Abstract
Introduction:
Observational studies have shown that body mass index (BMI) and waist-to-hip ratio (WHR) are both inversely associated with lung function, as assessed by forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). However, observational data are susceptible to confounding and reverse causation.
Methods:
We selected genetic instruments based on their relevant large-scale genome-wide association studies. Summary statistics of lung function and asthma came from the UK Biobank and SpiroMeta Consortium meta-analysis (n=400,102). After examining pleiotropy and removing outliers, we applied inverse-variance weighting to estimate the causal association of BMI and BMI-adjusted WHR (WHRadjBMI) with FVC, FEV1, FEV1/FVC, and asthma. Sensitivity analyses were performed using weighted median, MR-Egger and MRlap methods.
Results:
We found that BMI was inversely associated with FVC (effect estimate, −0.167; 95% confidence interval (CI), −0.203 to −0.130) and FEV1 (effect estimate, −0.111; 95%CI, −0.149 to −0.074). Higher BMI was associated with higher FEV1/FVC (effect estimate, 0.079; 95%CI, 0.049 to 0.110) but was not significantly associated with asthma. WHRadjBMI was inversely associated with FVC (effect estimate, −0.132; 95%CI, −0.180 to −0.084) but has no significant association with FEV1. Higher WHR was associated with higher FEV1/FVC (effect estimate, 0.181; 95%CI, 0.130 to 0.232) and with increased risk of asthma (effect estimate, 0.027; 95%CI, 0.001 to 0.053).
Conclusion:
We found significant evidence that increased BMI is suggested to be causally related to decreased FVC and FEV1, and increased BMI-adjusted WHR could lead to lower FVC value and higher risk of asthma. Higher BMI and BMI-adjusted WHR were suggested to be causally associated with higher FEV1/FVC.
1. Introduction
In the past few decades, there has been a steady trend of increasing obesity around the world. Obesity has been generally recognized as a major risk factor for many chronic diseases, for instance, diabetes, cardiovascular disease, and cancer1. Commonly used phenotypes of obesity include measures of overall obesity, such as body mass index (BMI), and measures of abdominal obesity, such as waist-to-hip ratio (WHR). Recently a growing number of studies have shown that obesity is associated with impaired pulmonary function and increased risk of asthma2. It is more challenging to treat asthmatic patients with obesity compared to those without obesity, and such cases involve more acute attacks and increased use of medications3. Therefore, it is worthwhile to pursue a better understanding of the relationship between obesity and both pulmonary function and asthma.
Findings from previous observational studies have been inconsistent. One study showed that BMI has a significant effect on lung volumes and has the greatest effect among people with severe obesity (BMI ≥ 40kg/m2)4. Another study also found that forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) are significantly reduced among people with severe obesity5. But a recent study indicated no evidence of an association between BMI and vital capacity6. As for abdominal obesity, WHR was associated with impaired spirometry and lung volumes in both mildly obese and severely obese persons7. Another study suggested that WHR is significantly associated with lung function in men only8. Due to this inconsistency in published studies, it remains uncertain whether there is a causal relationship between obesity and lung function or asthma. Further, published reports describe observational studies, commonly only adjusting for basic clinical characteristics including age, sex, height, and cigarette smoking status. The lack of consideration of more comprehensive confounders and the variety of study designs might explain the discrepancies in their findings.
Mendelian randomization (MR) analysis is an approach that uses genetic variants associated with exposures as instrumental variables to examine the causal association between exposure and the trait of interest9. MR studies provide more credible evidence than conventional observational studies because genetic variants are randomly allocated from parents to offspring during meiosis; thus, they are less susceptible to confounders, bias, and reverse causation. Two-sample MR is a branch of MR analysis in which the association between SNPs and exposure and the association between SNPs and outcome are estimated from two different samples from the same underlying population9. To the best of our knowledge, there are only a few two-sample MR studies exploring the effect of obesity on respiratory volume and respiratory diseases10 11. Therefore, we aimed to examine and provide further evidence using the MR approach. In this study, we implemented a two-sample MR analysis to investigate the causal relationship of obesity (BMI and BMI-adjusted WHR) with pulmonary function - specifically FEV1, FVC, and FEV1/FVC - and with asthma, utilizing publicly available summary statistics from large-scale genome-wide association studies (GWAS).
2. Methods
2.1. Study Design
We conducted two-sample MR analyses to investigate the causal relationship between obesity and lung function measurements and respiratory diseases. Specifically, exposures included BMI and BMI-adjusted WHR (WHRadjBMI), and outcomes included FVC, FEV1, FEV1/FVC and diagnosed asthma. GWAS summary statistics of all the exposures and outcomes were from European ancestry samples. After collecting GWAS summary statistics, we calculated the MR estimates of BMI and WHRadjBMI on FVC, FEV1, FEV1/FVC and asthma using various statistical methods as described in the statistical analysis section.
2.2. GWAS of Exposure Variables
GWAS Summary statistics of BMI were obtained from a GWAS and Metabochip meta-analysis from the GIANT consortium12. The study included association results on up to 322,154 individuals of European-descents. The phenotype BMI in the study was adjusted for age, age-squared, and necessary study-specific covariates including principal components. The inverse normal transformed residual was used for the genetic association analysis. It identified 97 BMI-associated SNPs (p-value<5×10−8) representing 97 independent loci.
We obtained summary statistics of WHRadjBMI from a meta-analysis of GWAS in up to 142,762 individuals of European ancestry and Metabochip studies in up to an additional 67,326 European- ancestry individuals13. The trait was also adjusted for age, age-squared, and any necessary study-specific covariates including principal components. Residuals were calculated for men and women separately and then inverse-normal-transformed for later genetic association analysis. The study identified 39 SNPs associated with WHRadjBMI (p-value<5×10−8) representing 39 independent loci among a combined-sex sample of European ancestry.
