Abstract
Background:
Despite the growing concern that people with HIV (PWH) will experience a disproportionate burden of dementia as they age, very few studies have examined the sex-specific prevalence of dementia, including Alzheimer’s disease and related dementias (AD/ADRD) among older PWH versus people without HIV (PWOH) using large national samples.
Methods:
We constructed successive cross-sectional cohorts including all PWH aged 65+ years from U.S. Medicare enrollees and PWOH in a 5% national sample of Medicare data from 2007 to 2019. All AD/ADRD cases were identified by ICD-9-CM/ICD-10-CM diagnosis codes. Prevalence of AD/ADRD was calculated for each calendar year by sex-age strata. Generalized estimating equations were used to assess factors associated with dementia and calculate the adjusted prevalence.
Results:
PWH had a higher prevalence of AD/ADRD which increased over time compared with PWOH, especially among female beneficiaries and with increasing age. For example, among those aged 80+ years, the prevalence increased from 2007 to 2019 (females with HIV: 31.4% to 44.1%; females without HIV: 27.4% to 29.9%; males with HIV: 26.2% to 33.3%; males without HIV: 21.0% to 23.5%). After adjustment for demographics and comorbidities, the differences in dementia burden by HIV status remained, especially among older age groups.
Conclusions:
Older Medicare enrollees with HIV had an increased dementia burden over time compared to those without HIV, especially females and older subjects. This underscores the need to develop tailored clinical practice guidelines that facilitate the integration of dementia and comorbidity screening, evaluation, and management into the routine primary care of aging PWH.
Keywords: HIV, dementia, prevalence, aging, sex differences
Introduction
Safe and effective combination antiretroviral therapy (ART) has prolonged life expectancy in people with HIV (PWH).1 PWH 50+ years comprise more than half of all adults with HIV in the U.S., with those 65+ years showing the greatest growth.2 Cognitive decline is more common in PWH than in people without HIV (PWOH). Studies have found that 20 to 50% of PWH have at least mild neurocognitive impairment, even when they are taking suppressive ART.3–8
Dementia is a major neurocognitive disorder that manifests as impairment in thinking, executive function, learning, social cognition, memory, language, and other cognitive functions severe enough to interfere with daily activities.9 Aging is the primary risk factor for dementia in the general population. The prevalence of Alzheimer’s disease (AD) and AD-related dementias (ADRD) doubles for every 5-year interval beyond age 65.10 Many aging-related comorbidities contributing to cognitive decline—such as cardiovascular disease, hypertension, diabetes mellitus, stroke, and depression—are more prevalent in PWH.11–14 Other risk factors, including substance use, low education, social isolation, and sedentary lifestyle, are also more common in PWH.15 In addition, PWH are exposed to HIV-related biomechanisms of neurological change, including a persistent state of low-grade inflammation,16,17 cumulative exposure to ART,18–21 and increased aging-related comorbidities,11–14 which may augment the progression of dementia. These factors increase the concern that PWH will experience a disproportionate burden of dementia as they age.
Very few studies have examined trends in the prevalence of dementia among older PWH compared to PWOH using large national samples. One recent study examined 13,296 older PWH on ART and demographically similar PWOH who received care at Kaiser Permanente (KP) healthcare systems between 2000 and 2016. Although both groups experienced increases in dementia prevalence, the overall prevalence of dementia was higher among PWH.22 The model adjusted for a selected set of comorbidities focusing on cardiovascular disease risk factors and depression. No sex-stratified data were provided.
Compared to men, AD/ADRD disproportionately affects older women23,24 and cognitive decline is also faster among females.25 The biological underlying mechanisms, mixed pathology, clinical presentation, and risk factors for dementia all differ by sex.24,26–28 Factors related to female reproductive history influence AD/ADRD risk and the disease experience.29,30 Considering and accounting for sex differences in AD/ADRD research is critical to both clinical practice and research.
The objective of this study was to evaluate the trend of AD/ADRD prevalence by sex in older U.S. Medicare enrollees with and without HIV, and further to examine the factors associated with AD/ADRD prevalence and the effect of HIV on time trends and sex differences. We hypothesize that the prevalence of AD/ADRD is higher and increasing among PWH, especially females, after adjusting for demographics and comorbidities. Understanding the trends and associated factors of dementia in this population will help guide the screening and management of dementia among older PWH and inform policymakers regarding the optimal distribution of healthcare resources.
Methods
Data source
We used Medicare data from 100% of beneficiaries with an HIV diagnosis and a 5% national sample of Medicare enrolees without an HIV diagnosis anytime in 2007–2019 from all 50 US states and the District of Columbia (DC) through access to the Centers for Medicare and Medicaid Services (CMS) Virtual Research Data Center. Data was integrated from the Master Beneficiary Summary Files (MBSF). The MBSF contains demographic and enrolment information about beneficiaries enrolled in Medicare during a calendar year, such as date of birth, sex, race/ethnicity, date of death, state, Medicaid Dual Eligibility, original entitlement for Medicare, and monthly coverage. The Chronic Conditions Data Warehouse (CCW) includes indicators of 62 chronic conditions, such as first ever occurrence date and flags of conditions in the calendar year based on algorithms requiring a multiple year look-back period. This study was approved by the University of Texas Medical Branch at Galveston Institutional Review Board (IRB # 20–0275). A Data Use Agreement was established with the CMS prior to all data analysis.
