Abstract
The coexistence of leucine-rich glioma-inactivated 1 (LGI1) and contactin-associated protein-like 2 (CASPR2) autoantibodies in the same individual is surprisingly often observed. We herein report the first case of LGI1 encephalitis followed by Isaacs syndrome in which LGI1 and CASPR2 antibodies in the serum and cerebrospinal fluid (CSF) were measured during the entire disease course. After the resolution of limbic encephalitis, LGI1 antibodies disappeared from the CSF simultaneously with the appearance of CASPR2 antibodies in the serum. The alternating presence of these pathogenic autoantibodies along with the clinical and phenotypic alternations suggested that LGI1 encephalitis was associated with CASPR2 autoantibody production in the peripheral tissue, leading to CASPR2-associated Isaacs syndrome.
Keywords: leucine-rich glioma-inactivated 1 (LGI1), contactin-associated protein-like 2 (CASPR2); limbic encephalitis; Isaacs syndrome; autoantibodies
Introduction
Leucine-rich glioma-inactivated 1 (LGI1) and contactin-associated protein-like 2 (CASPR2), two proteins that constitute the voltage-gated potassium channel (VGKC)-complex, were first identified in 2010 (1). Patients with autoantibodies targeting LGI1 have limbic encephalitis, often with hyponatremia, and half of such patients have faciobrachial dystonic seizures (1,2). CASPR2 autoantibodies cause a more variable constellation of peripheral nervous system and/or central nervous system (CNS) symptoms, including Isaacs syndrome, limbic encephalitis, and Morvan's syndrome (1-3).
To our knowledge, the present case is the first instance of LGI1 encephalitis followed by Isaacs syndrome in which an alternating presence of the pathogenic autoantibodies along with limbic encephalitis (anti-LGI1) and subsequent peripheral nerve hyperexcitability (anti-CASPR2) was observed.
Case Report
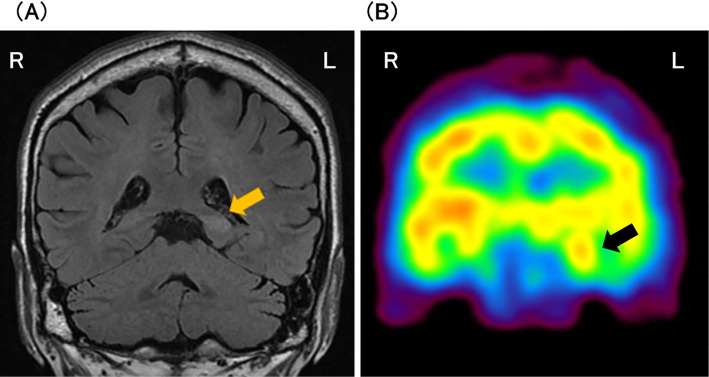
A 63-year-old man with a history of intractable sinusitis was admitted with a chief complaint of memory impairment of 2 days' duration. A neuropsychological examination revealed a Mini-Mental State Examination-Japanese (MMSE-J) score of 24 (30), retrograde amnesia for social and autobiographical memory with a temporal gradient, and verbal and visual anterograde amnesia. The patient had no symptoms or neurological findings suggestive of peripheral nerve dysfunction or dysautonomia. Cerebrospinal fluid (CSF) test findings were normal except for a single, abnormal immunoglobulin band on CSF electrophoresis. Electroencephalography found no epileptiform discharges. Blood sodium was normal. Head magnetic resonance imaging (MRI) showed high-intensity signals on fluid-attenuated inversion-recovery images of the left posterior hippocampus and parahippocampal gyrus with increased blood flow on I-123 iodoamphetamine-single photon emission computed tomography (CT) (Fig. 1).
Figure 1.
(A) Coronal views on head magnetic resonance imaging (MRI) and (B) I-123 iodoamphetamine-single photon emission computed tomography (CT). MRI showing high-intensity signals on fluid-attenuated inversion-recovery images of the left posterior hippocampus (orange arrow) and parahippocampal gyrus. I-123 iodoamphetamine-single photon emission CT showing increased blood flow of the left hippo-campus (black arrow).