2.3. GWAS of Pulmonary Function Variables
Summary genetic statistics of pulmonary function variables (FEV1, FVC and FEV1/FVC) were from UK Biobank and SpiroMeta Consortium14. The GWA analyses were conducted on 400,102 European samples, adjusting for covariates age, sex, ever-smoking status and principal components.
2.4. GWAS of Asthma
Summary genetic statistics of diagnosed asthma (Yes/No) was from the UK Biobank (downloaded from http://www.nealelab.is/uk-biobank). The study provided GWAS results of up to 4,236 phenotypes, including a history of diagnosed asthma (Yes/No). The GWA analyses of diagnosed asthma were conducted on European sub-sample including 80,070 controls and 11,717 cases, adjusting for covariates age, age-squared, sex, the interaction between age and sex, the interaction between age-squared and sex, and principal components.
2.5. Data Harmonization
Our MR analyses included SNPs that were available in the GWAS summary statistics of both the exposure and the outcome. We performed data harmonization to ensure that the effects of a SNP on the exposure and the outcome corresponded to the same allele. This is an essential step when utilizing datasets generated from different studies, as the effect allele of one SNP may differ between different GWA studies15. SNPs with flipped effect and reference alleles in two datasets were oriented and their effect values were additively inversed9.
2.6. Statistical Analysis
The absence of horizontal pleiotropy (i.e. the absence of evidence that a genetic instrument affects the outcome via mechanisms other than through the exposure) is a crucial assumption in MR analysis. Before performing the actual MR analyses, we first used the Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) to check horizontal pleiotropy of the genetic instruments and remove potential outliers16. MR-PRESSO is a unified approach based on an inverse-variance weighting (IVW) method that evaluates horizontal pleiotropy in multi-instrument MRs. It has better sensitivity and correction for pleiotropy than previous MR methods and helps to identify potential pleiotropic genetic instruments. It contains three components: 1) a global test to detect horizontal pleiotropy; 2) an outlier test to correct horizontal pleiotropy by removing outliers; 3) a distortion test to check the differences in the causal estimates before and after correction for outliers. If a significant pleiotropic effect exists, MR-PRESSO will provide a suggestion of outliers. Here we utilized the MR-PRESSO tests to filter out SNPs from genetic instruments that showed pleiotropic associations with the outcome.
After removing the potentially invalid genetic instruments using MR-PRESSO, we performed MR analyses using IVW estimation as our primary approach17. The Wald ratio was calculated for each SNP by dividing the SNP-outcome association by the SNP-exposure association. All ratios were combined to yield an overall causal effect using inverse variance weighted meta-analysis. The IVW method assumes that all genetic instruments associated with exposure are valid, and that no horizontal pleiotropy occurs. Thus the estimate of IVW is sensitive to violation of MR assumptions. In addition, we performed sensitivity analyses with weighted median and MR-Egger regression methods as comparable causal estimators. The weighted median method is an approach that provides a consistent estimate of the causal effect even when up to 50% of the information comes from invalid instrumental variables and thus can provide evidence to support the IVW18. MR-Egger regression is a weighted regression of the SNP-outcome on the SNP-exposure association with the intercept that is not constrained to pass through zero and provides a valid estimate under the potential of horizontal pleiotropy17 19. It allows the assumption of no horizontal pleiotropy to be relaxed20. However, weighted median and MR-Egger method have less statistical efficiency compared with the IVW method, especially MR-Egger method is expected to provide a wider confidence interval. Therefore, we focused on whether the magnitude and direction of effect estimates were consistent across methods. In addition, concerning the sample overlap between GWAS of the exposure and GWAS of the outcome, we performed a sensitivity analysis using MRlap software, which accounts for sample overlap via LD-score regression related technique21. We used a p-value threshold of 5×10−8, an LD threshold of 0.1, and a distance threshold of 500kb to select IVs in MRlap analysis.
All analyses were conducted using R Studio, version 1.1.456, with the MendelianRandomization, MR-PRESSO and MRlap Packages16 21 22. All statistical tests were performed using 5% overall significance level.
3. Results
3.1. Analyses between BMI and pulmonary function measurements
Among 97 SNPs associated with BMI, three of them were not available in summary statistics for FVC, FEV1 and FEV1/FVC, and thus were eliminated from the analysis. Since we identified a significant pleiotropy effect on the outcomes by MR-PRESSO global test, we decided to use MR-PRESSO outlier test to identify outliers at the beginning of each analysis.
In the analysis of the relationship between BMI and FVC, the MR-PRESSO outlier test revealed 14 potential outlier SNPs. Therefore, we remained with 80 SNPs and found that pleiotropy among the instruments was eliminated. In the subsequent IVW analysis, we identified an inverse causal association between BMI and FVC (p-value<0.001; effect estimate, −0.167; 95% CI, −0.203 to −0.130) (Table 1 and Figure 1A), indicating that a higher BMI is associated with a lower FVC. We observed consistent results from MR-Egger (p-value, <0.001 effect estimate, −0.173; 95% CI, −0.260 to −0.086) and weighted median (p-value<0.001; effect estimate, −0.145; 95% CI, −0.184 to −0.105).
Table 1.
