STRUCTURED ABSTRACT
Objective:
To determine if THC discontinuation mitigates THC-associated changes in male reproductive health using a rhesus macaque model of daily THC edible consumption.
Design:
Research animal study.
Setting:
Research institute environment.
Patient(s):
Adult male rhesus macaques (8–10 years of age; n=6).
Intervention(s):
Chronic daily THC edible administration at medically and recreationally relevant contemporary doses followed by cessation of THC
Main Outcome Measure(s):
Testicular volume, serum male hormones, semen parameters, sperm DNA fragmentation, seminal fluid proteomics, and whole genome bisulfite sequencing (WGBS) of sperm DNA
Result(s):
Chronic THC use resulted in significant testicular atrophy, increased gonadotropins, decreased serum sex steroids, changes in seminal fluid proteome, and increased DNA fragmentation with partial recovery following THC discontinuation. For every 1mg/7kg/day increase in THC dosing, there was a significant decrease in total testicular volume bilaterally by 12.6 cm3 (95% CI 10.6–14.5, p<0.001), resulting in a 59% decrease in volume. With THC abstinence, total testicular volume increased to 73% of its original volume. Similarly, with THC exposure there were significant decreases in mean total testosterone (p=0.002) and estradiol (p<0.001), and a significant increase in follicle stimulating hormone (FSH) (p=0.010). With increasing THC dose, there was a significant decrease in liquid semen ejaculate volume (p=0.032) and weight of coagulum (p=0.042); but no other significant changes to other semen parameters were present. After discontinuing THC, there was a significant rise in total serum testosterone by 1.3 ng/ml (95%CI: 0.1–2.4, p=0.038) and estradiol by 2.9 pg/ml (95%CI: 0.4–5.4, p=0.025), and FSH decreased significantly by 0.06 ng/ml (95%CI: 0.01–0.11, p=0.025). Seminal fluid proteome analysis revealed differential expression of proteins enriched for processes related to cellular secretion, immune response, and fibrinolysis. WGBS identified 23,558 CpGs differentially methylated in heavy-THC versus pre-THC sperm, with partial restoration of methylation after discontinuation of THC. Genes associated with altered differentially methylated regions (DMRs) were enriched for those involved in nervous system development and function.
Conclusion(s):
This is the first study demonstrating that discontinuation of chronic THC use in rhesus macaques partially restores adverse impacts to male reproductive health, THC-associated sperm DMRs in genes important for development, and expression of proteins important for male fertility.
Keywords: male fertility, cannabis, marijuana, THC, delta-9-tetrahydrocannabinol
GRAPHICAL ABSTRACT
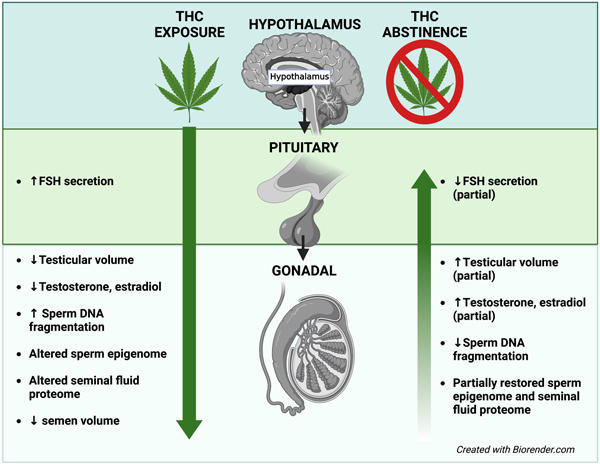
Capsule:
Discontinuing THC partially restores THC-associated impacts to male reproductive health and differential methylation in genes involved in development.
INTRODUCTION
Cannabis is the most commonly used psychoactive drug among reproductive age men in the United States and worldwide (1), which is extremely concerning given safety data is substantially lacking and cannabis users are often unaware of the potential adverse effects on fertility. Currently, the American Society of Reproductive Medicine discourages the use of cannabis in patients intending to conceive because the full impact on reproductive health has not been clearly established (2). Published studies examining the effect of cannabis exposure on male fertility and future offspring are conflicting. Most human studies are limited by small sample sizes, self-reporting, and co-use of other drugs (3). Rodent studies have largely focused on the effects of acute THC exposure, which was often delivered via intraperitoneal injection or oral gavage, not representative of typical human use (4, 5).
To overcome some of the limitations in prior studies, our group used a novel rhesus macaque model and demonstrated that chronic delta-9-tetrahydrocannabinol (THC) edible consumption results in testicular and epididymal atrophy, increased gonadotropin release, and decreased serum sex steroid concentrations suggestive of primary testicular failure (6). However, whether these effects are permanent or temporary remains unknown. A rhesus macaque model of chronic THC edible consumption (6, 7) is highly relevant and translatable to human use as edibles are one of the most popular forms of current cannabis use among young adult males (8, 9). Rhesus macaques and humans exhibit similar plasma disposition of THC, ~64 day spermatogenic cycle, physiologic, genetic, anatomic, and endocrine properties, all together suggesting that observations in rhesus macaques may be translated from bench to bedside in humans (10, 11).
Our group has also recently uncovered a highly concerning series of deleterious effects of cannabis exposure on sperm DNA methylation (12, 13). We reported that cannabis exposure in a small cohort of humans and rats was associated with widespread altered sperm DNA methylation (13). Genes affected by cannabis use were involved in early development, suggesting that preconception paternal cannabis use can impact long-term health in offspring (13). However, associated confounding variables such as route of administration and potency of the cannabis used were not determined in men, and administration of THC by oral gavage in rodents were not adjusted for in the interpretation of these results.
Using our rhesus macaque model, this study focuses on the impact of chronic THC use on sperm DNA methylation as a mechanism linking paternal cannabis use to reproductive and subsequent offspring health. Our study also examines the benefit of discontinuing THC on male fertility and the sperm epigenome after chronic use. This is the first study to provide a deeper understanding of the role and contributions of preconception THC use on male reproductive health and sperm epigenetics in a human-relevant, rhesus macaque model. Results from this study will help guide patient counseling and inform public health policies focused on cannabis use in the future as more states legalize cannabis use.
MATERIALS AND METHODS
Study Design
Our group has previously published on the effects of THC exposure in the NHP (12). This study focuses on extending our prior assessments of THC-associated effects on male fertility to include the seminal fluid proteome, sperm DNA integrity, and sperm epigenome in addition to determining the impact of discontinuing daily THC use in the same cohort of sexually mature, adult male rhesus macaques (Macaca mulatta) (n=6) ages 8–10 years old and weighing 9.3–12.7kg, with prior proven paternity. All procedures were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee (IACUC) and conformed to all applicable regulations (IP0001389).
The animals were socially-housed and maintained on a standard chow diet (LabDiet 5000, Purina Mills, St. Louis, Missouri, USA) with a daily cookie containing THC (THC edible), made using research-grade THC obtained directly from the National Institute on Drug Abuse (NIDA) Drug Supply Program as previously published (12, 14). Animals were all fed the same diet of fresh chow and produce enrichment, and only water was available ad libitum. All animals were slowly titrated up to 2.5mg/7kg/day of THC with a dose increase every 70 days (the NHP sperm life cycle is ~64 days) over approximately a 7-month time period to model published medical marijuana acclimation recommendations of 20–30mg/day for a 68kg adult (15, 16). Specifically, animals were initially maintained on a dose of 0.5mg/7kg/day of THC for days 1 to 70, 1mg/7kg/day (moderate-THC dose) for days 71–140, and 2.5mg/7kg/day (heavy-THC dose) for days 141 to 210. To minimize potential confounders and inter-animal variability, we utilized a longitudinal, single-case experimental design, where each male served as its own control during the study.
