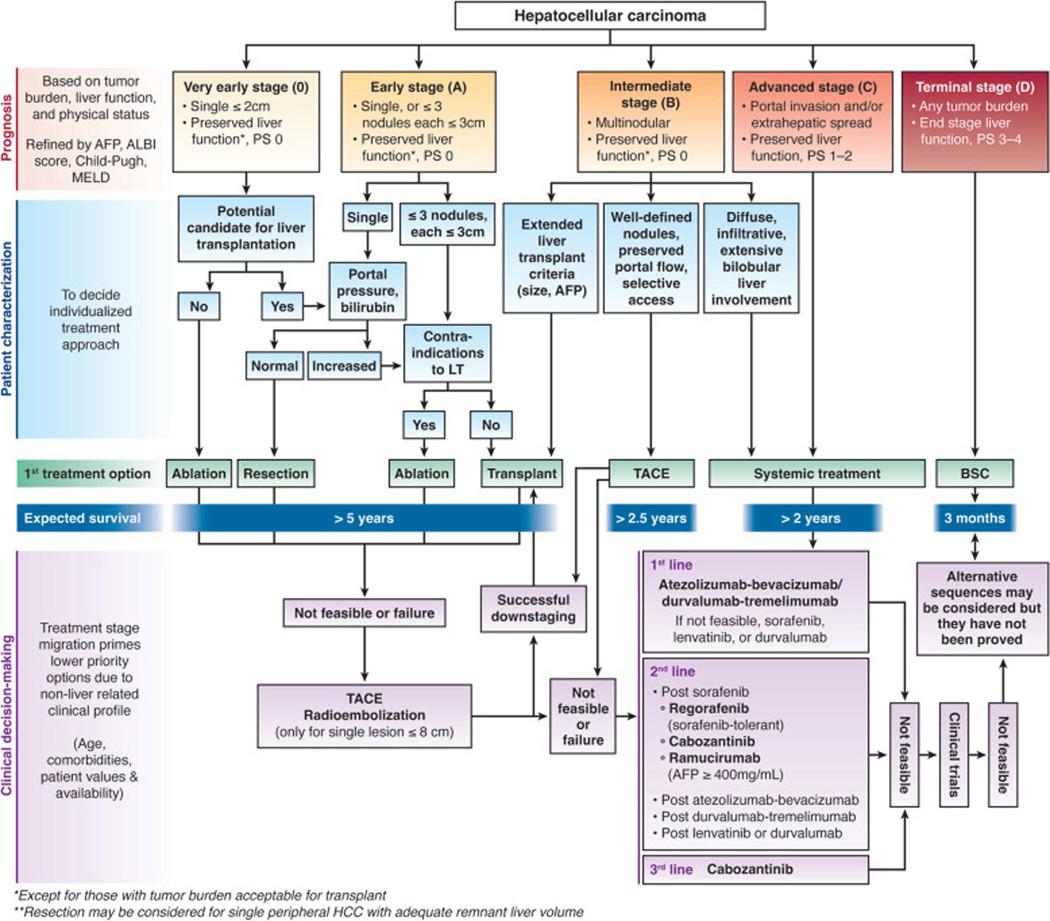
Hepatocellular carcinoma (HCC) is one of the few cancers with a 5-year survival below 20% and an incidence-to-mortality ratio near 1. However, we have witnessed several recent breakthroughs in therapy that have the potential to reverse this dismal prognosis (Table 1). These breakthroughs can be readily identified by examining changes in current treatment options as well as emerging novel therapies1. However, perhaps the most important turning point has been that HCC is no longer seen with a nihilistic perspective as a neoplasm for which few treatments can be efficacious. Better knowledge of the disease’s natural history and evolutionary stages has provided a clinically oriented stratification of patients and a treatment allocation paradigm to guide therapeutic decisions and optimize survival benefit. The Barcelona Clinic Liver Cancer (BCLC) model represents the most widely validated and endorsed staging and treatment allocation system. It has been updated to incorporate recent evidence, guiding evaluation of tumor stage and prognosis of patients at baseline, followed by an evidence-based treatment allocation proposal that accounts for individual patient’s clinical characteristics and preferences to recommend the optimal treatment strategy2 (Figure 1).
Table 1.
Breakthroughs in the field of Hepatocellular Carcinoma
|
• Incorporation of HCC screening in clinical practice |
|
• Development and validation of non-invasive diagnostic criteria |
|
• Stratification of patients into relevant evolutionary stages linked to treatment |
|
• Establishment of liver transplantation as curative treatment for HCC, including concept of downstaging |
|
• Improved selection criteria and introduction of minimally invasive techniques for surgical resection |
|
• Incorporation of ablation as an effective therapy competing with surgery for very-early-stage HCC |
|
• Proof of survival benefit of chemoembolization |
|
• Recognition and incorporation of concepts of TACE unsuitable and TACE refractory in clinical practice |
|
• Demonstration of objective responses with radioembolization, particularly with radiation segmentectomy technique for early-stage HCC |
|
• Availability of effective systemic therapy, including immunotherapy combinations, in sequential lines |
|
• Refined trial design to evaluate new interventions at any stage |
Figure 1.
Updated BCLC Staging and Treatment Algorithm. AFP, α-fetoprotein; ALBI, albumin-bilirubin; BSC, best supportive care; HCC, hepatocellular carcinoma; LT, liver transplantation; MELD, model for end-stage liver disease; PS, performance status; TACE, transarterial chemoembolization.
Herein, we describe major developments in the therapeutic realm using a stage-oriented sequence, although the traditional stage-specific treatment approach is likely outdated. There are increasing data about potential expansion of surgical therapies, including liver transplantation, to select patients with minimally impaired liver function and/or limited intermediate-stage HCC and increased use of systemic therapies for some patients with extensive, bilobar liver-localized disease. Instead, our presentation is meant to parallel clinical prioritization, first describing options with curative intent that provide longer disease-free survival, and then detailing non-curative locoregional and systemic therapy options.
Curative Surgical or Ablative Therapies
Liver transplantation, surgical resection, and local ablative therapies comprise the curative-intent treatment options for HCC, each providing a 5-year survival of approximately 70%. Although techniques for these therapies are mature, with minor advances in recent years compared to other treatments, there have been breakthroughs in our understanding of tumor biology and patient eligibility, increasing the proportion of patients who can benefit from each of these therapies.
Surgical Resection
Eligibility for surgical resection depends on several factors including tumor burden, the degree of liver dysfunction, portal hypertension, and planned extent of the hepatectomy including future liver remnant (FLR)2–4. Resection remains the treatment of choice for patients with localized HCC in the absence of cirrhosis, although non-cirrhotic HCC accounts for a minority of cases in the Western world despite higher proportions being reported in those with nonalcoholic steatohepatitis (NASH)5. In patients with underlying cirrhosis, resection had historically been largely limited to those with a unifocal tumor in the setting of compensated cirrhosis, lack of clinically significant portal hypertension, and an adequate FLR. Clinically significant portal hypertension [hepatic vein pressure gradient (HVPG) ≥ 10mmHg] is associated with risk of liver failure and is optimally measured by HVPG measurement6; however, lack of ascites, portosystemic varices, and a platelet count >100,000 are often used as non-invasive surrogates for lack of portal hypertension in routine clinical practice7. Introduction of minimally invasive surgical (MIS) approaches, in parallel with advances in intra-and peri-operative management, have expanded patient eligibility for surgical resection. MIS approaches, including laparoscopic and robotic-assisted hepatectomy, are widely used for limited minor resections in anatomically favorable locations, although use of these techniques for major hepatectomies is typically limited to high-volume centers. Meta-analyses of comparative studies, with or without propensity score matching, have reported MIS approaches are associated with decreased operative blood loss, shorter length of stay, and decreased 30-day morbidity compared to open resection; however, recurrence-free survival (RFS) or overall survival (OS) appear similar between the two techniques8,9. Given these differences, MIS approaches may extend resection criteria, allowing some patients with mild portal hypertension to safely undergo minor liver resection. Future studies are needed to define which patients with mild portal hypertension are best treated with MIS resection +/− salvage liver transplant versus up-front transplantation.
