Abstract
Glaucoma drainage devices (GDD) are increasingly utilized in the management of childhood glaucoma. This systematic review and meta-analysis assesses the efficacy of first-time Ahmed or Baerveldt implantation in children. PubMed, Embase, and Cochrane Library were searched for relevant English-language, peer-reviewed literature. Postoperative outcomes were pooled using random effects regression models with restricted maximum likelihood estimation. Thirty-two studies (1221 eyes, 885 children) were included. Mean ± standard deviation preoperative IOP was 31.8±3.4 mmHg. Pooled mean IOP at 12 and 24 months postoperatively were 16.5 mmHg (95% CI 15.5–17.6) and 17.6 mmHg (95% CI 16.4–18.7), respectively. Pooled proportions of success were 0.87 (95% CI 0.83–0.91) at 12 months, 0.77 (95% CI 0.71–0.83) at 24 months, 0.54 (95% CI 0.44–0.65) at 48 months, 0.60 (95% CI 0.48–0.71) at 60 months, and 0.37 (95% CI 0.32–0.42) at 120 months. There were no differences in proportion of success at 12 and 24 months among eyes that received Ahmed and Baerveldt tube shunts, nor between eyes with primary glaucoma, glaucoma following cataract surgery or other secondary glaucoma. Our findings show that Ahmed and Baerveldt shunts substantially reduced IOP for at least 24 months in childhood glaucoma, with similar findings among device types and glaucoma etiologies.
Keywords: Systematic review and meta-analysis, glaucoma drainage device, Ahmed glaucoma valve, Baerveldt glaucoma implant, childhood glaucoma
Introduction
Childhood glaucoma is a potentially blinding condition characterized by elevated intraocular pressure (IOP) leading to structural ocular damage, including optic neuropathy, corneal edema, and amblyopia. Management is primarily surgical, with medical management typically serving as a temporizing measure or adjunctive therapy.9 While anterior chamber angle procedures such as goniotomy and trabeculotomy have high initial success rates for primary childhood glaucoma, their efficacy in secondary glaucoma is somewhat limited, and even in successful cases, some eyes ultimately require additional surgical intervention with trabeculectomy or implantation of a glaucoma drainage device (GDD).48 GDDs are also used as primary therapy in cases expected to have poor response to goniotomy or trabeculotomy, particularly in secondary childhood glaucoma.16
The efficacy of GDDs in the adult population has been well-assessed through randomized-controlled trials and meta-analyses examining long-term effects on IOP, visual acuity, and visual field outcomes.6, 10, 23, 51 There are few comparable studies, however, in the pediatric population. The lower incidence of childhood glaucoma compared to glaucoma in adults limits the ability to conduct large interventional studies on efficacy of GDD implantation, and existing studies are additionally limited by small sample sizes and high rates of attrition.3, 4 Despite these challenges, the long-term outcomes of glaucoma interventions in children are important to characterize to assist in counseling parents, guiding clinical care, and ultimately, preventing lifelong vision loss.
The Ahmed glaucoma valve (New World Medical Inc, Rancho Cucamonga, CA) and the Baerveldt glaucoma implant (Advanced Medical Optics, Santa Ana, CA) are 2 of the most popular GDDs currently used. The Ahmed contains a flow-limiting mechanism and can provide immediate lowering of IOP, while the Baerveldt device does not contain such a mechanism and requires intraoperative flow restriction, delaying IOP control until capsular fibrosis has occurred around the plate; however, the Baerveldt has been show to provide greater IOP reduction than the Ahmed in adults.6This study consolidates available data in a systematic review and metaanalysis of the outcomes of first-time Ahmed or Baerveldt GDD implantation in the management of childhood glaucoma.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systemic Review and Meta-Analyses (PRISMA) statement.37 The PRISMA checklists are displayed in Supplementary Tables 1 and 2. A protocol of the methods was registered with PROSPERO (ID CRD42022306951).
Literature search
A comprehensive literature search of PubMed, Embase, and Cochrane databases was performed to identify relevant studies published on or before January 23, 2022. Searches were conducted using combinations of the following key terms: (glaucoma drainage device OR Ahmed glaucoma implant OR Baerveldt glaucoma implant OR aqueous drainage OR aqueous shunt) AND (pediatric OR childhood OR infant OR congenital OR child). Full search terms are shown in Supplementary Figure 1. The literature search was also supplemented by hand-searching the bibliographies of relevant studies.
Study selection and inclusion and exclusion criteria
The following inclusion criteria were used to select studies: (1) randomized controlled trial, prospective cohort study, or retrospective cohort study, (2) pediatric patients aged 0–18 years at time of device implantation, (3) specified GDD including Ahmed (S1, S2, S3, FP7, FP8) or Baerveldt (250, 350, 425), and (4) reporting of pre-operative and post-operative IOP and/or success with clearly defined success criteria.
Exclusion criteria were: (1) GDD implantation performed in combination with another intraocular surgery such as lensectomy or vitrectomy, (2) studies including eyes that had undergone previous GDD implantation, (3) case reports, abstract-only publications, letters, or review articles, or (4) full text not available in English.
Study screening and data extraction
Abstracts were screened by a single reviewer (JYS) to exclude irrelevant studies. Full text review was conducted to exclude studies based on inclusion and exclusion criteria. Data were extracted from eligible studies by a single reviewer (JYS). An independent validation of both the screening and data extraction process on a random 30% sample of studies was conducted by a second reviewer (JTO). Data extracted included year and location of study, sample size, glaucoma etiologies included, prior glaucoma and intraocular surgeries, IOP at each time point, number of medications used at each time point, proportion of success at each time point, definition of success, visual acuity at each time point, and complications and additional surgeries performed after GDD implantation.
Risk of bias and study quality assessment
Two reviewers (JYS and JTO) independently reviewed included studies for risk of bias according to the Risk of Bias In Non-Randomized Studies-of Interventions (ROBINS-I) tool for non-randomized studies,45 and the Cochrane risk of bias tool for randomized controlled trials (RoB2).46 Each study was graded on selection, attrition, measurement, performance, and reporting, and any discrepancies between reviewers were discussed to reach a consensus.
Statistical Analysis
The primary outcomes of interest were mean IOP and proportion of study eyes meeting the success definition at 12 and 24 months from implantation. While there were variations in the definition of success across studies, all definitions of success fell within the following constraints, which is the definition of success used in the current study: IOP 5–22 mmHg with or without glaucoma medications without an additional need for glaucoma surgery or vision-threatening complication. For studies that defined qualified and complete success separately, total success was extracted and used in the meta-analysis as a combination of qualified and complete success at each time point. This study used total success in order to include the fair amount of studies that did not report qualified and complete success separately. Secondary outcomes included longer term time points for mean IOP and proportion success, mean number of glaucoma medications at available time points, and the proportion of eyes experiencing complications or requiring additional surgery at any time point post-implantation.
