Abstract
Primary cystic duct carcinoma is a rare tumor. The curative treatment of cystic duct carcinoma is complete surgical resection, for which the evaluation of local extension is important. We herein report two cases of cystic duct carcinoma in which a preoperative examination was performed using per-oral cholangioscopy (POCS). Both patients underwent POCS due to suspicion of cystic duct carcinoma based on imaging findings. A visual analysis and biopsy were performed to evaluate local extension, which led to surgery. These cases suggest that POCS is useful for the preoperative assessment of local extension in advanced cystic duct carcinoma.
Keywords: cystic duct carcinoma, spyglass, cholangioscopy
Introduction
Primary cystic duct carcinoma is a rare malignancy defined by Farrar in 1951 (1). Its incidence has been reported to be 2.2-3.3% among all biliary carcinomas (2,3). The cystic duct, like the bile duct, is composed of three layers - mucosa, fibromuscular layer, and subserosa - and lacks the muscularis mucosa and submucosa. Therefore, cystic duct carcinoma spreads horizontally, and because of its thin wall, it tends to invade outside the wall. These two forms of local extension may extend from the hilar region of the liver to the pancreas (4).
The curative treatment of cystic duct carcinoma is complete surgical resection, for which the evaluation of local extension is important. However, reports on the preoperative diagnosis of cystic duct carcinoma using per-oral cholangioscopy (POCS) are rare.
We herein report two cases of cystic duct carcinoma in which a preoperative examination was performed using POCS.
Case Reports
Case 1
An 85-year-old woman was referred to our hospital for bile duct tumor on abdominal ultrasonography (US). She did not have any specific medical history. Her vital signs and physical examination results were unremarkable. Laboratory tests showed no abnormalities, including concerning hepatobiliary enzymes.
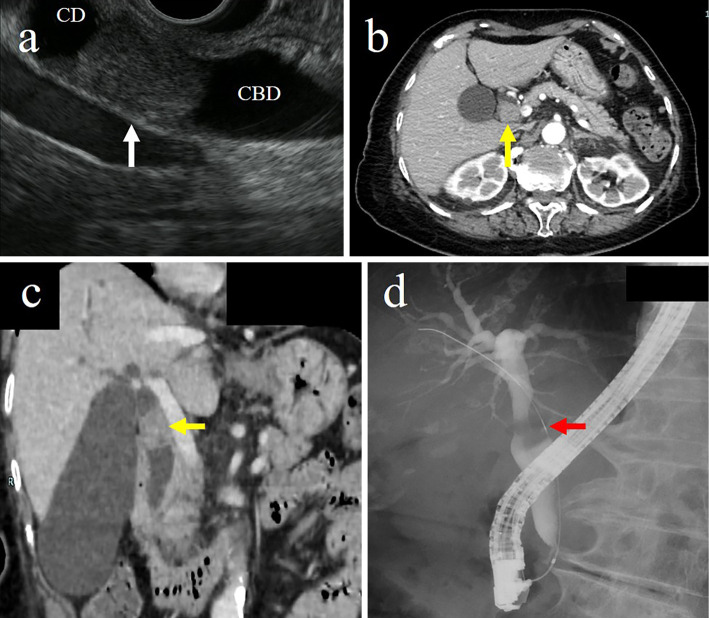
Endoscopic ultrasonography (EUS) showed a hypoechoic mass at the junction of the common hepatic duct and the cystic duct (Fig. 1a). Computed tomography (CT) showed a contrast-enhanced 22-mm tumor at the junction of the common hepatic duct and the cystic duct with no swelling of the surrounding lymph node (Fig. 1b, c). Endoscopic retrograde cholangiography (ERC) showed a contrast defect in the cystic duct and a tender image in the common hepatic duct (Fig. 1d). Subsequent POCS [SpyGlass DS Direct Visualization System (SpyDS); Boston Scientific Corporation, Natick, USA] showed a papillary tumor occupying the cystic duct, and the common hepatic duct was compressed by the tumor (Fig. 2). There was no superficial extension of the tumor, and the compressed common hepatic duct was covered with normal mucosa, with no evidence of invasion.
Figure 1.
