, Willis MS. Cessation of biomechanical stretch model of C2C12 cells models myocyte atrophy and anaplerotic changes in metabolism using non-targeted metabolomics analysis. Int J Biochem Cell Biol 79: 80–92, 2016. [Erratum in Int J Biochem Cell Biol 88: 238–240, 2017]. doi: 10.1016/j.biocel.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Csapo R, Gumpenberger M, Wessner B. skeletal muscle extracellular matrix – what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol 11: 253, 2020. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alameddine HS, Morgan JE. Matrix metalloproteinases and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles. J Neuromuscul Dis 3: 455–473, 2016. doi: 10.3233/JND-160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chou C-W, Huang Y-K, Kuo T-T, Liu J-P, Sher Y-P. An overview of ADAM9: structure, activation, and regulation in human diseases. Int J Mol Sci 21: E7790, 2020. doi: 10.3390/ijms21207790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Regn M, Laggerbauer B, Jentzsch C, Ramanujam D, Ahles A, Sichler S, Calzada-Wack J, Koenen RR, Braun A, Nieswandt B, Engelhardt S. Peptidase inhibitor 16 is a membrane-tethered regulator of chemerin processing in the myocardium. J Mol Cell Cardiol 99: 57–64, 2016. doi: 10.1016/j.yjmcc.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 48. Bevilacqua A, Bizzarri M. Inositols in insulin signaling and glucose metabolism. Int J Endocrinol 2018: 1968450, 2018. doi: 10.1155/2018/1968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Staunton CA, Owen ED, Hemmings K, Vasilaki A, McArdle A, Barrett-Jolley R, Jackson MJ. Skeletal muscle transcriptomics identifies common pathways in nerve crush injury and ageing. Skelet Muscle 12: 3, 2022. doi: 10.1186/s13395-021-00283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hitachi K, Nakatani M, Kiyofuji Y, Inagaki H, Kurahashi H, Tsuchida K. An analysis of differentially expressed coding and long non-coding RNAs in multiple models of skeletal muscle atrophy. Int J Mol Sci 22: 2558, 2021. doi: 10.3390/ijms22052558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yarrow JF, Phillips EG, Conover CF, Bassett TE, Chen C, Teurlings T, Vasconez A, Alerte J, Prock H, Jiron JM, Flores M, Aguirre JI, Borst SE, Ye F. Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J Neurotrauma 34: 2972–2981, 2017. doi: 10.1089/neu.2016.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med 37: 32–39, 2014. doi: 10.1179/2045772313y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, Spungen AM. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 43: 574–579, 2011. doi: 10.1055/s-0031-1280797. [DOI] [PubMed] [Google Scholar]
- 54. Petrie MA, Taylor EB, Suneja M, Shields RK. Genomic and epigenomic evaluation of electrically induced exercise in people with spinal cord injury: application to precision rehabilitation. Phys Ther 102: pzab243, 2022. doi: 10.1093/ptj/pzab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrie MA, Sharma A, Taylor EB, Suneja M, Shields RK. Impact of short- and long-term electrically induced muscle exercise on gene signaling pathways, gene expression, and PGC1a methylation in men with spinal cord injury. Physiol Genomics 52: 71–80, 2020. doi: 10.1152/physiolgenomics.00064.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murach KA, Dimet-Wiley AL, Wen Y, Brightwell CR, Latham CM, Dungan CM, Fry CS, Watowich SJ. Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell 21: e13527, 2022. doi: 10.1111/acel.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wen Y, Dungan CM, Mobley CB, Valentino T, von Walden F, Murach KA. Nucleus type-specific DNA methylomics reveals epigenetic “Memory” of prior adaptation in skeletal muscle. Function (Oxf) 2: zqab038, 2021. doi: 10.1093/function/zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mzoughi S, Fong JY, Papadopoli D, Koh CM, Hulea L, Pigini P, , et al. PRDM15 is a key regulator of metabolism critical to sustain B-cell lymphomagenesis. Nat Commun 11: 3520, 2020. doi: 10.1038/s41467-020-17064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Spinal Cord Injury Statistical Center. 2021 Annual Report: Complete Public Version (Online). https://www.nscisc.uab.edu/PublicDocuments/AR2021_public%20version.pdf [2021]
- 60. Curtin CM, Suarez PA, Di Ponio LA, Frayne SM. Who are the women and men in Veterans Health Administration’s current spinal cord injury population? J Rehabil Res Dev 49: 351–360, 2012. doi: 10.1682/jrrd.2010.11.0220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20260152.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.22581958.
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.20261475.
Supplemental Table S4: https://doi.org/10.6084/m9.figshare.20263659.
Supplemental Table S5: https://doi.org/10.6084/m9.figshare.20263668.
Supplemental Table S6: https://doi.org/10.6084/m9.figshare.20263674.
Supplemental Table S7: https://doi.org/10.6084/m9.figshare.21899421.
Supplemental Table S8: https://doi.org/10.6084/m9.figshare.21899430.
Supplemental File S1: https://doi.org/10.6084/m9.figshare.20419122.
Supplemental File S2: https://doi.org/10.6084/m9.figshare.20259897.
Supplemental File S3: https://doi.org/10.6084/m9.figshare.20259594.
Supplemental File S4: https://doi.org/10.6084/m9.figshare.20261460.
Supplemental File S5: https://doi.org/10.6084/m9.figshare.20419386.
Supplemental File S6: https://doi.org/10.6084/m9.figshare.20422227.
Supplemental File S7: https://doi.org/10.6084/m9.figshare.21899016.
Supplemental File S8: https://doi.org/10.6084/m9.figshare.21899394.
Supplemental File S9: https://doi.org/10.6084/m9.figshare.21899451.
Supplemental File S10: https://doi.org/10.6084/m9.figshare.21899457.
Supplemental File S11: https://doi.org/10.6084/m9.figshare.21899460.
Supplemental File S12: https://doi.org/10.6084/m9.figshare.21899466.
Data Availability Statement
Data will be made available upon reasonable request.