The primary outcomes were pooled across studies and examined using random effects regression models with restricted maximum likelihood estimation overall and by subgroups. Secondary outcomes were assessed similarly but without subgroup analysis as the number of studies with these outcomes was insufficient. Subgroup analysis was based on type of GDD (Ahmed or Baerveldt) and glaucoma etiology. Glaucoma etiology was classified using categories defined by the Childhood Glaucoma Research Network (CGRN) into the following groups: (1) primary glaucoma (primary congenital glaucoma [PCG] and juvenile open angle glaucoma), (2) glaucoma following cataract surgery (GFCS), and (3) all other secondary glaucomas excluding GFCS.49 Meta-regression was used to test for subgroup differences. Heterogeneity was assessed using the I2 statistic. A p-value of less than 0.05 was considered significant. All analyses were conducted in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Systematic review and study inclusion
The systematic database search produced a total of 1217 studies, with one additional study identified from citation searching (Figure 1). After removing duplicates, 475 unique records underwent screening, with 95 then undergoing full-text review. Thirty-eight were excluded on population (such as for including eyes that underwent previous GDD implantation or insufficient information on previous glaucoma surgeries), 15 were excluded on intervention (uncommon surgical technique or inclusion of alternate drainage implants such as the Molteno, Aurolab, or Susanna), and 7 were excluded by inadequate outcome measurement. Three studies were excluded because of an overlapping cohort of patients already described in another included study.1, 2, 44 A study that included one patient who was 18 years old at the time referral to glaucoma clinic, but 19 years old at the time of GDD implantation was included.47 After eligibility screening, 32 studies were included for qualitative synthesis (Figure 1).
Figure 1.
PRISMA flow diagram delineating search, screening, and eligibility assessment process.
Study demographics
Characteristics of the 32 included studies are summarized in Table 1.3, 4, 7, 8, 13–16, 18–22, 24–26, 28–32, 34–36, 38–44, 47 The studies were published between 2000 and 2021 and included patients from 14 countries. In total, 1221 eyes (885 children) were included; 55% were male. The mean ± standard deviation (SD) age at time of GDD implantation was 5.3±3.0 years. Mean ± SD length of follow-up was 45.2 ± 26.7 months. The most common glaucoma etiology was PCG (44%) followed by other secondary glaucoma (25%) and GFCS (25%). All but 4 studies included eyes that had previously undergone a glaucoma procedure, including goniotomy, trabeculotomy, trabeculectomy, deep sclerectomy, and cyclophotocoagulation. Of the 26 studies that specified this data, 447 of 736 eyes (60.7%) had previously undergone a glaucoma procedure (61.6% and 49% of eyes that subsequently received an Ahmed and Baerveldt, respectively, p = 0.87).
Table 1.
Characteristics of the 32 studies included in the meta-analysis of efficacy of Ahmed and Baerveldt drainage devices in childhood glaucoma
| First Author | Year | Study Design | Number of Eyes | Number of Patients | Age in Years at Time of Valve Implantation Mean (SD) | Follow-Up in Months Mean (SD) | Gender Male:Female | Valves Studied | Etiologies Studieda | Prior Glaucoma Surgery | Baseline IOP Mean (SD) | Baseline number of medications Mean (SD) | IOP, Final Follow-Up Mean (SD) | Number of Medications, Final Follow-Up Mean (SD) | Success Criteriab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balekudaru et al. | 2014 | Retrospective | 71 | 71 | 6.8 (4.9) | 37.8 (32.1) | 40:31 | Ahmed FP7, S2 | Primary, GFCS, Secondary | Yes | 35.86 (9.6) | 2.42 (1) | 16.4 (8.7) | 1.3 (1.1) | IOP 6–18 with (qualified) or without (complete) medications, without loss of LP vision or loss of vision due to complication |
| Banitt et al. | 2009 | Retrospective | 30 | 30 | 6.9 (5) | 29.8 (26.4) | 14:16 | Baerveldt 250, 350, 425 | Primary, GFCS, Secondary | Yes | 32.9 (7.9) | 3 (1.6) | IOP 5–21 with or without medications and without additional glaucoma surgery or loss of LP vision | ||
| Budenz et al. | 2000 | Retrospective | 10 | 9 | 6.6 (4.3) | 32.9 (12.1) | 6:3 | Baerveldt 250, 350, 425 | Secondary | Yes | 24.8 (6.2) | 1.8 (1) | 16.9 (2.3) | 1.1 (1.4) | IOP ≤ 21 with or without medications and without additional glaucoma surgery |
| Chen et al. | 2015 | Retrospective | 119 | 89 | 6.8 (5.7) | 73.2 (39.6) | 61:58 | Ahmed FP7, S2 | Primary, Secondary | Yes | 29.2 (9.7) | 2.2 (1.3) | IOP 5–21 and ≥ 20% reduction from baseline IOP with or without medications | ||
| Das et al. | 2005 | Retrospective | 30 | < 12 years | Ahmed S2 | Yes | 32.4 (4.5) | IOP 6–22 and ≥ 30% reduction from baseline IOP with (qualified) or without (complete) medications and without additional glaucoma surgery or visually devastating complications | |||||||
| Dave et al. | 2015 | Retrospective | 11 | 8 | 1.3 (0.4) | 17.9 (9.3) | 4:4 | Ahmed FP8 | Primary | Yes | 28 (5.7) | 2.6 (0.5) | 13.6 (3.4) | 1.6 (0.9) | IOP 6–18 with a maximum of 3 medications (qualified) or without medications (complete) |
| Djodeyre et al. | 2001 | Retrospective | 35 | 29 | 4.4 (4.7) | 12.6 (10.8) | 18:17 | Ahmed S2, S3 | Primary, GFCS, Secondary | Yes | 28.8 (4.5) | 18.1 (2.4) | IOP ≤ 21 with (qualified) or without (complete) medications without signs of progression, additional glaucoma surgery, or devastating complications. Surgical valve revision considered partial success | ||
| Eksioglu et al. | 2017 | Retrospective | 16 | 11 | 14.2 (3.3) | 64.5 (33.6) | 8:3 | Ahmed FP7, S2 | Secondary | Yes | 33.5 (7.3) | 3 (0) | 12.7 (3.2) | 1.0 (1.2) | IOP 6–21 with (qualified) or without (complete) medications and without additional glaucoma surgery or tube extraction surgery |