Steroid pulse therapy was started. Because these features did not meet the proposed diagnostic criteria for ‘definite autoimmune limbic encephalitis' (4), we did not exclude a diagnosis of herpes simplex encephalitis at that time. Therefore, acyclovir treatment was added until a negative polymerase chain reaction result for the detection of herpes simplex virus DNA was obtained.
After treatment, his neurological symptoms were immediately ameliorated. A diagnosis of LGI1 encephalitis was made based on a positive result for autoantibodies against LGI1 in the serum and CSF with no detectable autoantibodies against CASPR2 (Fig. 2). The patient was treated with oral prednisolone (PSL) 10 mg for 18 months, which was then tapered according to the recommendation for the treatment of LGI1 encephalitis (5).
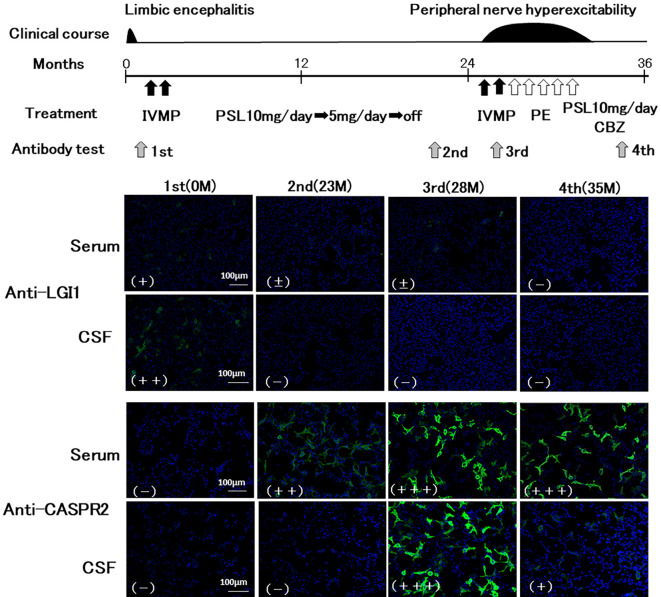
Figure 2.
Time course of the results of antibody testing for LGI1 and CASPR2 and the treatment course. LGI1 and CASPR2-antibodies were measured using cell-based assays. Images of HEK293 cells transfected with a plasmid containing human LGI1 or CASPR2 were captured with an epifluorescence microscope. Anti-human-IgG (green) FITC-conjugated secondary antibody was used. For fluorescence, DAPI was used as a nuclear counter-stain (blue). Based on fluorescence intensity, the results were classified as negative (-), faint (±), weakly positive (+), positive (++) or strongly positive (+++). After two cycles of IVMP, the patient’s anterograde and retrograde amnesia, caused by the LGI1 encephalitis, resolved within a few days. Furthermore, the patient received oral prednisolone (PSL) to prevent recurrence of the LGI1 encephalitis. At six months after the symptom onset, follow-up MRI showed improvement in the abnormal signals, and a neuropsychological examination yielded an MMSE-J score of 29 with normal near and distant memory. In contrast to the treatment for LGI1 encephalitis, aggressive immunotherapies, including two cycles of intravenous methylprednisolone (IVMP), five courses of plasma-exchange (PE) therapy, and carbamazepine (CBZ), were required to resolve the symptoms associated with Isaacs syndrome.
Twenty-three months later, head MRI demonstrated no abnormality, and neuropsychological tests found no deterioration. His modified Rankin Scale (mRS) was 0 at that time. Twenty-eight months later, the patient was again admitted for low back pain, numbness in the distal extremities, and body weight loss of 10 kg over the previous 2 months. He was markedly thinner with a body mass index of 15, hyperhidrosis, and tachycardia. No symptoms suggestive of limbic encephalitis were observed. He had bilateral lower leg myokymia with right predominance and decreased deep tendon reflexes. Blood and CSF tests, abdominal CT, upper gastrointestinal endoscopy, and MRI of the cervical and lumbar spine were unremarkable. Positron emission tomography-CT showed no findings suggestive of malignancy. A nerve conduction study of the tibial nerve revealed stimulus-induced repetitive discharge. Electromyography found myokymic discharge in the tibialis anterior and vastus medialis. Muscle echography showed myokymia in the lumbar paraspinal muscles, tibialis anterior, and vastus medialis. The serum was negative for anti-LGI1 antibody and positive for anti-CASPR2 antibody. Based on these findings, Isaacs syndrome was diagnosed.