(a) Endoscopic ultrasonography showed a hypoechoic mass at the confluence of the common bile duct and the cystic duct (white arrow). (b, c) Computed tomography showed a contrast-enhanced tumor at the confluence of the common bile duct and the cystic duct (yellow arrows). (d) Endoscopic retrograde cholangiography showed a contrast defect in the cystic duct and a tender image in the common hepatic duct (red arrow). CD: cystic duct, CBD: common bile duct
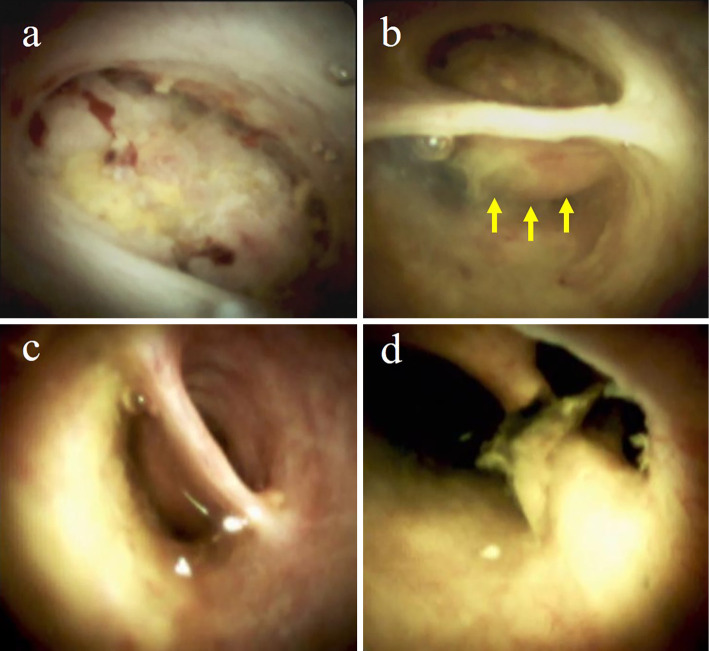
Figure 2.
Images visualized by POCS. (a) A papillary tumor occupying the cystic duct. (b) The common hepatic duct compressed by the tumor (yellow arrows). (c) A smooth surface was seen at the common bile duct. (d) A smooth surface and small stone were seen at the confluence of the hepatic ducts.
A tumor biopsy via POCS was performed, and the histology revealed adenocarcinoma. We performed a mapping biopsy using the SpyDS from the confluence of the hepatic ducts, the compressed common hepatic duct, and the common bile duct, but no malignant findings were found. Therefore, the patient underwent cholecystectomy and extrahepatic bile duct resection with hepaticojejunostomy. We determined that the lesion was confined to the cystic duct and therefore did not perform extended cholecystectomy.
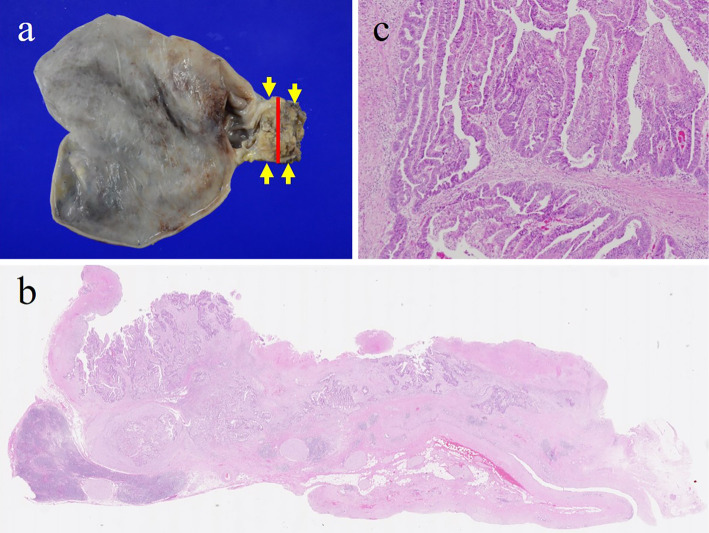
The excised specimen showed a papillary tumor in the cystic duct (Fig. 3). Histological findings revealed papillary adenocarcinoma of the cystic duct without lymph node metastasis (pT2aN0M0, stage IIA in the UICC TNM classification). The patient has been alive for five years since the surgery without recurrence.
Figure 3.
(a) The excised specimen showed a papillary tumor in the cystic duct (yellow arrow). The red line was the cut line of the split plane in b. (b) Hematoxylin and Eosin staining of the tumor (×5). (c) High-power view showing a papillary adenocarcinoma (×400).
Case 2
A 77-year-old man was referred for gallbladder swelling on US. He did not have any specific medical history. On a physical examination, there was no abdominal tenderness, but the gallbladder was palpated.