To determine peak THC concentrations with each increase in THC dosage, blood was sampled (2mL) at each dose adjustment time point during THC induction, three hours (17) following edible consumption. Immediately prior to each dosing increase and every 70 days after THC was discontinued, blood sampling, animal weight, scrotal ultrasound, and semen analysis were performed as previously published (12). A testicular biopsy was performed pre-THC, heavy-THC, and post-THC discontinuation. Seminal fluid was isolated from semen samples for proteomic analysis, and sperm genomic DNA was extracted from collected semen samples for DNA fragmentation analysis and whole genome bisulfite sequencing (WGBS) as described in the Supplemental Materials. All additional details and methods are included in the Supplemental Materials section.
RESULTS
Study Sample characteristics
A cohort of 6 male rhesus macaques (Macaca mulatta) of reproductive age (mean of 9.1 years, SD=0.6) with prior proven paternity and no history of known significant environmental exposures or drug exposure, including THC, were used in the study as previously described (12). During the THC induction period, average plasma THC concentrations increased by 2.54 ng/mol for each mg/7kg/day increase in THC (95% CI: 1.35–3.73 ng/mol, p<0.001) as previously published (Figure 1A) (12). Peak THC levels at the highest dosing regimen were within the expected reported contemporary range (e.g., 5–8ng/mL) reported in humans 3 hours following a similar oral THC dose (17, 18). The average baseline weight of all animals was 11.6kg (SD=1.4) pre-THC exposure, 11.9kg (SD=1.3) at the highest THC dose, and back to baseline at 11.6kg (SD=2.1) after 140 days of THC abstinence (Table 1).
Figure 1. (A) Study design overview.
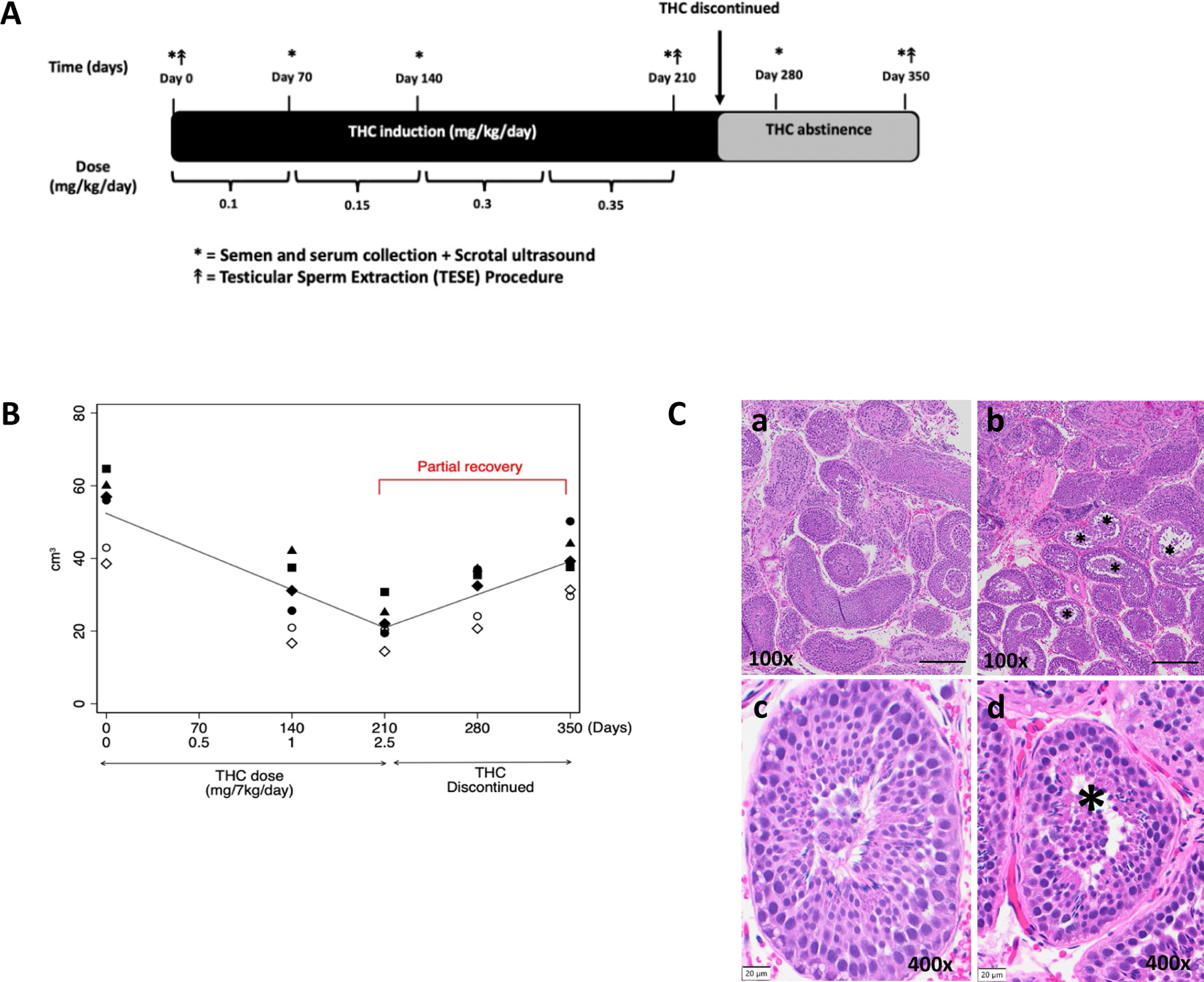
Adult male rhesus macaques (n=6) were used. Prior to THC induction, baseline semen collection, scrotal ultrasound (US) and blood and urine sampling were performed. THC induction occurred over ~30 weeks (~7 months) per published medical marijuana acclimation guidelines. The nonhuman primate (NHP) spermatogenic cycle is ~64 days (~10 weeks), so the THC dose was increased every 10 weeks to accommodate 1 cycle at each THC dose until the highest THC dose was reached (2.5mg/7kg/day, equivalent to a heavy human medical cannabis dose). At the end of each THC dosing period and after THC was discontinued, all males underwent serial plasma and semen collection in addition to scrotal US. Testicular sperm extraction (TESE) was performed for histologic assessment at baseline (pre-THC), during THC induction (heavy-THC), and after THC was discontinued for 140 days. (B) Total testicular volume significantly decreases with increasing THC dosing with partial recovery of total testicular volume after discontinuing THC. Individual (symbols) and average fixed effect (lines) testicular volume (cm3) in response to increasing oral THC dosage (0 to 2.5 mg/7kg/day) resulted in a 59% decrease in volume after 210 days. THC was then discontinued over 140 days with partial recovery to 73% of the original testicular volume in 6 rhesus macaques (p<0.001). (C) Reduced seminiferous tubule diameter and decreased germ cell layers with THC exposure. Representative rhesus macaque testicular histopathology from the same animal pre-THC exposure (a,c) and after THC exposure (b,d). Seminiferous tubules with reduced diameter and decreased germ cell layers as indicated by the asterisks (*), were observed in all animals. Scale bar for a,b = 200 µm, for c,d =20µm.
Table 1: Average weight, testicular anatomy, semen characteristics, and hormone levels (± standard deviation).
