Abstract
Background:
Acute ischemic stroke is one of the leading causes of death and disability globally. Recent advances in omics methodology enable lipidomic profiling, which may provide knowledge of the underlying pathology of acute ischemic stroke and its associated outcomes.
Objective:
This study aims to examine the longer-term relationships between symptoms and outcomes following acute ischemic stroke and the underlying lipidomic signatures over 6 months during recovery between acute ischemic stroke patients who received reperfusion therapies and those who did not.
Methods:
This prospective cohort study will enroll 104 participants post-acute ischemic stroke in two groups based on their receipt of reperfusion therapy (Group 1) or not (Group 2; n = 52/group). Peripheral plasma samples will be collected from both groups for lipidomic analysis over 6 months. Arterial blood samples will be collected during the procedure for those receiving reperfusion. Self-reported symptoms and outcome data will be collected from both groups.
Discussion:
We will compare and examine the associations among plasma lipidomic biomarkers and symptoms, cognitive, functional, and health-related quality of life outcomes over 6 months between acute ischemic stroke patients who did and did not receive reperfusion intervention.
Keywords: acute ischemic stroke, cohort study, lipidomics
Acute ischemic stroke (AIS) is the leading cause of mortality and disability globally and affects more than 116 million people in the world each year (Virani et al., 2021). In the United States, someone experiences AIS approximately every 40 s, with an annual cost of $34 billion, primarily due to functional changes and lost productivity (Virani et al., 2021). Approximately 50% of AIS survivors exhibit fatigue and depressive symptoms, cognitive deficits, and poorer functional and health-related quality of life (HRQOL) outcomes at 6 months (Ayerbe et al., 2014; De Ryck et al., 2013; Go et al., 2014; Virani et al., 2021).
AIS results from a thrombus obstructing a large cerebral artery in the brain, causing disruption in cerebral blood flow (Go et al., 2014), resulting in blockage of large arteries, tissue damage, and potential neuronal cell death. AIS triggers an ischemic cascade which causes injury to brain tissue; this cascade may persist for many days post-stroke. The pathophysiological processes following AIS are complex and involve ischemia, inflammation, and disturbances of lipid metabolism (Tobin et al., 2014). During AIS, phospholipases are activated, causing hydrolysis of cell membrane sphingomyelin and cholesterol, which can potentiate further brain injury (Adibhatla & Hatcher, 2008; Hussain et al., 2019). As neurons die, they produce an infarct core. The penumbra, the area surrounding the core, may be rescued if reperfusion occurs (Borgens & Liu-Snyder, 2012). Unfortunately, patients must meet specific criteria to receive treatment, and the outcome is time-dependent (Campbell et al., 2015). AIS treatments focus on removing the thrombus and re-establishing cerebral blood flow as quickly as possible. Current treatments dissolve (via tissue plasminogen activator [tPA]) or physically remove the thrombus (via mechanical thrombectomy [MT];(Berkhemer et al., 2015; Goyal et al., 2016; “Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group," 1995). MT provides a novel opportunity to examine pathophysiological changes during AIS by allowing researchers to isolate arterial blood within the occluded artery from the infarct zone.
About 90% of patients who might otherwise qualify for reperfusion therapies do not arrive at the hospital in time to receive either intervention, frequently resulting in severe disabilities at discharge (Bhole et al., 2017; Malhotra et al., 2017). Reperfusion interventions are associated with improved cognitive and functional outcomes, lower readmission hospital rates, and decreased mortality rates at 1 month post-AIS (Li et al., 2020). However, AIS patients treated with reperfusion interventions display variability in cognitive and functional outcomes and mortality rates at 1 month post-AIS (McCarthy et al., 2019). To date, what underlies the development of symptoms and poorer outcomes in those who do and do not receive reperfusion interventions is understudied.
Recent advances in omics methodology enable lipidomic profiling, which may provide knowledge of the underlying pathology of AIS and its associated outcomes. The purpose of the study is to examine the relationship among peripheral plasma lipidomic (fat) biomarkers and symptoms (fatigue and depression), cognitive, functional, and HRQOL outcomes over 6 months between acute ischemic stroke patients who received treatments to open their cerebral arteries (reperfusion therapies) and those who did not. The specific aims and hypotheses are:
Aim 1: Compare symptoms (fatigue and depressive), cognitive, functional, and HRQOL outcomes over 6 months between AIS patients who did and did not receive reperfusion intervention. Hypothesis 1: The non-reperfusion group will experience more severe fatigue and depressive symptoms and poorer cognitive, functional, and HRQOL outcomes post-AIS.