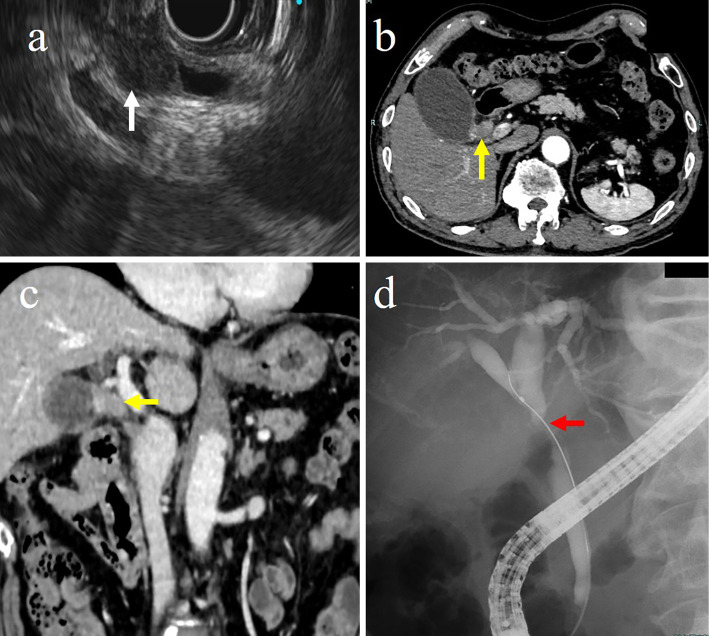
Laboratory tests showed a high level of CA19-9 at 101.9 U/mL. No other abnormalities were found. EUS and CT showed a tumor at the junction of the common hepatic duct and cystic duct (Fig. 4a, b). ERC showed a contrast defect of the cystic duct and stenosis of the common hepatic duct (Fig. 4c).
Figure 4.
(a) Endoscopic ultrasonography showed a hypoechoic mass at the confluence of the common bile duct and the cystic duct (white arrow). (b, c) Computed tomography showed a contrast-enhanced tumor at the confluence of the common bile duct and the cystic duct (yellow arrows). (d) ERC showed a contrast defect of the cystic duct and stenosis of the common hepatic duct (red arrow). CD: cystic duct, CBD: common bile duct
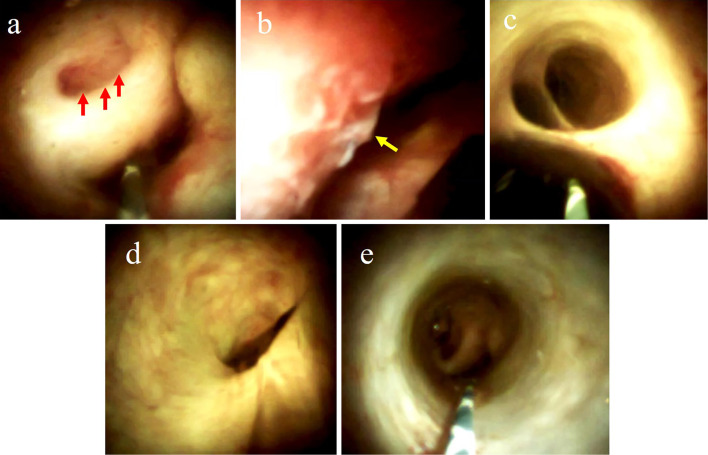
Subsequent POCS using the SpyDS revealed a granular nodular tumor in the cystic duct, the common hepatic duct was compressed by the tumor, and the mucosal surface of the right side of the common hepatic duct was irregular (Fig. 5). The mucosa at the confluence of the hepatic ducts and common bile duct was normal. We performed a mapping biopsy using the SpyDS from the confluence of the hepatic ducts, common hepatic duct, and common bile duct and found atypical epithelial cells only from the common hepatic duct. Based on the examination results, we determined that pancreaticoduodenectomy was not necessary.
Figure 5.
Images visualized by POCS. (a) A granular nodular tumor in the cystic duct (red arrow). (b) The common hepatic duct compressed by the tumor; the irregular mucosal surface of the right side of common hepatic duct (yellow arrow). (c) A smooth surface was seen at the confluence of the hepatic ducts. The right hepatic duct can be seen in the upper part of the image. (d) A smooth surface was seen at the left hepatic duct. (e) A smooth surface was seen at the common bile duct.
