Abstract
Environmental cues such as light and timing of food intake influence molecular clocks that produce circadian rhythmicity of many biological functions. The master circadian clock is entrained by light input and synchronizes with peripheral clocks in every organ of the body. Careers that require rotating shift work schedules predispose workers to a constant desynchronization of these biological clocks and are associated with increased risk of cardiovascular disease. We used a stroke-prone spontaneously hypertensive rat model exposed to a known biological desynchronizer, chronic environmental circadian disruption (ECD), to test the hypothesis that it would accelerate the time to stroke onset. We then investigated whether time-restricted feeding could delay stroke onset and evaluated its usefulness as a countermeasure when combined with the constant disruption of the light cycle. We found that phase advancing of the light schedule accelerated stroke onset. Restricting food access time to 5 h/day regardless of lighting profoundly delayed stroke onset in both standard 12-h:12-h light/dark or ECD-lighting conditions compared with ad libitum feeding; however, acceleration by ECD versus control lighting conditions was still observed. Since hypertension is a precursor to stroke in this model, we assessed blood pressure in a small cohort longitudinally using telemetry. Mean daily systolic and diastolic blood pressure increased in a similar manner across rats in control and ECD conditions, thus hypertension was not grossly accelerated to cause earlier strokes. However, we observed intermittent dampening of rhythms after each shift of the light cycle reminiscent of a relapsing-remitting nondipping state. Our results suggest that constant disruption of environmental rhythms may be associated with an increased risk of cardiovascular complications in the presence of cardiovascular risk factors.
NEW & NOTEWORTHY This stroke-prone spontaneously hypertensive rat model significantly delayed stroke onset with the timed food restriction intervention. Blood pressure recordings in this same model were continuous through the 3 mo and showed dampened systolic rhythms after each shift in the lighting schedule.
Keywords: circadian disruption, nondipping, shift work, stroke, time-restricted feeding
INTRODUCTION
The ability to predict environmental change evolved as a necessary function for survival (1). To support this need, circadian clocks are coupled to recurring 24-h rhythms of endogenous biochemical, physiological, and behavioral processes (2). Such rhythms have significance for cerebrovascular and cardiovascular health (3, 4). The master circadian clock is found in the hypothalamic suprachiasmatic nucleus (SCN) (5). This neuronal network synchronizes to environmental light/dark cycles and communicates with peripheral organ molecular clocks via hormonal and neuronal signals to regulate endogenous rhythms specific to organ function (6).
The established daily variations in physiological functions include metabolism, body temperature, and blood pressure, which coordinate by trough during rest versus peak during the active time of day. Synchrony of the blood pressure rhythm is necessary for adequate organ perfusion in response to changing environmental and endogenous demands. Prolonged desynchronization of these clocks can result in chronic health issues (5, 7, 8). In the United States, 16.4% of the labor force works on a shift schedule (9). Shift workers who were exposed to career-long rotating shift schedules showed an increased risk and incidence of cardiovascular disease (10, 11). We therefore seek to understand the consequences of a prolonged schedule of rotating light conditions as a model of a shift work environment.
Peripheral molecular clocks also receive daily cues, including feed-fast cycles and hormonal rhythms, which affect the cellular clocks as much as external cues of light and dark cycles. Previous studies have shown that restricting the time of food access does not decrease calorie consumption, but does increase rhythmicity, improves amplitude, and synchronizes phase oscillation of clock transcripts (12). Furthermore, intermittent fasting and adjusted diurnal rhythm of feeding have been shown to help prevent cardiovascular disease risk (13).
A long-term beat-to-beat measurement of blood pressure variability is not feasible for routine measurement in clinical and population studies. However, experimental animal models allow exploration of mechanisms of the consequences of molecular clock desynchrony. The stroke-prone spontaneously hypertensive rat (SP-SHR) is a well-characterized genetic model with hypertension as a precursor to progressive kidney disease and cerebrovascular injury (14, 15). We previously reported evidence that chronic phase advances of the light cycle in the SP-SHR misaligned and decreased the amplitude of urinary volume, concomitant with accelerated excretion of glomerular and tubular injury markers (16). To further understand the impact of chronic phase advancement, we evaluated the latency to stroke, daily blood pressure variation, and fixed time-restricted feeding schedule as a potential intervention in this animal model.
METHODS
General Methods
Male SP-SHR (n = 12 rats/group) obtained from Charles River Laboratories (Product 24104005), were used in this study. All rats were individually housed in a temperature-, light-, and humidity-controlled cabinets and fed a 4% NaCl diet (Zeigler, item 528801-12-01) ad libitum with free access to water from the first week of the experiment. Four groups of SP-SHR rats (n = 12 rats/group) were housed in either regular or continuous 6-h weekly phase advancing light cycles (Fig. 1). However, two of the groups, one regular and one phase advanced, had 5 h of access to food during the same clock time regardless of light conditions. Although the other two groups had 24 h access to food. Starting in week 4, to accelerate disease onset, 1% NaCl was added to the water for the duration of the study for all four groups. All animal procedures in this study were conducted in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Figure 1.