Cohort identification and study measures
We constructed successive cross-sectional cohorts for PWH and PWOH for each calendar year, including individuals ≥ 65 in the calendar year and with three years of fee-for-service (FFS) coverage (Medicare parts A and B with no Health Maintenance Organization enrollment). We created 8 strata based on age at the end of the calendar year (65–69, 70–74, 75–79, 80+ years) and sex (male, female) (eTable 1, Supplemental Digital Content for flow chart of cohort construction).
All diseases/conditions—such as HIV infection, AD/ADRD events, and comorbidities—were identified using physician diagnosis by International Classification of Diseases Ninth and Tenth Revision, Clinical Modification (ICD-9-CM/ICD-10-CM) codes based on CCW criteria.31 CCW uses combinations of inpatient claims and other non-drug claims of any service type during a reference period: for example, 2 years for HIV, 3 years for AD/ADRD, and 1 year for depression. Those who met the claims criteria were defined as having the condition. The primary outcome for this study was the occurrence of a physician clinical diagnosis of AD/ADRD including AD, vascular, frontotemporal, and unspecified dementia (eTable 2, Supplemental Digital Content for CCW AD/ADRD diagnosis codes and algorithm specification). Both earlier and recent validation studies using the Aging Demographics and Memory Study clinical assessments within the broader Health and Retirement Study have demonstrated that, with 3 years of data from the physician supplier and hospital outpatient claims files, investigators were able to correctly identify ~87% of patients with dementia using ICD-9-CM dementia codes32,33 and that ICD-10-CM codes for dementia diagnosis showed good discrimination, with an area under the curve of 0.86.34 We classified individual comorbidities relevant to dementia risk including psychiatric disorders, alcohol use disorders, tobacco use disorders, drug/opioid abuse disorder, hypertension, diabetes, cardiovascular disease, viral hepatitis, other liver conditions, stroke/transient ischemic attack [TIA], head injury, fibromyalgia/chronic pain/fatigue, obesity, visual impairment, hearing impairment, other (eTable 3, Supplemental Digital Content for classification of comorbidities).35,36 The number of other relevant comorbidities was categorized (0, 1, 2, 3, 4, 5+).
Other variables of interest included race (White, Black, Hispanic, Other/Unknown), original entitlement for Medicare enrollment (disabled, older age), and the time-varying covariates of Medicare-Medicaid dual eligibility status (Yes, No) and U.S. Census region (Northeast, South, Midwest, West). Observations with unknown region were excluded in multivariable analysis.
Statistical Analysis
All analyses were performed by stratifying age at each calendar year and sex (total of 8 strata). We first calculated the crude prevalence of AD/ADRD for each calendar year by HIV status. All individuals who reached 65 years in the calendar year and had at least 3 years of FFS coverage were included in the denominator. Those who met claims criteria for AD/ADRD and had sufficient FFS coverage were included in the numerator. Therefore, one individual might contribute multiple times over years across the age categories. We then examined the factors associated with the odds of AD/ADRD and whether the time trend differed between PWH and PWOH using generalized estimating equation (GEE) models with binomial distribution, logit link function, and an AR(1) working correlation structure. The GEE models included HIV diagnosis, calendar year as a categorical variable, and HIV-year interaction, adjusted for continuous age and other demographic variables and comorbidity classes. Due to significant HIV-year interactions, we present results further stratifying HIV status within the age-sex stratum. We estimated the adjusted prevalence of AD/ADRD by year and report the association between covariates and outcome using odds ratios (OR) and their 95% confidence intervals (CI). We further explored the interactions between HIV status and each demographic and comorbidity variable. The p-values were adjusted using the false discover rate (FDR). All tests were two-sided with a significance level of 0.05. All analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC).
Results
Demographics
We identified 87,216 PWH and 2,289,831 PWOH contributing at least a one-year assessment of AD/ADRD, with average person-years of 4.7 and 6.4, respectively (eTable 1, Supplemental Digital Content for flow chart of cohort construction). In the overall sample, 55.6% of beneficiaries were female. The mean age at first entering the cohort was 73.3 (standard deviation [SD] 8.1) years. More PWH were male compared with PWOH (65.7% vs. 43.6%). PWH were younger on average than PWOH, especially among males. The mean age (SD) was 70.0 (5.7), 72.8 (7.7), 72.6 (6.7), and 74.0 (7.8) years for males with HIV, females with HIV, males without HIV, and females without HIV, respectively. There were more Black beneficiaries among male and female PWH than among PWOH (30.7%, 37.1%, 7.6%, and 8.4%, respectively).
We observed a growing aging population among PWH, especially among males, which doubled over the span of 13 years, while the size of the PWOH cohort was relatively stable from 2007 to 2019 (Table 1). Demographic characteristics were similar between 2009 and 2019 for PWOH (Table 1). Among females with HIV, the proportion of Black beneficiaries grew (23.4% vs. 38.8%) and that of Hispanic beneficiaries shrank (10.8% vs. 5.3%) from 2007 to 2019. There was also a large increase in the proportion of PWH with disabled/end-stage renal disease (ESRD) as the original Medicare entitlement in both males (29.6% vs. 44.9%) and females (21.4% vs. 40.6%). The increase in the number of comorbidities over time was more pronounced in PWH and females, especially for psychiatric disorders, tobacco/drug/opioid use disorders, viral hepatitis/liver diseases, pain/fatigue, and obesity (Figure 1).