| El Sayed et al. - Silicone | 2013 | Prospective | 25 | 2.9 (5.4) | Ahmed FP7, FP8 | Primary, GFCS, Secondary | Yes | 33.8 (5.6) | 2.93 (0.7) | IOP ≤ 21 with (qualified) or without (complete) medications and no signs of glaucoma progression | |||||
| El Sayed et al. – Polypropylene | 2013 | Prospective | 25 | 2.8 (5.6) | Ahmed S2, S3 | Primary, GFCS, Secondary | Yes | 34.1 (5.9) | 2.8 (0.7) | IOP ≤ 21 with (qualified) or without (complete) medications and no signs of glaucoma progression | |||||
| Esfandiari et al. - Ahmed | 2019 | Retrospective | 16 | 16 | 4.4 (2.1) | 9:7 | Ahmed FP7 | GFCS | Yes | 28.6 (4.1) | 3 (0.2) | 17.6 (2.1) | IOP 5–21 and ≥ 20% reduction from baseline IOP with or without medications, without additional glaucoma surgery | ||
| Esfandiari et al. - Baerveldt | 2019 | Retrospective | 12 | 12 | 4.1 (1.2) | 7:5 | Baerveldt 350 | GFCS | Yes | 30 (4.1) | 3.1 (1.8) | 17.6 (1.7) | IOP 5–21 and ≥ 20% reduction from baseline IOP with or without medications, without additional glaucoma surgery | ||
| Geyer et al. | 2021 | Retrospective | 41 | 27 | 7.9 (7.3) | 61.1 (46.5) | Ahmed FP7 | GFCS | Yes | 35.8 (7.4) | 3.1 (1.3) | 18.7 (6.5) | 1.6 (1) | IOP ≤ 22 with (qualified) or without (complete) medications, without additional glaucoma surgery or visually devastating complications | |
| Helmy et al. | 2016 | RCT | 33 | 33 | 1.28 (0.5) | 17:16 | Ahmed FP8 | Primary | Yes | 33.4 (4.5) | 2.1 (0.4) | IOP 7–21 with (qualified) or without (complete) additional medications, without additional glaucoma surgery, with stable corneal diameter, cup to disc ratio, and stable or improving corneal edema or clarity | |||
| Huang et al. | 2015 | Retrospective | 14 | 11 | 4.3 (1.9) | 18.3 (11) | 7:4 | Ahmed FP7, FP8 | Primary | Yes | 39.5 (5.7) | IOP 6–21 and ≥ 30% reduction from baseline IOP, without additional glaucoma surgery, loss of LP vision, or serious complications | |||
| Karaconji et al. | 2020 | Retrospective | 6 | 5 | 6.6 (5.4) | 39.3 (25.6) | Baerveldt 350 | Secondary | Yes | 29 (1.7) | 3 | 15.6 (5.4) | IOP ≤ 21 with (qualified) or without (complete) medications and stable optic disc appearance. IOP ≤ 21 with stent removal also defined as qualified success | ||
| Khan et al. - Polypropylene | 2009 | Retrospective | 31 | 31 | 0.9 (0.5) | 17:14 | Ahmed S1, S2 | Primary, GFCS, Secondary | Yes | 33.4 (9.7) | 2.5 (0.7) | IOP ≤ 22 with (qualified) or without (complete) medications, without visually devastating complications | |||
| Khan et al. – Silicone | 2009 | Retrospective | 11 | 11 | 1.2 (0.5) | 5:6 | Ahmed FP7 | Primary | Yes | 33.9 (4.9) | 3.1 (1.1) | IOP ≤ 22 with (qualified) or without (complete) medications, without visually devastating complications | |||
| Kirwan et al. | 2005 | Retrospective | 19 | 13 | 8 | 32 | 7:6 | Ahmed S2 | GFCS | Yes | 31.1 (4.3) | 13 (3.5) | IOP ≤ 15 with (qualified) or without (complete) medications | ||
| Lee et al. | 2015 | Retrospective | 11 | 7.1 (7.2) | 94.6 (28.4) | Ahmed FP8, S3 | Primary | Yes | 26.9 (6.9) | 1.7 (1.1) | IOP 5–12 with (qualified) or without (complete) medications, without additional glaucoma surgery or loss of vision due to complication | ||||
| Mahdy et al. – Ahmed+MMC | 2011 | RCT | 20 | 14 | 4 (5) | 6:8 | Ahmed S3 | Primary | Yes | 32 (3.5) | IOP 10–21 with (qualified) or without (complete) medications, without additional glaucoma surgery, loss of LP vision, or devastating complications | ||||
| Mahdy et al. – Ahmed | 2011 | RCT | 20 | 18 | 5 (4) | 10:8 | Ahmed S3 | Primary | Yes | 35.1 (4) | IOP 10–21 with (qualified) or without (complete) medications, without additional glaucoma surgery, loss of LP vision, or devastating complications | ||||
| Mofti et al.c | 2020 | Retrospective | 178 | 178 | 5.8 (5.5) | 55.2 (37.2) | 89:89 | Ahmed F7, F8, S2 | Primary, GFCS, Secondary | Yes | 32 (27.8–36.3) | 3.5 (2.5–4.5) | 3 | IOP 5–21 without medications, without additional glaucoma surgery | |
| Mokbel et al. | 2019 | Retrospective | 14 | Ahmed, unspecified type | Primary | No | 36.6 (6.6) | IOP 6–21 with (qualified) or without (complete) medications, with stable ocular biometric measurements | |||||||
| Morad et al. | 2003 | Retrospective | 60 | 44 | 6 (4.9) | 24.3 (16) | 25:19 | Ahmed, unspecified type | Primary, GFCS, Secondary | Yes | 32.8 (6.2) | 4.5 (2.0) | 16.6 (8) | 2 (2) | IOP 5–21 with (qualified) or without (complete) medications, without signs of glaucoma progression or loss of > 2 Snellen lines of visual acuity secondary to implant |
| Novak-Laus et al. | 2016 | Retrospective | 10 | 9 | < 11 years | ≥ 12 months | 6:4 | Ahmed S3 | Primary, GFCS, Secondary | Yes | 31.4 (4.5) | 19.3 (5.6) | 1.1 (1.0) | IOP 6–21 with (qualified) or without (complete) medications, without loss of vision due to complications | |
| O’ Malley Schotthoefer et al.d | 2008 | Retrospective | 38 | 30 | 0.75 (0.2–15) | 66 (6–126) | Ahmed FP7, S2, Baerveldt 250, 350 | Primary | Yes | 28.5 (8–44) | 3 (0–5) | 17 (4–40) | 3 (0–4) | IOP ≤ 21 with (qualified) or without (complete) medications, without additional glaucoma surgery or severe complications | |
| Ou et al. | 2009 | Retrospective | 39 | 19 | 1.9 (2.6) | 57.6 (48) | 12:7 | Ahmed FP7, S2 | Primary | Yes | 28.4 (6.7) | 0.9 (0.7) | IOP 6–22 and ≥ 15% reduction from baseline IOP | ||
| Pakravan et al. | 2007 | RCT | 15 | 15 | 10.9 (5.1) | 13.1 (9.7) | 12:3 | Ahmed, unspecified type | GFCS | No | 32.8 (8.6) | 3.3 (0.5) | 14.1 (4.5) | 1.3 (1.0) | IOP 6–21 with no more than two (qualified) or without (complete) medications |
| Promelle et al. | 2021 | Retrospective | 81 | 63 | 6.4 (5.1) | 96 (60) | 35:28 | Ahmed FP7, S2 | Primary, GFCS, Secondary | Yes | 30.4 (8.1) | 3 (1.4) | 19.9 (6.8) | 1.5 (1.4) | IOP 5–21 with (qualified) or without (complete) medications |