Although surgical resection is primarily reserved for patients with BCLC stage 0/A HCC, increasing data highlight that it may also play a role in select patients with BCLC stage B HCC. A meta-analysis of 18 studies comparing surgical resection to TACE reported a significant survival advantage for surgical resection in patients with limited multifocal disease (HR 0.56, 95%CI 0.35–0.90), although available data are limited by channeling bias10. There are also data, primarily from Asia, suggesting resection can also be effective in select patients with PVTT11,12, although data from Western centers using this approach are more limited and less encouraging. Studies to better identify which patients beyond BCLC stage A who could benefit from resection instead of locoregional or systemic therapies is an area of need.
Although resection affords long-term survival, the risk of post-operative recurrence remains high, highlighting a need for (neo)adjuvant therapy. Factors associated with recurrence include older age, male sex, degree of liver dysfunction, and tumor size, number, grade/differentiation, micro- and macrovascular invasion, presence of satellite lesions, and AFP levels13–15. In hepatitis C-associated HCC, sustained virological response (SVR) after direct acting antiviral (DAA) therapy does not increase HCC recurrence risk and improves survival16–18. However, there are no proven (neo)adjuvant therapies for patients. Studies evaluating (neo)adjuvant tyrosine kinase inhibitors (TKIs) have failed to demonstrate improved RFS and OS19. Recent phase I-II studies have suggested neoadjuvant immune checkpoint inhibitors, such as cabozantinib plus nivolumab and nivolumab +/− ipilimumab, can induce major pathologic responses in 30–40% of patients, although we are awaiting phase III data to determine if these therapies can improve RFS and OS20,21.
Liver Transplantation
Liver transplantation (LT) is regarded as the optimal treatment for patients with early-stage HCC who are ineligible for resection due to liver dysfunction or tumor multifocality, as it provides a cure for both the HCC and the underlying liver disease. In a seminal article published in 1996, Mazzaferro and colleagues defined the Milan Criteria as providing optimal post-transplant outcomes, with 5-year survival exceeding 70% and ~10% five-year recurrence22. Since that time, several criteria with larger tumor burden including the UCSF criteria, up-to-seven criteria, extended Toronto criteria, and Kyoto criteria have also been shown to achieve acceptable post-transplant outcomes (Table 2)23–26. Beyond tumor burden at presentation, response to locoregional therapy and changes in tumor burden are increasingly used to identify those with favorable tumor biology. In patients exceeding Milan criteria but within defined limits of HCC tumor size and number, post-transplant outcome in those successfully downstaged to Milan criteria do not significantly differ from those who present within Milan criteria27–29. A randomized controlled trial (RCT) of 74 patients who presented beyond Milan criteria, were downstaged, and then subsequently randomized to LT versus non-transplant therapies, reported 5-year survival of 77% in the LT group versus 31% for others (HR 0.32, 95%CI 0.11–0.92)30. Based on these data, patients whose initial tumor burden exceeds Milan criteria can be considered for LT following successful downstaging to within Milan criteria. However, the downstaging strategy must pay attention to practicalities including accurate assessment of initial tumor staging and treatment response during follow-up, including a requirement for durable responses ≥6 months to ensure optimal outcomes31. In the United States, UNOS-DS criteria define the upper limits of downstaging to receive priority for deceased donor liver transplantation. Although patients beyond these criteria can still undergo living donor liver transplant, liberalizing criteria results in a higher risk of waitlist dropout, higher post-transplant recurrence, and lower post-transplant survival32. In addition to tumor burden, serum biomarkers including alpha fetoprotein (AFP) have prognostic value, with even low-level AFP elevations associated with increased post-LT recurrence33. Patients with marked elevations, such as AFP >1000 ng/mL, must achieve lower post-treatment levels for acceptable post-transplant outcomes, although the magnitude of required decrease needs validation34,35. Studies are ongoing to define the optimal LT criteria that achieve acceptable post-LT outcomes and maximize transplant benefit for the greatest number of patients with HCC.
Table 2.
Examples of Expanded Criteria for Liver Transplantation
| Expanded Criteria | |
|---|---|
| UCSF Criteria | One tumor ≤ 6.5 cm OR 2–3 tumors, each ≤ 4.5 cm, with total tumor volume ≤ 8 cm |
| Up-to-Seven Criteria | Diameter or largest tumor (cm) + number of tumors ≤ 7 |
| Extended Toronto Criteria | Biopsy demonstrating well to moderate differentiation for patients beyond Milan Criteria and ECOG performance status 0–1 |
| Total tumor volume <115 cm | Sum of volume for each tumor ≤ 115 cm3 |
| Kyoto Criteria | Number or tumors ≤ 10, maximum diameter of each tumor ≤ 5 cm, and serum DCP ≤ 400 mAU/mL |
| 5–5-500 Rule | Numbers of nodules ≤ 5, nodule size ≤ 5 cm, and AFP ≤ 500 ng/mL |
Neoadjuvant LRT such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), local ablation, and external beam radiation therapy (EBRT) is often used as a bridge to reduce the risk of waitlist dropout36,37. A single-center case series and selected case reports suggest that immune checkpoint inhibitors may also be safe as bridging therapy, although larger studies evaluating longer post-transplant outcomes and defining the optimal washout period are still needed38.
One of the limitations of transplantation is a shortage of available organs compared to those in need of LT39. To address this issue, living donor liver transplantation (LDLT) is an option for patients with HCC – including those beyond typical selection criteria. Initial concerns of increased HCC recurrence with LDLT for HCC were primarily related to patient selection, and recent reports have demonstrated improved survival for patients within Milan Criteria using LDLT compared to deceased donor LT when analyzed on an intention-to-treat basis, primarily due to reduced risk of wait-list dropout40,41.
Local ablative therapy
Local ablative therapy (LAT) offers a curative treatment option in patients with solitary HCC who are ineligible for surgery. Specifically, LAT may be considered for patients with centrally located tumors requiring major hepatectomy or those with very early-stage HCC, as RCTs demonstrate LAT affords similar survival and is cost-effective compared to resection in patients with BCLC stage 0 HCC42. LAT yields 3-year RFS and OS of ~46% and 76%, respectively, for unifocal HCC ≤3 cm43,44. Patients with HCC >3 cm experience lower objective response rates (ORR), higher recurrence rates, and worse OS compared to those with smaller tumors. Although initial radiologic objective response may be improved by combining ablation with TACE, survival benefit of this strategy is not proven. Contrast-enhanced ultrasound immediately after ablation can also assess for viable disease and enable retreatment as needed to optimize ORR.