Aggressive immunotherapy, including two cycles of intravenous methylprednisolone (IVMP), five courses of plasma-exchange (PE) therapy, and carbamazepine (CBZ) (Fig. 2), ameliorated his symptoms and neurophysiological findings. Although complete resolution of his symptoms was difficult, his mRS remained at 1 after aggressive immunotherapy. Fig. 2 shows the time course of the results of the autoantibody test using a cell-based assay (CBA) and the detailed treatment course.
Autoantibody tests for detecting LGI1 and CASPR2-antibodies
Anti-LGI1 and anti-CASPR2 antibodies were measured using both a tissue-based assay, which involves an immunohistochemical analysis of rat brain tissue, and CBAs following the previously described method (3,6). In brief, CBA was performed as follows: human embryonic kidney 293 cells (HEK293) were transiently transfected with a plasmid containing human CASPR2 or co-transfected with a plasmid containing human LGI1 and a plasmid containing ADAM23 using lipofectamine 2000 (Invitrogen, Carlsbad, USA). The patient's CSF (diluted 1:2) or sera (1:40) was then applied to the cells, followed by fluorescein isothiocyanate-conjugated anti-human IgG (1:1,000; Molecular Probes, Eugene, USA) (green). Images of the transfected HEK293 cells were captured using an epifluorescence microscope. For fluorescence, 4´,6-diamidino-2-phenylindole was used as a nuclear counter-stain (blue). The ImageJ software program (national institutes of health) was then used to convert the imaging file into an 8-bit file. After calibration of the background noise, the intensity levels of green fluorescence of CBA-positive cells were classified as follows: + (weakly positive, ≤50), ++ (positive, 51-100), +++ (strongly positive, 101-150). Negative and faint findings were judged by two independent raters.
The results of the autoantibody tests are summarized in Table. In total, 11 samples (serum: 7, CSF: 4) covering the entire disease course were tested for both anti-LGI1 and anti-CASPR2 antibodies. Fig. 2 shows the representative results of the antibody tests. At the onset of LGI1 encephalitis, a positive result for anti-LGI1 antibodies in the CSF, weak immunostaining for LGI1 in the serum, and no detectable immunostaining for CASPR2 were observed. After resolution of the LGI1 encephalitis, the anti-LGI1 antibodies disappeared from the CSF and showed much lower levels in the serum, simultaneously with the appearance of CASPR2 antibodies in the serum. After the onset of Isaacs syndrome, strong immunostaining for CASPR2 in the serum and no detectable immunostaining for LGI1 were continuously observed (Table).
Table.
Results of the Autoantibodies Testing for LGI1 and CASPR2.
| 0M | 6M | 23M | 28M | 29M | 30M | 35M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-LGI1 | Serum | + | + | ± | ± | - | - | - | ||||||||
| CSF | ++ | - | - | - | ||||||||||||
| Anti-CASPR2 | Serum | - | - | ++ | +++ | +++ | +++ | +++ | ||||||||
| CSF | - | - | +++ | + |
CASPR2: contactin-associated protein-like 2, CFS: cerebrospinal fluid, LGI1: leucine-rich glioma-inactivated 1
Discussion
The co-occurrence of autoantibodies against both anti-LGI1 and anti-CASPR2, which form the extracellular domains of the tightly packed VGKC-complex (1,2), in an individual is surprisingly often observed (2). From an immunological perspective, a common insult or immunological predisposition is thought to lead to epitope spreading and preferential presentation of the neighboring VGKC-complex antigens, LGI1 and CASPR2 (2). Therefore, a causal relationship between LGI1 encephalitis and Isaacs syndrome rather than an incidental co-occurrence of these rare diseases was assumed in our patient.