| Razeghinejad et al. | 2014 | Retrospective | 33 | 22 | 2.7 (3.1) | 32.6 (18.3) | 17:5 | Ahmed FP7, FP8, S2, S3 | Primary | Yes | 32.8 (7.3) | 2.5 (0.7) | 16.8 (4) | 2.2 (0.7) | IOP 6–21 with (qualified) or without (complete) medications |
| Rolim-de-Moura et al. | 2020 | RCT | 5 | 5 | 3.4 (2.6) | 4:1 | Baerveldt 250 | Primary | Yes | 22.8 (4.9) | 2.6 (0.5) | IOP 6–21 with (qualified) or without (complete) medications | |||
| Senthil et al. – PCGc | 2018 | Retrospective | 24 | 2 (1.5–3) | 26 (12–40) | Ahmed FP7, FP8 | Primary | Yes | 29 (28–33) | 3.5 (2.5–4) | 14.6 (11–18) | 2 (1.5–2) | IOP 6–21 with (qualified) or without (complete) medications | ||
| Senthil et al. – Secondary Glauomac | 2018 | Retrospective | 41 | 9 (2–13) | 27.8 (16.4–37.9) | Ahmed FP7, FP8 | Secondary | Yes | 30 (26–35) | 3 (3–4) | 15.4 (13–18) | 2 (1–2) | IOP 6–21 with (qualified) or without (complete) medications | ||
| Soyugelen et al. | 2019 | Retrospective | 3 | 3 | 9 (4.6) | 24 (10.8) | 3:0 | Ahmed FP7, S2 | Secondary | No | 34 (14.4) | 3.3 (1.2) | IOP 5–22 with or without medications | ||
| Speiss et al.c | 2021 | Retrospective | 29 | 23 | 85.4 (56.2) | 10:13 | Ahmed FP7, FP8, S2 | GFCS | No | 32.6 (6.7) | 2 (2–3) | IOP ≤ 21 with (qualified) or without (complete) medications, without loss of LP vision or devastating complications | |||
| Tai et al. | 2014 | Retrospective | 45 | 36 | 4.3 (4.8) | 26:19 | Baerveldt 350 | Primary, GFCS, Secondary | Yes | 31.6 (5) | 3 (1.2) | IOP 6–21 with or without medications, without additional glaucoma surgery or devastating complications |
Etiologies studied classified according to International Consensus Classification (Thau et al. 2018): “primary glaucoma” includes primary congenital glaucoma and juvenile open angle glaucoma. Glaucoma following cataract surgery (GFCS) was classified separately. All other secondary glaucomas aside from glaucoma following cataract surgery were classified into “secondary glaucoma”.
In studies where success criteria were defined separately for qualified or complete success, total success was extracted (combining rates of both qualified and complete success).
Data expressed in mean (standard deviation) unless otherwise specified
Data expressed in median (interquartile range)
Data expressed in median (range)
Abbreviations: SD (standard deviation); LP (light perception); IOP (intraocular pressure); RCT (randomized controlled trial); GFCS (glaucoma following cataract surgery)
The mean ± SD baseline IOP was 31.8 ± 3.4 mmHg. The mean ± SD number of glaucoma medications used prior to surgery was 2.7 ± 0.7. Owing to the limited data on visual acuity, this outcome was not synthesized. Of the studies that specified this data, the Ahmed FP7 was the most commonly studied device (426 eyes) followed by the Ahmed S2 (253 eyes) and Ahmed FP8 (138 eyes); the most common Baerveldt device included was the Baerveldt 350 (92 eyes).3, 4, 7, 8, 13–15, 18–21, 24–26, 29, 30, 35, 39–43, 47 In total, 1102 eyes received an Ahmed valve and 119 eyes received a Baerveldt implant. The Ahmed was more frequently implanted in eyes with primary glaucoma (98.1%) compared to GFCS (93.3%) (p = 0.01) and other secondary glaucoma (84%) (p < 0.01).
Of the 32 studies, 26 included data for the primary outcomes and thus were included in the quantitative synthesis. The number of studies and eyes included in the meta-analysis of each outcome is shown in Table 2.
Table 2. Random effects model estimates of mean intraocular pressure, proportion of success, and number of glaucoma medications.
Data were analyzed at 12 and 24 months after glaucoma drainage device implantation. Results are listed for all studies and separately for device type and etiology group. Meta-regression test for subgroup differences were performed when each subgroup had 2 or more studies quantitatively summarized. P-value less than 0.05 was considered significant.
| Overall | Device Type | Etiology | ||||||
|---|---|---|---|---|---|---|---|---|
| Ahmed | Baerveldt | Subgroup difference, device | Primary | GFCS | Other Secondary | Subgroup difference, etiology | ||
| IOP, mmHg | ||||||||
| 12 months | 16.5 [15.5, 17.6) | 16.4 [15.5–17.4] | 16.0 [8.9 – 23.2] | p = 0.89 | 15.4 [14.2–16.6] | -- | 18.1 [12.9–23.3] | p = 0.26 |
| I2 | 89.3% | 86.4% | 92.6% | 86.3% | -- | 60.3% | ||
| Studies (eyes) | 13 (414) | 11 (364) | 2 (50) | 7 (153) | 0 | 2 (19) | ||
| 24 months | 17.6 [16.4–18.7] | 17.5 [16.2–18.8] | 18.3 [16.9–19.7] | --a | 18.1 [17.8–18.5] | -- | 14.9 [13.7–16.1] | -- |
| I2 | 83.3% | 84.7% | -- | 0% | -- | -- | ||
| Studies (eyes) | 8 (297) | 7 (252) | 1 (45) | 2 (44) | 0 | 1 (16) | ||
| Proportion of Success | ||||||||
| 12 months | 0.87 [0.83–0.91] | 0.86 [0.81–0.90] | 0.92 [0.86 – 0.99] | p = 0.29 | 0.83 [0.74–0.93] | 0.91 [0.81–1.01] | 0.92 [0.84–1.00] | p = 0.34 |
| I2 | 78.6% | 82.2% | 42.3% | 83.6% | 83.8% | 0% | ||
| Studies (eyes) | 23 (1040) | 19 (920) | 4 (120) | 8 (205) | 4 (145) | 2 (47) | ||
| 24 months | 0.77 [0.71–0.83] | 0.75 [0.68–0.83] | 0.85 [0.77–0.92] | p = 0.34 | 0.75 [0.56–0.94] | 0.82 [0.73–0.92] | 0.70 [0.21–1.18] | p = 0.92 |
| I2 | 74.6% | 78.6% | 0% | 88.6% | 58.7% | 88.4% | ||
| Studies (eyes) | 16 (577) | 13 (490) | 3 (87) | 5 (127) | 4 (145) | 2 (47) | ||
| Medicatio ns | ||||||||
| 12 months | 1.2 [0.8–1.6] | 1.3 [0.9–1.7] | 0.80 [0.1–1.5] | -- | 1.1 [0.7–1.4] | -- | 1.8 [−0.9–4.6] | p = 0.53 |
| I2 | 72.7% | 78.3% | -- | 0% | -- | 93.9% | ||
| Studies (eyes) | 6 (130) | 5 (125) | 1 (5) | 3 (30) | 0 | 2 (19) | ||
| 24 months | 1.2 [0.8–1.7] | 1.2 [0.8–1.7] | -- | -- | 1.3 [0.5–2.0] | -- | 0.4 [−0.0–0.8] | -- |
| I2 | 78.9% | 78.9% | -- | |||||
| Studies (eyes) | 3 (108) | 3 (108) | 1 (11) | 0 | 1 (16) | |||
For subgroups that contained only one study, the data entry reflects the results from the study rather than result from a random effects model
For subgroups that contained only one study, test for subgroup differences was not performed.