The first LAT modality was percutaneous ethanol injection, although this has since been replaced in most centers by radiofrequency ablation, microwave ablation, and cryoablation– all of which induce superior ORR with fewer sessions. Although there are no randomized data showing superiority of one ablative modality over another, microwave ablation may be less susceptible to heat sink effects near large vessels and is now used as the primary LAT in many centers45–47. Irreversible electroporation (IRE), which uses high-current electrical pulses to induce cell death, has also been proposed as another form of LAT to avoid heat sink effects, although this approach is time consuming and has not been widely adopted. Early data suggest histotripsy, a sonic beam approach for LAT, may not only be another approach to induce ORR but also induce immunomodulatory effects to also induce abscopal effects in other untreated lesions, as also suggested for other LATs. Similarly, there are data that external beam radiation therapy, including stereotactic approaches, can induce high rates of local tumor control although larger cohorts evaluating clinically important outcomes including overall survival are still needed48–51.
Locoregional Therapies and Management of Intermediate Stage HCC
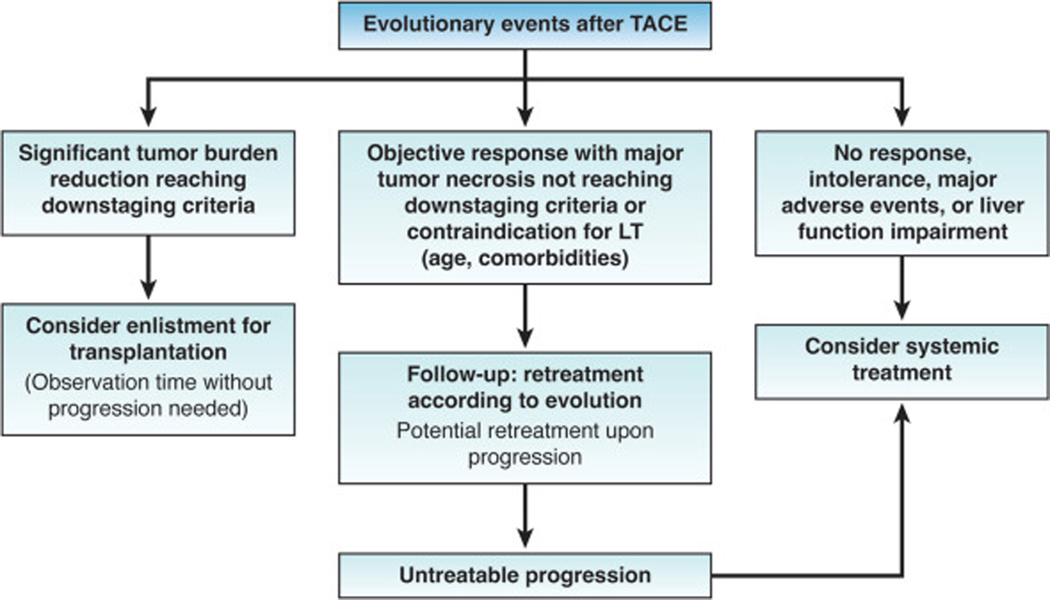
We are currently at the cusp of a paradigm shift in the treatment strategy for intermediate-stage HCC, i.e., liver-localized multifocal HCC beyond the Milan Criteria. Traditionally, AASLD and EASL had both recommended transarterial chemoembolization (TACE) as the only recommended treatment for patients with intermediate-stage HCC52,53, whereas guidelines from the Japan Society of Hepatology included a broader portfolio of treatment options including surgical resection, TACE, hepatic arterial infusion chemotherapy, and systemic therapy54. In recent years, recommendations regarding initial therapy for patients with intermediate stage disease have dramatically changed. Systemic therapy is now recommended for patients with large intrahepatic tumor burden by the latest AASLD guidance55, the European Society for Medical Oncology guideline56, and the updated version of the BCLC system2. The 2022 BCLC Update stratifies the BCLC-B stage into 3 groups of patients according to tumor burden and liver function: 1) the “Extended Liver Transplant” group can be candidates for liver transplantation because of expanded criteria and/or downstaging, 2) those with “well-defined nodules, preserved portal flow and selective access” can be candidates for selective TACE, and 3) patients with diffuse, infiltrative, extensive bilobular liver involvement who are recommended for systemic therapy2. Additionally, there is increasing recognition that post-TACE management can differ by response to therapy, with liver transplantation considered for those with sufficiently robust objective response and systemic therapy considered for those with inadequate response (Figure 2). This development is related to increasing adoption of two important concepts that push the management of these patients beyond a traditional TACE-only approach: “TACE refractory” and “TACE unsuitable”57–59.
Figure 2.
Response-guided treatment strategy after TACE.2 LT, liver transplantation; TACE, transarterial chemoem bolization.
Transarterial chemoembolization
TACE remains the primary recommended strategy with patients with limited tumor burden, without vascular invasion or extrahepatic spread, compensated liver disease, and no cancer-related symptoms44,60. In well selected patients, TACE induces objective responses in >50% of patients and significantly prolongs survival compared to no treatment. With optimal selection criteria, median survival of patient cohorts should be around 30 months61. While the potential benefits of TACE were long recognized and previously overemphasized in the absence of effective systemic therapies, the potential adverse effects of TACE are increasingly recognized in light of effective systemic therapies. The OPTIMIS is a global non-interventional study evaluating 1650 patients with HCC undergoing TACE in clinical practice highlighted that repeated TACE sessions not only had decreasing objective responses but also associated with risks of hepatic injury, manifested as increases in AST, ALT, bilirubin, and INR62. Therefore, repeated TACE is increasingly reserved for patients who tolerated the TACE with minimal adverse events and with evidence of objective tumor response (Figure 2).
TACE Refractory
Considering this risk-to-benefit ratio, the concept of TACE refractoriness was initially proposed in 201163, updated in 2014 64, and has now subsequently been adopted worldwide65–68. Whereas TACE failure was historically defined by stage migration from BCLC stage B to stage C, TACE refractoriness expands treatment failure to include patients without objective response after two TACE sessions. Therefore, these patients should then be considered for subsequent lines of therapy, with the idea to preserve liver function and allow patients to benefit from effective systemic therapy options. Retrospective clinical studies have supported this approach, with longer survival observed in patients who were promptly switched to systemic therapy after meeting TACE refractory criteria compared to those who were continued on repeated TACE69,70.
TACE Unsuitable
Another central concept for the management of patients with intermediate-stage HCC has been the concept of TACE unsuitability, i.e., patients who are not ideal candidates for TACE as initial therapy despite having liver localized disease and falling within the BCLC B stage. Similar to TACE refractory, the concept of TACE unsuitable is based on the risk of treatment-related hepatic dysfunction exceeding the incremental benefit of TACE over systemic therapy. Although there is widespread acceptance of TACE unsuitability as a concept, including by many expert interventional radiologists, there are multiple proposed criteria for TACE unsuitable. An Asia-Pacific Expert Consensus statement defined TACE unsuitable as HCC beyond up-to-seven criteria, ALBI grade 2 liver function, and tumors that are likely to be TACE resistant (e.g., extranodular growth pattern, confluent multinodular HCC, massive HCC, poorly differentiated)58; whereas the BCLC system uses terms such as diffuse, infiltrative, extensive bilobar involvement2. Similarly, patients beyond up-to-seven criteria but who are within downstaging criteria would often be treated with upfront locoregional therapy to maximize chance of objective response.
