Abstract
BACKGROUND:
Previous preclinical studies have demonstrated an altered gut microbiome after traumatic injury; however, the impact of sex on dysbiosis remains unknown. We hypothesized that the “pathobiome” phenotype induced by multicompartmental injuries and chronic stress is host sex specific with unique microbiome signatures.
METHODS:
Male and proestrus female Sprague-Dawley rats (n=8/group) aged 9–11 weeks were subjected to either multicompartmental injury (PT) (lung contusion, hemorrhagic shock, cecectomy, bifemoral pseudofractures), PT plus 2-hours daily chronic restraint stress (PT/CS) or naive controls. Fecal microbiome was measured on days 0 and 2 using high-throughput 16S rRNA sequencing and QIIME2 bioinformatics analyses. Microbial alpha diversity was assessed using Chao1 (number of different unique species) and Shannon (species richness and evenness) indices. Beta-diversity was assessed using principle coordinate analysis. Intestinal permeability was evaluated by plasma occludin and lipopolysaccharide binding protein (LBP). Histologic evaluation of ileum and colon tissues were scored for injury by a blinded pathologist. Analyses were performed in GraphPad and R, with significance defined as p<0.05 between males versus females.
RESULTS:
At baseline, females had significantly elevated alpha-diversity (Chao1, Shannon indices) compared to males (p<0.05) which was no longer present 2-days post injury in PT and PT/CS. Beta diversity also differed significantly between males and females after PT (p = 0.01). At day 2, the microbial composition in PT/CS females was dominated by Bifidobacterium; whereas PT males demonstrated elevated levels of Roseburia (p<0.01). PT/CS males had significantly elevated ileum injury scores compared to females (p=0.0002). Plasma occludin was higher in PT males compared to females (p=0.004); plasma LBP was elevated in PT/CS males (p=0.03).
CONCLUSIONS:
Multicompartmental trauma induces significant alterations in microbiome diversity and taxa, but these signatures differ by host sex. These findings suggest that sex is an important biological variable that may influence outcomes after severe trauma and critical illness.
LEVEL OF EVIDENCE:
Not applicable – basic science
Keywords: injury, microbiome, polytrauma, sex differences
Media Summary:
Severe traumatic injury in a rodent model alters the intestinal microbiome in a sex-specific manner. Females have increased diversity and unique microbial signatures compared to males. These sex-specific gut dysbiosis arrays may influence outcomes after multicompartmental trauma.
BACKGROUND
The intestinal microbiome is a complex system of microbial organisms which influences the immune system (1). Sepsis and critical illness induce changes in the microbiome such as a sharp decline in diversity and the dominance of pathogenic organisms which is collectively referred to as the pathobiome (2). Intestinal injury and subsequent breakdown of tight-junction barriers can result in increased intestinal permeability, which can perpetuate this state of dysbiosis (3). Plasma biomarkers such as occludin, a transmembrane component of tight junctions, and lipopolysaccharide binding protein (LBP), an acute-phase protein, have been linked to increased intestinal permeability (4, 5). Severe traumatic injury in both animal and human studies have been shown to acutely induce dysbiosis and intestinal injury; these changes have been correlated with hospital length of stay and even mortality in trauma patients (6–12). However, the influence of traumatic insults on the microbiome in females specifically remains unstudied.
Although not studied following severe injury, in animal models of sepsis, females demonstrated improved recovery of microbial diversity and commensal bacteria composition compared to males (13). There is a paucity of data investigating sexual dimorphism of intestinal injury after trauma and hemorrhagic shock; however, studies of ischemia-reperfusion have shown female resilience to intestinal injury and permeability compared to males (14, 15). Estrogen has been implicated as a potential contributing factor protecting against intestinal injury and permeability in females (16–18).
In order to control for factors such as age, diet, antibiotic administration, and blood transfusion, all of which can all alter the microbiome, we utilized a preclinical model of multicompartmental injury to evaluate for sex differences in the microbiome after trauma (19–22). This rodent model consisted of multiple injuries with or without replicated stress of a prolonged intensive care unit (ICU) stay. Our aim was to characterize acute changes in alpha-diversity, beta-diversity, microbial composition of the intestinal microbiome along with intestinal injury and permeability between males and females subjected to severe trauma with or without chronic stress. We hypothesized that females would retain more commensal bacteria and microbial diversity and demonstrate more resilience to intestinal injury and permeability after injury compared to their male counterparts.
METHODS
Animals
Male and female Sprague-Dawley rats (Charles River, Wilmington, MA) aged 9–11 weeks weighing 189 to 475 grams were housed in pairs on arrival in conventional rodent housing with ad lib access to standard irradiated pelleted diet and water. Prior to initiation of the experiment, rats were acclimated to a 12-hour light-dark cycle for at least 72 hours in their same cage for at least 10 days. Rats were housed individually after enrollment for the duration of the study to account for potential effects of coprophagy on the microbiome. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC Protocol 202011247). The ARRIVE guidelines were utilized to ensure proper reporting of methods, results, and discussion (see Supplemental Digital Content 1) (23).
Experimental Design
Rodents were randomly assigned to the following cohorts (n=8/group): naïve; multicompartmental injury (PT); and multicompartmental injury plus daily restraint stress (PT/CS) similar to previously described (24). A total of 60 rats were enrolled in the study: 48 assigned to groups (8 males and 8 females per cohort) and 12 as donors for pseudofracture. Based on a power analysis, assuming greater than 80% incidence in control rats at baseline, a 30% change would require a minimum of 8 rats per group. These injury models were chosen to represent common clinical scenarios: blunt chest trauma, hemorrhage, intestinal injury, and lower extremity fractures (PT) and the same multicompartmental injuries with chronic stress (PT/CS) (25, 26). Due to the presence of incisions on experimental groups and PT/CS rats requiring daily chronic stress, groups could not be blinded during care of the animals. All cohorts were euthanized on postoperative day two unless a humane endpoint was met prior; rats were evaluated and scored twice daily based on set criteria developed with animal care veterinarians (see Table in Supplemental Digital Content 2).
Only female rats in the proestrus phase were enrolled for the study to control for estrous cycle variability, given the protective effects of estrogen during hemorrhagic shock and injury (27). On the day of potential enrollment, female rats were subjected to vaginal lavage with sterile normal saline and cells evaluated on a under a Nikon Eclipse E200 microscope at 40X objective for predominance of nucleated epithelial cells consistent with the proestrus phase (28).
To create a pseudofracture solution to simulate bilateral femur fractures, age-, sex- and weight-matched donor rats were euthanized and bilateral femurs and tibias harvested under aseptic conditions. Female donors were only enrolled in the proestrus phase. All bones were crushed with mortar and pestle and 3mL of normal saline added to produce a pseudofracture solution as previously described (29).