The patient underwent cholecystectomy and extrahepatic bile duct resection with a plan to add right lobectomy of the liver if cancer was found on a rapid histological examination of the resected segment of the right hepatic duct. Since there was no wall thickening of the gallbladder on imaging, we assumed that even if the cancer had superficial extension, it had not invaded the subplasma layer of the gallbladder. In our facility, if the resection margin of the right hepatic duct was found to be negative, we did not perform extended cholecystectomy. We therefore performed additional right hepatic lobectomy, as a small tubular proliferation of atypical glandular epithelium around an adnexal gland deep in the bile duct was positive for resection in part (about 10%) of the resected margins of the right hepatic duct. Therefore, right lobectomy of the liver was added.
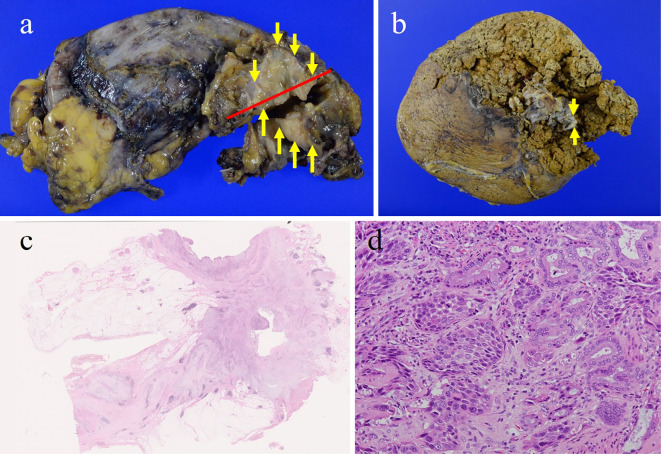
Histopathological findings revealed moderately differentiated tubular adenocarcinoma of the cystic duct with extraserosal invasion of the right hepatic duct and extension into the common hepatic duct (pT3N0M0, stage IIIA in the UICC TNM classification) (Fig. 6). Postoperative chemotherapy with S-1 was administered, but the disease recurred 12 months after surgery and the patient died 14 months after surgery.
Figure 6.
(a, b) The excised specimen showed granular nodular tumor in the cystic duct (yellow arrow). The red line was the cut line of the split plane in c. (c) Hematoxylin and Eosin staining of the tumor (×5). (d) High-power view showing a moderately differentiated tubular adenocarcinoma (×400).
Discussion
Classic primary cystic duct carcinoma is a rare tumor defined by Farrar (1). Farrar's definition proposed the following strict diagnostic criteria for primary cystic duct carcinoma: tumor must be confined to the cystic duct; tumor must be absent in the gallbladder, common bile duct, or hepatic duct; and there must be histologically confirmed cancer cells. Farrar's criteria refer only to “localized” cystic duct carcinoma, thereby excluding “advanced” cystic duct carcinoma. However, because of the short structure of the cystic duct, cystic duct carcinoma can easily invade adjacent organs. In clinical practice, we often encounter biliary tract carcinomas that are centered in the cystic duct but that have invaded the neck of gallbladder, common bile duct, or hepatic duct. These findings suggest that this tumor originates from the cystic duct, making it impractical to distinguish it from Farrar's cystic duct carcinoma. Since tumors grow concentrically in all directions, the center of the tumor may be considered the primary site. Siewert et al. used this concept to classify tumors located in the gastrointestinal tract (5). Ohtani et al. also defined the primary site of a tumor as its geometric center (6), located along the longitudinal diameter of the tumor grossly. Several new classification systems of cystic duct carcinoma have been proposed based on this concept (4,7-9), and the tumor is treated as primary cystic duct carcinoma in the broad sense, even if it invades other organs, as long as its primary site of occupancy is clearly within the cystic duct. In our cases, the diagnosis of advanced cystic duct carcinoma was made because the center of the tumor was located in the cystic duct.
The curative treatment of cystic duct carcinoma is complete surgical resection. Advanced primary cystic duct carcinoma invades the hilar region of the liver and extends superficially into the common bile duct, requiring pancreatoduodenectomy and/or hepatectomy (6). Therefore, it is important to evaluate the local development before surgery. Cholangiocarcinoma extends horizontally along the bile duct wall. Lateral extension consists of superficial mucosal extension and intramural extension. Superficial extension of the mucosa is mainly seen in papillary-expanding, papillary-infiltrating, and nodular-expanding types of cholangiocarcinoma. In contrast, intramural extension is more common in nodular-infiltrating, flat-expanding, and flat-infiltrating types (10). Tumors of the superficially extending type show granularity, feathering, or wall irregularity on direct cholangiography and are seen as nodular tumors on EUS or contrast-enhanced CT. In contrast, tumors with intramural extension present as narrowing, sclerosis, or stenosis on direct cholangiography and as thickening of the bile duct wall on intraductal ultrasonography (IDUS) or contrast-enhanced CT. Superficial extension is often difficult to evaluate by contrast-enhanced CT or IDUS but is observed as papillary or granular mucosa in POCS, and observation of the bile duct mucosa and bile duct biopsies are considered useful (11-13). Conversely, intramural extension has been reported to show mucosal irregularities, edema and stenosis, vascular irregularities, and tortuosity in POCS (14,15). However, the cancer may not be exposed on the mucosal surface and should be evaluated with contrast-enhanced CT or IDUS (16); in our cases, POCS proved useful for the preoperative extent diagnosis, as these were papillary- and nodular-expanding types.