Table 1.
Demographic shift over time among PWH and PWOH by sex
| PWOH | PWH | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||||||
| 2007 | 2019 | 2007 | 2019 | 2007 | 2019 | 2007 | 2019 | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total | 471,634 | 100 | 505,493 | 100 | 671,432 | 100 | 665,466 | 100 | 12,636 | 100 | 29,481 | 100 | 9,457 | 100 | 13,273 | 100 |
| Age Group (years) | ||||||||||||||||
| 65–69 | 73,570 | 15.6 | 83,991 | 16.6 | 83,389 | 12.4 | 100,017 | 15.0 | 3,881 | 30.7 | 11,013 | 37.4 | 1,881 | 19.9 | 3,623 | 27.3 |
| 70–74 | 134,112 | 28.4 | 162,135 | 32.1 | 159,860 | 23.8 | 197,231 | 29.6 | 4,020 | 31.8 | 9,445 | 32.0 | 2,633 | 27.8 | 3,302 | 24.9 |
| 75–79 | 111,482 | 23.6 | 113,574 | 22.5 | 148,283 | 22.1 | 139,249 | 20.9 | 2,553 | 20.2 | 4,833 | 16.4 | 2,130 | 22.5 | 2,297 | 17.3 |
| 80+ | 152,470 | 32.3 | 145,793 | 28.8 | 279,900 | 41.7 | 228,969 | 34.4 | 2,182 | 17.3 | 4,190 | 14.2 | 2,813 | 29.8 | 4,051 | 30.5 |
| Race/Ethnicity | ||||||||||||||||
| White | 419,354 | 88.9 | 432,275 | 85.5 | 591,166 | 88.1 | 573,359 | 86.2 | 8,526 | 67.5 | 18,902 | 64.1 | 5,989 | 63.3 | 6,886 | 51.9 |
| Black | 30,756 | 6.5 | 32,619 | 6.5 | 50,942 | 7.6 | 47,414 | 7.1 | 3,039 | 24.1 | 8,012 | 27.2 | 2,208 | 23.4 | 5,145 | 38.8 |
| Hispanic | 7,274 | 1.5 | 6,961 | 1.4 | 10,150 | 1.5 | 9,412 | 1.4 | 758 | 6.0 | 1,062 | 3.6 | 1,022 | 10.8 | 701 | 5.3 |
| Other/Unknown | 14,250 | 3.0 | 33,638 | 6.7 | 19,174 | 2.9 | 35,281 | 5.3 | 313 | 2.5 | 1,505 | 5.1 | 238 | 2.5 | 541 | 4.1 |
| Current Medicare & Medicaid dual coverage | ||||||||||||||||
| No | 420,014 | 89.1 | 457,598 | 90.5 | 544,473 | 81.1 | 573,432 | 86.2 | 8,230 | 65.1 | 18,833 | 63.9 | 5,309 | 56.1 | 6,749 | 50.9 |
| Yes | 51,620 | 10.9 | 47,895 | 9.5 | 126,959 | 18.9 | 92,034 | 13.8 | 4,406 | 34.9 | 10,648 | 36.1 | 4,148 | 43.9 | 6,524 | 49.2 |
| Current US Census Region | ||||||||||||||||
| Unknown | 2,969 | 0.6 | 2,391 | 0.5 | 3,420 | 0.5 | 2,524 | 0.4 | 93 | 0.7 | 150 | 0.5 | 56 | 0.6 | 91 | 0.7 |
| Northeast | 118,631 | 25.2 | 111,707 | 22.1 | 171,961 | 25.6 | 148,038 | 22.3 | 1,798 | 14.2 | 3,774 | 12.8 | 1,386 | 14.7 | 1,858 | 14.0 |
| South | 182,450 | 38.7 | 199,938 | 39.6 | 258,818 | 38.6 | 265,057 | 39.8 | 5,702 | 45.1 | 11,967 | 40.6 | 5,022 | 53.1 | 6,242 | 47.0 |
| Midwest | 87,215 | 18.5 | 87,436 | 17.3 | 133,283 | 19.9 | 121,310 | 18.2 | 2,740 | 21.7 | 7,003 | 23.8 | 1,819 | 19.2 | 3,241 | 24.4 |
| West | 80,369 | 17.0 | 104,021 | 20.6 | 103,950 | 15.5 | 128,537 | 19.3 | 2,303 | 18.2 | 6,587 | 22.3 | 1,174 | 12.4 | 1,841 | 13.9 |
| Original Medicare eligibility | ||||||||||||||||
| Disabled/ESRD | 54,164 | 11.5 | 65,495 | 13.0 | 51,190 | 7.6 | 65,463 | 9.8 | 3,743 | 29.6 | 13,236 | 44.9 | 2,020 | 21.4 | 5,382 | 40.6 |
| Old Age | 417,470 | 88.5 | 439,998 | 87.0 | 620,242 | 92.4 | 600,003 | 90.2 | 8,893 | 70.4 | 16,245 | 55.1 | 7,437 | 78.6 | 7,891 | 59.5 |
Abbreviations: PWH, People with HIV; PWOH, people without HIV; ESRD: end-stage renal disease.
Figure 1.