Characteristics of 6 male rhesus macaques during 3 doses of oral THC (0 to 2.5 mg/7kg/day), and following 70 and 140 days of THC discontinuation. Change with 1 mg/7kg/day increase in THC dose as well as change per 70 days of discontinuation was calculated (with 95% confidence intervals and associated p-values), from a random intercept mixed effects model with linear spline at point of THC discontinuation.
| Characteristic | Mean ± standard deviation at each time point | Marginal slopes from mixed effects models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0mg/7kg/day THC (pre-THC) | 1 mg/7kg/day THC (mod-THC) | 2.5 mg/7kg/day THC (heavy-THC) | 70 days post-THC | 140 days post-THC | Change per 1 mg/7kg/day THC dose | 95% CI | p-value | Change per 70 days post-THC | 95% CI | p-value | |
| Weight (kg) | 11.6±1.4 | 11.7±1.2 | 11.9±1.3 | 12.1±2.1 | 11.6±2.1 | 0.2 | −0.2, 0.5 | 0.323 | −0.1 | −0.5, 0.3 | 0.696 |
| Right epididymal head volume (cm3) | 0.70±0.11 | 0.37±0.05 | 0.34±0.06 | 0.38±0.03 | 0.42±0.04 | −0.15 | −0.18, −0.12 | < 0.001 | 0.06 | 0.03, 0.09 | < 0.001 |
| Left epididymal head volume (cm3) | 0.71±0.09 | 0.37±0.07 | 0.32±0.03 | 0.39±0.03 | 0.41±0.03 | −0.16 | −0.18, −0.13 | < 0.001 | 0.06 | 0.04, 0.09 | < 0.001 |
| Right testicular volume (cm3) | 27.3±5.5 | 15.1±4.9 | 10.2±2.1 | 16.1±3.6 | 19.5±3.4 | −6.7 | −7.8, −5.7 | < 0.001 | 4.9 | 3.6, 6.3 | < 0.001 |
| Left testicular volume (cm3) | 25.9±5.1 | 13.9±4.9 | 11.9±3.9 | 15.1±3.5 | 19.1±4.6 | −5.8 | −6.9, −4.8 | < 0.001 | 4.2 | 2.9, 5.5 | < 0.001 |
| Total testicular volume (cm3) | 53.2±10.2 | 29.0±9.7 | 22.0±5.5 | 31.1±7.0 | 38.7±7.7 | −12.6 | −14.5, −10.6 | < 0.001 | 9.1 | 6.8, 11.5 | < 0.001 |
| Weight of coagulum (g) | 0.80±0.31 | 0.47±0.13 | 0.54±0.16 | 0.55±0.21 | 0.72±0.50 | −0.12 | −0.24, 0.00 | 0.042 | 0.12 | −0.02, 0.26 | 0.105 |
| Liquid fraction volume (ml) | 0.45±0.14 | 0.26±0.16 | 0.32±0.18 | 0.30±0.14 | 0.33±0.13 | −0.06 | −0.12, −0.01 | 0.032 | 0.03 | −0.04, 0.10 | 0.426 |
| Sperm conc. (million/ml) | 795±326 | 1483±1005 | 1388±1464 | 1175±873 | 1188±757 | 241 | −75, 558 | 0.135 | −171 | −556, 213 | 0.382 |
| Total sperm count (million) | 338±135 | 274±166 | 269±189 | 347±230 | 348±202 | −24 | −73, 25 | 0.331 | 46 | −14, 105 | 0.133 |
| Percent motility | 88.9±6.2 | 86.3±8.4 | 87.0±7.9 | 86.9±10.4 | 86.5±6.6 | −0.8 | −3.3, 1.6 | 0.504 | 0.1 | −2.9, 3.1 | 0.950 |
| Total testosterone (ng/ml) | 5.76±3.72 | 3.68±3.27 | 1.96±1.64 | 3.26±1.60 | 4.67±2.17 | −1.5 | −2.5, −0.5 | 0.002 | 1.3 | 0.1, 2.4 | 0.038 |
| Estradiol (pg/ml) | 16.3±3.8 | 6.7±2.6 | 6.0±1.7 | 6.8±3.0 | 10.7±9.3 | −4.5 | −6.6, −2.4 | < 0.001 | 2.9 | 0.4, 5.4 | 0.025 |
| Prolactin (ng/ml) | 12.3±7.5 | 19.3±10.5 | 30.7±21.2 | 19.0±15.9 | 50.6±56.8 | 4.5 | −5.2, 14.1 | 0.364 | 10.0 | −1.6, 21.7 | 0.092 |
| Inhibin (pg/ml) | 1015±148 | 1048±479 | 1062±210 | 861±353 | 901±169 | 4 | −101, 109 | 0.936 | −87 | −214, 40 | 0.181 |
| Albumin (mg/ml) | 53.0±19.9 | 51.5±23.3 | 51.6±20.3 | 26.5±7.4 | 28.2±7.4 | −2.2 | −7.2, 2.8 | 0.383 | −12.3 | −18.3, −6.2 | < 0.001 |
| 17-OHP (ng/ml) | 1.47±0.28 | 1.09±0.34 | 0.93±0.37 | 1.17±0.52 | 1.16±0.59 | −0.20 | −0.32, −0.08 | 0.002 | 0.12 | −0.03, 0.27 | 0.109 |
| LH (ng/ml) | 0.69±0.18 | 0.92±0.38 | 1.10±0.35 | 0.85±0.39 | 0.98±0.60 | 0.14 | −0.03, 0.31 | 0.111 | −0.06 | −0.27, 0.14 | 0.557 |
| FSH (ng/ml) | 0.17±0.06 | 0.24±0.13 | 0.33±0.18 | 0.22±0.10 | 0.20±0.10 | 0.06 | 0.01, 0.10 | 0.010 | −0.06 | −0.11, −0.01 | 0.025 |
THC use results in primary testicular failure with partial recovery following abstinence
For every 1mg/7kg/day increase in THC dosing, there was a significant decrease in total testicular volume bilaterally by 12.6 cm3 (95% CI 10.6–14.5, p<0.001), resulting in a 59% decrease in volume after 280 days of THC use (approximately 4 spermatogenic cycles) (Figure 1B). A similar decrease was also observed in the left epididymal head width by 0.16 cm (95% CI: 0.13–0.18, p<0.001) and right epididymal head width by 0.15 cm (95% CI: 0.12–0.18, p<0.001), resulting in a 55% and 51% decrease in epididymal volume respectively (Table 1). No scrotal masses or varicoceles were noted on ultrasound or physical examination. On histologic exam, testicular volume loss appeared to be secondary to a reduction in seminiferous tubule diameter by an average of 51.3 µm (p<0.001) and decreased germ cell layers in all animals (Figure 1C).
Immunohistochemistry using the vascular endothelia marker erythroblast transformation-specific (ETS)-related gene (ERG) showed no significant difference in testicular tissue capillary density from THC exposure; there was an average of 10–10.5 blood vessels per high-powered-field at baseline (p=0.929), with THC exposure (p=0.668), and after THC was discontinued (p=0.798) (Supplemental Figure 1). With every 70 days of THC abstinence, total testicular volume increased significantly by 9.1 cm3 (95% CI 6.8–11.5, p<0.001) to 73% of its original volume by 140 days (Figure 1B). After discontinuation of THC, there was a significant increase in both left epididymal volume by 0.06cm3 (95% CI: 0.04–0.09, p<0.001) and right epididymal volume by 0.06cm3 (95% CI: 0.03–0.09, p<0.001) to approximately 60% of the original volume bilaterally at 140 days of THC abstinence (Table 1).
Similarly, with THC exposure there were significant decreases in mean total testosterone (p=0.002), intratesticular testosterone (17-OHP, p=0.002), and estradiol (p<0.001), and a significant increase in follicle stimulating hormone (FSH) (p=0.010). There was no statistically significant change in luteinizing hormone (LH) (p=0.111), prolactin (p=0.364), and albumin concentration (p=0.383). With increasing THC dose, there was a significant decrease in liquid semen ejaculate volume (p=0.032) and weight of coagulum (p=0.042); but no other significant changes to other semen parameters were present including sperm concentration (p=0.135), total sperm count (p=0.331), motility (p=0.504) (Table 1) and morphology (p=0.438). After discontinuation of THC, there was a significant rise in total serum testosterone by 1.3 ng/ml (95%CI: 0.1–2.4, p=0.038) and estradiol by 2.9 pg/ml (95%CI: 0.4–5.4, p=0.025), and FSH decreased significantly by 0.06 ng/ml (95%CI: 0.01–0.11, p=0.025). There were no other significant changes to male reproductive hormones and semen parameters, including liquid semen ejaculate volume, after THC discontinuation (Table 1).