For TACE unsuitable HCC, recently approved systemic therapy options provide an alternative strategy to induce responses in a large proportion of patients. Objective responses using RECIST criteria can be observed in 30% of patients using Atezolizumab plus Bevacizumab71 and ~20% using Durvalumab plus Tremelimumab72 or using Lenvatinib alone73. Subgroup analyses of clinical trial data74 and available retrospective cohort studies suggest even higher response rates may be observed in patients with intermediate-stage HCC; however, it should be noted that patients with >50% liver involvement were excluded from some clinical trials, including REFLECT 73. These therapies may be particularly beneficial in patients with larger intrahepatic tumor burden, for whom selective TACE is not possible. This concept was supported by a retrospective propensity-score matched analysis, in which upfront TACE versus Lenvatinib was compared in a group of patients with tumors exceeding up-to-seven criteria75. Those patients who received up-front Lenvatinib had significantly better overall survival (median 37.9 vs. 21.3 months; HR 0.48, 95%CI 0.16 – 0.79). In part, this differential survival was believed to be mediated by better preservation of liver function, with ALBI scores significantly degrading in the patients undergoing TACE but not those on lenvatinib. This proof-of-concept is now being evaluated in large phase II and phase III studies, including the ABC Trial (NCT04803994) randomizing patients with multifocal HCC beyond Milan Criteria to TACE or Atezolizumb plus Bevacizumab.
In the retrospective proof-of-concept study by Kudo and colleagues, there were some patients who had objective responses with lenvatinib which facilitated subsequent TACE (in a more selective fashion than initially possible), raising the concept of systemic-locoregional sequential therapy76. This practice is also possible in patients treated with immune checkpoint inhibitor combinations, such as atezolizumab plus bevacizumab, including in patients with positron emission tomography (PET)-positive HCC or those with large tumor burden77. In a multi-center retrospective cohort study including 110 patients with intermediate-stage HCC who were treated with atezolizumab plus bevacizumab, 38 (35%) achieved curative conversion and 24 (22%) achieved cancer-free, drug-free status. Among those who achieved curative conversion, seven underwent resection, 13 underwent radiofrequency ablation, 15 underwent curative TACE, and three received systemic therapy only78. These data highlight the potential for currently available systemic therapy options to achieve a high response rate79 (and “downstaging”, facilitating conversion to locoregional or surgical therapies. Notably, this concept is contrarian to traditional practice patterns in oncology, wherein once systemic therapy is initiated and achieves a response, it is continued as long as the regimen remains effective. However, for HCC, especially for patients with intermediate-stage HCC, extremely effective treatments (e.g., resection or ablation) could be available if objective responses are achieved. There are limited data at this time to guide these decisions and analysis of studies will need to be detailed to avoid flaws31. Thus, it seems reasonable that an option of curative conversion should always be considered, particularly given pathological CR can be rarely achieved by systemic therapy or locoregional therapy alone. Although clearly an extrapolation of data, this practice could also be potentially supported by the XXL Trial30, which demonstrated the benefit of conversion to potentially curative surgical therapy in those who achieved a response to locoregional therapy.
Combination Therapies
In addition to sequential therapies, there has also been interest in combining systemic and locoregional therapies to increase objective responses, PFS, and overall survival. Systemic therapy with tyrosine kinase inhibitors (TKIs) has several theoretical benefits: (i) inducing tumor necrosis, thereby potentially achieving “downstaging”; (ii) reducing hypoxic stress caused by TACE thus aiming to suppress the hypoxia-induced release of cytokines [e.g., vascular endothelial growth factor (VEGF)], that could prime progression and metastasis; and (iii) normalizing tumor vasculature that could enhance the effect of TACE80. These theoretical benefits prompted several trials evaluating the combination of sorafenib and TACE, with all failing to show any benefit in the primary outcomes of time-to-progression (TTP) or progression-free survival (PFS). Recently, the TACTICS trial demonstrated the combination of sorafenib plus TACE improved PFS (HR 0.59, 95% CI, 0.41–0.78) compared to TACE alone81, although this failed to translate into an OS benefit (36.2 vs. 30.8 months, respectively; HR 0.86, 95% CI 0.61–1.22)82. At this time, existing phase II and phase III studies have failed to demonstrate a benefit in proposed primary outcomes. However, there have been questions if overall survival is the optimal primary endpoint of locoregional therapies, particularly considering continued improvements in post-progression therapies, highlighting a need for validated, accurate surrogate measures that can be used in clinical trials83,84. Although a moderate correlation between PFS and overall survival has been suggested in systemic therapy trials, it is unclear if this is true for locoregional therapies as prior trials evaluating combination studies failed to show any correlation (r = 0.56).
With the introduction of immune checkpoint inhibitors in the advanced stage setting, there has been renewed interest in evaluating the combination of systemic and locoregional therapies. It is theorized that TACE may induce release of neoantigens which could then augment responses with systemic therapy, induced synergistic effects by using the two in combination. Accordingly, there are several ongoing phase III studies evaluating TACE in combination with immune checkpoint inhibitors.
Transarterial Radioembolization
Transarterial radioembolization (TARE) is an alternative intra-arterial therapy for early- and intermediate-stage HCC. Early studies with TARE failed to show a benefit compared to systemic therapy for patients with advanced-stage disease85–87; however, recent data have demonstrated efficacy in patients with BCLC stage A or B disease using a radiation segmentectomy approach88,89. In 2021, TARE using Y90 was granted FDA-approval based on results of the LEGACY Study, which reported objective responses in 88.3% of patients with solitary HCC up to 8 cm (median size 2.7 cm)90. The DOSISPHERE-01 trial also demonstrated the benefit of personalized dosimetry when performing TARE with a goal of >205Gy to the targeted area, with significantly improved objective responses and survival compared to standard dosimetry91. The choice between TACE and TARE is often determined by center expertise and availability given limited direct comparative data. A recent meta-analysis of comparative studies found TARE was associated with improved TTP but without significant difference in overall survival92. However, comparison of TTP between the two modalities can be difficult given post-radiation changes complicating interpretation of response.
Systemic therapy
While surgery and locoregional options were present for decades and have undergone progressive refinements and developments to increase effectiveness, the area of systemic therapy for HCC was lacking an effective intervention until 200793. Conventional chemotherapy was applied by default with more hope than evidence and adverse events due to toxicity often precluded any significant benefit. Neither intravenous administration, nor selective intraarterial administration, emulsified in lipiodol or not, were proven to offer survival benefit in large and robust trials with adequate sample size. Finally, a better understanding of the molecular mechanisms responsible for cancer progression and dissemination allowed generation of agents that would selectively act on specific targets driving HCC progression. Tyrosine-kinase inhibitors and antibodies aimed at angiogenesis and proliferation targets were tested in early phase development trials following successes in other neoplasms such as breast, lung, colorectal or renal cell cancer. Delay in these agents entering the field of HCC was related in part to the nihilist view about the likelihood of treatment efficacy versus safety concerns in the presence of underlying liver disease.
Tyrosine kinase inhibitors
Sorafenib was the sole agent that was tested in such manner after an initial phase 2 confirmed its safety94. Although the marginal rate of tumor response initially classified the study as negative, subsequent analysis of the data suggested a delay in tumor progression and improved survival as compared with natural history data. This experience paints a tail of caution for overreliance on non-validated surrogate outcomes83,84,95, i.e., objective responses in this case, as this nearly led to the discard of the first efficacious systemic therapy for advanced HCC. The pivotal phase 3 SHARP Trial had several important aspects that were applied to subsequent trials: 1) acceptance of a placebo-controlled trial with avoidance of any conventional chemotherapy in the control arm, 2) careful patient selection with avoidance of patients with decompensated liver disease, 3) introduction of stratification factors to secure a balance between arms, and 4) its use of overall survival as the primary endpoint given lack of validation for PFS as a valid surrogate outcome in patients with HCC96. SHARP also allowed treatment beyond progression as it was already envisioned that some progression would not have a major impact. Later studies confirmed that pattern of progression is a key aspect in prognosis prediction and trials analysis97. As now known, sorafenib significantly improved survival (median 10.7 vs. 7.9 months, HR 0.69, 95%CI 0.55 – 0.87) with a tolerable safety profile including low risk of liver dysfunction96. Adverse events, including hand-food skin reaction, were shown to suggest drug activity and predict a better outcome98,99.