After induction of anesthesia with isoflurane (Patterson Veterinary, Loveland, CA) via nose-cone, 1mg/kg sustained-release buprenorphine (ZooPharm, Laramie, WY) was administered subcutaneously. A unilateral right lung contusion was then performed using a manual nail gun (Arrow, Saddle River, NJ) applied directly to a 12mm plate placed over the rodent’s right axilla. Proposed incisions in the right neck, lower abdominal midline, and medial left thigh were injected with 4mg/kg ropivacaine (Akron, Lake Forest, IL). Next, the left femoral artery and right internal jugular vein were cannulated via cutdown using polyethylene-10 and polyethylene-50 tubing, respectively. The arterial line was connected to a BP-2 Digital Blood Pressure Monitor device (Columbus Instruments, Columbus, OH) for continuous measurement of mean arterial pressure (MAP). Blood was then withdrawn from the venous cannula at a rate of 1mL/minute to results in a MAP of 30–35mmHg for 45 minutes. A midline laparotomy and cecectomy was performed by exteriorizing the cecum, doubly clamping and cutting the cecum, and ligation with a 2–0 silk tie. The cecal stump was then returned to the abdomen and the abdominal wall closed in layers. After 45 minutes, 50% of the shed blood was reinfused and 150μL of pseudofracture bone marrow solution from age-, sex- and weight-matched rats was injected intramuscularly into each medial thigh. Rats were subsequently administered 3mL of subcutaneous normal saline subcutaneously.
Chronic restraint stress was performed in the designated group (PT/CS) starting the day after surgery. Rats were restrained in clear plastic rodent nose cone cylinders (Kent Scientific Corporation, Torrington, CT) for two hours daily; rats were subjected to two minutes of loud constant alarms and repositioning every thirty minutes.
All animals were handled daily after enrollment; clean catch stool was obtained on day zero (prior to intervention) and harvested from the distal descending colon at time of euthanasia on day two. Stool samples were immediately snap frozen in liquid nitrogen and then stored at −80°C until further processing.
Blood Studies
Blood was obtained via cardiac puncture on day of euthanasia in a 10mL syringe containing 0.1mL of heparinized saline (1000units/mL). Blood was aliquoted and centrifuged for plasma at 800g for ten minutes; plasma was then stored in a −80°C freezer. Plasma occludin and LBP levels were measured using an immunosorbent assay according to manufacturer’s protocols (MyBiosource, San Diego, CA).
Histology
Sections of terminal ileum and descending colon were obtained on day of euthanasia. Tissues were flushed and stored in formalin for 24 hours and then transferred to 70% ethanol. Tissues were sectioned, stained in hematoxylin and eosin (H&E), and embedded in paraffin. Histological slides of terminal ileum and descending colon were analyzed by a blinded veterinary pathologist and scored for injury similar to previously described; each category was graded from 0 (no injury) to 4 (severe changes) (see Tables in Supplemental Digital Content 3 and 4) (24).
Microbiome Analysis
Microbiome profiles were analyzed per our previously described methods (24, 30). Briefly, genomic DNA was extracted from fecal samples and quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the barcoded universal primers (515F/806R) (31, 32). The resulting amplicons were purified and quantified and the amplicon library was generated (33). The purified library was pooled in equimolar concentrations and sequenced on an Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA). Resulting sequences were processed using the Quantitative Insights Into Microbial Ecology (QIIME2) bioinformatics software suite (version 2.2021.2; https://qiime2.org/) in a miniconda environment and subjected to de-multiplexing (34). Subsequent quality-filtering, adapter-trimming, denoising, and removal of non-chimeric amplicons was performed with the DADA2 pipeline using the q2-dada2-plugin using default parameters (35). Alpha-rarefaction was performed at the lowest sequencing depth to avoid the bias of sequencing depth. Bacterial taxonomy was assigned to the amplicon sequence variants (ASV) by implementing the Naïve-Bayes classifier natively implemented in qiime2-dada2 and pre-trained on SILVA reference database (version 138.1, updated March 2021) (36). Community richness (alpha-diversity) metrices included Chao1 and Shannon indices. Community dissimilarities (beta-diversity) were quantitatively evaluated by the Bray-Curtis distance algorithm within QIIME2 and were represented by a principal coordinate analysis (PCoA) plot. The raw read counts were transformed to relative abundances by dividing each value by the total reads per sample and then collapsed to taxonomic levels by summing their respective relative abundances. All samples were batch-processed to avoid bias of DNA extraction or PCR primers/conditions on community composition obtained by amplicon sequencing.
Statistical Analysis
Analyses of alpha-diversity, terminal ileum injury scores, descending colon injury scores, and plasma occludin and LPB levels were performed in GraphPad Prism version 9.1.4 (GraphPad Software, La Jolla, CA) using Brown-Forsythe test and either ordinary one-way analysis of variance (ANOVA) with Tukey’s post hoc test for variables with equal variance or Welch’s ANOVA with Dunnett T3 test for those with unequal variance, with multiple comparisons. Comparisons between males and females were performed with student’s t-test with Welch’s correction for unequal standard deviations. Data are presented as mean±standard deviation with *p<0.05 considered statistically significant. Comparisons between cohorts of beta-diversity (PCoA), bacterial abundance and linear discriminatory analysis (LDA) effect size (LEfSe) were analyzed using the ‘R’ statistical package version 4.1.2 (37, 38). Alpha-diversity is defined as microbial community richness, or the distribution of microbial species in each sample. Community richness (alpha-diversity) metrics included the Chao1 index, which estimates the richness of microbial species present, and the Shannon index which accounts for both richness and evenness of microbial species. Beta-diversity, which represents community dissimilarities between two ecosystems (i.e., inter-individual variability), was assessed using the Bray-Curtis dissimilarity index and was visualized by PCoA plots; analysis was done by permutational analysis of variance (PERMANOVA) test. Unique bacterial taxa driving specific group-specific differences were determined using the biomarker discovery algorithm LEfSe with parameters set at LDA score of >3.0 and p-value <0.01 (37). The normalization method of taxon abundance data consisted of data transformation and scaling. Data transformation was done by generalized log-transformation (base 2) and data scaling by auto-scaling (mean-centered and divided by standard deviation of each variable).
RESULTS
Multicompartmental injury induces changes in microbial diversity and community composition
When assessing both males and females in each cohort at baseline, alpha-diversity demonstrated by Chao1 index and Shannon index were each not significantly different between cohorts (p>0.05). Beta-diversity, as assessed with principle coordinate analysis and PERMANOVA, was also not significantly different between groups at baseline (p=0.5). These data suggest that at baseline, there were no significant differences between rats assigned to experimental groups prior to intervention.
By day two, cohorts of both males and females demonstrated a significant decline in Chao1 index in both PT (45.3±8.3) and PT/CS (51.5±15.8) compared to the naïve group (66.7±17.5, p=0.0007 and p=0.045 respectively) (Fig. 1A). Similarly, the Shannon index was significantly decreased in PT (2.5±0.3) and PT/CS (2.7±0.4) cohorts compared to naïve rats (3.2±0.5, p=0.0005 and p=0.01 respectively) (Fig. 1B). There was no significant difference in the Chao1 or Shannon indices between PT and PT/CS (p=0.42 and p=0.40 respectively) (Fig. 1A–B). Beta-diversity was found to be significantly different between all cohorts (p=0.0001) (Fig. 1C). Together, this demonstrates a significant decline in alpha-diversity and alterations in beta-diversity and microbial composition between cohorts after injury with or without stress. At day zero, microbial composition was extremely similar between groups, with only naïve subjects showing dominance of a commensal bacteria, Ruminococcaceae. By day two, the naïve cohort had high abundances of commensal bacteria such as Lachnospiraceae, Ruminococcus, and Clostridia (p<0.01) (Supplemental Digital Content 5, showing microbiome taxa that are unique for each group.). PT was dominated by Blautia and Bacteroides while PT/CS had Parasutterella and Frisingicoccus (p<0.01) (Supplemental Digital Content 5).