THC exposure alters sperm DNA integrity with improvement after discontinuation
Average pre-THC sperm DNA fragmentation was 4.2% and within the normal range of Oregon National Primate Research Center (ONPRC) Assisted Reproductive Core male rhesus macaques (0.1–8.9%). All males had a THC-associated increase in sperm DNA fragmentation with an average 2.5-fold change at the moderate-THC dose and a 3.4-fold change at the heavy-THC dose. DNA-fragmentation improved toward pre-THC levels in half of the subjects after 70 days of THC cessation and in all males (n=6) after 140 days of THC abstinence.
Rhesus macaque and human seminal fluid proteins share high homology
Proteomic analysis of seminal plasma resulted in 1,395 quantifiable proteins (Figure 2A) in the tandem mass tag (TMT) experiment for the full set of samples (two 18-plex TMTpro kits). The 1,395 monkey protein sequences were aligned against a human canonical protein sequence set (20,588 proteins, UniProt v2022.01) to find reciprocal best matches, using BLAST (basic local alignment search tool; https://github.com/pwilmart/PAW_BLAST). The average percent identity was 88.6% (SD=14.9%). All rhesus macaque proteins were matched to human proteins with 99.4% of the rhesus macaque proteins (1,386) exceeding an identity cutoff of 44.3% (average minus 3 standard deviations). The 1,386 proteins accounted for 99.8% of the total measured TMT reporter ion intensity. The 10 highest expressed proteins in seminal fluid are shown in Supplemental Table 1.
Figure 2.
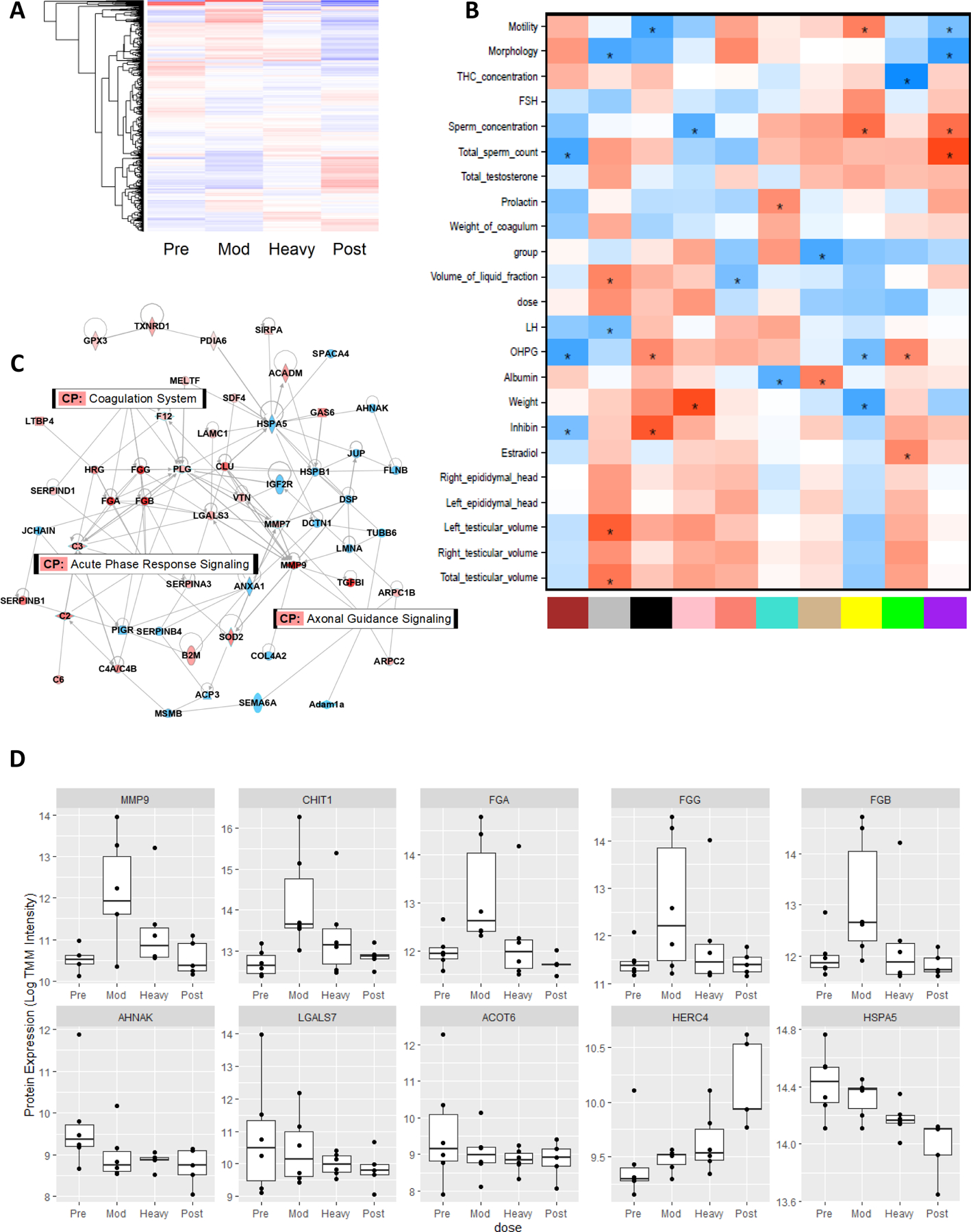
(A) Heatmap represents all 1,395 quantifiable proteins with mean per group log transformed and centered. Red color indicates higher mean expression and blue indicates lower expression relative to mean of all groups. (B) Seminal fluid proteome module-trait relationships. WGCNA was used to find correlation networks of co-expressed proteins. Each module (x-axis) containing multiple correlated proteins was reduced to a single eigenvalue (the first principal component) and then correlated with anatomical, seminal, hormonal, and dosing measurements (y-axis). The more positively correlated the module and the trait, the more red the square; the more negatively correlated, the more blue (* unadjusted p<0.05). (C) IPA merged network for proteins differentially expressed with either dose of THC (red= increased expression, blue = decreased expression) overlaid with top 3 most represented canonical pathways (CP). Orphan (disconnected) proteins were removed for visual clarity. (D) Top differentially expressed seminal fluid proteins with THC exposure. Boxplots represent all proteins with FDR p-value <=0.1 with either dose of THC (mod- or heavy-) relative to pre-THC. The top row contains proteins restored post-THC and the bottom row contains proteins not-restored, including 2 proteins with nominal dose-dependent association with THC exposure.
Seminal plasma proteins are associated with THC exposure and correlated with phenotypes
Weighted gene co-expression network analysis (WGCNA) was used to identify clusters of co-expressed proteins among all 1,395 quantifiable proteins in seminal fluid (19). The “green” module had the strongest correlation with THC concentration (r=−0.72; p=1e-05; Figure 2B) and was highly enriched for “binding of sperm to zona pellucida” and “epididymis tissue expression,” and contained several proteins previously identified as candidate biomarkers for male infertility and oxidative stress in seminal plasma (Supplemental Table 2) (20, 21). Several other modules were significantly correlated with sperm concentration, morphology, and other measures of reproductive health (Figure 2B). We performed pairwise analysis between treatment groups to detect differentially expressed proteins (DEP)s. Eight DEPs were detected with a false discovery rate (FDR) significance p<0.1 in any comparison. Thus, we focused downstream analyses on nominally significant DEPs. A total of 80 proteins were nominally differentially expressed with either dose of THC (mod- or heavy-) relative to pre-THC, with a larger number of DEPs and greater effect sizes observed with the moderate THC dose (Supplemental Table 3). Ingenuity Pathway Analysis (IPA) analysis revealed that THC exposure was associated with dysregulation of canonical pathways related to coagulation, axonal guidance, and acute phase response signaling (Supplemental Table 4; Figure 2C). STRING protein interaction analysis also highlighted enrichment of biological processes related to cellular secretion, immune response, fibrinolysis, and response to stimulus (Supplemental Table 5). Baseline expression in 60 of the 80 proteins dysregulated by THC exposure were restored after THC discontinuation for 140 days. The top restored DEPs were products of MMP9, CHIT1, FGA, FGG, and FGB genes (Figure 2D – top row), while products of AHNAK, LGALS7, and ACOT6 were not restored following THC-discontinuation (Figure 2D – bottom row). Additionally, proteins relevant to male fertility such as products of HSPA5 and HERC4 showed a trend for dose-associated dysregulation with THC exposure that was not restored (Figure 2D – bottom row).