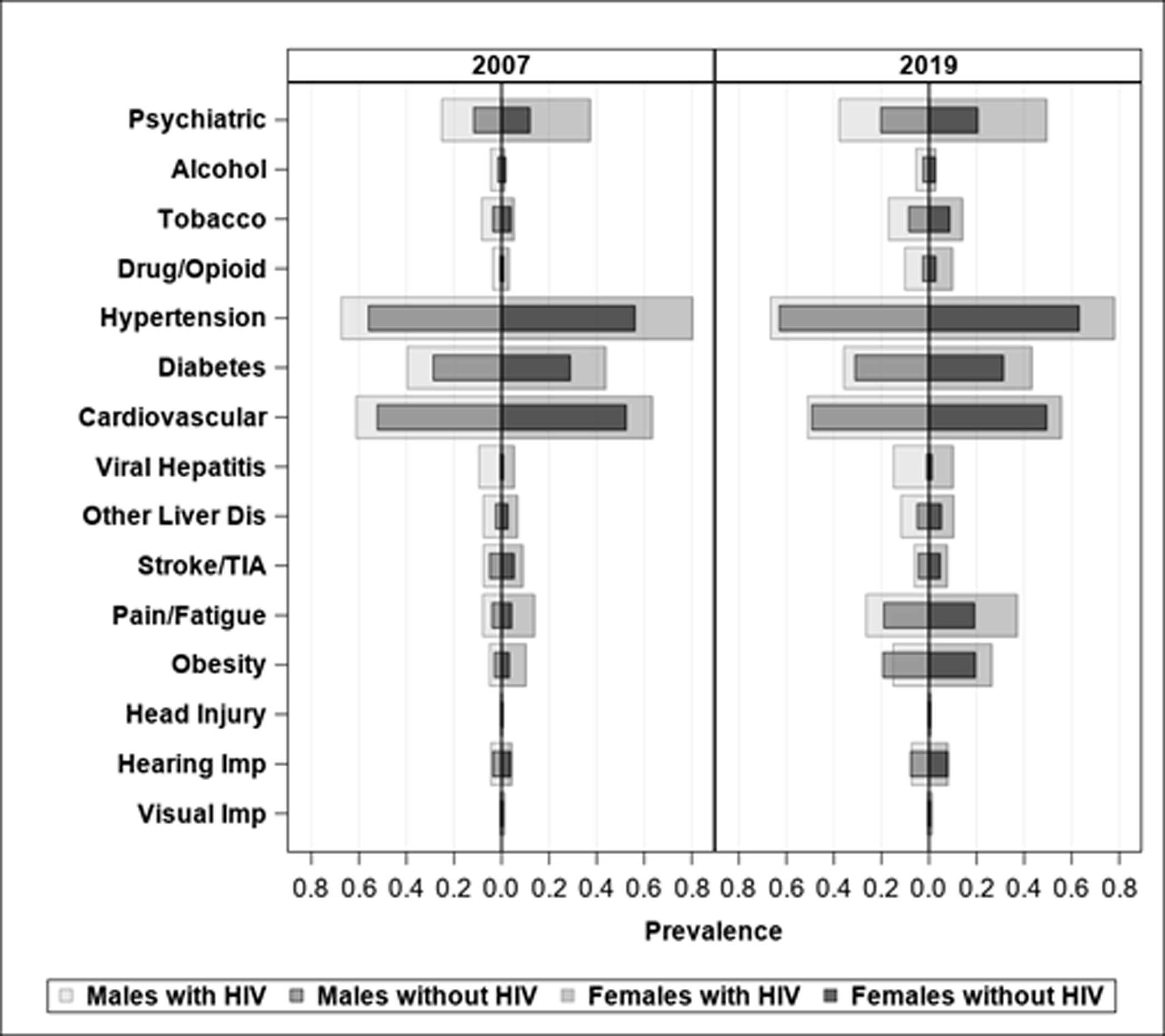
Increased crude prevalence of comorbidity over time among PWH and PWOH by sex
Abbreviations: PWH, People with HIV; PWOH, people without HIV; TIA, transient ischemic attack.
Crude prevalence of Alzheimer’s disease (AD) and AD-related dementias
We identified 26,887 and 608,141 AD/ADRD-prevalent cases in all years among PWH and PWOH, respectively. Both male and female PWH had a significantly higher overall prevalence of AD/ADRD across all years (males with HIV: 26.4%, 95% CI 26.0–26.7%; females with HIV: 39.4%, 95% CI 38.9–40.0%) compared to PWOH (males: 22.9%, 95% CI 22.8–23.0%; females: 29.4%, 95% CI 29.3–29.8%), with larger differences among females (Figure 2A). The gap between PWH and PWOH showed an increasing trend over time, was more prominent in females, and increased with age (Figure 2A). For example, among those aged 80+ years, the prevalence increased from 2007 to 2019 from 26.2% (95% CI: 24.3–28.0%) to 33.3% (95% CI: 31.9–34.7%) in males and from 31.4% (95% CI: 29.6–33.1%) to 44.1% (95% CI: 42.6–45.6%) in females with HIV, as compared to 21.0% (95% CI: 20.8–21.2%) to 23.5% (95% CI: 23.3–23.7%) in male PWOH and 27.4% (95% CI: 27.2–27.5%) to 29.9% (95% CI:29.7–30.1%) in female PWOH.
Figure 2.

Prevalence of AD/ADRD
Abbreviations: PWH, People with HIV; PWOH, people without HIV; AD/ADRD, Alzheimer’s disease (AD)/AD-related dementias (ADRD); GEE, generalized estimating equation.