Figure 1A-C.
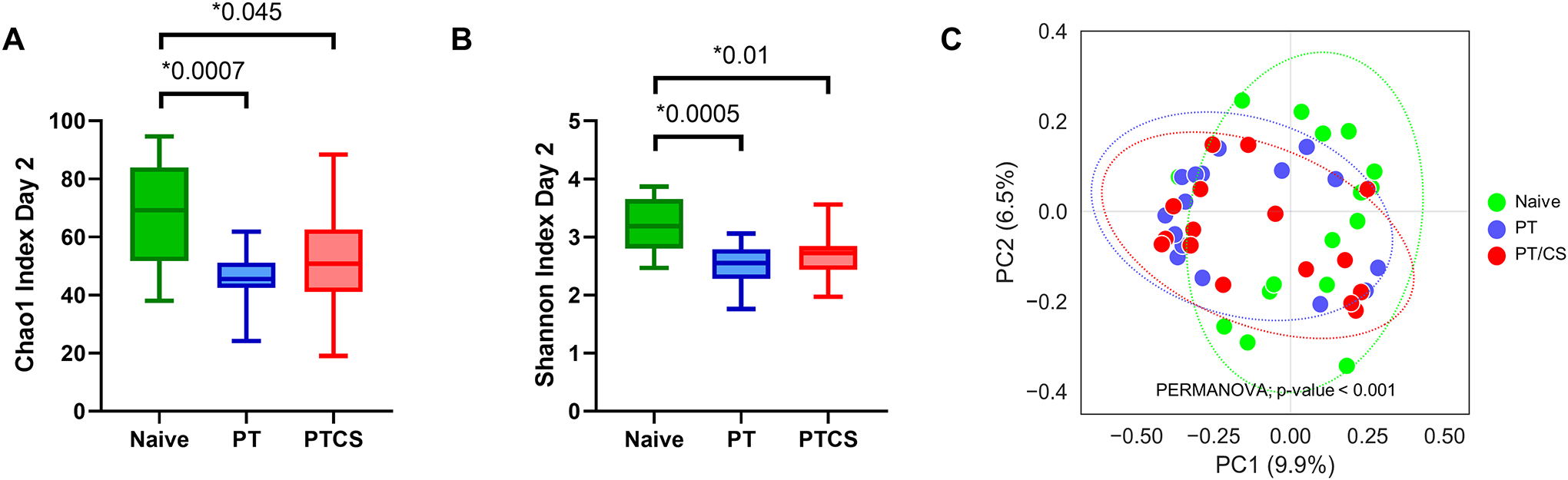
Changes in alpha-diversity represented by the A) Chao1 index at day 2 and B) Shannon index at day 2; and C) principal coordinate analysis (PCoA) plot showing changes in beta-diversity, the measure of differences in biodiversity across cohorts at day 2. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05).
Females demonstrate a sharp decline in microbial species diversity after injury
At day zero, females had a significantly elevated Chao1 indices and Shannon indices compared to males (Fig. 2A–B). At baseline, beta-diversity was not significantly different when comparing males and females (p=0.1) (Fig. 3A). This demonstrates that at baseline, there are differences in both the number and evenness of species between males and females.
Figure 2A-D.
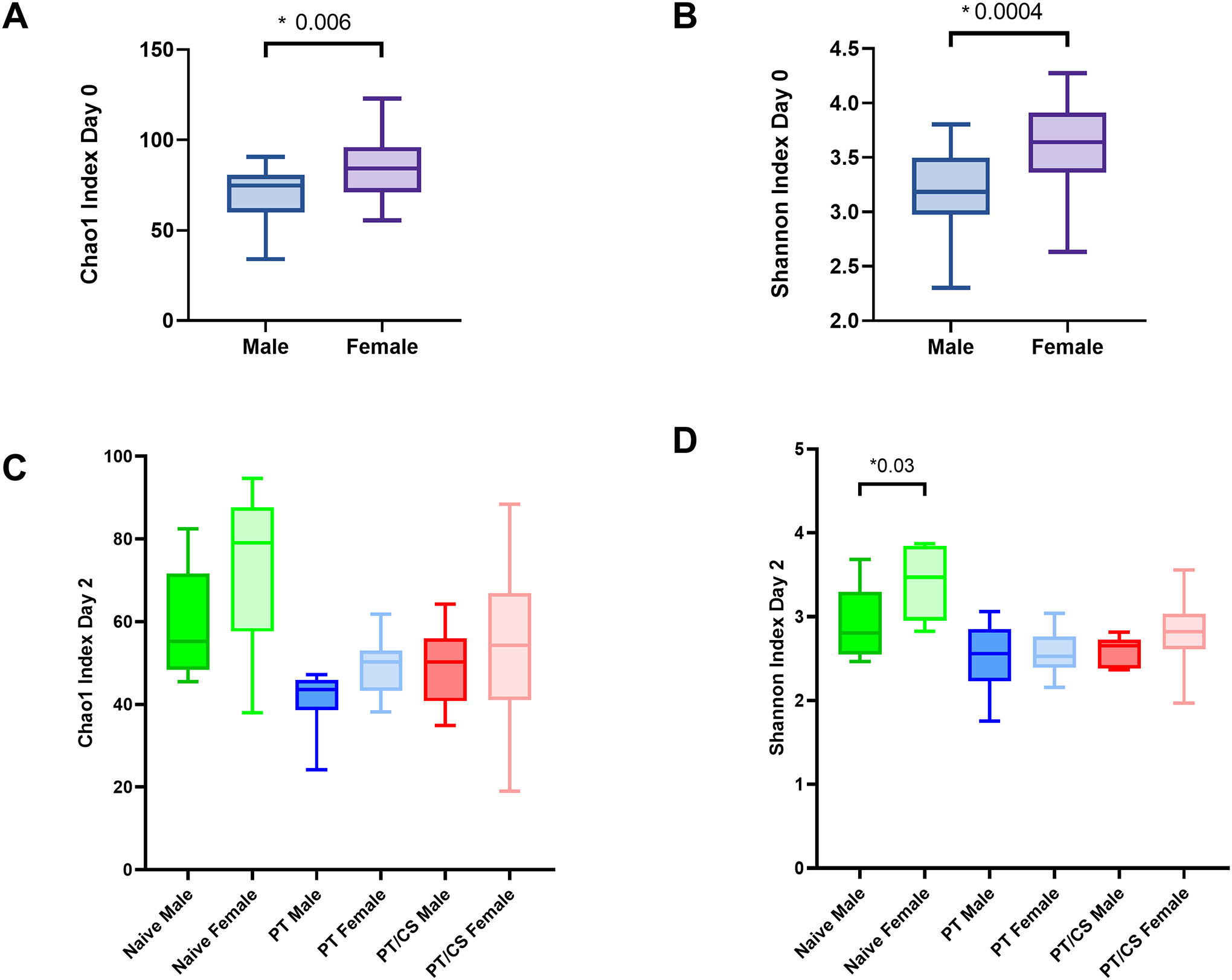
Changes in alpha-diversity between males and females represented by the A) Chao1 index at day 0, B) Shannon index at day 0, C) Chao1 index at day 2, and D) Shannon index at day 2. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05).
Figure 3A-D.