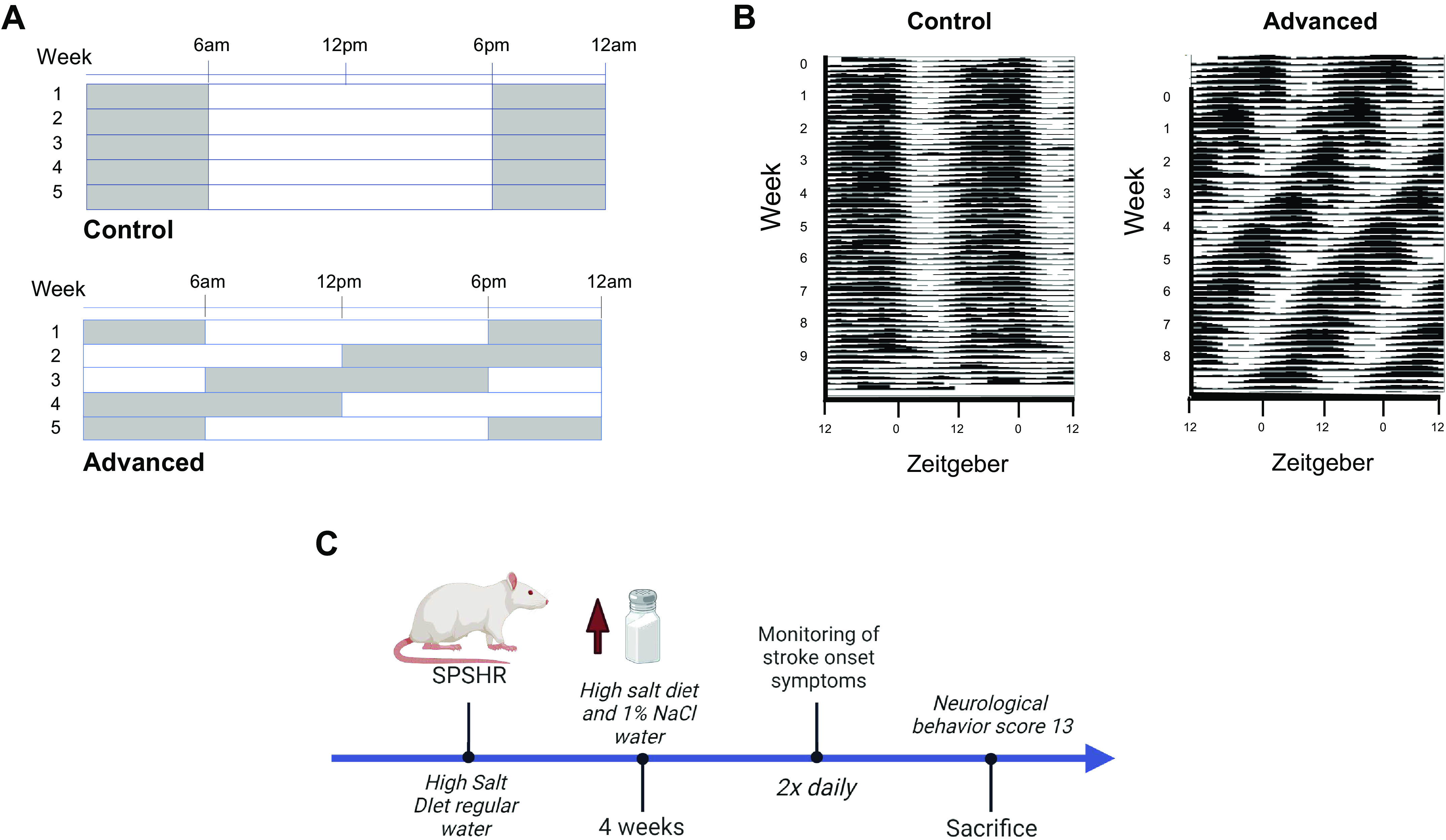
Light and time-restricted diet schedules. A: schematics of the lighting schedules for each week of the study. B: representative actograms illustrating rat locomotor activity in either control (static) or phase-shifted light cycles. C: summary of the experimental protocol. SP-SHR, stroke-prone spontaneously hypertensive rat. Images created with a licensed version of BioRender.
Stroke-Onset Evaluation Criteria
The occurrence of neurological signs of stroke in SP-SHR was evaluated daily by one blinded researcher and the primary investigator. Stroke onset was determined using neurobehavioral and pain scale scoring (Table 1) twice a day. Scores included a composite of the motor, sensory, weight, and appearance assigned values largely adapted from home cage monitoring (17, 18) and neurobehavioral scoring (19) methods. In this modified assessment scale, animals were euthanized when a predetermined score of 13 was reached. Animals were weighed weekly, and food and water intake were measured daily for the timed food-restricted animals.
Table 1.