Generalized Estimating Equation analysis
The GEE models estimate the adjusted odds of AD/ADRD which is the ratio of the adjusted probability that AD/ADRD occurs to the probability that AD/ADRD does not occur. The probability is adjusted for differential demographic and comorbidity characteristics between groups within each of the 8 strata at that stratum’s mean profile of each characteristic. To translate the odds to a more clinically interpretable scale, we presented the adjusted probability (i.e., adjusted prevalence) of AD/ADRD. The highly significant HIV-year interactions typically result in non-parallel curves on the probability scale. For example, in Figure 2D, the interaction p-value is 0.002 for females aged 80 and above. This means that the probability curves shown in Figure 2B diverge over time between PWH and PWOH for this stratum.
GEE analysis showed significant HIV-year interactions among 5 of the 8 age-by-sex strata, which indicates that the change in adjusted prevalence of AD/ADRD over time differed between PWH and PWOH (Figure 2D). Among PWOH, the covariate-adjusted AD/ADRD prevalence remained stable over time, except for the oldest group (80+), which showed an increasing trend, especially among females (Figure 2B). Females with HIV had a higher adjusted prevalence of AD/ADRD in all age categories and the increasing trend was more pronounced in those age 75+, reflecting differential trends between females with and without HIV, statistically shown as significant HIV-year interactions (p=0.01 and 0.002 respectively in Figure 2D). Despite the higher prevalence of AD/ADRD in females with HIV < 75 years of age, the change in AD/ADRD over time was statistically not different compared to females without HIV (Figures 2C and 2D). Although smaller differences in the adjusted prevalence were observed among males (Figures 2B and 2C), those with HIV who were 80 years and older had a higher adjusted prevalence of AD/ADRD, which increased faster than in males without HIV (Figure 2B).
The pattern of association between comorbidities and AD/ADRD was generally similar in PWH and PWOH among age-sex strata (Figure 3). The test for comorbidity-HIV interactions showed a modifying effect of HIV on the association between some comorbidities and AD/ADRD, such as psychiatric disorders and alcohol/drug/opioid use disorders (eTable 4, Supplemental Digital Content for p-values of interactions). Among all comorbidities, the top 5 conditions associated with AD/ADRD were psychiatric disorders, head injury, stroke/TIA, alcohol abuse disorder, and cardiovascular diseases in both PWH and PWOH. Particularly, for psychiatric disorders, the ORs (95% CI) ranged from 1.61 (1.55, 1.67) to 2.26 (2.14, 2.37) and 1.61 (1.60, 1.62) to 2.52 (2.45, 2.59) for PWH and PWOH respectively, with stronger associations in younger age groups and among females.
Figure 3.

Association between comorbidities and AD/ADRD among PWH and PWOH by sex and age strata
Abbreviations: PWH, People with HIV; PWOH people without HIV; AD/ADRD, Alzheimer’s disease (AD)/AD-related dementias (ADRD); NS, not significant (p ≥ 0.05); TIA, transient ischemic attack; Darker gradients indicate stronger association.
Discussion
This study found that PWH had a higher prevalence of AD/ADRD, which increased over time compared with PWOH, especially among female beneficiaries. The differences between PWH and PWOH largely decreased after adjustment for demographics and comorbidities. However, differences in dementia burden remained, especially among older age groups. We also found a spectrum of comorbidities associated with AD/ADRD, with psychiatric disorders the most prominent among these.
Given that older age is the main risk factor for dementia, the prolonged life expectancy among PWH will contribute to an increase in the prevalence of AD/ADRD in this population. Our findings are generally consistent with the recent study which showed overall increases in dementia prevalence over time.22 In contrast to our study, that study included PWH 50 years and older (mean age 54 years at baseline) among members of Kaiser Permanente health plans in Northern California, Southern California, and the Mid-Atlantic (Maryland, Virginia, Washington DC). That study compared HIV cases to frequency-matched controls and further adjusted for a selected set of comorbidities. The study found that that the overall prevalence of dementia was higher among PWH in 2000–2016 (adjusted prevalence ratio [aPR] 1.86, 95% CI 1.70–2.04) and also in 2015–2016 (aPR 1.75, 95% CI 1.56–1.97).22 Our study targeted an older population (≥ 65 years) and included Medicare beneficiaries from all U.S. states and DC. Thus, our analyses extend these findings to a much larger, more contemporary, older, national and rapidly growing population of PWH. Due to the expected high impact of age and sex on dementia prevalence, we examined the trend and associated factors by stratifying by age (in 5-year increments) and sex. We adopted the multivariable adjustment approach within each stratum and controlled for continuous age, other demographic variables, and a wide range of conditions. This process allowed us to account more effectively for differential age and sex effects and provide more specific information for the subgroups.
Within each age stratum, we found that PWH had a higher burden of dementia, which increased faster compared to PWOH, in both males and females. The oldest groups were most impacted, especially among females. These findings were confounded or moderated by differential demographic and comorbid characteristics and reflect the fact that older PWH increasingly develop age-related comorbidities and psychiatric disorders which impact cognitive function and are often more common in females. Alcohol/drug/opioid use disorders were also more common in PWH and increased over time. After adjusting for demographic factors and comorbidities, the differences between PWH and PWOH decreased in all strata, although significant gaps remained among those 80 years and older. That the gaps between PWH and PWOH were larger in females than in males for all age strata suggests that the excessive burden of AD/ADRD in females with HIV cannot be accounted by differences in demographics and comorbidities. We also found that many age-related comorbidities were highly associated with dementia among PWH. Compared to PWOH, the lower OR (i.e., smaller relative measure) in PWH reflects the observation that there were higher odds of AD/ADRD among PWH without a particular comorbidity.