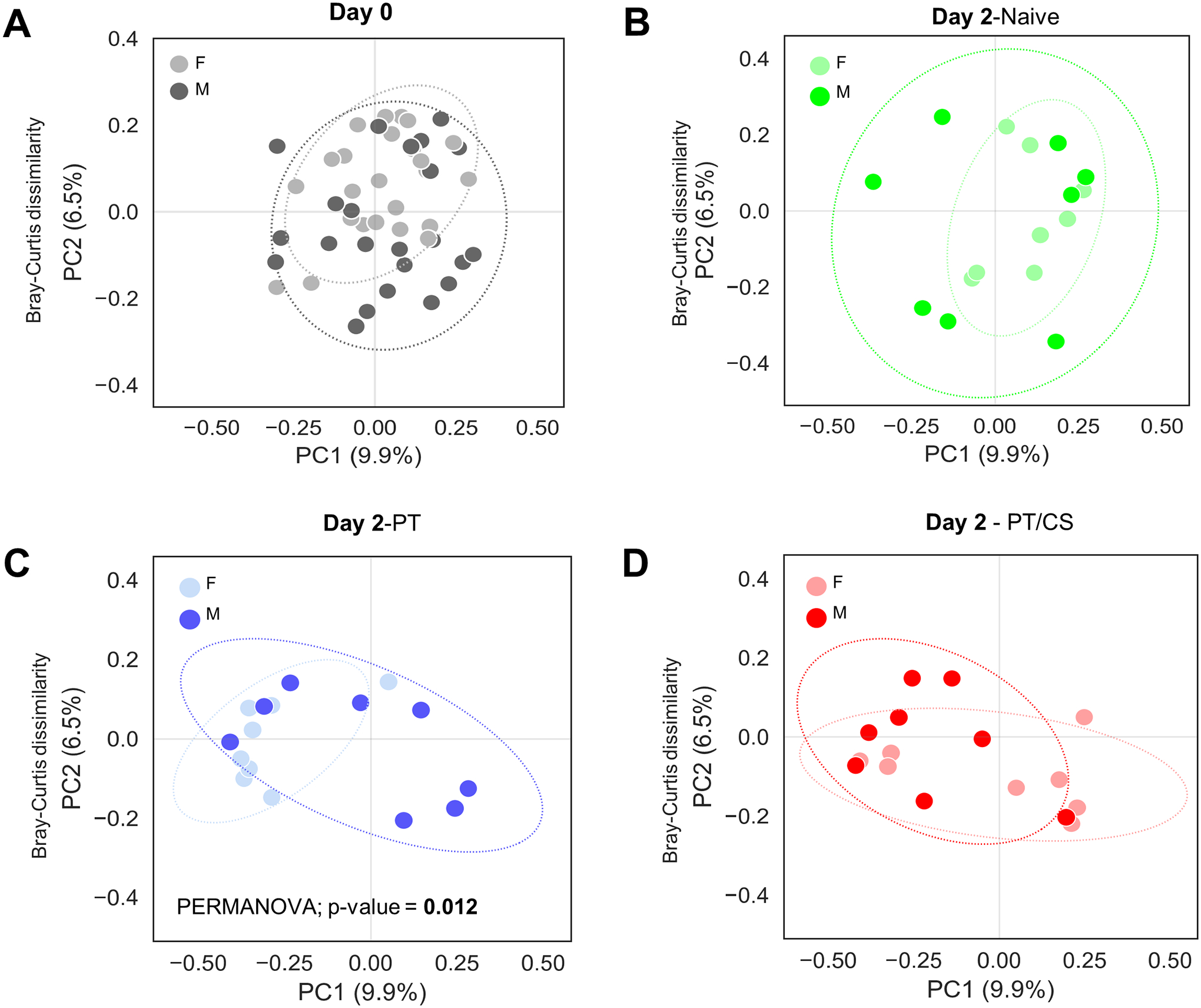
Principal coordinate analysis (PCoA) plots showing changes in beta-diversity, the measure of differences in biodiversity between males and females A) at day 0, and at day 2 for each cohort B) naïve, C) PT and D) PT/CS. Percent on each orthogonal axis represents the proportion of overall variance in the data. PT - polytrauma; PT/CS - polytrauma/chronic stress.
After two days, there were no significant differences in Chao1 index between males and females in the naïve group (Fig. 2C). Similarly, male and female Chao1 index also became similar in PT and PT/CS cohorts (Fig. 2C). Naïve females continued to have a significantly higher Shannon index than males, similar to baseline (Males: 2.9±0.4, Females: 3.4±0.4, p = 0.03) (Fig. 2D). In both PT and PT/CS, there was no longer significant differences between males and females within cohorts (Fig. 2D). Beta-diversity was not significantly different between males and females in the naïve cohort (Fig. 3B). However, there were differences in beta-diversity between sexes in the PT group (p=0.01); PT/CS also showed differences but this did not reach statistical significance (Fig. 3C–D). This indicates that acutely after injury, both males and females have a decline in the overall number and evenness of species, with females having a drastic decline compared to males, since at baseline females had significantly elevated Chao1 and Shannon indices. Differences in beta-diversity between the sexes persist after injury.
Intestinal microbiome composition after injury is unique between host sexes
At baseline, there were unique species among males and females in the intestinal microbiome composition. Evaluation at day zero showed that males had significantly more Lactobacillus, Bifidobacterium, Turicibacteri, and Romboutsia compared to females (p<0.01) (Fig. 4A). Whereas, females had significantly elevated levels of Lachnospiraceae, Murcibaculaceae, Bacteroides, Akkermansia, and Incertae Sedis compared to males (p<0.01) (Fig. 4A). This demonstrates that at baseline, males and females have unique microbiome composition with different commensal bacteria.
Figure 4A-B.