THC exposure is associated with differential methylation of sperm DNA
Sperm DNA extractions yielded >15,000 ng of genomic DNA per sample with high-quality (A260/A280 = 1.88–1.95). Whole genome bisulfite sequencing (WGBS) of the rhesus sperm DNA revealed a global pattern of hypermethylation (~80%; Supplemental Figure 2A), which is similar to previous reports in primates, pig, and cattle sperm (22–24). Principal component analysis of the unfiltered WGBS data revealed significant correlation of PC1 and PC2 with total sperm count, weight of coagulum, and measures of motility and morphology (Supplemental Figure 2B). We analyzed for differentially methylated CpGs (DMCs) and differentially methylated regions (DMRs) for each pairwise comparison of treatment groups (Supplemental Table 6). The relative frequency and methylation profiles of DMCs and annotated genes were highly similar to the DMR results (Supplemental Table 7), therefore we focus on DMRs and nearest genes for downstream analysis of functional enrichment. Overall, methylation levels increased with heavy-THC relative to pre-THC. Following the THC washout period, the mean level of methylation across DMRs was lower in post-THC sperm relative to heavy-THC exposed sperm while the profile of moderate-THC suggested dose-dependent hypermethylation with THC exposure (Figure 3A).
Figure 3.
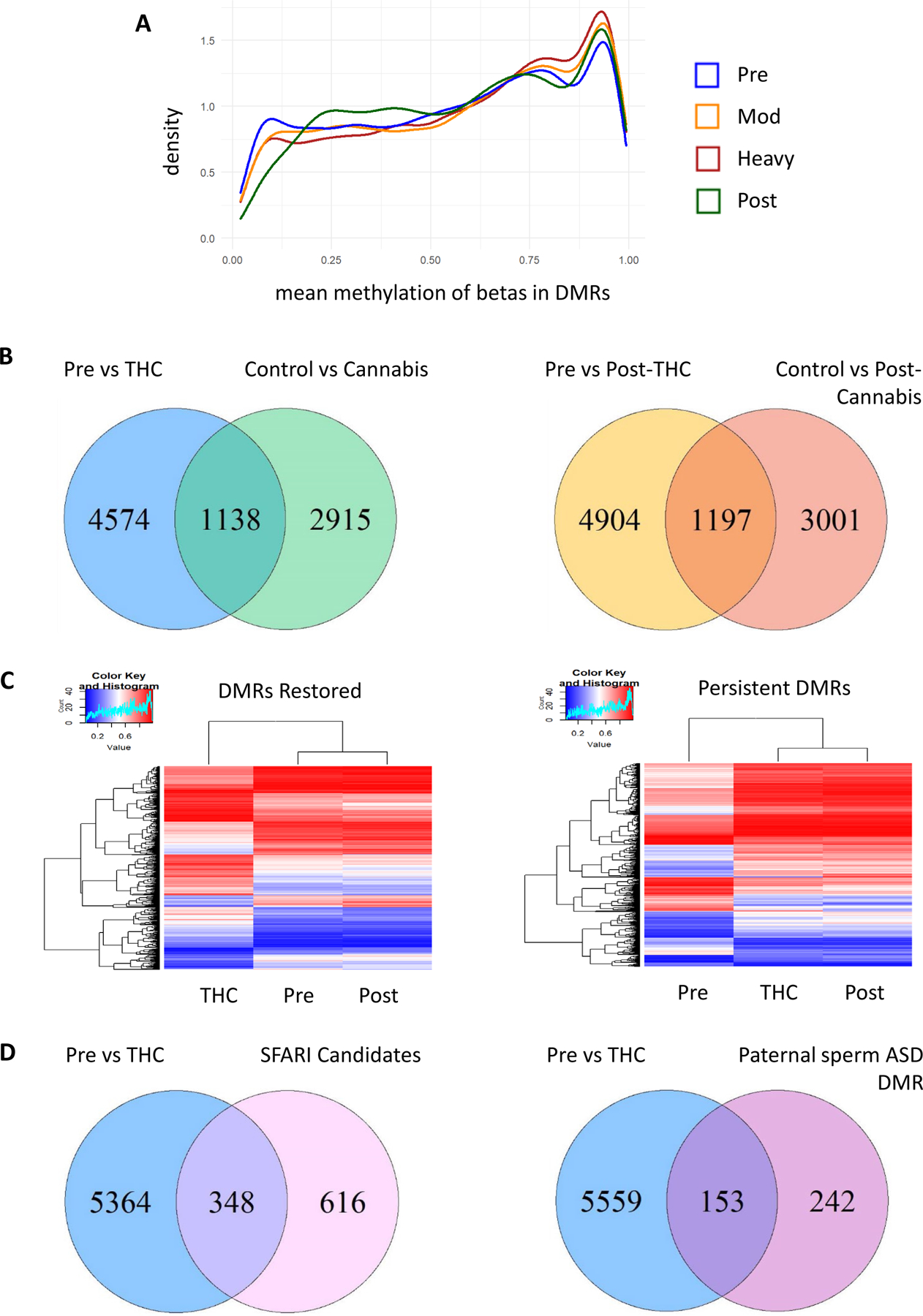
(A) Distribution of mean methylation across DMRs. (B) THC-exposed rhesus vs. human cannabis users. Rhesus pre-THC vs. heavy-THC DMRs which overlap genes nearest DMCs in human cannabis users vs control (Left). Post-THC DMR genes in rhesus macaques overlap with Post-cannabis DMC genes in humans (Right). (C) Identification of DMRs pre-THC versus heavy-THC restored with THC washout (Left) and persistent DMRs (Right). Heatmaps represent the log transformed and mean centered average methylation values per DMR which intersect DMRs between pre-THC and heavy-THC. Hierarchical clustering demonstrates overall similarity in means between groups. (D) Significant overlap of genes annotated to THC DMRs with candidate autism genes. Venn diagrams showing the number of genes that are differentially methylated in sperm after heavy-THC versus pre-THC which overlap (Left) genes included on the SFARI autism candidate list with homologs in rhesus macaques, and (Right) genes with DMRs in sperm from fathers of children with autism versus without (27).
Methylation changes with THC in rhesus sperm are consistent with effects of cannabis in humans
We tested for significant overlap of genes annotated to DMRs in our THC-exposed rhesus macaques with genes annotated to DMCs in our prior study of sperm from 18 cannabis users and 24 non-users using WGBS (25). The top 10K human cannabis DMCs annotated to 7,224 unique genes (rhesus homologs = 4,053 genes). Of these homologs, 1,138 overlapped with pre-THC versus heavy-THC DMR genes in this study, significantly more than expected by chance (p=1.7e-13; Figure 3B – left). Pre-THC vs post-THC rhesus DMRs were similarly enriched for genes annotated to DMCs post-cannabis discontinuation in humans (n=1,197 overlap; p=3.3e-08; Figure 3B – right) (25).
Discontinuation of THC in rhesus macaques restores methylation in a subset of DMRs
Out of 7,627 DMRs between pre-THC and heavy-THC, 1,613 DMRs overlapped DMRs in heavy-THC versus post-THC sperm samples and demonstrated an overall pattern of reversal post-THC toward the level of methylation pre-THC (Figure 3C – left). A similar number (1,601) of pre-THC versus heavy-THC DMRs overlapped with pre-THC versus post-THC DMRs, that did not significantly return to baseline levels after the THC washout period (Figure 3C – right).