Neurobehavioral stroke onset chart depicting scoring system used to evaluate stroke-prone spontaneously hypertensive rat
| Pain Scores |
|||||
|---|---|---|---|---|---|
| 0 | 2 | 3 | 5 | 10 | |
| Body weight, %decrease | <5 | 6–10 | 11–25 | >25 | |
| Appearance | Normal | Huddled Mild piloerection | Huddled Moderate piloerection | Huddled Not groomed Severe piloerection | |
| Behavior | Normal | Responsive when stimulated | Lethargic | Lethargic | Unresponsive Moribund |
| Decreased appetite | Mildly responsive | Mildly responsive | |||
| Decreased appetite and water intake | |||||
| Clinical signs | Normal | Ocular discharge | Mild respiratory distress | Moderate respiratory distress | Severe respiratory distress and/or severe hydration |
| Antaxia and/or hydration | |||||
Telemeter Surgical Implantation
In a separate cohort, male rats (n = 3 rats/group) were surgically implanted with a telemetry system sensor (HD-S10 Data Sciences International (DSI), Minneapolis, MN). Transmitter platforms continuously monitored systolic (SBP), diastolic blood (DBP) pressure activity, and body temperature until stroke onset. In brief, 12-wk-old SHRSP rats were anesthetized in a chamber of 5% isoflurane in pure oxygen 600 mL/min and subsequently placed on a heated surface and fitted with a nose mask with anesthesia maintained at 1.5–3% isoflurane. Hair was removed from the abdominal region and the area was disinfected with iodine using a soaked gauze. Blood flow to the abdominal aorta was temporarily occluded using a suture thread around the proximal and distal ends of the vessel. A needle puncture in the aorta between the bifurcation and the left renal artery was used to insert the catheter of the telemetry device. The insertion site was then reperfused, and the transmitter was secured in the abdominal wall with a nonabsorbable 5-0 suture. An injection of 0.25% bupivacaine and meloxicam (5 mg/kg) was administered at the end of the surgery to minimize pain. During the postoperative recovery period, rats were housed individually in cages and kept warm with a heating pad. Additional pain relief was given as a subcutaneous injection twice daily of buprenorphine (0.1 mg/kg) and meloxicam (1 mg/kg) for 4 days and accompanied by ad libitum access to an additional drinking bottle containing 15% glucose and 4 oz of moistened food. A total of 2 wk of recovery time was given before initiating experimental protocols. After recovery, the transmitter was turned on for continuous recording of physiological data until the end of the individual experiment. Blood pressure, body temperature, and activity were recorded every 10 s over the experimental period using computer-based acquisition hardware and software (Ponemah v6.2, Data Sciences International, Minneapolis, MN).
Statistical Analysis
Time-to-event analysis was performed using a graphical representation of occurrence with probabilities estimated by the Kaplan–Meier method with GraphPad Prism version 6.04 for Windows, GraphPad Software (La Jolla, CA). Right censoring was used to include a lack of occurrences in surviving time-restricted-fed animals. Plotted estimated survival probability was plotted against time where each vertical drop indicates an occurrence of daily events (20).
Longitudinal daily blood pressure data (Fig. 2, C and D) were analyzed using the 24-h mean for each rat and averaged together as (n = 3 rats) and reported as means ± SE per group. Rhythmicity of BP (amplitude and relative amplitude error) was analyzed with the fast Fourier transform–nonlinear least squares (FFT-NLLS) function in BioDare2 software. Data were further analyzed using the Student’s t test (for comparison of 2 groups with equal variance), one-way analysis of variance (ANOVA) followed by Dunnett’s test, or repeated-measures ANOVA followed by Bonferroni post hoc comparisons by day. In all analyses, P < 0.05 was taken to indicate statistical significance, and we also noted P < 0.1 as trending in some repeated-measures post hoc analyses.