The strong association between psychiatric disorders (e.g., depression) and AD/ADRD may be explained by several reasons. Depression can impair cognitive function leading to a “pseudodementia” presentation.37 Loneliness, social isolation and heavy alcohol use,38,39 major psychosocial consequences of depression and especially prominent among PWH, are risk factors for dementia.35 Depression and cognitive impairment may also represent the same underlying pathological process, perhaps due to direct and indirect effects of HIV replication in the central nervous system (CNS).40 Toxoplasmosis is a common central nervous system infection in PWH41 and some studies have demonstrated negative effects of latent toxoplasmosis on mental health42 and dementia.43
After accounting for demographics and comorbidities, PWH still had excess risks that differed among subgroups, which could be explained by unmeasured factors, such as HIV clinical factors (disease stage, treatment), education, and social support. CD4 nadir is a predictor of HIV neurocognitive impairment and initiation of ART as early as possible might reduce the risk of developing HIV-associated neurocognitive disorders.44 In contrast, it is possible that long-term exposure to certain ART drugs may cause chronic CNS toxicity.45–47 For example, a clinicopathological study of PWH demonstrated that darunavir or ritonavir use was associated with higher likelihood of cerebral degenerative changes, such as neuronal phospho-tau lesions and marked microgliosis in the putamen.46 Human herpes viruses are another possible contributor to excessive risk in PWH.48,49 Further studies are needed to investigate these factors.
This study has several limitations. We focused on the overall dementia burden over time and adopted a consecutive cross-sectional study design; thus, the results demonstrate association rather than causation. Further studies are needed to evaluate the incidence of dementia and investigate factors that impact the development of dementia. While we used a validated method to identify AD/ADRD in claims data, the accuracy and completeness of Medicare claims to identify patients with dementia is not perfect.28–30 The sensitivity and specificity of Medicare claims is reported to be 0.85 and 0.89 for AD/ADRD.32 Also, since most dementias are mixed,26,50 especially at later ages, and it is challenging to classify them into distinct categories based on the clinical diagnosis codes alone, our analyses considered all types of dementia together including AD and AD-related dementia. Therefore, the analysis could not study Alzheimer disease itself. Furthermore, the population of patients with dementia may differ from the population of those given a diagnosis of dementia by their physicians. We studied the latter, which underrepresents individuals with early or milder dementia and those with poor access to medical care.51 The pathogenesis of AD may differ in PWH. For example, the appearance of amyloid burden and association between the apolipoprotein E (APOE4) and AD in general populations were not observed in some studies of PWH.52,53 Also, a recent study examined biomarkers of age-related neurodegeneration in the CSF in relation to neurocognitive impairment in older PWH. Poorer neurocognitive performance was associated with higher CSF tTau, a marker of age-related neuronal injury, but not with biomarkers of amyloid metabolism.54 Further a small cases series demonstrated the complexity of AD diagnosis among older PWH.55 Finally, the data available were collected for processing claims, with no detailed clinical, psychological, or behavioral information, so these factors cannot be accounted for in this analysis.
Strengths of the present study included using data from 100% of older PWH and a 5% national sample of PWOH enrolled in the U.S. Medicare program. Data extended up to 13 years with recent data included. The large sample size made it possible to evaluate temporal trends, observe interactions, and conduct stratified analysis. We examined the time trend of prevalent dementia and a spectrum of comorbidities associated with dementia among 8 age-sex strata, then compared these between older PWH and PWOH, which was beyond the scope of previous studies.
These results are generalizable to the older fee-for-service Medicare population and have important implications for the management and treatment of dementia among patients with HIV. Given the increasingly high burden of dementia among older Medicare enrollees with HIV, enhanced regular screening for dementia among PWH and comprehensive management of dementia are indicated. The significantly higher burden of dementia among females with HIV and older age subgroups highlights the necessity of closely monitoring treatment and supporting engagement in the care of these vulnerable patients. In addition, early treatment of comorbidities, especially psychiatric disorders, alcohol and drug use disorders, and cardiovascular diseases in PWH is critical, given the additional risk of dementia in this population. Implementing these strategies will not only extend the lifespan of PWH but also improve their healthspan and quality of life.56
In conclusion, older Medicare enrollees with HIV had an increased dementia burden over time compared to those without HIV, especially females and older subjects. Age-related comorbidities—especially psychiatric disorders, which are more prominent in females—were strongly associated with this trend. These findings underscore the need to develop clinical practice guidelines that facilitate the integration of dementia and comorbidity screening, evaluation, and management into the routine primary care of aging people with HIV.