Abbreviations: IOP (intraocular pressure), GFCS (glaucoma following cataract surgery)
Meta-analysis of IOP and success
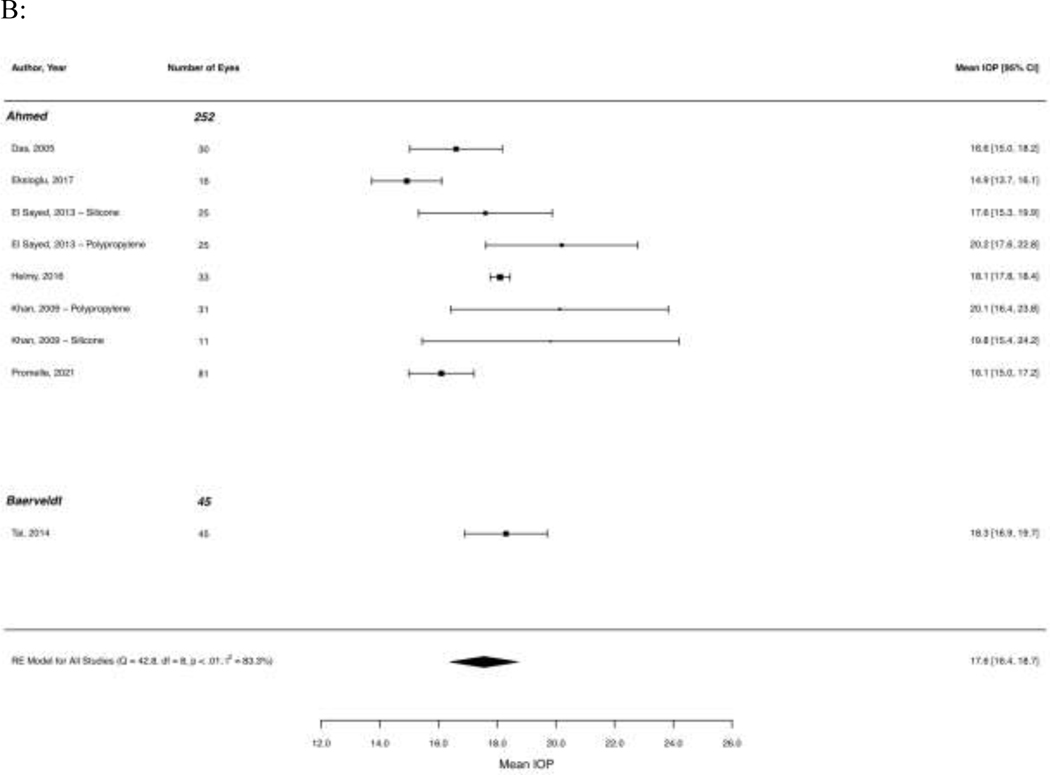
At 12 months after GDD implantation, the mean IOP was 16.5 mmHg (95% CI 15.5–17.6, I2 = 89.3%). Mean IOP at 24 months was 17.6 mmHg (95% CI 16.4–18.7, I2 = 83.3%). At 48 months, IOP was 17.3 mmHg (95% CI 12.7 – 21.9, I2 = 92.7%; 3 studies, 60 eyes), 15.9 mmHg at 60 months (95% CI 12.1 – 19.7, I2 = 93.3%; 3 studies, 136 eyes), and 15.1 mmHg at 120 months (95% CI 14.6 – 15.7, I2 = 0%; 2 studies, 200 eyes). Figure 2 shows forest plots of mean IOP at 12 and 24 months across studies.
Figure 2. Forest plots of mean intraocular pressure (A) 12 months and (B) 24 months after implantation of Ahmed and Baerveldt glaucoma drainage devices.
Results of random effects regression models for each subgroup and test for subgroup differences are shown, except where a subgroup contained less than two studies.
Abbreviation: CI (confidence interval); MMC (mitomycin-C); RE (random effects)
The proportion of eyes meeting study criteria for total success (including both qualified and complete success) at 12 months was 0.87 (95% CI 0.83–0.91, I2 = 78.6%) and decreased slightly at 24 months to 0.77 (95% CI 0.71–0.83, I2 = 74.6%). Success proportion was 0.54 (95% CI 0.44–0.65, I2 = 52.7%, 6 studies, 200 eyes) at 48 months, 0.60 (95% CI 0.48–0.71, I2 = 84.5%, 7 studies, 477 eyes) at 60 months, and 0.37 (95% CI 0.32–0.42, I2 = 0.0%, 3 studies, 335 eyes) at 120 months. Forest plots of proportion of success across studies are shown in Figure 3.All studies demonstrated a substantial drop in IOP after surgery that was largely sustained over the first 60 months. The final mean IOP in all studies was less than 20 mmHg.
Figure 3. Forest plots of proportion of success (A) 12 months and (B) 24 months after implantation of Ahmed and Baerveldt glaucoma drainage devices.
Results of random effects regression models for each subgroup and test for subgroup differences are shown.