Figure 2.
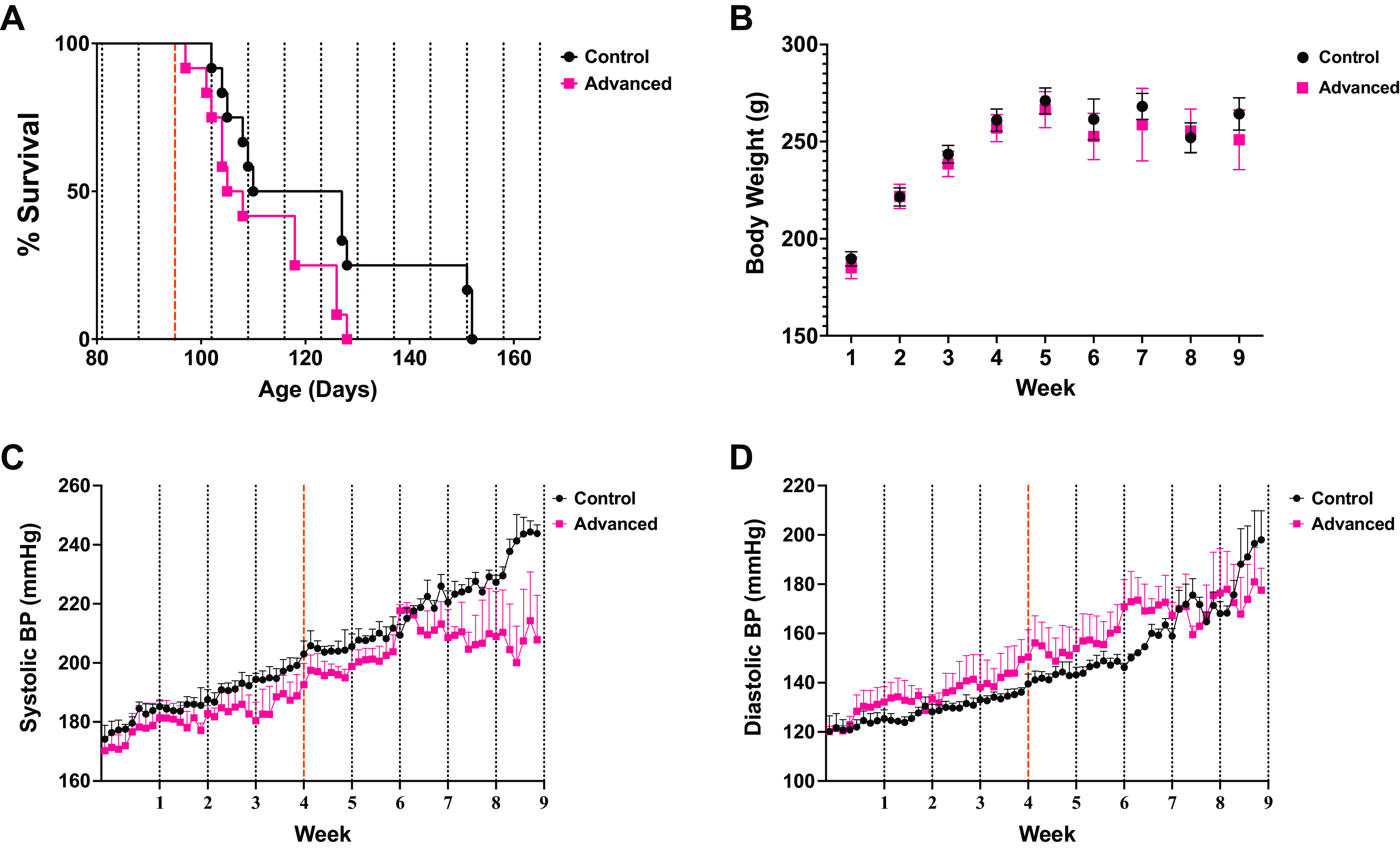
Environmental circadian disruption (ECD) accelerates time to stroke onset. Chronic phase advances of the light-cycle accelerated time to stroke onset without diverging systolic or diastolic blood pressure readings. Log-rank (Mantel–Cox) test survival analysis showed (P < 0.05) with a median survival of the control group (n = 12 rats) of 93.5 days to phase-advanced group (n = 12 rats) of 81.50 days (A). Body weight between the 2 groups (B) showed no significance [P = 0.6129; F(1,10) = 0.2727]. The phase advancing group (n = 3 rats; M = 194.2) compared with the control group (n = 3 rats; M = 204.6) difference between means (SE = −10.41 ± 2.921) demonstrated a significant attenuation of systolic blood pressure (SBP) rise during chronic phase advancing toward the end of the experiment (t = 3.565, df = 126, P = 0.0005) (C). However, diastolic blood pressure (DBP) was not significantly different between the 2 groups at any point (D). Dotted lines indicate shift day for rats in phase-advancing light conditions. Dashed red line indicates the point at which water was switched to 1% salt water for all animals.
RESULTS
Environmental Circadian Disruption Accelerates Time to Stroke Onset
Log-rank (Mantel–Cox) test survival analysis shows that the onset of stroke symptoms was significantly accelerated in the group housed in phase advancing light conditions (n = 12 rats) than that of the group housed in regular light conditions (n = 12 rats) (P < 0.05) (Fig. 2A). However, the mixed-effects Model shows that the body weight of the group housed in regular light conditions (n = 12 rats) was not statistically different from that of the phase advancing group (n = 12 rats) [P = 0.6129 F(1,10) = 0.2727] (Fig. 2B).
Diurnal Rhythmicity of Blood Pressure Is Lost during Prolonged Environmental Circadian Disruption
The onset/development of spontaneous hypertension was evaluated as a potential mechanism for accelerated stroke in environmental circadian disruption (ECD)-exposed rats (21). In general, BP increased at the same rate across groups in the study except for systolic pressure with the main effect for a time near the conclusion of the experiment (Fig. 2C), at which point many had already died and fewer contributed to the mean. Thus, basal BP does not increase faster in ECD rats, and cannot account for earlier stroke onsets. However, the daily rhythms in ECD rats did differ from controls in that ECD rats exhibited an intermittent decrease in the amplitude of the rhythm as defined by the daily percent variation from the Cosinor mesor. Systolic blood pressure (Fig. 3A) for the phase advancing group had a significantly dampened amplitude when collapsed across weeks [main effect of group: F(1,4) = 78.89; P = 0.0009]. It was also dynamic over time [main effect of time: F(31,124) = 7.609; P < 0.0001]. As there was no significant time × group interaction (P = 0.074), this might be interpreted as a consistent feature across the 6 wk of BP monitoring, but we are mindful that the small sample size precludes firm conclusions here. Post hoc pairwise analysis suggested that the loss of amplitude for ECD rats compared with controls was most pronounced on 1–2 days each following light cycle shifts 1, 3, 5, and 7. Similar results were observed for diastolic BP (Fig. 3B). DBP was again changing across the 6 wk of the study [main effect of time: F(30,120) = 10.72; P < 0.0001], and was lower overall for ECD rats [main effect of group: F(1,4) = 55.05; P = 0.0018]. DBP ANOVA did reveal an interaction [group × time: F(30,120) = 1.903; P = 0.0079], which we interpret to mean that the decreased amplitude in ECD rats was progressive over time. Post hoc analysis suggested that the most pronounced differences in diurnal amplitude of this measure across groups were observed after shifts 4, 5, 6, and 7; thus, later in the study once ECD exposure was prolonged.