Mendelian Randomization results for BMI and WHRadjBMI on pulmonary related traits
| IVW | MR-Egger | Weighted Median | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | FVC (80 SNPs) |
−0.167 | (−0.203, −0.130) | <0.001 | −0.173 | (−0.260, −0.086) | <0.001 | 0.000 (0.871) | −0.145 | (−0.184, −0.105) | <0.001 |
| FEV1 (86 SNPs) |
−0.111 | (−0.149, −0.074) | <0.001 | −0.050 | (−0.139, 0.039) | 0.268 | −0.002 (0.139) | −0.080 | (−0.120, −0.039) | <0.001 | |
| FEV1/FVC (72 SNPs) |
0.079 | (0.049, 0.110) | <0.001 | 0.140 | (0.068, 0.213) | <0.001 | −0.002 (0.071) | 0.095 | (0.051, 0.139) | <0.001 | |
| Asthma (94 SNPs) |
0.004 | (−0.010, 0.017) | 0.616 | 0.024 | (−0.009, 0.058) | 0.154 | −0.001 (0.181) | 0.008 | (−0.013, 0.030) | 0.441 | |
| WHR adjBMI | FVC (28 SNPs) |
−0.132 | (−0.180, −0.084) | <0.001 | 0.052 | (−0.223, 0.327) | 0.713 | −0.005 (0.185) | −0.120 | (−0.176, −0.064) | <0.001 |
| FEV1 (31 SNPs) |
−0.024 | (−0.083, 0.034) | 0.417 | 0.084 | (−0.234, 0.403) | 0.604 | −0.003 (0.496) | 0.017 | (−0.040, 0.075) | 0.557 | |
| FEV1/FVC (28 SNPs) |
0.181 | (0.130, 0.232) | <0.001 | 0.100 | (−0.146, 0.346) | 0.417 | 0.002 (0.510) | 0.211 | (0.153, 0.270) | <0.001 | |
| Asthma (39 SNPs) |
0.027 | (0.001, 0.053) | 0.040 | 0.050 | (−0.08, 0.181) | 0.448 | −0.001 (0.722) | 0.040 | (0.010, 0.071) | 0.010 | |
CI indicates confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; IVW, inverse-variance weighting; SNP, single-nucleotide polymorphism.
Figure 1.
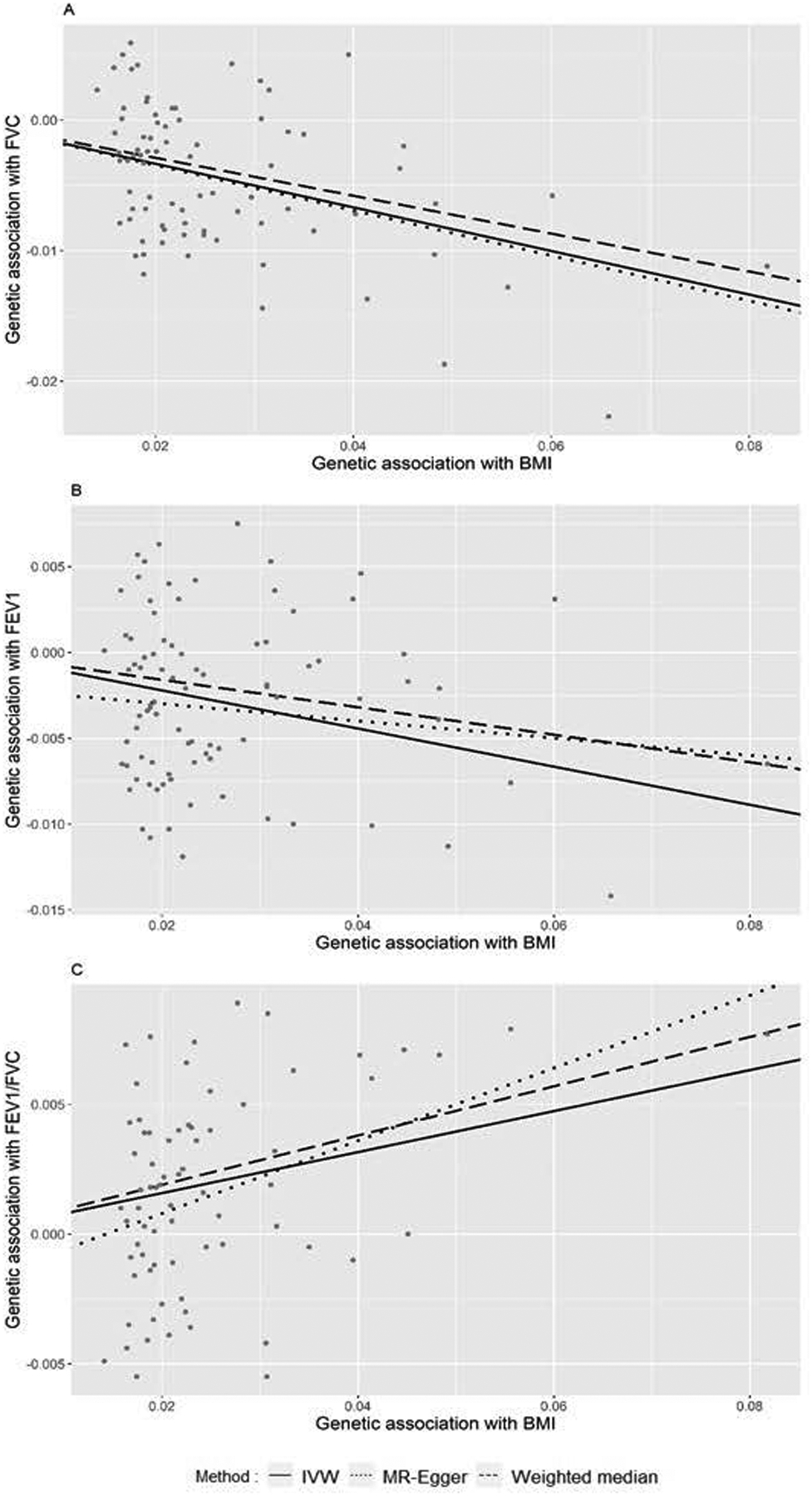
A. Plot of association between BMI and FVC using 80 BMI-associated SNPs. B. Plot of association between BMI and FEV1 using 86 WHR-associated SNPs. C. Plot of association between BMI and FEV1/FVC using 72 WHR-associated SNPs.