Abbreviation: CI (confidence interval); MMC (mitomycin-C); PCG (primary congenital glaucoma); RE (random effects)
Subgroup analysis
Table 2 displays the results of the meta-analysis of mean IOP and proportion of success at 12 and 24 months after GDD implantation, including results of subgroup analyses. Mean IOP at 12 months was similar for Ahmed (16.5 mmHg, 95% CI 15.5–17.4, I2 = 86.4%) and Baerveldt tube shunts (16.1 mmHg, 95% CI 8.9–23.2, I2 = 92.6%) (p = 0.89). Subgroup analysis was not performed for individual device models due to small sample size. There were no significant differences in mean IOP at 12 months between eyes with primary glaucoma (15.4 mmHg, 95% CI 14.2–16.6 I2 = 86.3%) and secondary glaucoma apart from GFCS (18.1 mmHg, 95% CI 12.9–23.3, I2 = 60.3%) (p = 0.26), though this was limited by sample size in the secondary glaucoma group (19 eyes, 2 studies). Subgroup comparisons at the 24-month time point were not conducted due to low number of studies within subgroups. There were no significant differences in proportion of success at 12 or 24 months in sub-analyses between device types (12 month, p = 0.29; 24 month, p = 0.34) and glaucoma etiology (12 month, p = 0.34; 24 month, p = 0.92).
Post-operative glaucoma medications and complications
The average number of post-operative glaucoma medications after GDD implantation was 1.22 at 12 months (95% CI 0.84–1.59, I2 = 72.7%) and 1.23 at 24 months (95% CI 0.76–1.70, I2 = 78.9%). There was no significant difference in number of medications at 12 months between eyes with primary and other secondary glaucoma (p = 0.53); tests for other subgroup comparisons were deferred due to low number of studies within each subgroup.
Shallowing of the anterior chamber was the most commonly reported complication, occurring in 13.6% (95% CI 8.7–18.6%) of eyes, followed by hypotony (11.7%, 95% CI 6.2–17.2%), and choroidal effusion (8.3%, 95% CI 5.2–11.3%). Endophthalmitis (1.7%, 95% CI 0.8–2.6%), suprachoroidal hemorrhage (1.6%, 95% CI 0.4–2.7%), and progression to no light perception vision (2.8%, 95% CI 0.9–4.8%) were uncommon. The most frequently performed surgery after GDD implantation was tube adjustment or repositioning in 11.1% (95% CI 8–14.2%) of eyes, followed by implantation of a second GDD (9.5%, 95% CI 3.7–15.2%) for uncontrolled pressure or following tube explant. Other post-operative complications and additional surgeries performed are summarized in Supplementary Table 3.
Risk of bias assessment
The results of bias assessment are displayed in Supplementary Table 4. Risk of selection bias was generally low as most studies reviewed all eyes within a given time-constrained cohort. However, any studies that included stricter inclusion criteria such as a particular age group or follow-up length were considered to have greater risk of selection bias. Five studies were noted to have greater than 50% of eyes lost to follow up by the end of the study period and were noted to have serious risk of attrition bias. Risk of measurement and performance biases were low in most studies given the fairly standardized methods of IOP measurement and retrospective nature of most studies. Lastly, the majority of papers were graded to have low to moderate risk of reporting bias given clear selection and analytical plans.
Discussion
This study integrates all available evidence on the efficacy of first-time implantation of Ahmed and Baerveldt GDDs in the treatment of childhood glaucoma. Overall, mean IOP decreased substantially after GDD implantation for at least 24 months. While quantitative analysis relied on fewer studies for longer follow-up intervals past 24 months, IOP remained substantially lower than pre-operative IOP up to 120 months post-operatively. The proportion of eyes meeting criteria for success was high at both 12 and 24 months (87% and 77% respectively). We found no significant differences in success at 12 and 24 months between eyes that received Ahmed or Baerveldt devices, nor between eyes with primary glaucoma, GFCS, or other secondary glaucoma.
The pooled estimates of mean IOP and proportion of success over time reported in our quantitative summary are similar to early studies of GDD implantation in children. In 1993, Netland and coworkers reported success proportion of 80% at an average follow-up time of 25 months post-operatively for the Baerveldt shunt.33 GDDs are generally thought to have a higher risk of failure in children due to a robust wound healing response and the higher prevalence of eye rubbing which may predispose the GDD to bleb encapsulation or tube malposition. Despite this, our study results are also comparable to those of randomized controlled studies in adults that have reported success of 57% - 86% at 1 year and 51% - 63% at 5 years.5, 11, 12 This suggests that despite these challenges, tube shunts retain their efficacy in children although analysis of long-term success relies on data from fewer studies.
Comparison of Ahmed and Baerveldt glaucoma implants in the pediatric population has, to date, relied on retrospective studies. Amongst eyes with primary and secondary glaucoma, El Gendy and coworkers reported better IOP control with the Baerveldt compared to Ahmed.17 More recent comparisons have found similar outcomes between Baerveldt and Ahmed in eyes with GFCS and juvenile open angle glaucoma.19, 27 In the current meta-analysis, we found no significant differences between Ahmed or Baerveldt devices in post-operative IOP at 12 months or proportion of success at 12 and 24 months. It is important to note, however, that the majority of studies in our analysis studied the Ahmed glaucoma implant.
Prior studies have shown that eyes with PCG have higher success after GDD implantation compared to those with secondary causes such as uveitic glaucoma,16, 25 though the literature is conflicting and others have reported similar success of GDD implantation in PCG compared to GFCS.35 Similarly, Senthil and coworkers found no significant difference in success of Ahmed implantation in PCG and secondary pediatric glaucoma with the latter group containing a substantial number of eyes with GFCS.42 While GFCS is nominally contained within secondary childhood glaucoma, our study separated GFCS into its own subgroup to better discern etiology-based differences in outcomes. Subgroup analysis by etiology found no significant differences in success at 12 or 24 months, although the number of studies in each subgroup was low and long-term outcomes could not be quantitatively compared. Regardless, our results present useful information as many studies on childhood glaucoma include heterogeneous etiologies that are often studied together.
This meta-analysis focuses on first-time implantation of GDD. Studies including eyes with prior GDD implantation were excluded in order to isolate the effect of first-time GDD implantation. The efficacy of subsequent GDD implantation should be studied separately as these reports would necessarily reflect the increased complexity of cases that warrant additional surgery. Additionally, to isolate the effect of Ahmed or Baerveldt implantation, studies that included implantation of tube shunt with concurrent surgery such as penetrating keratoplasty or retina surgery, implantation of second tube shunt, and use of alternative tube shunts such as Molteno or Aurolab were excluded. Thus, generalizability of these results may be limited to less complex cases.