Supplementary Material
Yu_JAIDS_Supplemental Digital Content.docx
eTable 1. Flow chart of cohort construction
eTable 2. CCW AD/ADRD diagnosis codes and algorithm specification
eTable 3. Classification of comorbidities
eTable 4. Comorbidity-HIV interactions
Funding support:
Drs. Yu and Berenson are supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH; Berenson, PI) from the National Institutes of Health/Office of the Director (OD)/National Institute of Allergy and Infectious Diseases (NIAID), and Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD). Dr. Yu is also supported by Sustained Training in Aging and HIV Research (R25MH108389: Dilip V. Jeste/Scott L. Letendre Co-PIs; 2R25MH108389-06: Scott L. Letendre/ Dilip V. Jeste/Erin Elizabeth Sundermann). Dr. Giordano is supported by the MD Anderson Foundation Chair at Baylor College of Medicine and Texas Developmental Center for AIDS Research (P30AI161943; Giordano, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
Conflict of Interest:
There are no conflicts of interest.
Part of the work was presented at virtual conference The Lancet Summit: HIV and Healthy Longevity on March 17–18, 2022.
References
- 1.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. Aug 2017;4(8):e349–e356. doi: 10.1016/s2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV Surveillance Report, 2019; vol.32. Updated Published May 2021. Accessed November 2, 2022, https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- 3.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011/02/01 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackiewicz MM, Overk C, Achim CL, Masliah E. Pathogenesis of age-related HIV neurodegeneration. J Neurovirol. Oct 2019;25(5):622–633. doi: 10.1007/s13365-019-00728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastrorosa I, Pinnetti C, Brita AC, et al. Declining Prevalence of Human Immunodeficiency Virus (HIV)-Associated Neurocognitive Disorders in Recent Years and Associated Factors in a Large Cohort of Antiretroviral Therapy-Treated Individuals With HIV. Clin Infect Dis. Feb 8 2023;76(3):e629–e637. doi: 10.1093/cid/ciac658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wubetu AD, Asefa KK, Gebregiorgis BG. Prevalence of Neurocognitive Impairment and Associated Factors Among People Living with HIV on Highly Active Antiretroviral Treatment, Ethiopia. HIV AIDS (Auckl). 2021;13:425–433. doi: 10.2147/hiv.S298141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flatt A, Gentry T, Kellett-Wright J, et al. Prevalence and 1-year incidence of HIV-associated neurocognitive disorder (HAND) in adults aged ≥50 years attending standard HIV clinical care in Kilimanjaro, Tanzania. Int Psychogeriatr. Mar 24 2021:1–12. doi: 10.1017/s1041610221000156. [DOI] [PubMed] [Google Scholar]
- 8.Focà E, Magro P, Motta D, et al. Screening for Neurocognitive Impairment in HIV-Infected Individuals at First Contact after HIV Diagnosis: The Experience of a Large Clinical Center in Northern Italy. Int J Mol Sci. Mar 24 2016;17(4):434. doi: 10.3390/ijms17040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. 2013. Washington, DC.
- 10.Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. Jan 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities Among US Patients With Prevalent HIV Infection-A Trend Analysis. J Infect Dis. Dec 19 2017;216(12):1525–1533. doi: 10.1093/infdis/jix518. [DOI] [PubMed] [Google Scholar]
- 12.Serrao R, Pinero C, Velez J, et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int J Infect Dis. Feb 2019;79:94–100. doi: 10.1016/j.ijid.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. Dec 2011;53(11):1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 14.Cole MB, Galarraga O, Rahman M, Wilson IB. Trends in Comorbid Conditions Among Medicaid Enrollees With HIV. Open Forum Infect Dis. Apr 2019;6(4):ofz124. doi: 10.1093/ofid/ofz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vance DE, Lee L, Munoz-Moreno JA, et al. Cognitive Reserve Over the Lifespan: Neurocognitive Implications for Aging With HIV. J Assoc Nurses AIDS Care. Sep-Oct 2019;30(5):e109–e121. doi: 10.1097/JNC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 16.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. Jun 1 2013;27(9):1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. Aug 15 2011;57(5):371–9. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cody SL, Vance DE. The neurobiology of HIV and its impact on cognitive reserve: A review of cognitive interventions for an aging population. Neurobiol Dis. Aug 2016;92(Pt B):144–56. doi: 10.1016/j.nbd.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Funes HA, Apostolova N, Alegre F, et al. Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis. Nov 1 2014;210(9):1385–95. doi: 10.1093/infdis/jiu273. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez AB, Kaul M. Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND. Brain Sci. Feb 23 2017;7(3)doi: 10.3390/brainsci7030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A. Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res. Nov 2016;30(4):677–697. doi: 10.1007/s12640-016-9646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam JO, Lee C, Gilsanz P, et al. Comparison of dementia incidence and prevalence between individuals with and without HIV infection in primary care from 2000 to 2016. AIDS. Mar 1 2022;36(3):437–445. doi: 10.1097/qad.0000000000003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J Alzheimers Dis. 2018;64(4):1077–1083. doi: 10.3233/jad-180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative I. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. Apr 2014;75(4):563–73. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). Sep 1 2015;1(2):103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duara R, Barker W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics. Jan 27 2022;doi: 10.1007/s13311-022-01185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley RF, Mormino EC, Rabin JS, et al. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. May 1 2019;76(5):542–551. doi: 10.1001/jamaneurol.2018.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. Dec 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schelbaum E, Loughlin L, Jett S, et al. Association of Reproductive History With Brain MRI Biomarkers of Dementia Risk in Midlife. Neurology. 2021;97(23):e2328–e2339. doi: 10.1212/wnl.0000000000012941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J, Harris K, Peters SAE, Woodward M. Reproductive factors and the risk of incident dementia: A cohort study of UK Biobank participants. PLoS Med. Apr 2022;19(4):e1003955. doi: 10.1371/journal.pmed.1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chronic Condition Data Warehouse. Accessed Octobor 30, 2022. https://www2.ccwdata.org/web/guest/condition-categories.