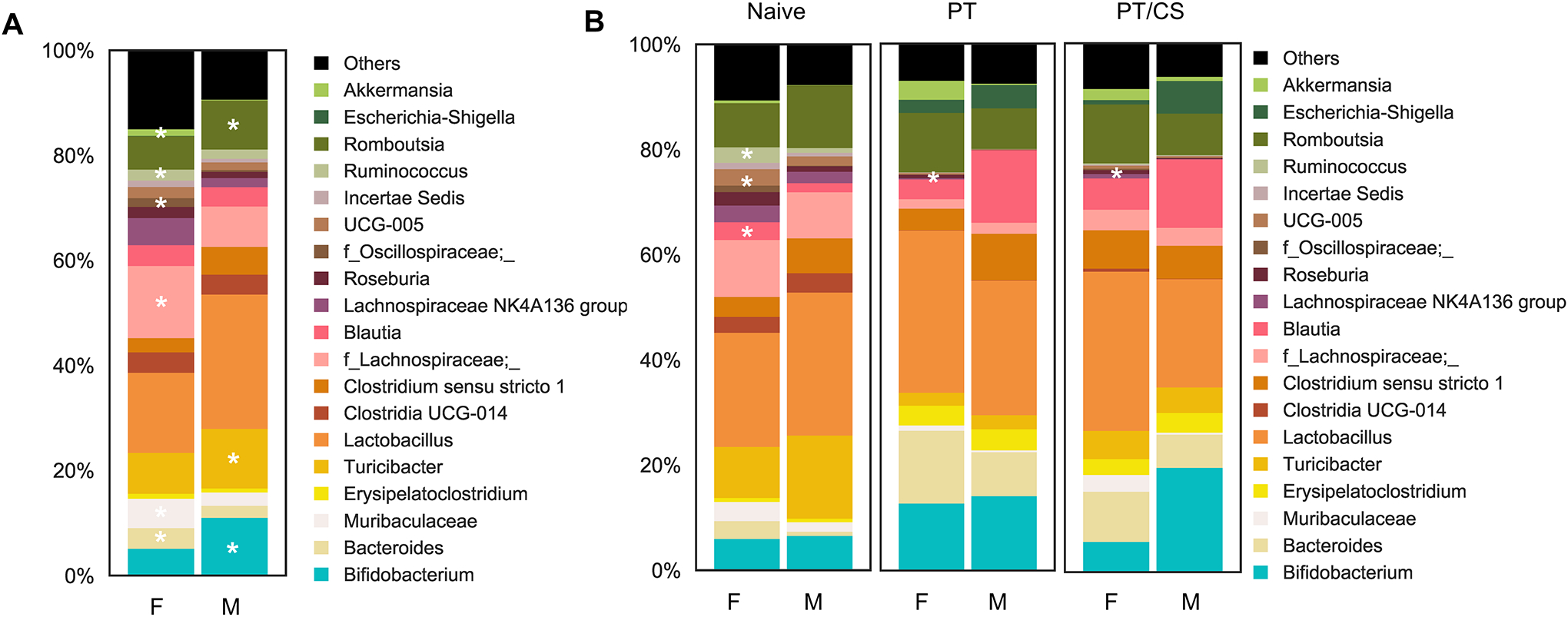
Genus-level microbiome composition between males and females A) at day 0 and B) between cohorts at day 2 [naïve, polytrauma (PT), and polytrauma with chronic stress (PT/CS)]. Unique microbial species identified with *p<0.01.
By day two, naïve rats continued to have unique microbial composition, with males having elevated levels of Oscillospiraceae, Blautia, Ruminococcus, and Bacteroides (p<0.01) (Fig. 4B). PT males were dominated by Ruminococcus and Roseburia compared to their female counterparts (p<0.01) (Fig. 4B). Finally, in the PT/CS cohort, males had elevated levels of Lacnospiraceae and Muribaculaceae compared to females (p<0.01) (Fig. 4B). Females subjected to PT/CS had higher levels of Bifidobacterium than male counterparts (p<0.01) (Fig. 4B).
Intestinal injury is sex-specific after multicompartmental injury and stress
Representative terminal ileum tissue can be found in Supplemental Digital Content 6 (see figures in Supplemental Digital Content 6, showing images of naïve, PT and PT/CS ileum for males and females). There were no significant differences in terminal ileum injury scores between pooled groups (containing both males and females) (p≥0.05). With subgroup analysis comparing total ileum injury scores between sexes within each experimental cohort at day two, there was no significant difference between males and females in the PT group (Fig. 5A). However, there were significantly higher terminal ileum injury scores in males compared to females in the PT/CS group (PT/CS Males: 8.8±1.8, PT/CS Females: 4.1±1.7, p=0.0002) (Fig. 5A). Male PT/CS terminal ileum tissue demonstrated congestion, villi blunting and sloughing, edema of the lamina propria, and leukocytes in the lamina propria.
Figure 5A-B.
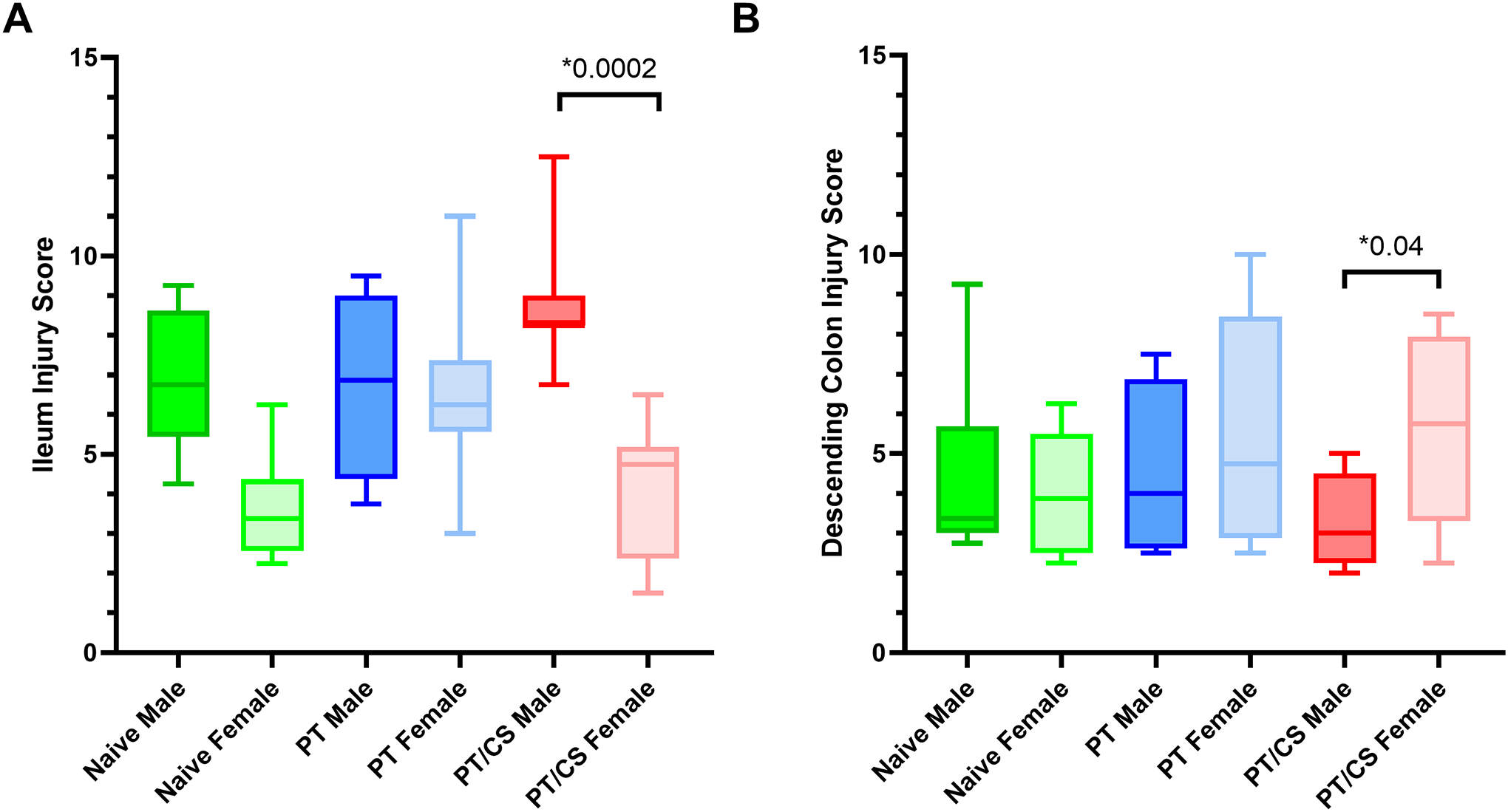
Histologic injury scores in the A) terminal ileum between sexes and D) descending colon between sexes. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05).