Chronic THC use alters methylation in genes related to nervous system development and function
We next examined the potential functional relevance of genes annotated to DMRs following THC exposure. Among the pre-THC versus heavy-THC DMRs, the top canonical pathways associated with THC were the “synaptogenesis signaling pathway”, “signaling by Rho family GTPases”, and “3-phosphoinositide biosynthesis” (Supplemental Table 8). Top canonical pathways enriched among DMRs restored post-THC discontinuation included several terms related to nervous system signaling such as the “BEX2 (brain-expressed X-linked 2) signaling pathway”, “Ephrin receptor signaling”, and the “synaptogenesis signaling pathway”. We also observed large activity scores in the “HIPPO signaling”, “AMPK signaling”, and “sperm motility” canonical pathways. The non-restored DMRs were similarly enriched for genes in the “synaptogenesis signaling pathway”, as well as the “PPAR Signaling” canonical pathway.
Exposure to THC is associated with altered DNA methylation at loci enriched for candidate autism spectrum disorder (ASD) genes
We mapped candidate autism spectrum disorder (ASD) genes from the Simons Foundation Autism Research Initiative (SFARI) database to homologs in rhesus macaques (26). Of 964 candidate ASD gene homologs, 348 were annotated to pre-THC versus heavy-THC sperm DMRs (hypergeometric test p=1.8e-19; Figure 3D – left). Furthermore, 208 out of these 348 genes were near DMRs between heavy-THC and post-THC sperm, suggesting potential reversal of methylation in some ASD candidate genes following THC abstinence. We also observed significant enrichment of pre-THC versus heavy-THC DMR genes in genes previously identified as differentially methylated in sperm from fathers of children with autism versus those without (Figure 3D – right; p=3.6e-12) (27).
DISCUSSION
This is the first study to examine the impact of chronic THC use on measures of reproductive health in a translational rhesus macaque model and whether discontinuation of THC mitigates these effects. Chronic THC use resulted in significant testicular atrophy, increased gonadotropins, decreased serum sex steroids, and increased DNA fragmentation, suggestive of primary testicular failure. Discontinuation of THC resulted in partial recovery of testicular volume, sex steroids and sperm DNA integrity. The underlying mechanism for the observed testicular effects is in part secondary to reduced seminiferous tubule diameter and germ cell layers. However, it does not appear to be secondary to decreased vascularity as capillary density was maintained during THC exposure.
We performed proteomic analysis of collected seminal fluid to better understand the molecular mechanisms underlying the impact of THC exposure on male reproductive health. Proteins in the seminal fluid are involved in sperm protection (28), capacitation (29), acrosome reaction, and sperm-egg binding and fertilization (30). Given the increasing prevalence of assisted reproductive technologies (ART) and limited diagnostic tools to assess male infertility, there is a growing focus on proteomic analysis of the seminal fluid to potentially identify specific protein biomarkers for infertility, and as a prediction of ART success (31). Analysis of the seminal fluid proteome revealed differential expression of 80 proteins following any THC exposure, of which 60 were restored following THC discontinuation. Among the top DEPs, CHIT1 has been previously associated with oligozoospermia (low sperm count) (32), and MMP9 with sperm concentration (33) and motility (34). We also observed a trend for dose-dependent effect of THC exposure on several proteins relevant to male fertility that were not restored post-THC. For example, HSPA5, heat shock protein 5, is widely expressed in male reproductive tissues (35) and is decreased in patients with idiopathic asthenospermia (reduced sperm motility) (36). Loss of the ubiquitin ligase HERC4 in mice is associated with decreased sperm maturation and motility, resulting in reduced fertility (37). Additionally, several proteins related to “fibrinolytic” activity were increased with THC exposure, which may increase seminal clotting and ultimately affect fertility (38). This finding is supported by our observation of decreased liquid fraction and increased concentration of sperm per volume of seminal fluid following THC consumption. We also found dysregulation of pathways related to cellular stress and immune-related signaling which is similar to a prior study focused on the impact of oxidative stress on the seminal fluid proteome (39). THC dose was significantly correlated with the WGNCA “green” module, which contained several proteins previously reported as candidate seminal plasma biomarkers related to male fertility and oxidative stress such as TEX101 (Testis-Expressed Protein 101) (Supplemental Table 2) (20, 21).
Chronic THC exposure in rhesus macaques was associated with altered methylation in genes involved in neurodevelopment and ASD. WGBS identified 30,464 CpG sites with significantly different DNA methylation in heavy-THC-exposed sperm versus pre-THC-exposed sperm (p<0.00001; delta>0.1), and discontinuation of THC restored methylation in a subset of loci toward pre-THC exposure levels. Genes annotated to DMRs (Supplemental Table 6) following THC exposure were enriched in genes involved in nervous system development and function including brain-derived neurotropic factor (BDNF), several members of the cadherin family, ephrin receptor family, glutamate receptor genes, and multiple synapsin genes. In addition, DMRs were enriched for genes involved in sperm motility (e.g., adenylate cyclase 10) and endocannabinoid signaling canonical pathways (e.g., several calcium voltage-gated channel subunit genes). Additionally, there was overlap of candidate ASD genes with genes that were differentially methylated following THC exposure in our study. These results from rhesus macaques are similar to prior studies (40) in rats and humans and suggests the need for better understanding the contributions of paternal cannabis use to offspring neurodevelopment given emerging literature demonstrating a positive association between prenatal cannabis exposure and increased ASD incidence in offspring (41, 42).
Similar to our findings, a prior study of 18 cannabis users and 24 cannabis non-users in humans found significantly different DNA methylation in sperm between groups (43). Genes associated with altered CpG sites were enriched with those involved in development, including cardiogenesis and neurodevelopment. When cannabis was discontinued for one spermatogenic cycle, many of the alterations in sperm DNA methylation between groups were restored. The partial mitigation of DNA methylation changes observed after cannabis abstinence may be because the sperm in the ejaculate after one spermatogenic cycle represent a mixture of sperm formed after the cannabis use was stopped in addition to sperm formed prior to cannabis discontinuation that has not cleared. However, our rhesus macaque study had a longer period of THC abstinence, approximately two spermatogenic cycles, and similarly reported an amelioration of cannabis-associated methylation changes in sperm, but not full resolution. Thus, the residual persistent methylation following cannabis and THC abstinence observed in humans and our rhesus macaque study respectively may reflect alterations that originated in the spermatogonia.
The current lack of understanding regarding the effects of cannabis on male fertility is due in part to the paucity of relevant preclinical models with strong translation to human health. Our study demonstrated the translational strength of the rhesus macaque model to study the impact of cannabis on male reproductive health and the sperm epigenome. This study demonstrated that seminal fluid proteome homology was 99.4% between humans and rhesus macaques, and an overlap of genes annotated to DMRs in rhesus macaques following THC with published gene lists annotated to DMCs between human cannabis users and control males. In addition, we found similar functional enrichment in this rhesus macaque study with exposure and after THC abstinence compared to a prior human study of cannabis users versus non-users (43). Top categories in both species were in terms associated with nervous system development and cardiovascular system development.
Our study has many strengths; it is the first study examining the impact of THC on the sperm epigenome in the rhesus macaque and reveals methylation loci impacting genes in sperm involved in potential transmission of neurobehavioral and health outcomes to future offspring. Our translational rhesus macaque model also allowed for standardization of subject variability, THC exposure and experimental manipulation, aspects not achievable or ethical in humans, to elucidate direct biological consequences of chronic cannabis use while controlling for potential confounders. Moreover, this model avoids the confounding effects of polysubstance use, different modes of cannabis delivery, and reliance on patient self-reporting, all of which have plagued human studies(44, 45). In addition, the use of THC edibles ensured rigor and reproducibility by allowing exposure to precisely measured THC without confounding from cannabis smoke. Limitations of this study include the small animal cohort size, which was addressed by using a single-case longitudinal experimental design, where each male served as its own control during the study to minimize inter-animal variability. Although non-sedated collaborative semen sample collection was performed early in the morning to try to avoid self-ejaculation prior, it is possible that we were not always successful in doing so and that can impact the semen parameter and sperm count in the collected sample. Future studies are planned to increase the animal cohort size, over a longer time interval in order to glean more information relating to chronic THC use. Moreover, we plan to expand our studies to include other THC delivery modalities (e.g., vaping and smoking) and to assess the impact on offspring outcomes, including neurodevelopment.