Figure 3.
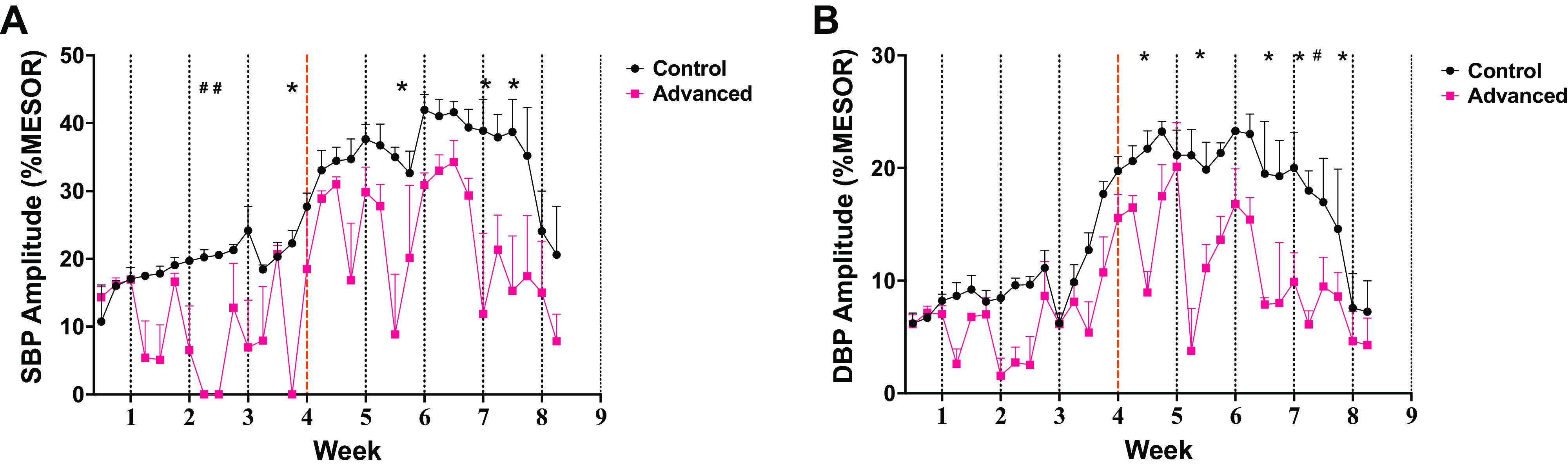
Diurnal rhythmicity of blood pressure is dampened during prolonged environmental circadian disruption (ECD) pressures were continuously measured by telemetry and averaged in 1-h bins through the end of the experiment (n = 3 rats/group). Cosinor (rhythm) daily amplitude, expressed as percentage of mesor for systolic (SBP; A) and diastolic (DBP; B) blood pressures are plotted for each day of the study (means ± SE of n = 3 rats/group). Post hoc statistical daily comparisons are indicated by *P < 0.05 and #P < 0.1 and suggest that the amplitude (day/night change) is suppressed intermittently after each shift of the light/dark cycle.
Time-Restricted Food Access Delays Stroke Onset during ECD
We next investigated to see if time-restricted access to food would delay the time to stroke onset in SHRSP. A timed restriction to food access was used in both regular and phase-advancing light conditions. Food was accessible during the same clock time daily for both time-restricted groups for 5 h correlating with the beginning of the active period of rats housed in regular light conditions. This restriction to food access resulted in a dramatic delay of time to stroke onset under both regular 12-h:12-h light/dark conditions and phase advanced light conditions (Fig. 4A). Notably, rats in the time-restricted feeding phase advancing group had a prolonged time to stroke onset (50% time to events median = 120 days) when compared with phase advancing rats given 24-h food access (50% time to events = 80 days). This intervention is also significantly attenuated stroke onset in SHRSP housed in regular lighting conditions. Note that only 3/12 rats in this group had clinically relevant stroke events even out to 170 days. Despite such lengthened survival, the analysis showed a significantly longer life span for the control group versus the phase-advancing group during time-restricted food (log-rank Mantel–Cox test; P < 0.0001). Weight gain was similar among the two groups (2-way ANOVA; P = 0.1286).
Figure 4.