Representative descending colon tissue can be found in Supplemental Digital Content 7 (see figures in Supplemental Digital Content 7, showing images of naïve, PT and PT/CS ileum for males and females). There were no significant differences in colon injury scores between pooled groups (containing both males and females) (p≥0.05).
Subgroup analysis of experimental groups to identify differences between descending colon injury scores between males and females did not reveal statistically significant differences in the PT group (Fig. 5B). In the PT/CS group, however, females had significantly elevated descending colon injury scores compared to their male counterparts (PT/CS Males: 3.4±1.2, PT/CS Females: 5.6±2.4, p=0.04) (Fig. 5B). In the PT/CS group, female descending colon tissues were characterized by crypt alterations and inflammatory cell infiltrate.
Intestinal permeability is more prominent in males compared to females
Plasma occludin levels at day two between cohorts of both males and females demonstrated significantly elevated levels in both PT and PT/CS compared to naïve (Naïve: 7.6±1.3, PT: 34.4±16.2*, PT/CS: 25.42±12.8*) (Fig. 6A). There was no significant difference in plasma occludin levels in PT and PT/CS (Fig. 6A). Performance of subgroup analysis to evaluate sex differences within each cohort demonstrated no sex differences in plasma occludin levels between naïve males and females (Fig. 6C). In the PT group, males had significantly elevated plasma occludin compared to their female counterparts (PT Males: 45.0±13.9*, PT Females: 23.7±10.4) (Fig. 6C).
Figure 6A-D.
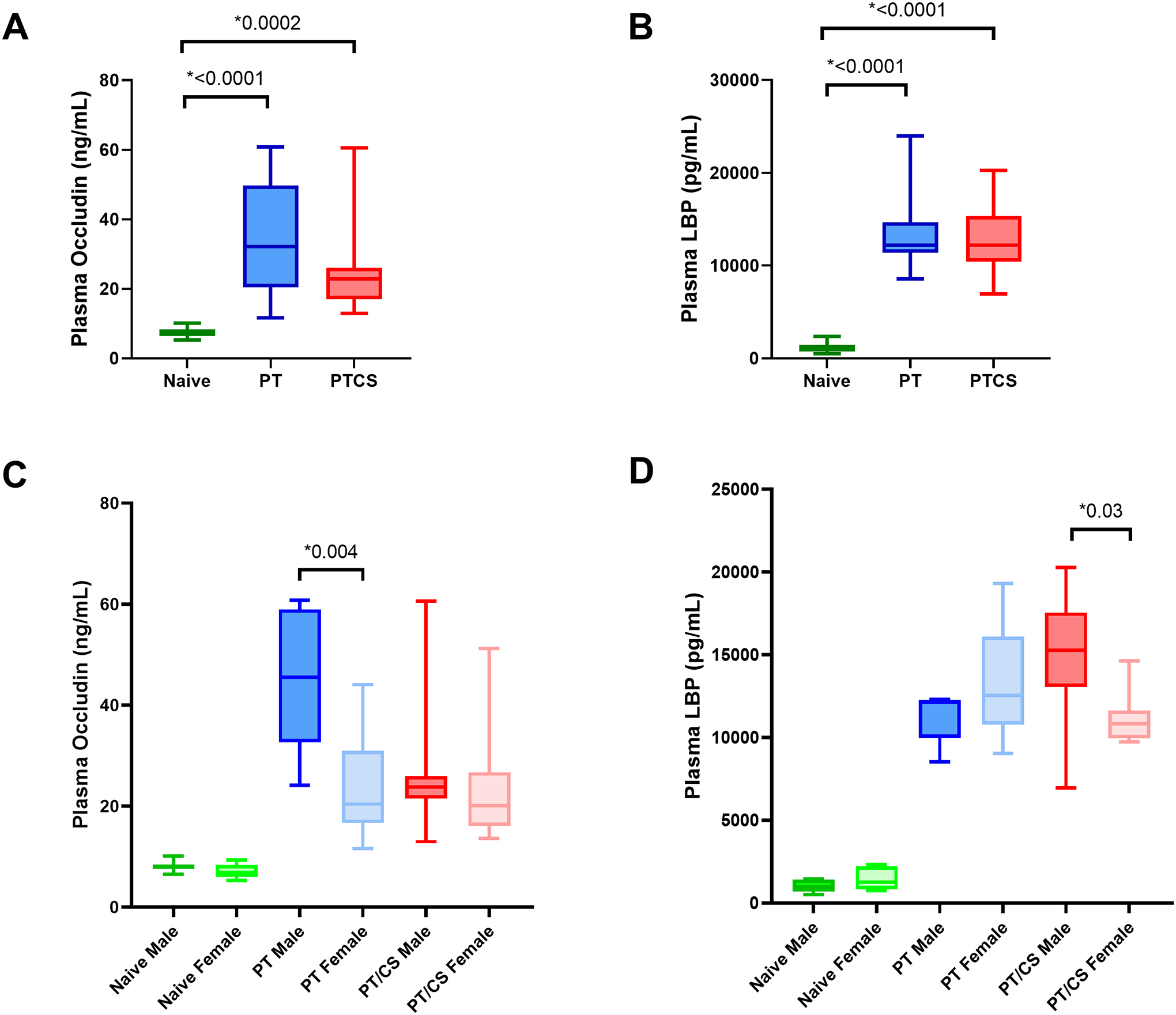
Intestinal permeability represented by plasma levels at day 2 of A) occludin across cohorts, B) lipopolysaccharide binding protein (LBP) across cohorts, C) occludin between sexes, D) LBP between sexes. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05).
Plasma LBP levels at day two between cohorts of both males and females were significantly higher in both PT and PT/CS compared to naïve (Fig. 6B). Subgroup analysis between sexes in the naïve cohort did not show significant differences between males and females (Fig. 6D). Plasma LBP levels were similar in PT males and females (Fig. 6D). However, PT/CS males had significantly elevated plasma LBP compared to females (Fig. 6D).
DISCUSSION
Multicompartmental injury with or without stress acutely results in a drastic decline in alpha-diversity and alterations in beta-diversity and microbial composition in both males and females. Sex-specific differences in alpha-diversity at baseline were no longer apparent by postinjury day two. Beta-diversity, although not different at baseline, was different between males and females after multiple injuries with or without stress. Microbial composition also remained unique between males and females within cohorts. Injured males subjected to stress had higher ileum injury scores compared to their female counterparts; surprisingly, PT/CS females had more descending colonic injury compared to males. Finally, plasma biomarkers suggestive of intestinal permeability were elevated in males compared to females after injury with or without chronic stress. As a whole, the evidence provided in this study demonstrates that overall there are differences in the way the microbiome, intestinal permeability, and intestinal histology changes after injury between sexes.
We were surprised to find that after severe injury with or without chronic stress, differences in alpha-diversity were no longer evident between sexes, suggestive that females had a sharper decline in alpha-diversity compared to males in both PT and PT/CS groups. PT induced significant changes in beta diversity. Finally, females had a unique microbial composition compared to males at day zero, consisting of different commensal bacteria such as Lachnospiraceae and Murcibaculaceae. After severe injury (PT), the microbiome in males consisted of dominance of the commensal bacteria Roseburia but also the pro-inflammatory Ruminococcus which has been associated with inflammatory bowel disease (39, 40). In the PT/CS group, females were dominated by the commensal bacteria such as Bifidobacterium compared to male counterparts even after one stress period (40). We did not observe significant differences in alpha or beta diversity between PT and PT/CS groups, suggesting that a short duration of stress may not significantly impact the intestinal microbiome. Together, this suggests that despite a decline in diversity, females retain commensal bacteria compared to males after severe injury.