In the analysis between BMI and FEV1, the MR-PRESSO outlier test suggested 8 potential outliers for removal. As a result, we retained 86 SNPs for MR analysis. BMI was inversely associated with FEV1 according to the IVW model (p-value<0.0001; effect estimate, −0.111; 95% CI, −0.149 to −0.074) (Table 1 and Figure 1B). The results of weighted median (p-value<0.001; effect estimate, −0.080; 95%CI, −0.120 to −0.039) and MR-Egger (p-value, 0.268; effect estimate, −0.050; 95%CI, −0.139 to 0.039) were directionally consistent with IVW. Overall, the results demonstrated that BMI has inverse causal associations with both FVC and FEV1.
In the analysis between BMI and FEV1/FVC, the MR-PRESSO outlier test suggested the removal of 22 potential outliers, and therefore 72 SNPs were retained for analysis. The IVW analysis showed that higher BMI is associated with higher FEV1/FVC ratio (p-value <0.001; effect estimate, 0.079; 95% CI, 0.049 to 0.110) (Table 1 and Figure 1C). The MR-Egger and weighted median analysis also provided directionally consistent evidence of their association.
3.2. Analyses between WHRadjBMI and pulmonary function measurements
Among 39 SNPs associated with WHRadjBMI, one of them was not available in summary statistics for FVC, FEV1 and FEV1/FVC, and thus was eliminated from the analysis. The MR-PRESSO global test also indicated pleiotropy among the instruments of outcomes and MR-PRESSO outlier test was performed in each analysis.
For the WHRadjBMI and FVC analysis, the MR-PRESSO outlier test suggested 10 potential outliers, and therefore, 28 SNPs remained in the analysis. Similar to BMI, WHRadjBMI was inversely associated with FVC in the IVW model (p-value, <0.001; effect estimate, −0.132; 95%CI, −0.180 to −0.084) (Table 1 and Figure 2A). The weighted median also provided evidence of inverse association between WHRadjBMI and FVC (p-value, <0.001; effect estimate, −0.120; 95%CI, −0.176 to −0.064), while the MR-Egger method showed no significant association (p-value, 0.713; effect estimate, 0.052; 95% CI, −0.223 to 0.327).
Figure 2.
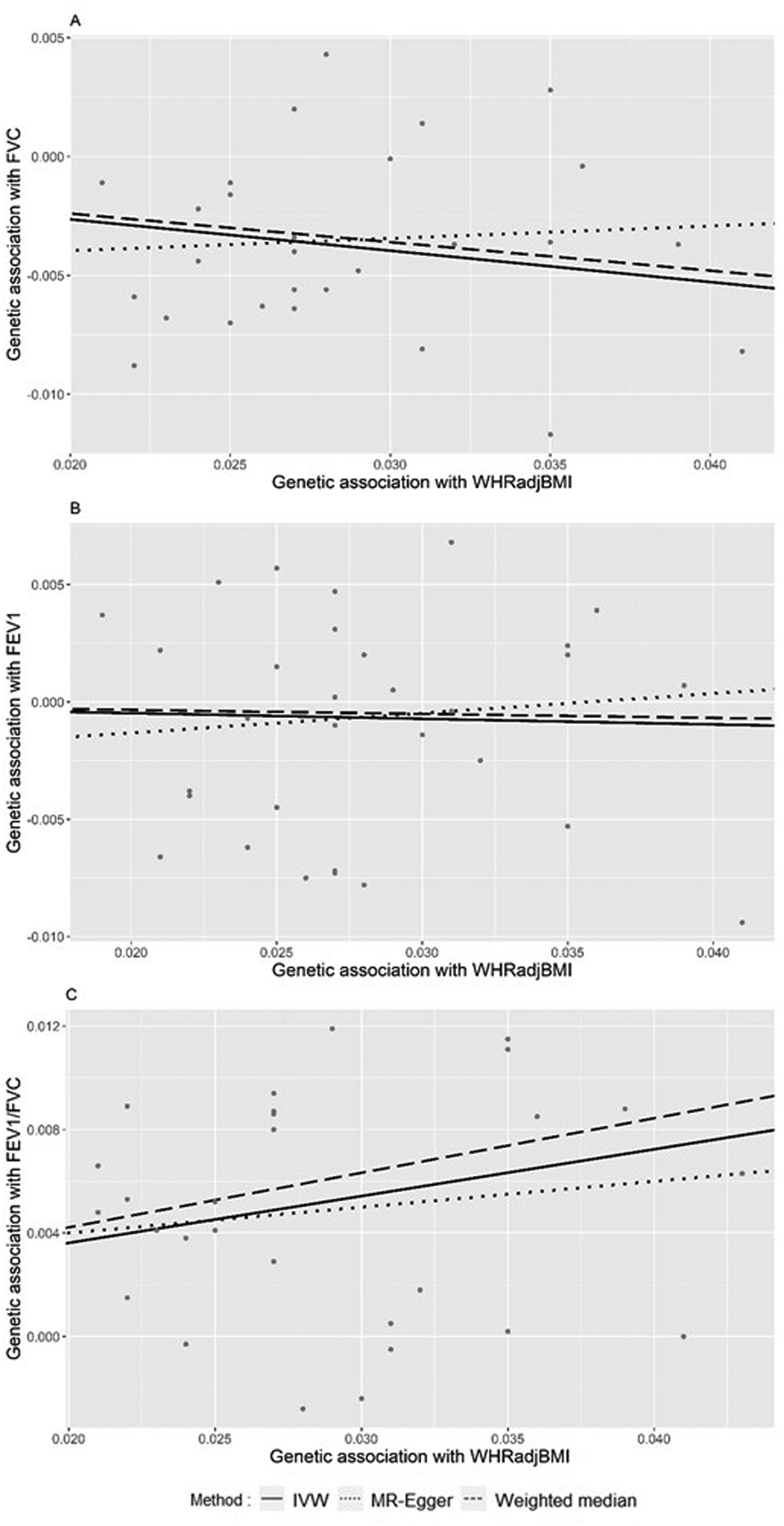
A. Plot of association between WHRadjBMI and FVC using 28 WHRadjBMI-associated SNPs. B. Plot of association between WHRadjBMI and FEV1 using 31 WHRadjBMI-associated SNPs. C. Plot of association between WHRadjBMI and FEV1/FVC using 28 WHR-associated SNPs.
