Abstract
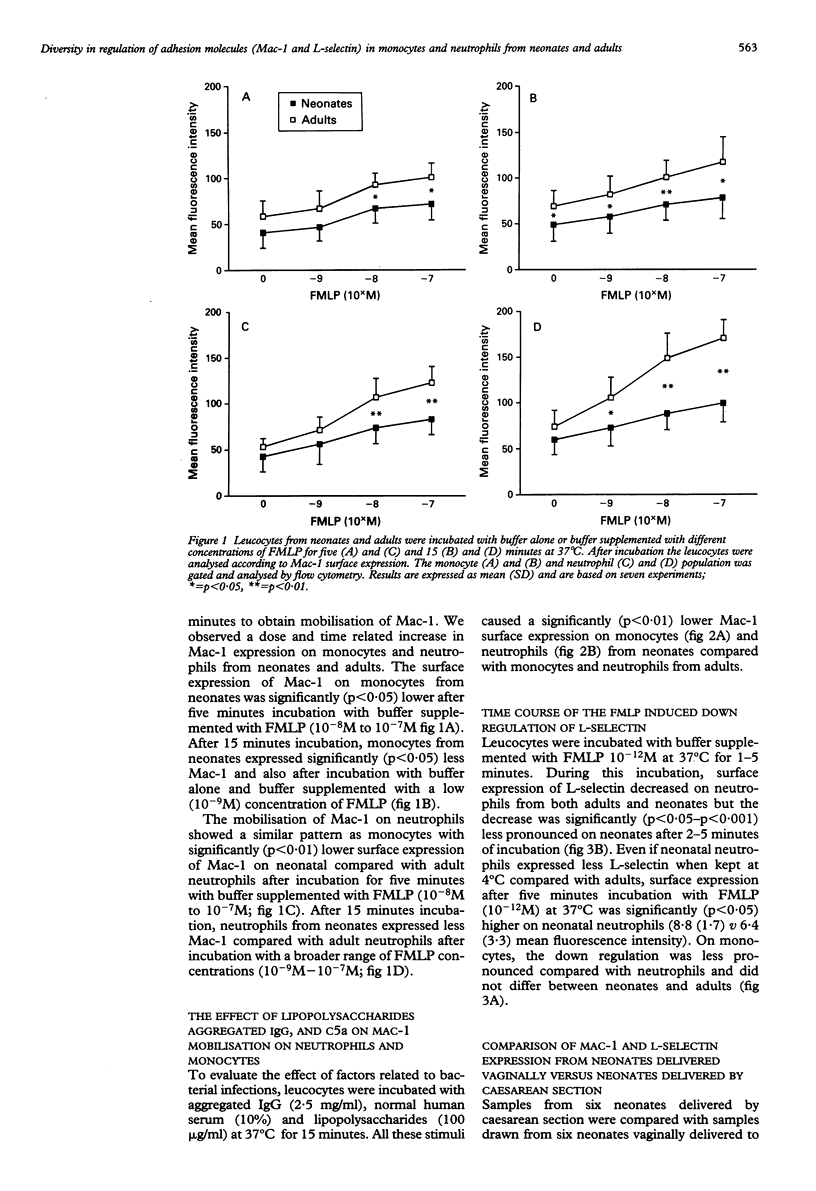
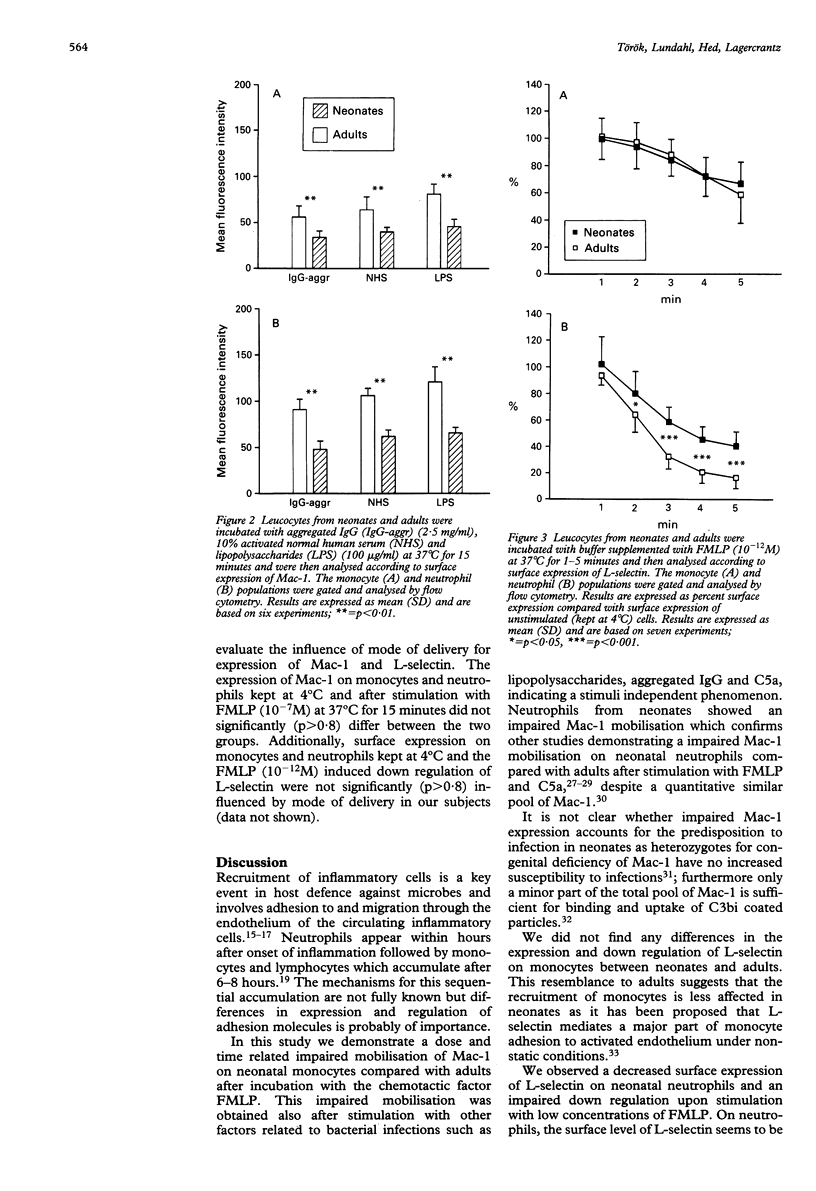
The surface expression and regulation of the adhesion promoting glycoproteins Mac-1 and L-selectin was measured on monocytes and neutrophils from neonates and adults. A significant decrease in Mac-1 up regulation on both monocytes and neutrophils was found in neonates after both high (10(-7)M) and low (10(-9)M) concentrations of the chemotactic factor N-formyl-methionyl-phenylalanine (FMLP). A significant difference was obtained after incubation for five minutes, which was further enhanced after incubation for 15 minutes. Factors related to bacterial infections, lipopolysaccharides, activated sera (C5a), and aggregated IgG induced an impaired Mac-1 up regulation on both monocytes and neutrophils from neonates compared with adults. The expression of L-selectin was significantly lower on neutrophils from neonates and was less down regulated upon stimulation with a low concentration (10(-12)M) of FMLP. On monocytes from neonates, the expression and down regulation of L-selectin did not differ from monocytes from adults. Mode of delivery did not influence the regulation of Mac-1 and L-selectin in neonates. Diversity in expression and regulation of Mac-1 and L-selectin on monocytes and neutrophils may contribute to the increased susceptibility to infections observed in neonates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Abbassi O., Kishimoto T. K., Koenig J. M., McIntire L. V., Smith C. W. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J Immunol. 1991 May 15;146(10):3372–3379. [PubMed] [Google Scholar]
- Anderson D. C., Freeman K. L., Heerdt B., Hughes B. J., Jack R. M., Smith C. W. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. Blood. 1987 Sep;70(3):740–750. [PubMed] [Google Scholar]