CONCLUSIONS
In summary, chronic THC use adversely impacts male reproductive health and alters methylation of genes related to nervous system development and function, including those linked to ASD that may impact long term offspring outcomes in a translational rhesus macaque model. Discontinuation of THC for two spermatogenic cycles resulted in partially restored reproductive health parameters and methylation in only a subset of DMRs. Our study’s findings are the first to provide a comprehensive understanding of the benefit and minimum duration of abstinence needed after chronic THC use. These data can be translated directly to the clinical setting to guide healthcare providers when counseling patients and couples regarding cannabis use prior to attempting to conceive.
Supplementary Material
Supplemental Table 1. Top 10 abundance-ranked rhesus macaque proteins. These 10 abundance-ranked proteins make up 59.2% of the total proteome (based on summed reporter ion signal).
Supplemental Table 2. STRING Terms enriched in the proteome WGCNA MEgreen module.
Supplemental Table 3. Seminal fluid nominally significant (p<0.05) DEPs THC vs Pre-THC sorted by minumum p-value.
Supplemental Table 4. IPA Canonical Pathways enriched among THC-DEPs (filtered for - log(p-value)>1.3 and sorted by significance).
Supplemental Table 5. STRING Terms enriched among THC-Differentially expressed proteins (DEPs)
Supplemental Table 6. Significant differentially methylated regions (DMRs) from WGBS sorted by genomic location.
Supplemental Table 7. Differentially methylated CpGs (DMCs) sites and differentially methylated regions (DMRs) for each THC pairwise dosing comparison. The largest absolute number of DMCs and DMRs was demonstrated between heavy-THC relative to pre-THC and post-THC.
Supplemental Table 8. IPA Canonical Pathways enriched among DMRs (filtered for -log(p-value)>1.3 and sorted by significance).
Supplemental Figure 1. No difference in capillary density at baseline, during THC exposure, and after THC is discontinued. Representative high-power fields (100X) of testis showing ERG positive endothelial cell nuclei (arrowheads) at baseline (A) and after THC is discontinued (B).
Supplemental Figure 2. Sperm WGBS summary. (A) Density plot of raw CpG methylation rates calculated as the number of methylated reads/total reads per CpG. (B) Analysis of WGBS principal components correlated with phenotypes and experimental conditions.
Acknowledgments:
We would like to thank the veterinary, husbandry and Behavioral Services Unit staff who provided excellent care for the animals used in this study, in particular Dr. Lauren Drew Martin, Dr. Heather Sidener, Travis Hodge, Trent Crowley, Lisa Houser and Breanna Kolwitz. Additionally, we would like to thank the Endocrine Technologies Core, Assisted Reproductive Core, and Flow Cytometry Core at the Oregon National Primate Research Center as well as the Bioanalytical Shared Resource/Pharmacokinetics Core and the Proteomics Shared Resource at Oregon Health & Science University. Lastly, we would like to thank Thomas O’Leary, director of the Oregon Health & Science University Andrology Lab, and the National Institutes of Health NIDA Drug Supply Program. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Funding:
American Society for Reproductive Medicine Pilot and Exploratory grant, NIH P51 OD011092, NIH R01 OD028223–01, RSDP NIH/NICHD K12 HD000849, NIH/NIDA DP1 DA056493–01, Oregon Health & Science University Medical Research Foundation Award, Oregon Health & Science University Exploratory Research SEED Grant, and Silver Family Faculty Excellence and Innovation Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None of the authors have financial or other relationships that could result in a conflict of interest.
Presentation: Presented as an oral presentation at the 78th American Society for Reproductive Medicine Annual Meeting in Anaheim, California, October 24, 2022.
Attestation: Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Data and materials availability: All data are available in the main text or the supplementary materials. The raw and processed whole genome bisulfite sequencing datasets used and/or analyzed during the current study are publicly accessible through NCBI Sequence Read Archive (SRA) via accession series PRJNA910175.
Article type: Cohort study
References:
- 1.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health 2020.
- 2.Optimizing natural fertility: a committee opinion. Fertil Steril 2022;117:53–63. [DOI] [PubMed] [Google Scholar]
- 3.Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW. Cannabis and Male Fertility: A Systematic Review. J Urol 2019;202:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalterio S, Badr F, Bartke A, Mayfield D. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science 1982;216:315–6. [DOI] [PubMed] [Google Scholar]
- 5.Symons A, Teale J, Marks V. Proceedings: Effect of delta9-tetrahydrocannabinol on the hypothalamic-pituitary-gonadal system in the maturing male rat. The Journal of endocrinology 1976;68:43P–4P. [PubMed] [Google Scholar]
- 6.Hedges JC, Hanna CB, Bash JC, Boniface ER, Burch FC, Mahalingaiah S et al. Chronic delta-9-tetrahydrocannabinol exposure impacts testicular volume and male reproductive health in rhesus macaques Fertility and sterility 2022. [DOI] [PMC free article] [PubMed]
- 7.Ryan KS, Mahalingaiah S, Campbell LR, Roberts VH, Terrobias JJD, Naito CS et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F&S Science 2021. [DOI] [PMC free article] [PubMed]
- 8.Steigerwald S, Wong PO, Cohen BE, Ishida JH, Vali M, Madden E et al. Smoking, vaping, and use of edibles and other forms of marijuana among US adults. Annals of internal medicine 2018;169:890–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick ME, Miech RA, Kloska DD, Wagner AC, Johnston LD. Trends in Marijuana Vaping and Edible Consumption From 2015 to 2018 Among Adolescents in the US. JAMA Pediatrics 2020;174:900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf DP, Thomson JA, Zelinski‐Wooten MB, Stouffer RL. In vitro fertilization‐embryo transfer in nonhuman primates: The technique and its applications. Molecular reproduction and development 1990;27:261–80. [DOI] [PubMed] [Google Scholar]
- 11.Sibal LR, Samson KJ. Nonhuman primates: a critical role in current disease research. ILAR journal 2001;42:74–84. [DOI] [PubMed] [Google Scholar]
- 12.Hedges JC, Hanna CB, Bash JC, Boniface ER, Burch FC, Mahalingaiah S et al. Chronic exposure to delta-9-tetrahydrocannabinol impacts testicular volume and male reproductive health in rhesus macaques. Fertility and sterility 2022;117:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018;13:1208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan KS, Mahalingaiah S, Campbell LR, Roberts VHJ, Terrobias JJD, Naito CS et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F S Sci 2021;2:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannabis Clinicians Colorado. Medical marijuana new patient success guide Available from: http://coscc.org/wp-content/uploads/2016/07/CCC-General-CannabisAsMedicine-2017DRAFT.pdf Last accessed October 1, 2022.