The success of sorafenib represented a revolution in the field and opened the era where several agents would be tested in the first-line and second-line settings. However, in all instances for the following 10 years, studies turned negative, and the HCC field was classified as a graveyard100–104. We had to wait 10 years to see another agent come to market, with the landscape changing in a positive manner in 2017. Data from positive phase 3 trials in the first- and second-line setting are detailed in Table 3. In the first-line setting, Lenvatinib showed non-inferiority to sorafenib in terms of overall survival but demonstrated significant improvements in secondary outcomes including PFS and objective response rates74. Second-line therapies demonstrated as superior to placebo in phase 3 RCTs included regorafenib105 and cabozantinib106. Although ramucirumab initially failed to demonstrate a benefit in all-comers in the second-line setting, a subsequent RCT (REACH-2) showed a survival benefit in patients with AFP >400ng/mL107.
Table 3.
Outcomes reported in systemic treatment positive phase III trials
| LINE | Study | Systemic Therapy | n | FU m, median (IQR) | ORR RECIST 1.1, %(95CI) | CR RECIST 1.1, n(%) | TTP (m), median (95CI) | PFS (m), median (95CI) | OS (m), median (95CI) |
|---|---|---|---|---|---|---|---|---|---|
| 1L | Llovet JM, et al. NEJM 2008 | Sorafenib | 299 | n=7 (2%) PR | 0 | 5.5 (4.1–6.9) | 10.7 (9.4–13.3) | ||
| Kudo M, et al. Lancet 2018 | Lenvatinib | 478 | 27.7 (23.3–32.8) | n=90 18.8 % (15.3–22.3) | 2 (<1) | 8.9 (7.4–9.2) | 7.4 (6.9–8.8) | 13.6 (12.1–14.9) | |
| Sorafenib | 476 | 27.2 (22.6–31.3) | n=31 6.5 % (4.3–8.7) | 1 (<1) | 3.7 (3.6–5.4) | 3.7 (3.6–4.6) | 12.3 (10.4–13.9) | ||
| Finn RS, et al. NEJM 2020 Cheng, et al. J Hepatol 2022 |
Atezolizumab-bevacizumab | 336 | 17.6 (0.1–28.6) | 30% (22.5–32.5) | 25 (8.0) | - | 6.9 (5.7 – 8.6) | 19.2 (17.0–23.7) | |
| Sorafenib | 165 | 10.4 (0–27.9) | 11 % (7–17) | 1 (<1.0) | - | 4.3 (4.0–5.6) | 13.4 (11.4–16.9) | ||
| Ren Z, et al. Lancet Oncol 2021 | Sintilimab + IBI305 (Bevacizumab biosimilar) | 380 | 10.0 (8.5–11.7) | 21% (17–25) PR | 0 | 5.2 (4.2–5.8) | 4.6 (4.1–5.7) | Not reached | |
| Sorafenib | 191 | 10.0 (8.4–11.7) | 4 % (2–8) PR | 0 | 2.8 (2.7–3.2) | 2.8 (2.7–3.2) | 10.4 (8.5-Not reached) | ||
| Abou-Alfa, et al. NEJM evidence 2022 | Tremelimumab + Durvalumab (STRIDE) | 393 | 33.18 | 20.1% | 12 (3.1) | 5.4 (3.8–5.6) | 3.78 (3.75.3) | 16.43 (14.2–19.6) | |
| Durvalumab | 389 | 32.56 | 17.0% | 6 (1.5) | 3.8 (3.7–5.4) | 3.65 (3.2–3.7) | 16.56 (14.1–19.1) | ||
| Sorafenib | 389 | 32.23 | 5.1% PR | 0 | 5.6 (5.1–5.8) | 4.07 (3.8–5.5) | 13.77 (12.3–16.1) | ||
| Qin S. et al ESMO 2022 | Camrelizumab + Rivoceranib | 272 | 14.5 (for OS) | 25.4 (20.3–31.0) | 3 (1.1) | 7.2 (5.6–8.2) | 5.6 (5.5–6.3) | 22.1 (19.1–27.2) | |
| Sorafenib | 271 | 5.9 (3.4–9.4) | 1 (0.4) | 3.7 (3.6–3.7) | 3.7 (2.8–3.7) | 15.2 (13.0–18.5) | |||
| 2L | Bruix J, et al. Lancet 2017 | Regorafenib | 379 | 7.0 (3.7–12.6) | n=25 (7%) PR | 0 | 3.2 (2.9–4.2)§ | 3.1 (2.8–4.2)§ | 10.6 (3.1–12.1) |
| Abou-Alfa, et al.NEJM 2018 | Cabozantinib* | 470 | n=18 (4%) PR | 0 | - | 5.2 (4.0–5.5) | 10.2 (9.1–12.0) | ||
| X Zhu et al. Lancet Oncol 2019 | Ramucirumab | 197 | 7.6 (4.0–12.5) | n=9 4.6 % (1.7–7.5) PR | 0 | - | 2.8 (2.8–4.1) | 8.5 (7.0–10.6) | |
| Qin S, et al. Journal of Oncology (Presented at ASCO feb 2022) | Pembrolizumab | 300 | 33.8 (18.7–49.0) | 12.7% | Not specified | 2.7 | 2.6 (1.5–2.8) | 14.6 (12.6–18.0) |
Abbreviatons: FU: Follow-up; ORR: Overall response rate; CR: Complete response; PR: Partial response; TTP: Time-to-progression; PFS: Progression-free survival; m: Months; OS: Overall survival; 95CI: 95% Confidence Interval.
by mRECIST.
includes data as 3L option.
Immune Checkpoint Inhibitors
Unexpectedly, single agent immune checkpoint inhibitors failed to improve overall survival in the first-line setting (nivolumab vs sorafenib)102 or second-line setting (pembrolizumab vs. placebo)103 despite durable response rates of ~15–20%. Subsequent RCTs from China have demonstrated non-inferiority of tislelizumab vs. sorafenib in the first-line setting [RATIONALE-301 (NCT03412773) study] and superiority of pembrolizumab vs placebo in the second-line setting108.
The prominent position of targeted therapy was finally lost in 2020 when the combination of Atezolizumab (a checkpoint inhibitor of the programmed cell death receptor) and the antibody bevacizumab (a powerful inhibitor of the vascular endothelial growth factor receptor) was proven superior to sorafenib in the 1st line setting in the IMBRAVE150 phase 3 trial109. VEGF inhibition is not only cytotoxic but also has immunomodulatory effects including increased cytotoxic T lymphocytes and dendritic cells as well as decreased regulators T cells, tumor associated macrophages and myeloid derived suppressor cells110. The combination of Atezolizumab plus Bevacizumab provided a median survival of 19.2 months, compared to 13.4 months for sorafenib, and was well tolerated with minimal grade 3–4 adverse events71. The risks of bleeding due to bevacizumab were minimized by restricting to patients with well-preserved liver function and confirming absence of high-risk varices or other stigmata on an upper endoscopy within 6 months prior to randomization. The improvement in survival was statistically significant and clinically relevant, and the safety of the treatment was evident. Therefore, Atezolizumab-bevacizumab was immediately established as the preferred treatment option in patients considered for systemic therapy2,111,112. Patients with high risk of bleeding, severe vascular disorders, or autoimmune conditions (including post-transplant patients) would not be optimal candidates for the combination, thus leaving some patient subgroups to still be considered for sorafenib or Lenvatinib, although these agents also have some risk of GI bleeding. More success was around the corner with positive data obtained by the combination of Tremelimumab (CTLA-4 inhibitor) and Durvalumab (a PD-L1 inhibitor) in the 1st line vs sorafenib72. CTLA-4 inhibition is an independent mechanism to block negative inhibition enhance priming of T lymphocytes in lymphoid organs113. Interesting, this combination was the first successful treatment that did not act upon the VEGF pathway. It offered a significant survival benefit vs sorafenib, providing a median overall survival of 16.4 months and 3-year survival of 30.7%, compared to 13.8 months and 20.2%, respectively for sorafenib. The HIMALYA Trial also showed non-inferiority of durvalumab as single agent compared to sorafenib72.