In the analysis of the association between WHRadjBMI and FEV1, the MR-PRESSO outlier test suggested 7 potential outliers for removal, releasing 31 SNPs for analysis. The IVW method (p-value, 0.417; effect estimate, −0.024; 95%CI, −0.083 to 0.034), weighted median method (p-value, 0.557; effect estimate, 0.017; 95% CI, −0.040 to 0.075) and MR-Egger method (p-value, 0.604; effect estimate, 0.084, 95%CI, −0.234 to 0.403) showed no significant associations. In Figure 2B, the genetic variants associated with WHR were distributed along the IVW regression line, representing the various effects of WHRadjBMI on FEV1. In summary, higher WHRadjBMI was associated with lower FVC but was not significantly associated with FEV1.
For the association between WHRadjBMI and FEV1/FVC, 10 SNPs were identified as potential outliers and were suggested to remove by MR-PRESSO outlier test, releasing 28 SNPs for analysis. The IVW method showed WHRadjBMI is associated with FEV1/FVC (p-value, <0.001; effect estimate, 0.181; 95% CI, 0.130 to 0.232). Weighted median method also indicated that higher WHRadjBMI was associated with higher FEV1/FVC (p-value, <0.001; effect estimate, 0.211; 95%CI, 0.153 to 0.270), while the MR-Egger method showed no significant association (p-value, 0.417; effect estimate, 0.100; 95% CI, −0.146 to 0.346).
3.3. Analyses between Obesity Exposures and Asthma
All 94 SNPs associated with BMI were identified in the GWAS summary statistics of asthma. In the association between BMI and asthma, MR-PRESSO global test did not identify evidence of pleiotropy, and thus all 94 SNPs were used in the analysis. There was no significant association between BMI and asthma based on the IVW model (p-value, 0.616; effect estimate, 0.004; 95%CI, −0.010 to 0.017), MR-Egger (p-value, 0.154; effect estimate, 0.024; 95%CI, −0.009 to 0.058) and weighted median (p-value, 0.441; effect estimate, 0.008; 95%CI, −0.013 to 0.03) (Table 1 and Figure 3A).
Figure 3.
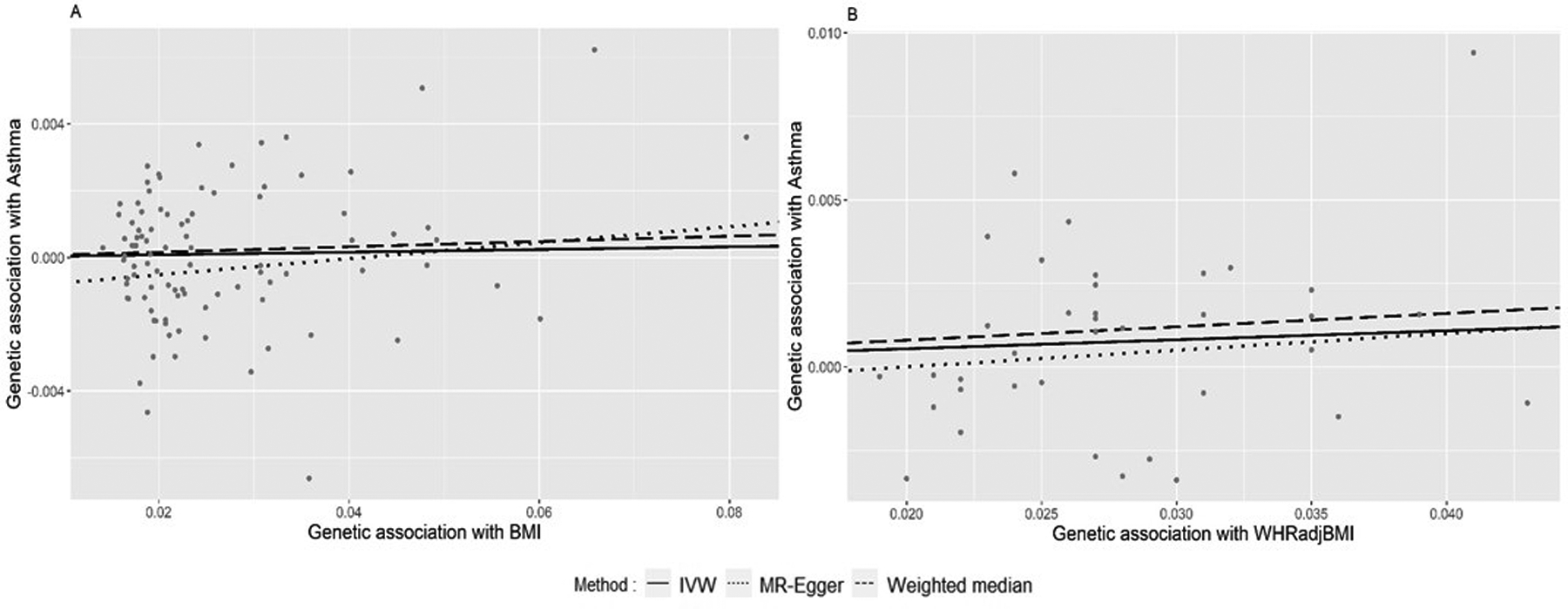
A. Plot of association between BMI and asthma using 94 BMI-associated SNPs. B. Plot of association between WHRadjBMI and asthma using 39 WHRadjBMI-associated SNPs.