- 32.Taylor DH Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–15. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy EP, Chang CH, Tilton N, Kabeto MU, Langa KM, Bynum JPW. Validation of Claims Algorithms to Identify Alzheimer’s Disease and Related Dementias. J Gerontol A Biol Sci Med Sci. Dec 17 2021; doi: 10.1093/gerona/glab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moura L, Festa N, Price M, et al. Identifying Medicare beneficiaries with dementia. J Am Geriatr Soc. Aug 2021;69(8):2240–2251. doi: 10.1111/jgs.17183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. Aug 8 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. Apr 25 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. May 3 2011;7(6):323–31. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emlet CA. An examination of the social networks and social isolation in older and younger adults living with HIV/AIDS. Health Soc Work. Nov 2006;31(4):299–308. doi: 10.1093/hsw/31.4.299. [DOI] [PubMed] [Google Scholar]
- 39.Khan MR, Young KE, Caniglia EC, et al. Association of Alcohol Screening Scores With Adverse Mental Health Conditions and Substance Use Among US Adults. JAMA Netw Open. Mar 2 2020;3(3):e200895. doi: 10.1001/jamanetworkopen.2020.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alford K, Vera JH. Cognitive Impairment in people living with HIV in the ART era: A Review. Br Med Bull. Sep 1 2018;127(1):55–68. doi: 10.1093/bmb/ldy019. [DOI] [PubMed] [Google Scholar]
- 41.Ayoade F, Stevenson A, Chandranesan J. HIV-1 Associated Toxoplasmosis. Updated September 20, 2022. Accessed December 20, 2022, https://www.ncbi.nlm.nih.gov/books/NBK441877/#_NBK441877_pubdet_
- 42.Flegr J, Horáček J. Negative Effects of Latent Toxoplasmosis on Mental Health. Front Psychiatry. 2019;10:1012. doi: 10.3389/fpsyt.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang HY, Chien WC, Chung CH, et al. Risk of dementia in patients with toxoplasmosis: a nationwide, population-based cohort study in Taiwan. Parasit Vectors. Aug 28 2021;14(1):435. doi: 10.1186/s13071-021-04928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. Sep 10 2011;25(14):1747–51. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Q, Vaida F, Wong J, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. Apr 2016;22(2):170–8. doi: 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soontornniyomkij V, Umlauf A, Soontornniyomkij B, et al. Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS. Sep 10 2018;32(14):2005–2015. doi: 10.1097/QAD.0000000000001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamkwalala AR, Wang K, O’Halloran J, et al. Starting or Switching to an Integrase Inhibitor-Based Regimen Affects PTSD Symptoms in Women with HIV. AIDS Behav. Jan 2021;25(1):225–236. doi: 10.1007/s10461-020-02967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itzhaki RF. Overwhelming Evidence for a Major Role for Herpes Simplex Virus Type 1 (HSV1) in Alzheimer’s Disease (AD); Underwhelming Evidence against. Vaccines (Basel). Jun 21 2021;9(6)doi: 10.3390/vaccines9060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianella S, Massanella M, Wertheim JO, Smith DM. The Sordid Affair Between Human Herpesvirus and HIV. J Infect Dis. Sep 15 2015;212(6):845–52. doi: 10.1093/infdis/jiv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cysique LA, Brew BJ. Vascular cognitive impairment and HIV-associated neurocognitive disorder: a new paradigm. J Neurovirol. Oct 2019;25(5):710–721. doi: 10.1007/s13365-018-0706-5. [DOI] [PubMed] [Google Scholar]
- 51.Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer’s disease solely from Medicare claims records. J Am Geriatr Soc. Feb 1999;47(2):215–9. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 52.Cooley SA, Paul RH, Fennema-Notestine C, et al. Apolipoprotein E ε4 genotype status is not associated with neuroimaging outcomes in a large cohort of HIV+ individuals. J Neurovirol. Oct 2016;22(5):607–614. doi: 10.1007/s13365-016-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Becker JT, Martinson JJ, Penugonda S, et al. No association between Apoε4 alleles, HIV infection, age, neuropsychological outcome, or death. J Neurovirol. Feb 2015;21(1):24–31. doi: 10.1007/s13365-014-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis RJ, Chenna A, Petropoulos CJ, et al. Higher cerebrospinal fluid biomarkers of neuronal injury in HIV-associated neurocognitive impairment. J Neurovirol. Jun 2022;28(3):438–445. doi: 10.1007/s13365-022-01081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calcagno A, Celani L, Trunfio M, et al. Alzheimer Dementia in People Living With HIV. Neurol Clin Pract. Oct 2021;11(5):e627–e633. doi: 10.1212/cpj.0000000000001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaeberlein M How healthy is the healthspan concept? Geroscience. Aug 2018;40(4):361–364. doi: 10.1007/s11357-018-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Yu_JAIDS_Supplemental Digital Content.docx
eTable 1. Flow chart of cohort construction
eTable 2. CCW AD/ADRD diagnosis codes and algorithm specification
eTable 3. Classification of comorbidities
eTable 4. Comorbidity-HIV interactions