While we have not discussed negative trials in depth, it is worth noting recent failure in 1st line of Lenvatinib plus pembrolizumab (LEAP-002114), cabozantinib plus atezolizumab (COSMIC-312)115, and TARE vs. sorafenib in the first-line setting85–87. These trials failed to improve overall survival despite promising signals for high objective responses or PFS, highlighting that these are imperfect surrogates for overall survival. Further, these agents may significantly improve outcomes for a subgroup of patients (i.e., improving the tail of the survival curve), whereas many others do not benefit, providing similar median survival estimates. These negative trials highlight the continued need for surrogate outcomes for overall survival as well as a need for treatment response biomarkers that can help maximize likelihood of objective response and improved survival.
Systemic Therapy Options in Child Pugh B
Although there have been notable advances in systemic therapy options, it is notable that all were evaluated in patients with preserved liver function. There remains a need for safe and effective therapies for patients with Child Pugh B cirrhosis, which comprise a sizable portion of patients seen in clinical practice. There are real world data demonstrating safety of TKIs, primarily sorafenib, as well as data for single-agent immune checkpoint inhibition in select patients with Child Pugh B cirrhosis116. The CheckMate 040 phase II study included a subset of 49 patients with Child Pugh B7 disease who were safely treated with Nivolumab, with no unexpected safety signals. A recent metanalysis shows that the heterogeneity of published data excessive and prevents a valid recommendation117. Although these therapies may be safe, it is important to consider that the competing risk of liver-related mortality likely mitigates any survival benefit of HCC-directed therapy. The competing risk of liver-related mortality varies among patients with Child Pugh B cirrhosis, with higher risk in patients with CTP score 9 compared to those with CTP score 7, so the former patient would likely derive less benefit than the latter patient. Other factors including patient performance status and goals of care should also be taken into account when considering treatment in these patients.
Future Developments
With recent breakthroughs, there has been a breakdown of previously existing treatment silos, with patients transitioning across the BCLC system from left to right and vice versa. As above, we are becoming increasing cognizant of treatment failure or ineligibility, most evident with TACE in the intermediate stage space. We are also observing high response rates with locoregional and systemic treatments, allowing downstaging of some patients to liver transplantation or surgical resection despite being ineligible at first presentation. This complexity highlights the importance of multidisciplinary care when managing HCC patients in the contemporary landscape118,119.
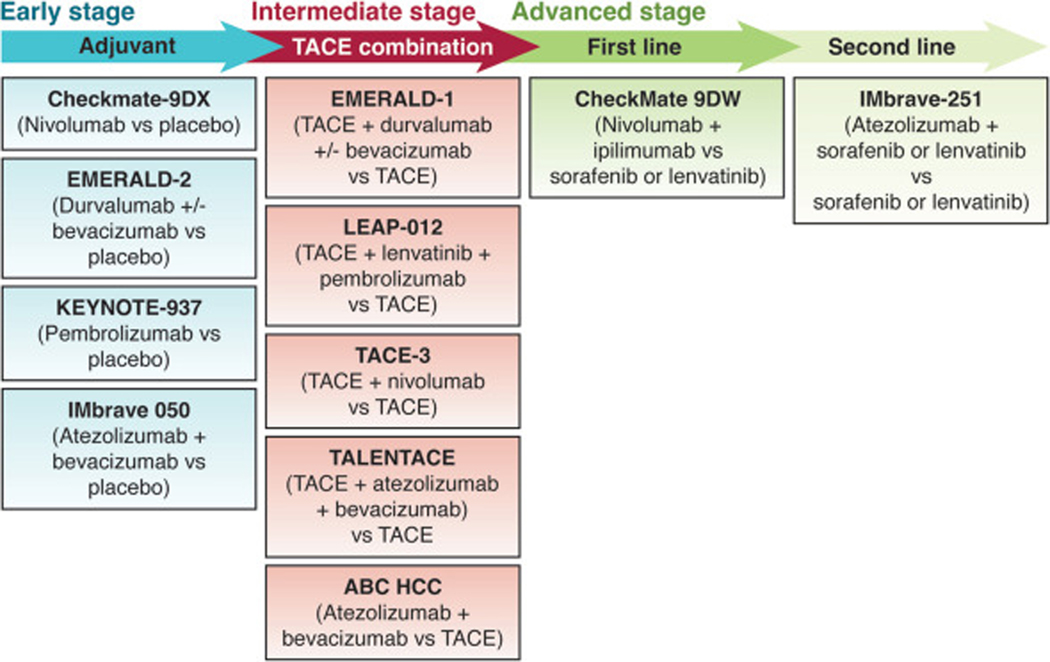
In this vein, combination studies using TKIs with TACE failed to improve PFS or OS, although combinations are now being revisited with immune checkpoint inhibitors. Several phase 3 trials examining combinations of immune checkpoint inhibitors +/− TKIs are currently underway for both early-stage and intermediate-stage HCC (Figure 3). Based on the favorable outcomes in advanced HCC, there is a fair chance that these trials will be successful, which would transform our approach for early- and intermediate-stage HCC.
Figure 3.
Ongoing phase III trials. ABC, Atezolizumab plus Bevacizumab versus transarterial Chemoembolization; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; TALENTACE, Tecentriq plus Avastin Liver Envision Tomorrow-TACE (A phase III, open-label, randomized study of on-demand TACE combined with atezolizumab plus bevacizumab [atezo/bev] or on-demand TACE alone in patients with untreated hepatocellular cacinoma).
Despite these breakthroughs, there continues to be work that needs to be performed (Table 4). First, there remains a stark difference in survival between patients with early-stage HCC and those with larger tumor burden, highlighting a need for efforts to promote surveillance and early tumor detection. In parallel with continued research evaluating novel therapeutics, we must continue to promote surveillance effort to maximize the proportion of patients detected at an early stage120,121. Second, as discussed above, most therapies were evaluated in patients with preserved liver function and there remains a need for therapies that are safe and ideally prolong survival in patients with Child Pugh B cirrhosis116,117. Third, despite major advancements in the stratification of patients according to molecular profile, we still lack the data to link a specific profile with a clinical decision about the best treatment option to propose or the treatment that should be avoided1,122. There is a strong need for treatment response biomarkers to better select patients123. In parallel, the potential role of clinical factors including underlying liver disease etiology as potential moderators of treatment response should be properly investigated124–126. This critical knowledge gap remains an area of immense need, leaving room for continued breakthroughs over the next couple decades. Ongoing trials using novel designs such as umbrella trials not only have the potential to identify new treatment combinations but also to potentially identify treatment response biomarkers that could be used to tailor therapy decisions.
Table 4.