Unfortunately, there is a paucity of data investigating the influence of sex on the microbiome in trauma and critical illness. Efron et al. found that both murine males and females subjected to cecal ligation and puncture had a decline in alpha-diversity and differences in beta diversity compared to controls after seven days which recovered by day 14 only in females (13). This group also demonstrated that after seven days, male microbiota were dominated by Bacteroidales while females had high abundance of Clostridiales (13). While this study was of a model of surgical sepsis, this suggests that critical illness induces a decline in alpha-diversity and changes in beta-diversity similar to our study within one week (13). Given the findings by this group, additional studies on the longer-term effects of severe trauma on the microbiome in both sexes are warranted to elucidate microbiome resilience and recovery after insult.
When evaluating the effects of injury with or without stress on both sexes, there were no statistically significant differences in colonic injury between naïve, PT and PT/CS cohorts after two days despite evident trends of increasing injury scores. We were surprised to find on further analysis that PT/CS females had elevated colonic injury scores compared to male counterparts, with crypt alterations and inflammatory cell infiltrate. Other studies have not yet investigated colonic histological changes after severe injury in females; studies in male rodents have shown colonic injury such as breakdown of the mucosal barrier acutely (8, 41). Estrogen has been shown to have a protective effect on the intestine in studies of other diseases such as inflammatory bowel disease; however, some other studies have suggested a more complex role of estrogen in ulcerative colitis, potentiating inflammation (42). Therefore, further study on the effects of severe injury and hemorrhagic shock on the colon in females is warranted, including estrogen levels and colonic estrogen receptor expression.
Despite increasing trends of terminal ileum injury in PT and PT/CS groups consisting of both males and females, this did not reach statistical significance. However, subgroup analysis revealed that males who underwent PT/CS had significantly more injured terminal ileum compared to female counterparts consisting of congestion, edema, villi blunting and sloughing, and the presence of leukocytes. Our findings align with others demonstrating that females are more resistant to small intestinal injury in trauma and ischemia-reperfusion. A human study of ischemia-reperfusion demonstrated that females had a blunted inflammatory response and less extensive epithelial damage in the jejunum compared to males (14). A murine model of intestinal ischemia-reperfusion demonstrated sex-specific differences in complement activation as well (15). It can be postulated that estradiol may have protective effects on the small intestine in injury. Multiple animal studies of either ischemia-reperfusion or trauma with hemorrhagic shock on proestrus female rats, ovariectomized female rats, and male rats administered estradiol all demonstrated blunted small intestinal injury and a reduction in the post-injury inflammatory cytokine response compared to ovariectomized females and male rats (16–18). This may suggest a role of estradiol as a protective factor in small intestinal injury, however further study in the mechanisms of estrogen in multicompartmental injury should be performed.
Both males and females showed significantly elevated plasma occludin and LBP levels in both PT and PT/CS compared to naïve. On subgroup analysis, both PT and PT/CS males showed elevated plasma occludin and PT/CS males had significantly elevated plasma LBP. Other animal studies support increased intestinal permeability after trauma and hemorrhagic shock acutely, but these were all only in male rodents (8, 41, 43). Estrogen deficiency has been linked to acutely increased permeability in ovariectomized female rats (44). Therefore, additional experiments are required to better understand the role of estrogen in maintenance of the intestinal barrier in trauma.
This study has limitations. Despite individual housing for animals after enrollment, subjects were co-housed in pairs of the same sex before the initial stool sample was obtained. However, our day zero results of no significant differences in alpha- or beta-diversity suggest that there were not significant differences between rats assigned to experimental groups prior to intervention. For anesthesia in the multicompartmental traumatic injury model, isoflurane was utilized which has been shown in other rodent studies to affect the microbiome alone, which may be a confounding variable in our results when comparing injured groups to the naïve cohort (45, 46). Part of our multicompartmental injury model involves intestinal surgery with cecectomy; while this may contribute to changes in microbial diversity, other studies of traumatic injury without intestinal manipulation demonstrate changes in the microbiome diversity and composition (47). In addition, plasma biomarkers such as occludin and LBP are not direct measures of intestinal permeability; more direct methods could include the administration of oral agents for measurement in the peripheral blood. We also only utilized females in the proestrus phase; additional investigation into the effects of injury on the microbiome in females at all stages of the estrous cycle should be pursued. Finally, age is known to influence the microbiome and this study only utilized rats aged 9–11 weeks; further evaluation of this model in a variety of age groups is warranted to understand the impact of severe trauma on the microbiome in different populations (22).
In summary, despite a decline in alpha-diversity after injury, females retain dominance of commensal bacteria similar to pre-injury and have less evidence of intestinal permeability and terminal ileum injury. This study suggests that sex is an important biological variable which may impact outcomes after severe trauma and critical illness. While complex and yet to be fully understood, it has been suggested that differences in the intestinal microbiome composition and diversity between sexes could be a result of sex hormone levels which may contribute to the sexual dimorphism observed in the intestinal microbiome after severe injury (48). Future studies should evaluate the role of sex hormones on the microbiome, intestinal permeability, and intestinal injury after trauma to understand underlying mechanisms behind female resilience after multicompartmental injury.
Supplementary Material
Supplemental Digital Content 1. ARRIVE Guidelines checklist.
Supplemental Digital Content 2. Table showing humane endpoint scoring and associated plan of care.
Supplemental Digital Content 3. Table showing ileum injury scoring scheme.
Supplemental Digital Content 4. Table showing descending colon injury scoring scheme.
Supplemental Digital Content 5. Linear discriminatory analysis (LDA) score plot showing microbiome taxa that are unique for each group. Percent on each orthogonal axis represents the proportion of overall variance in the data. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05)
Supplemental Digital Content 6. Representative histology sections of terminal ileum for A) naïve male, B) polytrauma (PT) male, C) polytrauma with chronic stress (PT/CS) male, D) naïve female, E) PT female, and F) PT/CS female.
Supplemental Digital Content 7. Representative histology sections of descending colon for A) naïve male, B) polytrauma (PT) male, C) polytrauma with chronic stress (PT/CS) male, D) naïve female, E) PT female, and F) PT/CS female.
Acknowledgements:
The authors would like to acknowledge the Animal Care Services for their assistance with rodent care for this study.
Funding:
This research was supported by the National Institutes of Health. AMM was supported by NIH NIGMS R01 GM105893. JAM, GSG and LSK were supported by postgraduate training grant NIH NIGMS T32 GM-008721 in burns, trauma, and perioperative injury.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
This study was presented at the 36th Annual Meeting of the Eastern Association for the Surgery of Trauma, January 17–21, 2023 in Orlando, Florida.