The absence of CSF lymphocytosis or oligoclonal bands and serum-predominant anti-LGI1 antibodies in a considerable number of patients with LGI1 encephalitis suggests an immune response of peripheral origin (7). However, a recent study of deep B-cell immune repertoire sequencing using paired CSF and peripheral blood (PB) mononuclear cells from patients with LGI1 encephalitis provided strong evidence for an intrathecal, antigen-driven immune response (8). The authors of the study also demonstrated that restricted pools of clonally related B cells participate in both the CSF and PB, suggesting that antigen-stimulated B cells migrate from the CNS to the periphery (8,9). In contrast, a summary of the core features of 46 patients with coexistent anti-LGI1 and anti-CASPR2 antibodies demonstrated that these patients had clinical features associated more prominently with CASPR2 antibody-associated disease than LGI1 (2). Furthermore, they also demonstrated a frequent association with an underlying peripheral tumor, particularly a thymoma, and myasthenia gravis (2), suggesting that antigen recognition mainly occurs in peripheral tissues. Thus, these findings suggest that the immune response against LGI1 antigen may occur in both CNS and the periphery, wherein ectopic expression of LGI1 may be associated with autoimmunity.
In our case, based on the concentration of IgG in the primary antibody solution of the CBA (CSF: 1.7 mg/dL, serum: 24.8 mg/dL), the results of CBAs (Table) showed CSF-predominant anti-LGI1 antibodies. Furthermore, the most unique feature of our patient was the alternating presence of pathogenic autoantibodies to LGI1 and subsequently to CASPR2. After the resolution of LGI1 encephalitis, the LGI1 antibodies disappeared from the CSF simultaneously with the appearance of CASPR2 antibodies in the serum. These findings suggest the transition of the main site of the immune response from the CNS to the periphery. If LGI1 epitope recognition in our patient began in the peripheral tissue, the continuous presence of anti-LGI1 antibodies in the serum after the appearance of anti-CASPR2 antibodies in the serum might be expected, as in patients with concurrent anti-LGI1 and anti-CASPR2 antibodies, rather than the disappearance of the former (Fig. 2). LGI1 is mainly expressed in the CNS, while CASPR2 is expressed in both the CNS and peripheral tissues (10,11). In addition to intrathecal synthesis of anti-LGI1 antibodies, migration of antigen-stimulated B cells from the central to peripheral region and differential expression of the target antigen in the CNS and peripheral tissues may result in the alternating presence of autoantibodies against LGI1 and CASPR2. Given the multifaceted roles of microglia in the CNS for innate and adaptive immune responses, microglia may play a pivotal role in the alternative presence of the pathogenic autoantibodies in our case. Microglia can act as an efficient antigen-presenting cell and a regulator of the blood-brain barrier integrity through secretion of proinflammatory cytokines and chemokines (12). Our case further supports a close association of the anti-LGI1 antibody with the anti-CASPR2 antibody and suggests that the dysregulation of immune system underlying LGI1 encephalitis may be associated with anti-CASPR2 antibody production in the peripheral tissue, leading to CASPR2-associated Isaacs syndrome.
The patient provided his written informed consent.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 133: 2734-2748, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binks SNM, Klein CJ, Waters P, Pittock SJ, Irani SR. LGI1, CASPR2 and related antibodies: a molecular evolution of the phenotypes. J Neurol Neurosurg Psychiatry 89: 526-534, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87: 521-528, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15: 391-404, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Sonderen A, Mar Petit-Pedrol, Josep Dalmau, Maarten JTitulaer. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol 13: 290-301, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Hara M, Martinez-Hernandez E, Ariño H, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology 90: e1386-e1394, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Sonderen A, Thijis RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87: 1449-1456, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Lehmann-Horn K, Irani SR, Wang S, et al. Intrathecal B-cell activation in LGI1 antibody encephalitis. Neurol Neuroimmunol Neuroinflamm 7: e669, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Büdingen HC, Kuo TC, Sirota M, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest 122: 4533-4543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Head K, Gong S, Joseph S, et al. Defining the expression pattern of the LGI1 gene in BAC transgenic mice. Mamm Genome 18: 328-337, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Saint-Martin M, Joubert B, Pellier-Monnin V, Pascual O, Noraz N, Honnorat J. Contactin-associated protein-like 2, a protein of the neurexin family involved in several human diseases. Eur J Neurosci 48: 1906-1923, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40: 218-231, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]