Unmet needs in the field of Hepatocellular Carcinoma
|
• Validation of blood-based biomarkers for HCC surveillance and diagnosis |
|
• Validation of biomarkers and molecular profiles to predict prognosis and treatment response |
|
• Increase the application of surgery through liver function preservation/improvement |
|
• Increase the access to liver transplantation |
|
• Develop tools to predict treatment failure prior to clinic-radiologic progression |
|
• Effective adjuvant therapy and co-interventions to improve recurrence-free and progression-free survival for early- and intermediate stage HCC, respectively |
|
• Increased efficacy of locoregional and systemic treatments to achieve complete response |
|
• Efficacious and safe treatments for patients with Child Pugh B cirrhosis |
|
• Further data to support inclusion of external beam radiation therapy into guidelines |
|
• Valid surrogate endpoints of survival that can be used for clinical trials |
Summary
As exposed, the field of HCC has experienced a major improvement in management, and we have witnessed the dawn of a new area in all areas of treatment. Earlier diagnosis, better tumor staging, and improved evaluation of liver function have primed a better selection of patients to be proposed for any intervention, as well as development of novel approaches. This has been especially intense in the field of systemic therapy that has evolved from an absence of effective options to a plethora of agents that improve survival. Further improvements will take place with novel agents or combinations, but to bring HCC to precision oncology, there is need to further investigate the oncogenic mechanisms that govern tumor progression and dissemination in order to ultimately, treat patients according to such mechanisms and abandon the era of one size fits all.
Financial Source:
Dr. Singal research is supported in part by NIH R01 MD012565 and R01 CA256977. Dr. Kudo research is supported in part by Japan Agency for Medical Research and Development (AMED) 21444735. Dr. Bruix research is supported by grant from Instituto de Salud Carlos III (PI18/00768) and from CIBEREHD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Conflict of Interest:
Amit Singal has served as a consultant or on advisory boards for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, Freenome, and GRAIL.
Masatoshi Kudo has served as a consultant or on advisory boards for Eli Lilly, Bayer, Eisai, Chugai, Roche, AstraZeneca, Takeda, MSD, Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie and GE Healthcare.
Jordi Bruix has served in advisory boards for Arqule, Bayer-Shering Pharma, Novartis, BMS, BTG-Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance, Roche, AbbVie, MSD, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly, Basilea, Nerviano, Sanofi, Taiho; and received research/educational grants from Bayer, and lecture fees from Bayer-Shering Pharma, BTG-Biocompatibles, Eisai, Terumo, Sirtex, Ipsen;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345–1362. [DOI] [PubMed] [Google Scholar]
- 2.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citterio D, Facciorusso A, Sposito C, et al. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg 2016;151:846–853. [DOI] [PubMed] [Google Scholar]
- 4.Tsilimigras DI, Bagante F, Moris D, et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: A multi-institutional analysis of 1,010 patients. Surgery 2019;166:967–974. [DOI] [PubMed] [Google Scholar]
- 5.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526–536. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - Renewing consensus in portal hypertension. J Hepatol December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciria R, Gomez-Luque I, Ocaña S, et al. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol 2019;26:252–263. [DOI] [PubMed] [Google Scholar]
- 9.Witowski J, Rubinkiewicz M, Mizera M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc 2019;33:1491–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology 2018;68:977–993. [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Guo WX, Chen MS, et al. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine 2016;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938–943. [DOI] [PubMed] [Google Scholar]
- 13.Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284–1293. [DOI] [PubMed] [Google Scholar]
- 14.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947–955. [DOI] [PubMed] [Google Scholar]
- 15.Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg 2014;38:2910–2918. [DOI] [PubMed] [Google Scholar]
- 16.Sapena V, Enea M, Torres F, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut 2022;71:593–604. [DOI] [PubMed] [Google Scholar]
- 17.Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology 2019;157:1253–1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019;156:1683–1692.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344–1354. [DOI] [PubMed] [Google Scholar]
- 20.Kaseb AO, Hasanov E, Cao HST, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho WJ, Zhu Q, Durham J, et al. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat Cancer 2021;2:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–700. [DOI] [PubMed] [Google Scholar]
- 23.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587–2596. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Sapisochin G, Goldaracena N, Laurence JM, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology 2016;64:2077–2088. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Pavel M, Rimola J, et al. Pilot study of living donor liver transplantation for patients with hepatocellular carcinoma exceeding Milan Criteria (Barcelona Clinic Liver Cancer extended criteria). Liver Transpl 2018;24:369–379. [DOI] [PubMed] [Google Scholar]
- 27.Mehta N, Frenette C, Tabrizian P, et al. Downstaging Outcomes for Hepatocellular Carcinoma: Results From the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology 2021;161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kardashian A, Florman SS, Haydel B, et al. Liver Transplantation Outcomes in a U.S. Multicenter Cohort of 789 Patients With Hepatocellular Carcinoma Presenting Beyond Milan Criteria. Hepatology 2020;72:2014–2028. [DOI] [PubMed] [Google Scholar]
- 29.Mehta N, Dodge JL, Grab JD, et al. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020;71:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020;21:947–956. [DOI] [PubMed] [Google Scholar]
- 31.Tran NH, Muñoz S, Thompson S, et al. Hepatocellular carcinoma downstaging for liver transplantation in the era of systemic combined therapy with anti-VEGF/TKI and immunotherapy. Hepatology 2022;76:1203–1218. [DOI] [PubMed] [Google Scholar]
- 32.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128–139. [DOI] [PubMed] [Google Scholar]
- 33.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143. [DOI] [PubMed] [Google Scholar]
- 34.Halazun KJ, Rosenblatt RE, Mehta N, et al. Dynamic α-Fetoprotein Response and Outcomes After Liver Transplant for Hepatocellular Carcinoma. JAMA Surg 2021;156:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N, Dodge JL, Roberts JP, et al. Alpha-Fetoprotein Decrease from > 1,000 to < 500 ng/mL in Patients with Hepatocellular Carcinoma Leads to Improved Posttransplant Outcomes. Hepatology 2019;69:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018;67:381–400. [DOI] [PubMed] [Google Scholar]
- 37.Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients From the US Multicenter HCC Transplant Consortium. Ann Surg 2017;266:525–535. [DOI] [PubMed] [Google Scholar]
- 38.Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant 2021;21:1979–1980. [DOI] [PubMed] [Google Scholar]
- 39.Navasa M, Bruix J. Multifaceted perspective of the waiting list for liver transplantation: The value of pharmacokinetic models. Hepatology 2010;51:12–15. [DOI] [PubMed] [Google Scholar]
- 40.Ivanics T, Wallace D, Claasen MPAW, et al. Low utilization of adult-to-adult LDLT in Western countries despite excellent outcomes: international multicenter analysis of the US, UK, and Canada. J Hepatol September 2022. [DOI] [PubMed] [Google Scholar]
- 41.Goldaracena N, Gorgen A, Doyle A, et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol 2019;70:666–673. [DOI] [PubMed] [Google Scholar]
- 42.Takayama T, Hasegawa K, Izumi N, et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2021;11:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol 2018;68:783–797. [DOI] [PubMed] [Google Scholar]
- 44.Kloeckner R, Galle PR, Bruix J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology 2021;73 Suppl 1:137–149. [DOI] [PubMed] [Google Scholar]
- 45.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122–130. [DOI] [PubMed] [Google Scholar]
- 47.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235–240. [DOI] [PubMed] [Google Scholar]
- 48.Apisarnthanarax S, Barry A, Cao M, et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2022;12:28–51. [DOI] [PubMed] [Google Scholar]
- 49.Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol 2018;36:600–608. [DOI] [PubMed] [Google Scholar]
- 50.Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92–99. [DOI] [PubMed] [Google Scholar]
- 51.Parikh ND, Marshall VD, Green M, et al. Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma: An analysis of SEER-medicare. J Med Imaging Radiat Oncol 2018;62:673–681. [DOI] [PubMed] [Google Scholar]