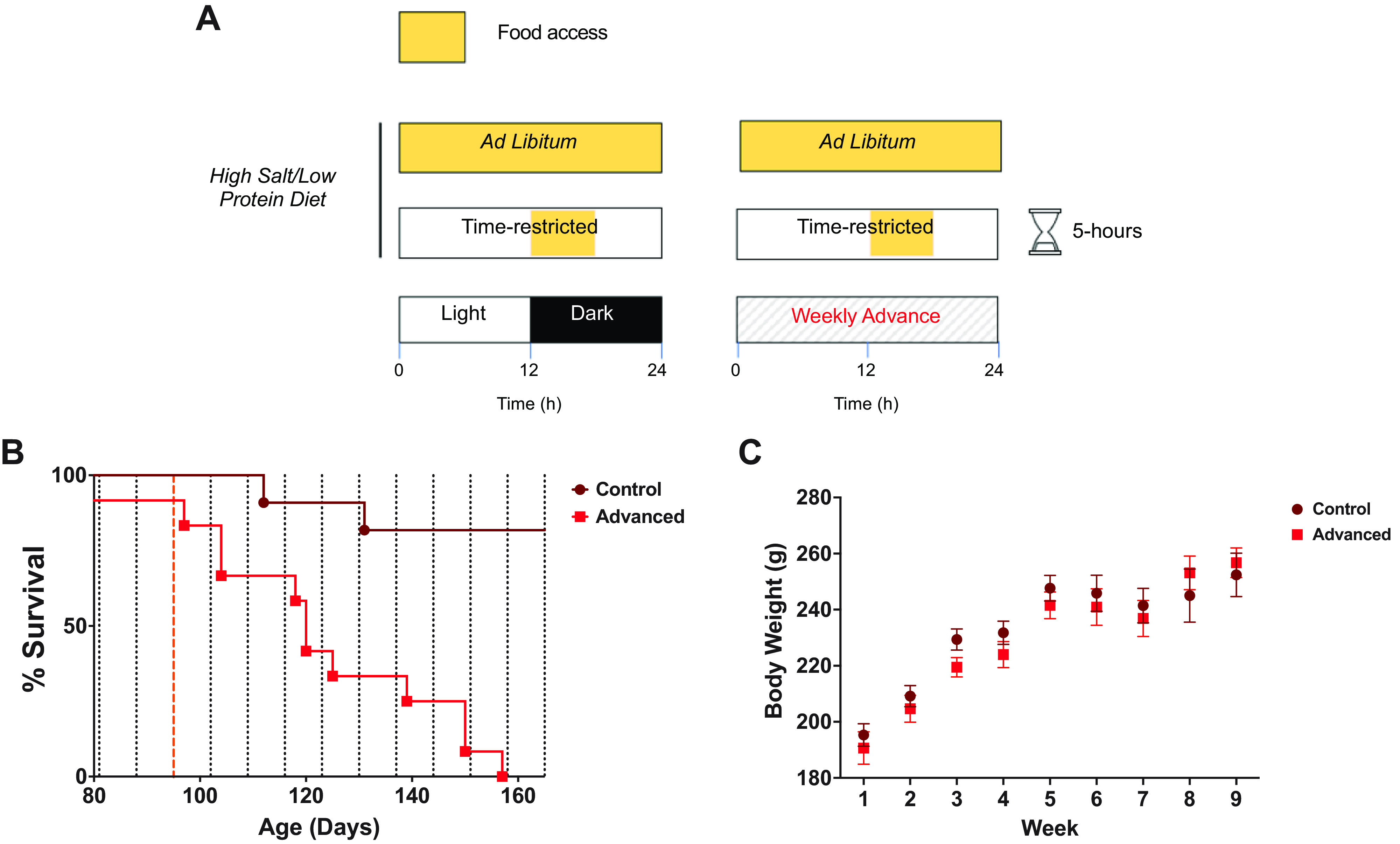
Time-restricted food access delays stroke onset but does not prevent environmental circadian disruption (ECD) effects. A: rats were fed a high-salt diet and water ad libitum. At the beginning of week 4, the water was switched to 1% salt water. Time-restricted feeding protocol allowed ad libitum access to food for 5 h during the same clock time daily as the rats in the regular light conditions regardless of their phase advancing schedule starting at 12 wk of age. B: while fixed time-restricted feeding lengthened life span overall, survival analysis showed a significantly longer life span of the control group (n = 12 rats) vs. the phase advancing group (n = 12 rats; log-rank Mantel–Cox test; P < 0.0001). C: body weights were similar across these 2 groups (2-way ANOVA; P = 0.1286) Dashed red line indicates the point at which water was switched to 1% salt water for all animals.
DISCUSSION
In this study, we observed accelerated onset of spontaneous stroke due to ECD in a stroke-prone rat model. These results add to a growing list of health risks identified in shift-work epidemiology that can be modeled in rodents, allowing for mechanistic study. In this model, we show that intermittent nondipping hypertension may be a causal factor in such effects, and also that time-restricted feeding, a noninvasive behavioral intervention, can partially offset these effects by delaying stroke onset.
Epidemiological studies have demonstrated that chronic health issues including hypertension, cardiovascular, and compromised metabolic function are frequently seen in patient populations who work on shift-work schedules (22–25), but causality and mechanism are difficult to establish in human research. Our previous work demonstrates that 6-h weekly phase advances of the light cycle create ECD and can accelerate death in rodent models (26). More recently, we and others have shown that chronic exposure to such schedules increases stroke severity (27, 28), likely due to changes in inflammatory signaling. We have also recently reported that the same continuous phase advancing of the light cycle accelerated kidney injury in salt-loaded SHRSP (16) and that kidney rhythm disruption perhaps decreased resilience to kidney disease. Here, we expand on these results to show that these rats, in the same lighting conditions, also exhibit a shorter latency to a fatal stroke.