Aim 2: Compare peripheral plasma lipid levels over 6 months between AIS patients who did and did not receive reperfusion intervention. Hypothesis 2: Those who do not receive reperfusion intervention will have higher sphingomyelin, ceramides, and cholesterol levels post-AIS than those who received reperfusion intervention.
Aim 3: Examine early and late peripheral plasma lipid levels and symptoms, cognitive, functional, and HRQOL outcomes over time in patients who do and do not receive reperfusion intervention. Hypothesis 3.1: Higher sphingomyelin, ceramides, and cholesterol levels (Days 1, 3, 5) will predict higher severity of fatigue and depressive symptoms and poorer cognitive, functional, HRQOL outcomes (Months 1, 3, 6). Hypothesis 3.2: At Months 1, 3, 6, higher sphingomyelin, ceramides, and cholesterol levels will be associated with higher severity of fatigue and depressive symptoms, poorer cognitive, functional, HRQOL outcomes (Months 1, 3, 6).
Aim 4: Explore the relationships of 13 lipid classes with symptoms, cognitive, functional, and HRQOL outcomes over 6 months in (4a) distal and proximal arterial plasma lipid levels in reperfusion intervention group (4b/c) peripheral plasma lipid levels in AIS patients (4b) who did and (4c) did not receive reperfusion intervention.
Methods
Design
A single-center, prospective, cohort study design will be used to characterize longer-term associations of symptoms and outcomes following AIS and the underlying lipidomic signatures at specific time points in the trajectory of recovery. This study includes two groups: (a) AIS patients who did and (b) AIS patients who did not receive reperfusion intervention.
AIS study participants (Groups 1 and 2) will be recruited within 48 hr of AIS diagnosis from the University of Illinois (UI) Health Hospital, Chicago, IL, USA, an urban regional medical center. The Institutional Review Board of the University of Illinois at Chicago (protocol #2021-0615) approved the study.
Conceptual Framework
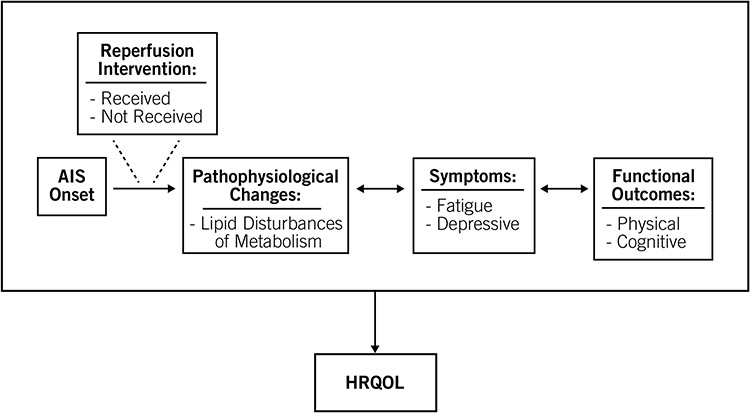
This study has adapted the Institute of Medicine’s (IOM) Disability Framework to develop a new conceptual framework (Figure 1; (Institute of Medicine, 1991). Reperfusion intervention (received or not received) influences the pathophysiological changes, symptoms, functional outcomes (and vice versa), and HRQOL outcomes following AIS. An increase in lipid disturbances of metabolism correlates to increased symptoms and poorer functional and HRQOL outcomes post-AIS. Understanding the trajectory of recovery is important in predicting outcomes and developing timely, tailored interventions for managing symptoms and reducing long-term disability post-AIS.
Figure 1.
Conceptual Framework of Pathophysiology, Symptoms, and Outcomes Following AIS
Study Site and Recruitment
A target of up to 104 AIS survivors will be recruited. Adults (≥ 18 years of age) with AIS will be recruited from UI Health hospital in the neuroscience units (e.g., neurosurgery intensive care, neurological step down) and enrolled in one of two groups: participants who have received reperfusion therapy (Group 1) and participants who have not received reperfusion therapy (Group 2). Participant eligibility criteria are specified in Table 1. We expect patients to be able to complete self-report questionnaires on study Day 5 and Months 1, 3, and 6.
Table 1.