- 16.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018;49:12–9. [DOI] [PubMed] [Google Scholar]
- 17.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 1980;28:409–16. [DOI] [PubMed] [Google Scholar]
- 19.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candenas L, Chianese R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannarella R, Crafa A, Barbagallo F, Mongioì LM, Condorelli RA, Aversa A et al. Seminal Plasma Proteomic Biomarkers of Oxidative Stress. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molaro A, Hodges E, Fang F, Song Q, McCombie WR, Hannon GJ et al. Sperm methylation profiles reveal features of epigenetic inheritance and evolution in primates. Cell 2011;146:1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang L, Zhou Y, Liu S, Jiang J, Bickhart DM, Null DJ et al. Comparative analyses of sperm DNA methylomes among human, mouse and cattle provide insights into epigenomic evolution and complex traits. Epigenetics 2019;14:260–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Liu S, Mi S, Li W, Zhang S, Ding X et al. Comparative Analyses of Sperm DNA Methylomes Among Three Commercial Pig Breeds Reveal Vital Hypomethylated Regions Associated With Spermatogenesis and Embryonic Development. Front Genet 2021;12:740036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrott R, Murphy SK, Modliszewski JL, King DE, Hill B, Itchon-Ramos N et al. Refraining from use diminishes cannabis-associated epigenetic changes in human sperm. Environ Epigenet 2021;7:dvab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee-Basu S, Packer A. SFARI Gene: an evolving database for the autism research community. In: The Company of Biologists Limited, 2010. [DOI] [PubMed]
- 27.Garrido N, Cruz F, Egea RR, Simon C, Sadler-Riggleman I, Beck D et al. Sperm DNA methylation epimutation biomarker for paternal offspring autism susceptibility. Clin Epigenetics 2021;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 1994;16:259–67. [DOI] [PubMed] [Google Scholar]
- 29.Manjunath P, Thérien I. Role of seminal plasma phospholipid-binding proteins in sperm membrane lipid modification that occurs during capacitation. J Reprod Immunol 2002;53:109–19. [DOI] [PubMed] [Google Scholar]
- 30.Viana AGA, Martins AMA, Pontes AH, Fontes W, Castro MS, Ricart CAO et al. Proteomic landscape of seminal plasma associated with dairy bull fertility. Scientific Reports 2018;8:16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samanta L, Parida R, Dias TR, Agarwal A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reproductive Biology and Endocrinology 2018;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kratz EM, Zurawska-Plaksej E, Solkiewicz K, Kokot I, Faundez R, Piwowar A. Investigation of seminal plasma chitotriosidase-1 and leukocyte elastase as potential markers for ‘silent’ inflammation of the reproductive tract of the infertile male - a pilot study. J Physiol Pharmacol 2020;71. [DOI] [PubMed] [Google Scholar]
- 33.Kareskoski M, Vakkamäki J, Laukkanen K, Palviainen M, Johannisson A, Katila T. Matrix metalloproteinase (MMP)-2, MMP-9, semen quality and sperm longevity in fractionated stallion semen. Theriogenology 2021;164:93–9. [DOI] [PubMed] [Google Scholar]
- 34.Kurzawski M, Kaczmarek M, Kłysz M, Malinowski D, Kazienko A, Kurzawa R et al. MMP2, MMP9 and TIMP2 polymorphisms affect sperm parameters but not fertility in Polish males. Andrologia 2017;49. [DOI] [PubMed] [Google Scholar]
- 35.Sadeghi N, Tavalaee M, Shahverdi A, Sengupta P, Leisegang K, Saleh R et al. Vulnerability of The Male Reproductive System to SARS-CoV-2 Invasion: Potential Role for The Endoplasmic Reticulum Chaperone Grp78/HSPA5/BiP. Cell J 2022;24:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol 2013;31:1395–401. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez CI, Stewart CL. Disruption of the ubiquitin ligase HERC4 causes defects in spermatozoon maturation and impaired fertility. Dev Biol 2007;312:501–8. [DOI] [PubMed] [Google Scholar]
- 38.Lwaleed BA, Greenfield R, Stewart A, Birch B, Cooper AJ. Seminal clotting and fibrinolytic balance: a possible physiological role in the male reproductive system. Thromb Haemost 2004;92:752–66. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol 2013;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrott R, Greeson KW, King D, Symosko Crow KM, Easley CAt, Murphy SK. Cannabis alters DNA methylation at maternally imprinted and autism candidate genes in spermatogenic cells. Syst Biol Reprod Med 2022:1–13. [DOI] [PMC free article] [PubMed]
- 41.Corsi DJ, Donelle J, Sucha E, Hawken S, Hsu H, El-Chaâr D et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nature medicine 2020;26:1536–40. [DOI] [PubMed] [Google Scholar]
- 42.Sajdeya R, Brown JD, Goodin AJ. Perinatal Cannabis Exposures and Autism Spectrum Disorders. Med Cannabis Cannabinoids 2021;4:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schrott R, Murphy SK, Modliszewski JL, King DE, Hill B, Itchon-Ramos N et al. Refraining from use diminishes cannabis-associated epigenetic changes in human sperm. Environmental epigenetics 2021;7:dvab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassan FL, Arvizu M, Mínguez-Alarcón L, Williams PL, Attaman J, Petrozza J et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Human Reproduction 2019;34:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise LA, Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Sørensen HT et al. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health 2018;72:208–15. [DOI] [PubMed] [Google Scholar]
- 46.Houser LA, Ramsey C, de Carvalho FM, Kolwitz B, Naito C, Coleman K et al. Improved Training and Semen Collection Outcomes Using the Closed Box Chair for Macaques. Animals (Basel) 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hedges JC, Hanna CB, Bash JC, Boniface ER, Burch FC, Mahalingaiah S et al. Chronic exposure to delta-9-tetrahydrocannabinol impacts testicular volume and male reproductive health in rhesus macaques. Fertil Steril 2022;117:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NISWENDER GD, SPIES HG. Serum levels of luteinizing hormone, follicle-stimulating hormone and progesterone throughout the menstrual cycle of rhesus monkeys. The Journal of Clinical Endocrinology & Metabolism 1973;37:326–8. [DOI] [PubMed] [Google Scholar]
- 49.Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Infor 2009;2:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eng JK, Jahan TA, Hoopmann MR. Comet: an open-source MS/MS sequence database search tool. Proteomics 2013;13:22–4. [DOI] [PubMed] [Google Scholar]
- 51.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 2013;41:D808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greeson KW, Fowler KL, Estave PM, Kate Thompson S, Wagner C, Clayton Edenfield R et al. Detrimental effects of flame retardant, PBB153, exposure on sperm and future generations. Scientific reports 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evenson DP. Sperm Chromatin Structure Assay (SCSA®): 30 years of experience with the SCSA®. In: Sperm chromatin: Springer, 2011:125–49. [Google Scholar]
- 55.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011;27:1571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mordaunt CE, Mouat JS, Schmidt RJ, LaSalle JM. Comethyl: a network-based methylome approach to investigate the multivariate nature of health and disease. Brief Bioinform 2022;23. [DOI] [PMC free article] [PubMed]
- 58.Fortin JP, Triche TJ Jr., Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017;33:558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian Y, Morris TJ, Webster AP, Yang Z, Beck S, Feber A et al. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017;33:3982–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng H, Wu H. Differential methylation analysis for bisulfite sequencing using DSS. Quant Biol 2019;7:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM et al. Ensembl 2022. Nucleic Acids Res 2022;50:D988–d95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 2011;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krämer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014;30:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Top 10 abundance-ranked rhesus macaque proteins. These 10 abundance-ranked proteins make up 59.2% of the total proteome (based on summed reporter ion signal).
Supplemental Table 2. STRING Terms enriched in the proteome WGCNA MEgreen module.
Supplemental Table 3. Seminal fluid nominally significant (p<0.05) DEPs THC vs Pre-THC sorted by minumum p-value.
Supplemental Table 4. IPA Canonical Pathways enriched among THC-DEPs (filtered for - log(p-value)>1.3 and sorted by significance).
Supplemental Table 5. STRING Terms enriched among THC-Differentially expressed proteins (DEPs)
Supplemental Table 6. Significant differentially methylated regions (DMRs) from WGBS sorted by genomic location.
Supplemental Table 7. Differentially methylated CpGs (DMCs) sites and differentially methylated regions (DMRs) for each THC pairwise dosing comparison. The largest absolute number of DMCs and DMRs was demonstrated between heavy-THC relative to pre-THC and post-THC.
Supplemental Table 8. IPA Canonical Pathways enriched among DMRs (filtered for -log(p-value)>1.3 and sorted by significance).
Supplemental Figure 1. No difference in capillary density at baseline, during THC exposure, and after THC is discontinued. Representative high-power fields (100X) of testis showing ERG positive endothelial cell nuclei (arrowheads) at baseline (A) and after THC is discontinued (B).
Supplemental Figure 2. Sperm WGBS summary. (A) Density plot of raw CpG methylation rates calculated as the number of methylated reads/total reads per CpG. (B) Analysis of WGBS principal components correlated with phenotypes and experimental conditions.