In humans, blood pressure changes in a circadian manner, such that a 10–20% change in arterial pressure between day and night is associated with optimal cardiovascular function (29, 30). The blunting of nocturnal blood pressure rhythm is associated with an increased risk of death and cardiorenal, and cardiovascular disease (31, 32). In both normotensive and hypertensive populations, an absence of this rhythmicity is associated with increased cardiovascular mortality and end-organ damage (33, 34). Because of these known risk factors in humans, we measured arterial pressures in a small number of (n = 3 rats) our rats longitudinally in both normal and advancing light schedules to determine if BP rhythms are blunted before the stroke. We observed that mean daily BP rises similarly in both groups across the study timeline and thus does not account for a more rapid stroke onset. However, the BP rhythms in shifted rats were intermittently suppressed, reminiscent of nondipping hypertension. Rats housed in control lighting conditions showed an extreme dipping diurnal blood pressure phenotype. Abnormal nocturnal blood pressure, whether extreme or nondipping, is linked to negative cardiovascular outcomes (35). Although an extreme dip in blood pressure has been shown with increased sodium intake in rats (36), it is noteworthy that in spite of this, the phase-advancing group still lost this diurnal pattern. After each shift of the lights, BP rhythms were suppressed for several days, then rebounded, only to be again suppressed after the next lighting cycle shift. Although in our previous study in this rat model (16), we could not measure rhythms in kidney function each day as we did here for blood pressure, we indeed observed that kidney rhythms were suppressed in these rats. It is tempting to speculate that loss of kidney rhythms contributed to a loss of BP rhythms and is causally related to acceleration of both kidney damage and stroke in these rats. The small sample size and lack of female rats in this study is a limitation in assessing the full impact of exposure to disrupted circadian light cycle. Additional studies are needed to verify our observations.
Timed food restriction has been shown to improve insulin sensitivity, reduce blood pressure, and lower heart rate (37). Clinical studies also suggest that timed-restricted feeding may be one component of a lifestyle that decreases stroke risk (38) and that timing of food intake drives blood pressure circadian rhythmicity (39). Therefore, we tested this intervention to offset the acceleration of fatal strokes by circadian disruption. Although this feeding schedule did lengthen the life span in both shifted rats and controls in a fixed 12-h:12-h light/dark cycle (40), the circadian disruption group still eventually succumbed to stroke while controls were nearly completely protected. Our interpretation is that fixed mealtime did not rescue the powerful consequences of ECD. It is possible that had we shifted the mealtime along with the lights rather than keep it at a fixed clock time, the circadian disruption would have been ameliorated and the effects of ECD prevented.
Best practices to offset the long-term health consequences of shift work and other lifestyles that compromise circadian timing remain elusive but are desperately needed. Given the prevalence of 24/7 business and service needs, and the ubiquity of artificial light, such lifestyles are clearly not going away, so additional efforts are needed to identify protective strategies. Although meal timing appears to have protective effects in this rat model of stroke risk, such interventions in humans remain impractical and the beneficial effects we have observed are nonspecific to circadian disruption.
DATA AVAILABILITY
Data will be made available upon request.
GRANTS
This work was supported by National Institutes of Health Grants R21NS108197 and R35GM136661 (to A.J.D.) and P01HL136267 (to D.M.P.) and American Heart Association Grants 908953 (to A.M.R) and 827566 (to M.K.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.R. conceived and designed research; A.M.R., A.H., and I.E. performed experiments; A.M.R. analyzed data; A.M.R., A.S., M.K.R., D.M.P., and A.J.D. interpreted results of experiments; A.M.R. prepared figures; A.M.R. drafted manuscript; D.M.P. and A.J.D. edited and revised manuscript; A.M.R., A.S., A.H., I.E., M.K.R., D.M.P., and A.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Morehouse School of Medicine Center for Laboratory Animal Resources for technical assistance.