For the association between WHRadjBMI and asthma, MR-PRESSO did not reveal significant evidence of pleiotropy; therefore, all 39 SNPs were included in the analysis. Both IVW (p-value, 0.040; effect estimate, 0.027; 95%CI, 0.001 to 0.053) and the weighted median (p-value, 0.010; effect estimate, 0.040; 95% CI, 0.010 to 0.071) showed significant positive associations between WHR and risk of asthma, while the MR-Egger showed no significant association but a similar effect estimate (p-value, 0.448; effect estimate, 0.05; 95%CI, −0.080 to 0.181) (Table 1 and Figure 3B). Overall, higher WHRadjBMI was significantly associated with the risk of asthma.
3.4. Sensitivity analyses using MRlap
The results of sensitivity analyses are presented in Supplementary Table 1. The BMI-related analyses had 97 SNPs as instrumental variables selected by MRlap. Like regular MR analyses above, MRlap also detected significant associations between higher BMI and lower FEV1 (p-value, <0.001; effect estimate, −0.146; 95% CI, −0.214 to −0.077), lower FVC (p-value, <0.001; effect estimate, −0.236; 95% CI, −0.306 to −0.166), and higher FEV1/FVC (p-value, <0.001; effect estimate, 0.147; 95% CI, 0.085 to 0.209). Meanwhile, it did not reveal a significant association between BMI and asthma (p-value, 0.480; effect estimate, 0.022; 95% CI, −0.039 to 0.083).
The WHRadjBMI and pulmonary function analyses had 47 SNPs selected as IVs, and 48 SNPs were selected for WHRadjBMI and asthma analysis. MRlap identified a significant association between higher WHRadjBMI and higher FEV1/FVC (p-value, 0.003; effect estimate, 0.169, 95% CI, 0.059 to 0.279. There was no significant association between WHRadjBMI and FEV1 or FVC. And higher WHRadjBMI was significantly associated with a higher risk of asthma (p-value, 0.016; effect estimate, 0.105; 95% CI, 0.020 to 0.190).
4. Discussion
In this study, we examined the causal relationship between obesity, measured as BMI or WHRadjBMI, and lung function measurements (FVC, FEV1 and FEV1/FVC) as well as asthma through two-sample Mendelian Randomization methods which include a robust approach of MR-PRESSO to select IV instruments and IVW method with the support of weighted median and MR-Egger for analysis. Using publicly available GWAS summary data from the GIANT consortium and the UK Biobank, we found significant evidence that higher BMI was associated with lower FVC and FEV1 but higher FEV1/FVC, and WHRadjBMI also has an inverse association with FVC and positive association with FEV1/FVC. We also found a significant positive association between WHRadjBMI and risk of asthma while there was no significant association between BMI and asthma. The sensitivity analyses accounting for sample overlap using MRlap provided supportive evidence for the findings from the primary analyses.
The finding of the negative association between BMI and FVC and FEV1 is consistent with the majority of previous studies. A recent MR study by Wielscher et al. observed that higher BMI is causally associated with lower FEV1 and FVC10. Another MR study by Skaaby et al. using 26 BMI-associated SNPs in 162,124 participants also found the inverse causal association between BMI with FEV1 and FVC11. On the other hand, an observational study showed that WHRadjBMI was significantly inversely associated with FVC and FEV123, while we found no causal relationship between WHRadjBMI and FEV1, a result worthy of further exploration. Furthermore, our result of the positive association between obesity BMI and FEV1/FVC was consistent with MR study by Wielscher et al. which also found that higher BMI is causally associated with increased FEV1/FVC10. However, the findings from observational studies indicated an opposite association. A meta-analysis found that FEV1/FVC was significantly lower among obese subjects24. Two studies not included in that meta-analysis also reported a negative association between BMI and FEV1/FVC25 26. MR method might provide a different perspective on the association between obesity and FEV1/FVC compared to prior studies based on phenotype alone.
The finding of no significant association between BMI and asthma in our study was different from previous studies. A meta-analysis of prospective epidemiologic studies showed that higher BMI was significantly associated with a higher risk of asthma27. BMI is usually considered the measure of general obesity, and it does not reflect body fat distribution. The results of our study suggest that abdominal obesity may be a better predictor of asthma, compared to BMI.
Although the mechanisms underlying the association between obesity and pulmonary function and lung diseases remain largely unknown, hypotheses can be suggested. Most studies have demonstrated that obesity can lead to a reduction in lung and chest wall compliance, as lung compliance is exponentially related to BMI28. This reduction may explain why higher BMI results in slightly lower FVC and FEV1. The abdominal fat deposition may directly limit the expansion of the diaphragm, probably by encroaching into the chest by the chest wall or diaphragm or by impeding the descent of the diaphragm during FVC maneuver23. This reduced expansion may reduce expiratory reserve volume via compressing the lungs and diaphragm29. This will result in lower FVC measurements, consistent with our results of the strong inverse association between WHR and FVC. Another possible mechanism is that abdominal adiposity might lead to a redistribution of blood to the thoracic compartment that reduces vital capacity8. Furthermore, obesity may also affect respiratory function through systemic inflammation. Obesity has a significant impact on the immune cells in adipose tissue which is an endocrine and paracrine organ producing many cytokines and inflammatory mediators, leading to a pro-inflammatory state that is associated with lung volume and increased risk of asthma in obese individuals30.