- 52.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 53.Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 54.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109–1113. [DOI] [PubMed] [Google Scholar]
- 55.Singal AG, Llovet JM, Yarchoan M, et al. AASLD Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. . Hepatology (In press) 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogel A, Martinelli E, Cervantes A, et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801–805. [DOI] [PubMed] [Google Scholar]
- 57.Bruix J, Reig M, Rimola J, et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology 2011;54:2238–2244. [DOI] [PubMed] [Google Scholar]
- 58.Kudo M, Han KH, Ye SL, et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020;9:245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kudo M, Kawamura Y, Hasegawa K, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Baere T, Ronot M, Chung JW, et al. Initiative on Superselective Conventional Transarterial Chemoembolization Results (INSPIRE). Cardiovasc Intervent Radiol 2022;45:1430–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012;56:1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peck-Radosavljevic Markus, Lee Han Chu, Kudo Masatoshi, et al. Practice patterns and outcomes of transarterial chemoembolization in patients with hepatocellular carcinoma who were either ineligible or eligible for transarterial chemoembolization at inclusion: Global OPTIMIS exploratory analysis. J Hepatol 2019;70:FRI-494. [Google Scholar]
- 63.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339–364. [DOI] [PubMed] [Google Scholar]
- 64.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87 Suppl 1:22–31. [DOI] [PubMed] [Google Scholar]
- 65.Lu SN, Wang JH, Su CW, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc 2018;117:381–403. [DOI] [PubMed] [Google Scholar]
- 66.Cheng AL, Amarapurkar D, Chao Y, et al. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: Consensus recommendations and review by an International Expert Panel. Liver Int 2014;34:174–183. [DOI] [PubMed] [Google Scholar]
- 67.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238–iv255. [DOI] [PubMed] [Google Scholar]
- 68.Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer 2014;3:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014;87:330–341. [DOI] [PubMed] [Google Scholar]
- 70.Arizumi T, Ueshima K, Chishina H, et al. Validation of the criteria of transcatheter arterial chemoembolization failure or refractoriness in patients with advanced hepatocellular carcinoma proposed by the LCSGJ. Oncology 2014;87 Suppl 1:32–36. [DOI] [PubMed] [Google Scholar]
- 71.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862–873. [DOI] [PubMed] [Google Scholar]
- 72.Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence 2022;1. [DOI] [PubMed] [Google Scholar]
- 73.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 74.Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer 2019;8:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kudo M. Atezolizumab plus Bevacizumab Followed by Curative Conversion (ABC Conversion) in Patients with Unresectable, TACE-Unsuitable Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kudo M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer 2021;10:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kudo M, Finn RS, Galle PR, et al. IMbrave150: Efficacy and safety of atezolizumab plus bevacizumab vs sorafenib in patients with Barcelona Clinic Liver Cancer stage B unresectable hepatocellular carcinoma—An exploratory analysis of the phase III study. Liver Cancer (In press) 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58–62. [DOI] [PubMed] [Google Scholar]
- 81.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kudo M, Ueshima K, Ikeda M, et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022;11:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruix J. Endpoints in clinical trials for liver cancer and their value in evidence-based clinical decision making: An unresolved Gordian knot. J Hepatol 2021;74:1483–1488. [DOI] [PubMed] [Google Scholar]
- 84.Cabibbo G, Bruix J. Radiological endpoints as surrogates for survival benefit in Hepatocellular Carcinoma trials: all that glitters is not gold. J Hepatol October 2022. [DOI] [PubMed] [Google Scholar]
- 85.Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164–1174. [DOI] [PubMed] [Google Scholar]
- 86.Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018;36:1913–1921. [DOI] [PubMed] [Google Scholar]
- 87.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624–1636. [DOI] [PubMed] [Google Scholar]
- 88.Dhondt E, Lambert B, Hermie L, et al. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022;303:699–710. [DOI] [PubMed] [Google Scholar]
- 89.Kim E, Sher A, Abboud G, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol 2022;7:843–850. [DOI] [PubMed] [Google Scholar]
- 90.Salem R, Johnson GE, Kim E, et al. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021;74:2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17–29. [DOI] [PubMed] [Google Scholar]
- 92.Brown AM, Kassab I, Massani M, et al. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 94.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293–4300. [DOI] [PubMed] [Google Scholar]
- 95.Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2019;16:617–630. [DOI] [PubMed] [Google Scholar]
- 96.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 97.Reig M, Rimola J, Torres F, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: Rationale for second-line trial design. Hepatology 2013;58:2023–2031. [DOI] [PubMed] [Google Scholar]
- 98.Díaz-González Á, Sanduzzi-Zamparelli M, Sapena V, et al. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther 2019;49:482–491. [DOI] [PubMed] [Google Scholar]
- 99.Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol 2014;61:318–324. [DOI] [PubMed] [Google Scholar]
- 100.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509–3516. [DOI] [PubMed] [Google Scholar]
- 101.Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57–67. [DOI] [PubMed] [Google Scholar]
- 102.Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77–90. [DOI] [PubMed] [Google Scholar]
- 103.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 104.Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;19:682–693. [DOI] [PubMed] [Google Scholar]
- 105.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 106.Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282–296. [DOI] [PubMed] [Google Scholar]
- 108.Qin S, Chen Z, Fang W, et al. Pembrolizumab Versus Placebo as Second-Line Therapy in Patients From Asia With Advanced Hepatocellular Carcinoma: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol December 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New England Journal of Medicine 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 110.Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nature Reviews Gastroenterology & Hepatology 2021 18:8 2021;18:525–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vogel A, Martinelli E, Cervantes A, et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Annals of Oncology 2021;32:801–805. [DOI] [PubMed] [Google Scholar]
- 112.Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960–974. [DOI] [PubMed] [Google Scholar]
- 113.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 2020;20:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finn RS, Kudo M, Merle P, et al. Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annals of Oncology 2022;808–869:33-(suppl_7). [Google Scholar]
- 115.Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995–1008. [DOI] [PubMed] [Google Scholar]
- 116.Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol 2021;75:600–609. [DOI] [PubMed] [Google Scholar]
- 117.el Hajra I, Sanduzzi-Zamparelli M, Sapena V. Outcome of patients with hepatocellular carcinoma and liver dysfunction under immunotherapy: A Systematic review and meta-analysis. . Hepatology (In press) 2022. [DOI] [PubMed] [Google Scholar]
- 118.Byrd K, Alqahtani S, Yopp AC, et al. Role of Multidisciplinary Care in the Management of Hepatocellular Carcinoma. Semin Liver Dis 2021;41:1–8. [DOI] [PubMed] [Google Scholar]
- 119.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;77:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singal AG, Lok AS, Feng Z, et al. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin Gastroenterol Hepatol 2022;20:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paradis V, Zucman-Rossi J. Pathogenesis of primary liver carcinomas. J Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 123.Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021;160:2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heinrich B, Brown ZJ, Diggs LP, et al. Steatohepatitis Impairs T-cell-Directed Immunotherapies Against Liver Tumors in Mice. Gastroenterology 2021;160:331–345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021;592:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pinter M, Pinato DJ, Ramadori P, et al. NASH and hepatocellular carcinoma: immunology and immunotherapy. Clin Cancer Res October 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]