REFERENCES
- 1. Pilorz V, Helfrich-Forster C, Oster H. The role of the circadian clock system in physiology. Pflugers Arch 470: 227–239, 2018. doi: 10.1007/s00424-017-2103-y. [DOI] [PubMed] [Google Scholar]
- 2. Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 25: 85–93, 2001. [PMC free article] [PubMed] [Google Scholar]
- 3. Thosar SS, Shea SA. Circadian control of human cardiovascular function. Curr Opin Pharmacol 57: 89–97, 2021. doi: 10.1016/j.coph.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castilla-Guerra L, Fernandez-Moreno MC. Chronic management of hypertension after stroke: the role of ambulatory blood pressure monitoring. J Stroke 18: 31–37, 2016. doi: 10.5853/jos.2015.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol 26: R432–R443, 2016. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 6. Brown AJ, Pendergast JS, Yamazaki S. Peripheral circadian oscillators. Yale J Biol Med 92: 327–335, 2019. [PMC free article] [PubMed] [Google Scholar]
- 7. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA 113: E1402–E1411, 2016. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. West AC, Smith L, Ray DW, Loudon ASI, Brown TM, Bechtold DA. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun 8: 417, 2017. doi: 10.1038/s41467-017-00462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Bureau of Labor Statistics. Workers by Shift Usually Worked and Selected Characteristics, Averages for the Period 2017-2018. Economic News Release, 2018. https://www.bls.gov/news.release/flex2.t07.htm. [Google Scholar]
- 10. Abu Farha R, Alefishat E. Shift work and the risk of cardiovascular diseases and metabolic syndrome among Jordanian employees. Oman Med J 33: 235–242, 2018. doi: 10.5001/omj.2018.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown DL, Feskanich D, Sánchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol 169: 1370–1377, 2009. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23: 1048–1059, 2016. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fontana L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat Rev Cardiol 15: 566–577, 2018. doi: 10.1038/s41569-018-0026-8. [DOI] [PubMed] [Google Scholar]
- 14. Smeda JS. Hemorrhagic stroke development in spontaneously hypertensive rats fed a North American, Japanese-style diet. Stroke 20: 1212–1218, 1989. doi: 10.1161/01.str.20.9.1212. [DOI] [PubMed] [Google Scholar]
- 15. Nagaoka A, Iwatsuka H, Suzuki Z, Okamoto K. Proceedings: cerebral lesions in the spontaneously hypertensive rats. Effects of NaCl-loading, sex-related difference and genetic analysis. Jpn Heart J 15: 216–217, 1974. doi: 10.1536/ihj.15.216. [DOI] [PubMed] [Google Scholar]
- 16. Hill AM, Crislip GR, Stowie A, Ellis I, Ramsey A, Castanon-Cervantes O, Gumz ML, Davidson AJ. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am J Physiol Renal Physiol 320: F224–F233, 2021. doi: 10.1152/ajprenal.00421.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tappe-Theodor A, King T, Morgan MM. Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci Biobehav Rev 100: 335–343, 2019. doi: 10.1016/j.neubiorev.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aubert A. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev 23: 1029–1036, 1999. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 19. Foroud A, Whishaw IQ. Changes in the kinematic structure and non-kinematic features of movements during skilled reaching after stroke: a Laban Movement Analysis in two case studies. J Neurosci Methods 158: 137–149, 2006. doi: 10.1016/j.jneumeth.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20. Bewick V, Cheek L, Ball J. Statistics review 12: survival analysis. Crit Care 8: 389–394, 2004. doi: 10.1186/cc2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472–476, 1986. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 22. Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet 2: 89–92, 1986. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 23. Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra143, 2012. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63: 1860–1869, 2014. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 99: 2008–2019, 2005. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 26. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: R914–R916, 2006. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsey AM, Stowie A, Castanon-Cervantes O, Davidson AJ. Environmental circadian disruption increases stroke severity and dysregulates immune response. J Biol Rhythms 35: 368–376, 2020. doi: 10.1177/0748730420929450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Earnest DJ, Neuendorff N, Coffman J, Selvamani A, Sohrabji F. Sex differences in the impact of shift work schedules on pathological outcomes in an animal model of ischemic stroke. Endocrinology 157: 2836–2843, 2016. doi: 10.1210/en.2016-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet 2: 397, 1988. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 30. Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119: 108–114, 2018. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res 35: 695–701, 2012. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- 32. Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol 22: 598–604, 2011. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Myredal A, Friberg P, Johansson M. Elevated myocardial repolarization lability and arterial baroreflex dysfunction in healthy individuals with nondipping blood pressure pattern. Am J Hypertens 23: 255–259, 2010. doi: 10.1038/ajh.2009.252. [DOI] [PubMed] [Google Scholar]
- 34. Soylu A, Yazici M, Duzenli MA, Tokac M, Ozdemir K, Gok H. Relation between abnormalities in circadian blood pressure rhythm and target organ damage in normotensives. Circ J 73: 899–904, 2009. doi: 10.1253/circj.cj-08-0946. [DOI] [PubMed] [Google Scholar]
- 35. Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens 23: 645–653, 2009. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 36. Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, Titze JM, Pollock DM. Diurnal pattern in skin Na+ and water content is associated with salt-sensitive hypertension in ET(B) receptor-deficient rats. Am J Physiol Regul Integr Comp Physiol 314: R544–R551, 2018. doi: 10.1152/ajpregu.00312.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J 20: 631–637, 2006. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 38. DeBoer MD, Filipp SL, Sims M, Musani SK, Gurka MJ. Risk of ischemic stroke increases over the spectrum of metabolic syndrome severity. Stroke 51: 2548–2552, 2020. doi: 10.1161/STROKEAHA.120.028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang D, Colson JC, Jin C, Becker BK, Rhoads MK, Pati P, Neder TH, King MA, Valcin JA, Tao B, Kasztan M, Paul JR, Bailey SM, Pollock JS, Gamble KL, Pollock DM. Timing of food intake drives the circadian rhythm of blood pressure. Function (Oxf) 2: zqaa034, 2021. doi: 10.1093/function/zqaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song M-Y, Yi F, Xiao H, Yin J, Huang Q, Xia J, Yin X-M, Wen Y-B, Zhang L, Liu Y-H, Xiao B, Gu W-P. Energy restriction induced SIRT6 inhibits microglia activation and promotes angiogenesis in cerebral ischemia via transcriptional inhibition of TXNIP. Cell Death Dis 13: 449, 2022. doi: 10.1038/s41419-022-04866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.