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Rothlein R., Marlin S. D., Krater S. S., Smith C. W. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990 Dec 15;76(12):2613–2621. [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Bruce M. C., Baley J. E., Medvik K. A., Berger M. Impaired surface membrane expression of C3bi but not C3b receptors on neonatal neutrophils. Pediatr Res. 1987 Mar;21(3):306–311. doi: 10.1203/00006450-198703000-00022. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Christensen R. D., MacFarlane J. L., Taylor N. L., Hill H. R., Rothstein G. Blood and marrow neutrophils during experimental group B streptococcal infection: quantification of the stem cell, proliferative, storage and circulating pools. Pediatr Res. 1982 Jul;16(7):549–553. doi: 10.1203/00006450-198207000-00011. [DOI] [PubMed] [Google Scholar]
- Erdman S. H., Christensen R. D., Bradley P. P., Rothstein G. Supply and release of storage neutrophils. A developmental study. Biol Neonate. 1982;41(3-4):132–137. doi: 10.1159/000241541. [DOI] [PubMed] [Google Scholar]
- Fleit H. B. Fc and complement receptor (CR1 and CR3) expression on neonatal human polymorphonuclear leukocytes. Biol Neonate. 1989;55(3):156–163. doi: 10.1159/000242911. [DOI] [PubMed] [Google Scholar]
- Hed J., Berg O., Forslid J., Halldén G., Lärka-Rafner G. The expression of CR1 and CR3 on non-modulated and modulated granulocytes of healthy blood donors as measured by flow cytofluorometry. Scand J Immunol. 1988 Sep;28(3):339–344. doi: 10.1111/j.1365-3083.1988.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Hill H. R. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res. 1987 Oct;22(4):375–382. doi: 10.1203/00006450-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Hogg N. Roll, roll, roll your leucocyte gently down the vein.... Immunol Today. 1992 Apr;13(4):113–115. doi: 10.1016/0167-5699(92)90103-E. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Schmalstieg F. C., Dempsey K., Krater S. S., Nannen D. D., Smith C. W., Anderson D. C. Subcellular distribution and mobilization of MAC-1 (CD11b/CD18) in neonatal neutrophils. Blood. 1990 Jan 15;75(2):488–498. [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Lundahl J., Halldén G., Hed J., Johansson S. G. A flow cytometric method to measure the stimulated mobilization and the intracellular pool of the adhesion promoting glucoprotein Mac-1. APMIS. 1991 Feb;99(2):139–146. doi: 10.1111/j.1699-0463.1991.tb05131.x. [DOI] [PubMed] [Google Scholar]
- Mease A. D. Tissue neutropenia: the newborn neutrophil in perspective. J Perinatol. 1990 Mar;10(1):55–59. [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. E. Phagocytosis in the newborn infant: humoral and cellular factors. J Pediatr. 1969 Feb;74(2):255–259. doi: 10.1016/s0022-3476(69)80073-9. [DOI] [PubMed] [Google Scholar]
- Quie P. G. Antimicrobial defenses in the neonate. Semin Perinatol. 1990 Aug;14(4 Suppl 1):2–9. [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Campbell D. E., Ludomirsky A., Polin R. A., Douglas S. D., Garty B. Z., Harris M. C. Expression of the complement receptors CR1 and CR3 and the type III Fc gamma receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediatr Res. 1990 Aug;28(2):120–126. doi: 10.1203/00006450-199008000-00009. [DOI] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Gimbrone M. A., Jr, Tedder T. F. Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-selectin) under nonstatic conditions. J Exp Med. 1992 Jun 1;175(6):1789–1792. doi: 10.1084/jem.175.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stein M., Keshav S. The versatility of macrophages. Clin Exp Allergy. 1992 Jan;22(1):19–27. doi: 10.1111/j.1365-2222.1992.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986 Jan;108(1):1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]