The main strength of our study is that it is the first systematic review and meta-analysis of GDD outcomes in the pediatric population to date. There are several limitations, however, to this study. First, almost all of the summarized literature was retrospective in nature rather than prospective or randomized and controlled, though this is an inherent limitation given the low incidence of pediatric glaucoma. Additionally, overall attrition was relatively high, which may limit the generalizability of long-term success results. This may bias the results towards those with better compliance to care with better IOP, or conversely may bias towards worse outcomes due to more complex cases requiring close follow-up. Another limitation is the paucity of visual outcomes with which to correlate IOP and GDD success, reflecting the challenges of measuring visual acuity in this population. Furthermore, the smaller number of studies that examined the Baerveldt glaucoma implant, GFCS, and other secondary glaucoma limited the power of our subgroup comparisons. Specifically, that 90% of eyes in this study received the Ahmed glaucoma valve compared to only 10% receiving a Baerveldt biases the results of this study toward the efficacy of the Ahmed valve and may reflect global trends of device availability. Additionally, at time points after 24 months, the number of reports and eyes summarized is relatively small, limiting the strength of our conclusions at longer follow-up points.
A final limitation of this study is that considerable heterogeneity across studies was identified in the analyses. The included reports span two decades and may reflect changes in surgical practice over time. Additionally, there were differences in surgical technique across studies such as the addition of augmentation with anti-fibrotic agents; however, these studies were included to reflect a “real world” view of the range in surgical practice in GDD implantation. Variations in study follow up time also introduce heterogeneity into the pooled estimates of complications, which may have occurred at different post-operative time points across studies. IOP measurement likely represents a significant source of heterogeneity as well, with varying devices and settings, such as with or without anesthesia, or variable instruments used for IOP measurement, reflecting the challenges of consistently and accurately obtaining this metric in children. We attempted to reduce heterogeneity associated with device type or etiology through subgroup analyses, and sources of heterogeneity were partially accounted for in the pre-specification of random effects models. Given small numbers of studies in some analyses, however, the level of heterogeneity expressed by the I2 statistic may be incorrect as I2 has been reported to be biased in small meta-analyses.50
Lastly, there has yet to be a consensus on the ideal definition of success in children, and as such, analyses of glaucoma outcomes in this study and its included reports relies on success definitions extracted from adult studies. Further research is necessary to develop ideal glaucoma outcome parameters for the pediatric population.
Conclusion
Our meta-analysis of 32 studies found that first-time implantation of an Ahmed or Baerveldt GDD produced significant lowering of IOP and high proportion of success at 12 and 24 months. Longer-term results, although limited by fewer studies, suggest continued IOP-lowering effect up to 120 months post-operatively, although cumulative success declined significantly by 48 months. This information may help surgeons counsel patients about the long-term effects of surgery and lay the groundwork for future prospective studies assessing surgical outcomes in childhood glaucoma.
Method of Literature Search
Pubmed, Embase, and Cochrane databases were searched using combinations of the following key terms: (glaucoma drainage device OR Ahmed glaucoma implant OR Baerveldt glaucoma implant OR aqueous drainage OR aqueous shunt) AND (pediatric OR childhood OR infant OR congenital OR child). All relevant studies published on or before January 23, 2022 were included in the initial search. Hand-search of bibliographies of relevant studies was also performed to supplement database searching. International literature was included. Literature without full-text available in English were excluded. See methods section for detailed inclusion and exclusion criteria.
Supplementary Material
Financial Support:
This study was funded, in part, by the NIH-NEI EY002162 Core Grant for Vision Research and K12EY031372. The funding organization had no role in the design or conduct of this research.
Footnotes
Conflicts of Interest: The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Mobarak F, Khan AO. Complications and 2-year valve survival following Ahmed valve implantation during the first 2 years of life. Br J Ophthalmol 2009;93(6):795–8. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mobarak F, Khan AO. Two-year survival of Ahmed valve implantation in the first 2 years of life with and without intraoperative mitomycin-C. Ophthalmology 2009;116(10):1862–5. [DOI] [PubMed] [Google Scholar]
- 3.Balekudaru S, Vadalkar J, George R, Vijaya L. The use of Ahmed glaucoma valve in the management of pediatric glaucoma. J aapos 2014;18(4):351–6. [DOI] [PubMed] [Google Scholar]
- 4.Banitt MR, Sidoti PA, Gentile RC, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. J Glaucoma 2009;18(5):412–7. [DOI] [PubMed] [Google Scholar]
- 5.Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 2011;118(3):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 2015;122(2):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budenz DL, Sakamoto D, Eliezer R, et al. Two-staged Baerveldt glaucoma implant for childhood glaucoma associated with Sturge-Weber syndrome. Ophthalmology 2000;107(11):2105–10. [DOI] [PubMed] [Google Scholar]
- 8.Chen A, Yu F, Law SK, et al. Valved glaucoma drainage devices in pediatric glaucoma: retrospective long-term outcomes. JAMA Ophthalmol 2015;133(9):1030–5. [DOI] [PubMed] [Google Scholar]
- 9.Chen TC, Chen PP, Francis BA, et al. Pediatric glaucoma surgery: a report by the American Academy Of Ophthalmology. Ophthalmology 2014;121(11):2107–15. [DOI] [PubMed] [Google Scholar]
- 10.Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed Versus Baerveldt Study: five-year treatment outcomes. Ophthalmology 2016;123(10):2093–102. [DOI] [PubMed] [Google Scholar]
- 11.Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology 2011;118(11):2180–9. [DOI] [PubMed] [Google Scholar]
- 12.Christakis PG, Zhang D, Budenz DL, et al. Five-year pooled data analysis of the Ahmed Baerveldt Comparison Study and the Ahmed Versus Baerveldt Study. Am J Ophthalmol 2017;176:118–26. [DOI] [PubMed] [Google Scholar]
- 13.Das JC, Chaudhuri Z, Sharma P, Bhomaj S. The Ahmed Glaucoma Valve in refractory glaucoma: experiences in Indian eyes. Eye (Lond) 2005;19(2):183–90. [DOI] [PubMed] [Google Scholar]