Conventional POCS was introduced to provide a direct view of the biliary system for a diagnosis and treatment in the mid-1970s (17,18) but was limited by several factors, including low durability, suboptimal irrigation capacity, limited scope operability, small instrument channels, and inadequate optical resolution. In cystic duct diseases, conventional POCS may have been difficult to perform because of the limited maneuverability of the scope with its tip angled in only two directions. Since cystic ducts are also difficult to biopsy under fluoroscopy by ERC, many cases have been reported where surgery was performed without POCS or histology.
Recently, a new type of oral digital endoscope, the SpyDS, has been widely used in clinical settings. The SpyDS has a higher optical resolution than conventional bile duct scopes and has a perfusion channel to maintain a good field of view. In addition, the SpyDS has a four-way angle, which allows POCS to easily visualize narrow and intricate ducts, such as the cystic and intrahepatic bile ducts. This has made it possible to observe minute changes in the mucosal surface and evaluate tumor extension and invasion. Recently, there have been several reports regarding the use of the SpyDS for the diagnosis of indeterminate biliary stricture (19-21). In a meta-analysis, the sensitivity of the visual impression was 95%, the specificity was 92%, and the positive diagnosis rate was 94%. In addition, the use of the Spybite (Boston Scientific Corporation), a dedicated biopsy device, enabled a visual examination followed by a direct visual biopsy under cholangioscopy. The sensitivity of the biopsy was reported to be 74%, the specificity 98%, and the positive detection rate 85% (22). Especially on the hilar side, an additional cholangioscopic mapping biopsy has been reported to be more diagnostically accurate than cholangioscopy alone (16). In our cases, cholangioscopic findings and a mapping biopsy with the SpyDS were performed, with the findings proving useful for a preoperative extent diagnosis.
There have been a few reports of cystic duct tumors diagnosed using POCS (23-26). They described the preoperative diagnosis of stage 0 or I tumors or the diagnosis of advanced cystic duct carcinoma that did not lead to surgery. We found no reports of the preoperative evaluation of local extension of advanced cystic duct carcinoma using POCS. In our cases, POCS enabled the determination that pancreaticoduodenectomy was unnecessary and that resection of the right lobe of the liver might be necessary in case 2, which was an advanced case. A more extensive cholangioscopic diagnosis of the local progression of cystic duct carcinoma using POCS can help avoid over- or under-operation.
In conclusion, the SpyDS was useful for the preoperative diagnosis of cystic duct carcinoma. In our cases, the findings on POCS were particularly useful with regard to the surgical decision concerning advanced cystic duct cancer. Although primary cystic duct carcinoma is a rare disease, its incidence is expected to increase in the future due to new classifications and advances in imaging. If cystic duct carcinoma is suspected, an examination should be performed using the SpyDS.
The authors state that they have no Conflict of Interest (COI).