Many literatures have discussed the influence of sample overlap on the type I error rate and bias in an MR analysis31–33. Therefore, it is necessary to properly account for sample overlap when performing an MR analysis. In our study, the GWAS of the exposures were from the GIANT consortium while the GWAS of asthma was from UK Biobank, and these GWAS data had no sample overlap. The GWAS of pulmonary function measurements were from a meta-analysis of UK Biobank (n= 321,047) and Spirometa consortium (n= 79,055, consisting of 22 studies). And 10 of those 22 studies were also in the GIANT consortium, leading to an approximate overlapped sample of n=18,757. We have performed sensitivity analysis using MRlap to account for sample overlap, and the significance and effect direction remained unchanged in general, so our findings were stable under a sample overlap rate of about 4.6%.
Our study has the strengths of utilizing a more robust MR method that carries out a stricter IV selection, as well as providing a comprehensive evaluation of causal relationships between obesity indices and pulmonary related traits. The previous MR study by Skaaby et al. applied IVW, MR-Egger, and weighted median to analyze the causal relationship between BMI with lung function and asthma11. Another MR study by Wielscher et al. applied MR-PRESSO for IV selection and IVW, MR-Egger, and weighted median to detect the causal relationship between BMI with FVC, FEV1, and FEV1/FVC. In our study, we were also able to detect pleiotropy effects and remove outliers in genetic instruments to improve the robustness of results by utilizing MR-PRESSO. Meanwhile, our study provided additional evidence for the relationship between obesity indices, especially WHRadjBMI, and pulmonary function and asthma, hence can offer a more comprehensive understanding of their causality. Moreover, our study utilized GWAS datasets of exposures and outcomes from large European samples, thereby increasing the reliability of our findings.
However, this study also has limitations. First, we could not perform stratification analysis by sex since the sex-specific summary data for BMI was unavailable online. Several observational studies showed that the effect of WHR on FVC and FEV1 is different between males and females8 23 29. Although the latter two studies used unadjusted WHR in the model instead of WHRadjBMI, which may result in incomparability, it is worth the effort for further sex-stratified analysis to investigate the relationship between WHR and lung function. Second, smoking status is a strong environmental factor and a potential confounder in the relationship between obesity and respiratory diseases like asthma. Many observational studies found smoking status could significantly modify the association between obesity and asthma when controlling for smoking status as a confounder34–36. However, the data of outcomes from UK Biobank we used were not adjusted for smoking status. Third, our study utilized data from the European ancestry, and thus, inference beyond the European ancestry requires further investigation. Also, due to the lack of a third source of GWAS summary statistics of exposures, we were not able to perform a three-sample MR in order to take care of the selection bias of instrumental variables37 38. Finally, the instrumental variables used in MRlap analyses were not exactly the same as those used in the primary analyses. That was because MRlap could not input a user-defined IV list, it could only select IVs based on p-value, LD, and distance thresholds.
In conclusion, we found statistically significant evidence of a causal relationship between BMI and lung function that suggested higher genetically determined BMI leading to lower lung function (FVC and FEV1) and higher FEV1/FVC. Increased BMI-adjusted WHR could also lead to lower FVC value and a higher risk of asthma. Interventions to lower body weight or central body fat may serve as a potential target to enhance lung function and lower the risk of respiratory diseases. Further study to validate our results may provide additional evidence and insight into the causal mechanism between obesity and lung function and respiratory diseases.
Supplementary Material
Acknowledges:
This research was supported by the grant NIH R01DK122503.
Footnotes
Conflict of Interest Statement:
The authors declare that there is no conflict of interests.
Data Availability Statement:
All data used in the analysis presented in the manuscript are publicly available and were downloaded using the following links:
The GWAS of BMI and WHRadjBMI: https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files
The GWAS of pulmonary functions: https://www.ebi.ac.uk/gwas/publications/30804560
The GWAS of asthma: http://www.nealelab.is/uk-biobank
Reference:
- 1.Rippe JM, Crossley S, Ringer R. Obesity as a Chronic Disease. Journal of the American Dietetic Association 1998;98(10):S9–S15. doi: 10.1016/s0002-8223(98)00704-4 [DOI] [PubMed] [Google Scholar]
- 2.Bantula M, Roca-Ferrer J, Arismendi E, et al. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J Clin Med 2021;10(2) doi: 10.3390/jcm10020169 [published Online First: 2021/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med 2010;3:335–43. doi: 10.2147/IJGM.S11926 [published Online First: 2010/12/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006;130(3):827–33. doi: 10.1378/chest.130.3.827 [published Online First: 2006/09/12] [DOI] [PubMed] [Google Scholar]
- 5.Schachter LM, Salome CM, Peat JK, et al. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001;56(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Ye Z, Lu H, et al. Association between body mass index (BMI) and vital capacity of college students of Zhuang nationality in China a cross-section study. Oncotarget 2017;8(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LC, Hoberty PD, Walker JF, et al. The effect of body fat distribution on pulmonary function tests. Chest 1995;107(5):1298–302. doi: 10.1378/chest.107.5.1298 [published Online First: 1995/05/01] [DOI] [PubMed] [Google Scholar]
- 8.Harik-Khan R, Wise RA, Fleg JL. The effect of gender on the relationship between fat distribution and lung function. Journal of Clinical Epidemiology 2001;54 (2001):399–406. [DOI] [PubMed] [Google Scholar]