- 14.Dave P, Senthil S, Choudhari N, Sekhar GC. Outcomes of Ahmed valve implant following a failed initial trabeculotomy and trabeculectomy in refractory primary congenital glaucoma. Middle East Afr J Ophthalmol 2015;22(1):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djodeyre MR, Peralta Calvo J, Abelairas Gomez J. Clinical evaluation and risk factors of time to failure of Ahmed Glaucoma Valve implant in pediatric patients. Ophthalmology 2001;108(3):614–20. [DOI] [PubMed] [Google Scholar]
- 16.Eksioglu U, Yakin M, Sungur G, et al. Short- to long-term results of Ahmed glaucoma valve in the management of elevated intraocular pressure in patients with pediatric uveitis. Can J Ophthalmol 2017;52(3):295–301. [DOI] [PubMed] [Google Scholar]
- 17.El Gendy NM, Song JC. Long term comparison between single stage Baerveldt and Ahmed glaucoma implants in pediatric glaucoma. Saudi J Ophthalmol 2012;26(3):323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Sayed Y, Awadein A. Polypropylene vs silicone Ahmed valve with adjunctive mitomycin C in paediatric age group: a prospective controlled study. Eye (Lond) 2013;27(6):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esfandiari H, Kurup SP, Torkian P, et al. Long-term clinical outcomes of Ahmed and Baerveldt drainage device surgery for pediatric glaucoma following cataract surgery. J Glaucoma 2019;28(10):865–70. [DOI] [PubMed] [Google Scholar]
- 20.Geyer O, Segal A, Melamud A, Wolf A. Clinical outcomes after Ahmed glaucoma valve implantation for pediatric glaucoma after congenital cataract surgery. J Glaucoma 2021;30(1):78–82. [DOI] [PubMed] [Google Scholar]
- 21.Helmy H. Combined trabeculotomy-trabeculectomy versus Ahmed valve implantation for refractory primary congenital glaucoma in Egyptian patients: a long-term follow-up. Electron Physician 2016;8(2):1884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Lin J, Wu Z, et al. Outcomes of Ahmed glaucoma valve implantation in advanced primary congenital glaucoma with previous surgical failure. Clin Ophthalmol 2015;9:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junoy Montolio FG, Müskens R, Jansonius NM. Influence of glaucoma surgery on visual function: a clinical cohort study and meta-analysis. Acta Ophthalmol 2019;97(2):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karaconji T, Ting ER, Zagora SL, et al. Surgical treatment for SWS glaucoma: experience from a tertiary referral pediatric hospital. J Glaucoma 2020;29(12):1132–7. [DOI] [PubMed] [Google Scholar]
- 25.Khan AO, Almobarak FA. Comparison of polypropylene and silicone Ahmed valve survival 2 years following implantation in the first 2 years of life. Br J Ophthalmol 2009;93(6):791–4. [DOI] [PubMed] [Google Scholar]
- 26.Kirwan C, O’Keefe M, Lanigan B, Mahmood U. Ahmed valve drainage implant surgery in the management of paediatric aphakic glaucoma. Br J Ophthalmol 2005;89(7):855–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le PH, Nguyen M, Humphrey KA, Klifto MR. Ahmed and Baerveldt Drainage Implants in the Treatment of Juvenile Open-angle Glaucoma. J Glaucoma 2021;30(3):276–80. [DOI] [PubMed] [Google Scholar]
- 28.Lee N, Ma KT, Bae HW, et al. Surgical results of trabeculectomy and Ahmed valve implantation following a previous failed trabeculectomy in primary congenital glaucoma patients. Korean J Ophthalmol 2015;29(2):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahdy RA. Adjunctive use of bevacizumab versus mitomycin C with Ahmed valve implantation in treatment of pediatric glaucoma. J Glaucoma 2011;20(7):458–63. [DOI] [PubMed] [Google Scholar]
- 30.Mofti A, Alharbi A, Alsuhaibani M, et al. Long-term outcomes of the Ahmed glaucoma valve surgery in childhood glaucoma. J aapos 2020;24(6):346.e1-.e8. [DOI] [PubMed] [Google Scholar]
- 31.Mokbel TH, El Hefney EM, Hagras SM, et al. Launching a paradigm for first and redosurgery in primary congenital glaucoma: institutional experience. Int J Ophthalmol 2019;12(2):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morad Y, Donaldson CE, Kim YM, et al. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol 2003;135(6):821–9. [DOI] [PubMed] [Google Scholar]
- 33.Netland PA, Walton DS. Glaucoma drainage implants in pediatric patients. Ophthalmic Surg 1993;24(11):723–9. [PubMed] [Google Scholar]
- 34.Novak-Lauš K, Škunca Herman J, Šimić Prskalo M, et al. Initial clinical experience with Ahmed valve implantation in refractory pediatric glaucoma. Acta Clin Croat 2016;55(4):555–9. [DOI] [PubMed] [Google Scholar]
- 35.O’Malley Schotthoefer E, Yanovitch TL, Freedman SF. Aqueous drainage device surgery in refractory pediatric glaucomas: I. Long-term outcomes. J aapos 2008;12(1):33–9. [DOI] [PubMed] [Google Scholar]
- 36.Ou Y, Yu F, Law SK, et al. Outcomes of Ahmed glaucoma valve implantation in children with primary congenital glaucoma. Arch Ophthalmol 2009;127(11):1436–41. [DOI] [PubMed] [Google Scholar]
- 37.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pakravan M, Homayoon N, Shahin Y, Ali Reza BR. Trabeculectomy with mitomycin C versus Ahmed glaucoma implant with mitomycin C for treatment of pediatric aphakic glaucoma. J Glaucoma 2007;16(7):631–6. [DOI] [PubMed] [Google Scholar]
- 39.Promelle V, Lyons CJ. Long-term results of Ahmed valve implantation with mitomycin-C in pediatric glaucoma. J Glaucoma 2021;30(7):596–605. [DOI] [PubMed] [Google Scholar]
- 40.Razeghinejad MR, Kaffashan S, Nowroozzadeh MH. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. J aapos 2014;18(6):590–5. [DOI] [PubMed] [Google Scholar]
- 41.Rolim-de-Moura C, Esporcatte BLB, C FN, Paranhos A Jr. Baerveldt implant versus trabeculectomy as the first filtering surgery for uncontrolled primary congenital glaucoma: a randomized clinical trial. Arq Bras Oftalmol 2020;83(3):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senthil S, Turaga K, Mohammed HA, et al. Outcomes of silicone Ahmed glaucoma valve implantation in refractory pediatric glaucoma. J Glaucoma 2018;27(9):769–75. [DOI] [PubMed] [Google Scholar]
- 43.Soyugelen Demirok G, Ekşioğlu Ü, Yakın M, et al. Short- and long-term results of glaucoma valve implantation for aniridia-related glaucoma: a case series and literature review. Turk J Ophthalmol 2019;49(4):183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiess K, Calvo JP. Clinical characteristics and treatment of secondary glaucoma after pediatric congenital cataract surgery in a tertiary referral hospital in spain. Journal of Pediatric Ophthalmology and Strabismus 2020;57(5):292–300. [DOI] [PubMed] [Google Scholar]
- 45.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 47.Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J aapos 2014;18(6):550–3. [DOI] [PubMed] [Google Scholar]
- 48.Tanimoto SA, Brandt JD. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol 2006;17(2):132–7. [DOI] [PubMed] [Google Scholar]
- 49.Thau A, Lloyd M, Freedman S, et al. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol 2018;29(5):385–94. [DOI] [PubMed] [Google Scholar]
- 50.von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YW, Wang PB, Zeng C, Xia XB. Comparison of the Ahmed glaucoma valve with the Baerveldt glaucoma implant: a meta-analysis. BMC Ophthalmol 2015;15:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.