References
- 1. FARRAR DA. Carcinoma of the cystic duct. Br J Surg 39: 183-185, 1951. [DOI] [PubMed] [Google Scholar]
- 2. Glenn F, Hill MR Jr. Extrahepatic biliary tract cancer. Cancer 8: 1218-1225, 1955. [DOI] [PubMed] [Google Scholar]
- 3. Neibling HA, Dockerty MB, Waugh JM. Carcinoma of the extrahepatic bile ducts. Surg Gynecol Obstet 89: 429-438, 1949. [PubMed] [Google Scholar]
- 4. Nakata T, Kobayashi A, Miwa S, Soeda J, Uehara T, Miyagawa S. Clinical and pathological features of primary carcinoma of the cystic duct. J Hepatobiliary Pancreat Surg 16: 75-82, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85: 1457-1459, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Ohtani T, Shirai Y, Tsukada K, Hatakeyama K, Muto T. The association between extrahepatic biliary carcinoma and the junction of the cystic duct and the biliary tree. Eur J Surg 160: 37-40, 1994. [PubMed] [Google Scholar]
- 7. Kim WC, Lee DH, Ahn SI, Kim JM. A case of cystic duct carcinoma treated with surgery and adjuvant radiotherapy: a proposal for new classification. J Gastrointestin Liver Dis 16: 437-440, 2007. [PubMed] [Google Scholar]
- 8. Yokoyama Y, Nishio H, Ebata T, et al. New classification of cystic duct carcinoma. World J Surg 32: 621-626, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Nan L, Wang C, Dai Y, et al. Cystic duct carcinoma: a new classification system and the clinicopathological features of 62 patients. Front Oncol 11: 696714, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 227: 405-411, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawakami H, Kuwatani M, Etoh K, et al. Endoscopic retrograde cholangioscopy versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy 41: 959-964, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Osanai M, Itoi T, Igarashi Y, et al. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancernous extension: a prospective multicenter study. Endoscopy 45: 635-641, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Nishikawa T, Tsuyuguchi T, Sakai Y, et al. Preoperative assessment of longitudinal extention of cholangiocarcinoma with peroral videocholangioscopy: a prospective study. Dig Endosc 26: 450-457, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Itoi T, Osanai M, Igarashi Y, et al. Diagnostic peroral video cholangioscopy is an accurate diagnostic tool for patients with bile duct lesions. Clin Gastroenterol Hepatol 8: 934-938, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Ishida Y, Itoi T, Okabe Y, et al. Can image enhanced cholangioscopy distinguish benign from malignant lesions in the biliary duct? Best Pract Res Clin Gastroenterol 29: 611-625, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Ogawa T, Ito K, Koshita S, et al. Usefulness of cholangioscopic-guided mapping biopsy using Spy-Glass DS for preoperative evaluation of extrahepatic cholangiocarcinoma. Endosc Int Open 6: E199-E204, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakajima M, Akasaka Y, Fukumoto K, Mitsuyoshi Y, Kawai K. Peroral cholangiopancreatosocopy (PCPS) under duodenoscopic guidance. Am J Gastroenterol 66: 241-247, 1976. [PubMed] [Google Scholar]
- 18. Urakami Y, Seifert E, Butke H. Peroral direct cholangioscopy (PDCS) using routine straight-view endoscope: first report. Endoscopy 9: 27-30, 1977. [DOI] [PubMed] [Google Scholar]
- 19. Shah RJ, Raijman I, Brauer B, Gumustop B, Pleskow D. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy 49: 651-658, 2017. [DOI] [PubMed] [Google Scholar]
- 20. Ogura T, Imanishi M, Kurisu Y, et al. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Digestive Endosc 29: 782-789, 2017. [DOI] [PubMed] [Google Scholar]
- 21. Bang JY, Navaneethan U, Hasan M, Sutton B, Hawes R, Varadarajulu S. Optimizing outcomes of single-operator cholangioscopy-guided biopsies based on a randomized trial. Clin Gastroenterol Hepatol 18: 441-448, 2020. [DOI] [PubMed] [Google Scholar]
- 22. Wen LJ, Chen JH, Xu HJ, Yu Q, Liu K. Efficacy and safety of digital single-operator cholangioscopy in the diagnosis of indeterminate biliary strictures by targeted biopsies: a systematic review and meta-analysis. Diagnostics (Basel) 10: 666, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiba H, Misawa T, Ito R, et al. Preoperative diagnosis of early cystic duct cancer using endoscopic ultrasonography and endocholangioscopy: report of a case. J Gastrointest Surg 15: 1477-1479, 2011. [DOI] [PubMed] [Google Scholar]
- 24. Miyazawa M, Matsuda S, Fuchizaki U. Primary cystic duct carcinoma diagnosed by targeted biopsy with digital cholangioscopy. Digest Endosc 30: 690-691, 2018. [DOI] [PubMed] [Google Scholar]
- 25. Anderloni A, Fugazza A, Di Leo M, et al. A case of cystic duct carcinoma successfully diagnosed with a novel digital cholangioscope. Gastrointest Endosc 85: 854-855, 2017. [DOI] [PubMed] [Google Scholar]
- 26. Miyabe K, Notohara K, Asano G, et al. Early detection of high-grade biliary intraepithelial neoplasia (BilIN-3) in the cystic duct visualized by SpyGlass DS cholangioscopy. Intern Med 60: 47-52, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]