- 9.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23(R1):R89–98. doi: 10.1093/hmg/ddu328 [published Online First: 2014/07/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wielscher M, Amaral AFS, van der Plaat D, et al. Genetic correlation and causal relationships between cardio-metabolic traits and lung function impairment. Genome Med 2021;13(1):104. doi: 10.1186/s13073-021-00914-x [published Online First: 2021/06/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaaby T, Taylor AE, Thuesen BH, et al. Estimating the causal effect of body mass index on hay fever, asthma and lung function using Mendelian randomization. Allergy 2018;73(1):153–64. doi: 10.1111/all.13242 [published Online First: 2017/07/05] [DOI] [PubMed] [Google Scholar]
- 12.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518(7538):197–206. doi: 10.1038/nature14177 [published Online First: 2015/02/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518(7538):187–96. doi: 10.1038/nature14132 [published Online First: 2015/02/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrine N, Guyatt AL, Erzurumluoglu AM, et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet 2019;51(3):481–93. doi: 10.1038/s41588-018-0321-7 [published Online First: 2019/02/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwig FP, Davies NM, Hemani G, et al. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol 2016;45(6):1717–26. doi: 10.1093/ije/dyx028 [published Online First: 2017/03/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50(5):693–98. doi: 10.1038/s41588-018-0099-7 [published Online First: 2018/04/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Au Yeung SL, Borges MC, Lawlor DA. Association of Genetic Instrumental Variables for Lung Function on Coronary Artery Disease Risk: A 2-Sample Mendelian Randomization Study. Circ Genom Precis Med 2018;11(4):e001952. doi: 10.1161/CIRCGEN.117.001952 [published Online First: 2018/04/14] [DOI] [PubMed] [Google Scholar]
- 18.Bowden J, Davey Smith G, Haycock PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 2016;40(4):304–14. doi: 10.1002/gepi.21965 [published Online First: 2016/04/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44(2):512–25. doi: 10.1093/ije/dyv080 [published Online First: 2015/06/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S, Foley CN, Allara E, et al. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun 2020;11(1):376. doi: 10.1038/s41467-019-14156-4 [published Online First: 2020/01/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. 2022. doi: 10.1101/2021.03.26.437168 [DOI] [PubMed] [Google Scholar]
- 22.Burgess S, Yavorska O. MendelianRandomization v0.3.0: an R package for performing Mendelian randomization analyses using summarized data. International Journal of Epidemiology 2017;46(6):1734–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canoy D, Luben R, Welch A, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. American Journal of Epidemiology 2004;159(12):1140–49. [DOI] [PubMed] [Google Scholar]
- 24.Forno E, Han YY, Mullen J, et al. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J Allergy Clin Immunol Pract 2018;6(2):570–81 e10. doi: 10.1016/j.jaip.2017.07.010 [published Online First: 2017/10/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee J, Roy A, Singhamahapatra A, et al. Association of Body Mass Index (BMI) with Lung Function Parameters in Non-asthmatics Identified by Spirometric Protocols. J Clin Diagn Res 2014;8(2):12–4. doi: 10.7860/JCDR/2014/7306.3993 [published Online First: 2014/04/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatti U, Laghari ZA, Syed BM. Effect of Body Mass Index on respiratory parameters: A cross-sectional analytical Study. Pak J Med Sci 2019;35(6):1724–29. doi: 10.12669/pjms.35.6.746 [published Online First: 2019/11/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007;175(7):661–6. doi: 10.1164/rccm.200611-1717OC [published Online First: 2007/01/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108(1):206–11. doi: 10.1152/japplphysiol.00694.2009 [published Online First: 2009/10/31] [DOI] [PubMed] [Google Scholar]
- 29.Ochs-Balcom HM, Grant BJB, Muti P, et al. Pulmonary Function and Abdominal Adiposity in the General Population. Chest 2006;129(4):853–62. doi: 10.1378/chest.129.4.853 [DOI] [PubMed] [Google Scholar]
- 30.Mafort TT, Rufino R, Costa CH, et al. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med 2016;11:28. doi: 10.1186/s40248-016-0066-z [published Online First: 2016/07/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 2016;40(7):597–608. doi: 10.1002/gepi.21998 [published Online First: 2016/10/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry C, Liu J, Richmond R, et al. Exploiting collider bias to apply two-sample summary data Mendelian randomization methods to one-sample individual level data. PLoS Genet 2021;17(8):e1009703. doi: 10.1371/journal.pgen.1009703 [published Online First: 2021/08/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Zhao J, Lin Z, et al. Mendelian randomization for causal inference accounting for pleiotropy and sample structure using genome-wide summary statistics. Proc Natl Acad Sci U S A 2022;119(28):e2106858119. doi: 10.1073/pnas.2106858119 [published Online First: 2022/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behrens G, Matthews CE, Moore SC, et al. Body size and physical activity in relation to incidence of chronic obstructive pulmonary disease. Canadian Medical Association Journal 2014;186(12):E457–E69. doi: 10.1503/cmaj [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Censin JC, Peters SAE, Bovijn J, et al. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet 2019;15(10):e1008405. doi: 10.1371/journal.pgen.1008405 [published Online First: 2019/10/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjellvik V, Tverdal A, Furu K. Body mass index as predictor for asthma: a cohort study of 118,723 males and females. Eur Respir J 2010;35(6):1235–42. doi: 10.1183/09031936.00192408 [published Online First: 2010/01/16] [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Han S. Effect of selection bias on two sample summary data based Mendelian randomization. Sci Rep 2021;11(1):7585. doi: 10.1038/s41598-021-87219-6 [published Online First: 2021/04/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q, Chen Y, Wang J, et al. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int J Epidemiol 2019;48(5):1478–92. doi: 10.1093/ije/dyz142 [published Online First: 2019/07/13] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the analysis presented in the manuscript are publicly available and were downloaded using the following links:
The GWAS of BMI and WHRadjBMI: https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files
The GWAS of pulmonary functions: https://www.ebi.ac.uk/gwas/publications/30804560
The GWAS of asthma: http://www.nealelab.is/uk-biobank