REFERENCES
- 1.Miller WD, Keskey R, Alverdy JC. Sepsis and the Microbiome: A Vicious Cycle. J Infect Dis. 2021;223(12 Suppl 2):S264–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alverdy JC, Krezalek MA. Collapse of the Microbiome, Emergence of the Pathobiome, and the Immunopathology of Sepsis. Crit Care Med. 2017;45(2):337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons JD, Coopersmith CM. Pathophysiology of the Gut and the Microbiome in the Host Response. Pediatr Crit Care Med. 2017;18(3_suppl Suppl 1):S46–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–53. [DOI] [PubMed] [Google Scholar]
- 5.Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol. 2021;12:686240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson SE, Merrill D, Zhu C, Burmeister DM, Zou Y, Lai Z, et al. Polytrauma independent of therapeutic intervention alters the gastrointestinal microbiome. Am J Surg. 2018;216(4):699–705. [DOI] [PubMed] [Google Scholar]
- 7.Appiah SA, Foxx CL, Langgartner D, Palmer A, Zambrano CA, Braumuller S, et al. Evaluation of the gut microbiome in association with biological signatures of inflammation in murine polytrauma and shock. Sci Rep. 2021;11(1):6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrba L, Ohmann JJ, Eisele P, Chakraborty S, Braumuller S, Braun CK, et al. Remote Intestinal Injury Early After Experimental Polytrauma and Hemorrhagic Shock. Shock. 2019;52(4):e45–e51. [DOI] [PubMed] [Google Scholar]
- 9.Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, et al. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2(1):e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56(8):2361–5. [DOI] [PubMed] [Google Scholar]
- 11.Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, et al. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141(4):481–9. [DOI] [PubMed] [Google Scholar]
- 12.Burmeister DM, Johnson TR, Lai Z, Scroggins SR, DeRosa M, Jonas RB, et al. The gut microbiome distinguishes mortality in trauma patients upon admission to the emergency department. J Trauma Acute Care Surg. 2020;88(5):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efron PA, Darden DB, Li EC, Munley J, Kelly L, Fenner B, et al. Sex Differences Associate with Late Microbiome Alterations after Murine Surgical Sepsis. J Trauma Acute Care Surg. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundscheid IHR, Schellekens D, Grootjans J, Derikx JPM, Buurman WA, Dejong CHC, et al. Females Are More Resistant to Ischemia-Reperfusion-induced Intestinal Injury Than Males: A Human Study. Ann Surg. 2020;272(6):1070–9. [DOI] [PubMed] [Google Scholar]
- 15.Wu M, Rowe JM, Fleming SD. Complement Initiation Varies by Sex in Intestinal Ischemia Reperfusion Injury. Front Immunol. 2021;12:649882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricardo-da-Silva FY, Fantozzi ET, Rodrigues-Garbin S, Oliveira-Filho RM, Vargaftig BB, Breithaupt-Faloppa AC, et al. Estradiol Modulates Local Gut Injury Induced by Intestinal Ischemia-Reperfusion in Male Rats. Shock. 2017;48(4):477–83. [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137(1):56–65. [DOI] [PubMed] [Google Scholar]
- 18.Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu DZ, et al. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS One. 2010;5(2):e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yracheta J, Muraoka W, Wu X, Burmeister D, Darlington D, Zhao D, et al. Whole blood resuscitation restores intestinal perfusion and influences gut microbiome diversity. J Trauma Acute Care Surg. 2021;91(6):1002–9. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer M, Mendez-Garcia C, Rojo D, Barbas C, Moya A. Antibiotic use and microbiome function. Biochem Pharmacol. 2017;134:114–26. [DOI] [PubMed] [Google Scholar]
- 21.Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martino C, Dilmore AH, Burcham ZM, Metcalf JL, Jeste D, Knight R. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol. 2022. [DOI] [PubMed] [Google Scholar]
- 23.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci. 2020;4(1):e100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munley JA, Kelly LS, Pons EE, Kannan KB, Coldwell PS, Whitley EM, et al. Multicompartmental Traumatic Injury and the Microbiome: Shift to a Pathobiome. J Trauma Acute Care Surg. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loftus TJ, Thomson AJ, Kannan KB, Alamo IG, Ramos HN, Whitley EE, et al. Effects of trauma, hemorrhagic shock, and chronic stress on lung vascular endothelial growth factor. J Surg Res. 2017;210:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller ES, Apple CG, Kannan KB, Funk ZM, Plazas JM, Efron PA, et al. Chronic stress induces persistent low-grade inflammation. Am J Surg. 2019;218(4):677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24 Suppl 1:101–6. [DOI] [PubMed] [Google Scholar]
- 28.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–14. [DOI] [PubMed] [Google Scholar]
- 29.Darwiche SS, Kobbe P, Pfeifer R, Kohut L, Pape HC, Billiar T. Pseudofracture: an acute peripheral tissue trauma model. J Vis Exp. 2011(50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagpal R, Indugu N, Singh P. Distinct Gut Microbiota Signatures in Mice Treated with Commonly Used Food Preservatives. Microorganisms. 2021;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17(19):7843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105(46):17994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 39.Nie K, Ma K, Luo W, Shen Z, Yang Z, Xiao M, et al. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front Cell Infect Microbiol. 2021;11:757718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hills RD Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu R, Ma XC. Role of metabolic changes of mucosal layer in the intestinal barrier dysfunction following trauma/hemorrhagic shock. Pathol Res Pract. 2018;214(11):1879–84. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Villatoro EL, Allred CD. Estrogen receptor actions in colitis. Essays Biochem. 2021;65(6):1003–13. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Li J, Zhang S, Chen G, Chi S, Li X, et al. Metabolomics analysis of gut barrier dysfunction in a trauma-hemorrhagic shock rat model. Biosci Rep. 2019;39(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. 2017;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serbanescu MA, Mathena RP, Xu J, Santiago-Rodriguez T, Hartsell TL, Cano RJ, et al. General Anesthesia Alters the Diversity and Composition of the Intestinal Microbiota in Mice. Anesth Analg. 2019;129(4):e126–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian X, Zhu Q, Sun L, Cheng Y. Effect of Anesthesia/Surgery on Gut Microbiota and Fecal Metabolites and Their Relationship With Cognitive Dysfunction. Front Syst Neurosci. 2021;15:655695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly LS, Apple CG, Gharaibeh R, Pons EE, Thompson CW, Kannan KB, et al. Stress-related changes in the gut microbiome after trauma. Journal of Trauma and Acute Care Surgery. 2021;91(1):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.d’Afflitto M, Upadhyaya A, Green A, Peiris M. Association Between Sex Hormone Levels and Gut Microbiota Composition and Diversity-A Systematic Review. J Clin Gastroenterol. 2022;56(5):384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. ARRIVE Guidelines checklist.
Supplemental Digital Content 2. Table showing humane endpoint scoring and associated plan of care.
Supplemental Digital Content 3. Table showing ileum injury scoring scheme.
Supplemental Digital Content 4. Table showing descending colon injury scoring scheme.
Supplemental Digital Content 5. Linear discriminatory analysis (LDA) score plot showing microbiome taxa that are unique for each group. Percent on each orthogonal axis represents the proportion of overall variance in the data. PT - polytrauma; PT/CS - polytrauma/chronic stress. Only statistically significant comparisons displayed (*p<0.05)
Supplemental Digital Content 6. Representative histology sections of terminal ileum for A) naïve male, B) polytrauma (PT) male, C) polytrauma with chronic stress (PT/CS) male, D) naïve female, E) PT female, and F) PT/CS female.
Supplemental Digital Content 7. Representative histology sections of descending colon for A) naïve male, B) polytrauma (PT) male, C) polytrauma with chronic stress (PT/CS) male, D) naïve female, E) PT female, and F) PT/CS female.