Eligibility Criteria for Participants
| Eligibility Criteria for AIS Participants Who Received Reperfusion Therapy (Group 1) |
Eligibility Criteria for AIS Participants Who Did Not Received Reperfusion Therapy (Group 2) |
|---|---|
| Inclusion Criteria: | Inclusion Criteria: |
|
|
|
|
|
|
|
|
|
|
| Exclusion Criteria: | Exclusion Criteria: |
|
|
|
|
|
Note. AIS = acute ischemic stroke; CT = computed tomography; MRI = magnetic resonance imaging; MT = mechanical thrombectomy
The research team will identify potential participants by screening electronic health records (EHR) of potentially eligible AIS participants against eligibility criteria. Research personnel (e.g., principal investigator [PI], research assistant) will communicate with the neurology and neurosurgery teams regarding potential candidates based on reperfusion and non-reperfusion therapy participant status. The research personnel will discuss the study with the patients and/or legally authorized representative (LAR), after which potential participants or their LARs will have the opportunity to ask questions. If they decide to participate in the study, informed consent will be obtained. Participants will be enrolled in the study within 48 hr of hospital admission post-AIS (Study Day 1). Additional visits will occur on Study Days 3, 5 and Months 1, 3, 6. If the LAR initially provides consent, we will obtain continuing consent from the patient who had the AIS once determined stable by a medical team.
Procedures
Acute Ischemic Stroke Participants (Group 1 and Group 2)
Blood samples.
The peripheral blood samples on Study Day 1 will be collected at the time of obtaining informed consent. Neurosurgeons will collect arterial blood samples (distal and proximal blood to the cerebral thrombus) during the MT procedure and bank the specimens under an emergency waiver of consent. Due to the emergent nature of AIS patients undergoing radiographic imaging, reperfusion interventions, and the consequences of stroke itself (i.e., swallowing issues or dysphasia), these patients are typically fasting the first 24–48 hr of hospital admission (during Study Day 1). Peripheral blood samples will be collected before 10 a.m. on study Days 3 and 5 and Months 1, 3, and 6; patients will be asked to fast after 12 a.m. on the day of sample collection to reduce the effect of dietary variability on lipidomic profiling.
Peripheral venous blood samples from Study Days 1, 3, and 5 will be collected inpatient and initially processed in the University of Illinois at Chicago (UIC) College of Nursing (CON) Biobehavioral Core Laboratory. Approximately 6mL of blood will be collected from each participant and placed into EDTA-anticoagulated blood tubes (Becton Dickinson, Franklin Lakes, NJ) for plasma isolation. EDTA tubes will be centrifuged at 1000g for 10 min at room temperature to separate plasma from the buffy coat. All plasma samples will be aliquoted in 250μL and stored at −80°C until lipidomic analysis. For Months 1, 3, and 6, the venous blood draws will be completed at participants’ residences, rehabilitation facilities, or the clinic, then processed and stored at −80°C for batch processing. All plasma samples will be transported using standard protocols for lipidomic analysis.
Questionnaires.
At Study Day 5 and Months 1, 3, and 6, participants will complete questionnaires regarding fatigue and depressive symptoms, physical and cognitive function, HRQOL, and Columbia Suicide Severity Rating Scale (C–SSRS; Table 2). On Study Day 5, participants may still be in the hospital, and the study team will assist them with questionnaires. Participants are likely to have been discharged at 1, 3, and 6 months post-AIS. Some participants may be still in rehabilitation facilities for 1 and 3 months. Procedures will be conducted at either participants’ residences, rehabilitation facilities, or in the patient exam room. These visits take approximately 1 hr to complete all procedures.
Table 2.
Measures and Time Points
| Days | Months | ||||||
|---|---|---|---|---|---|---|---|
| Group | 1 | 3 | 5 | 1 | 3 | 6 | |
| Lipid Biomarkers - Distal and Proximal Arterial Plasma: | MT | X | |||||
| Lipid Biomarkers - Peripheral Plasma: | RI, nRI | X | X | X | X | X | X |
| Symptoms: | |||||||
| Fatigue: Neuro-QOL (fatigue) | RI, nRI | X | X | X | X | ||
| Depressive: Neuro-QOL (depressive) | RI, nRI | X | X | X | X | ||
| Functional Outcomes: | |||||||
| Physical: Neuro-QOL (physical) | RI, nRI | X | X | X | X | ||
| Cognitive: Neuro-QOL (cognitive) | RI, nRI | X | X | X | X | ||
| HRQOL: | |||||||
| HRQOL: Neuro-QOL (quality of life) | RI, nRI | X | X | X | X | ||
| Suicide/Suicidal Ideation: | |||||||
| Columbia Suicide Severity Rating Scale (C-SSRS) | RI, nRI | X | X | X | X | ||
| Covariates: Demographics | RI, nRI | X | |||||
Note. RI = reperfusion intervention is mechanical thrombectomy (MT); nRI = non-reperfusion intervention; RI = reperfusion intervention; HRQOL = health-related quality of life
Measures
Demographic and Clinical Characteristics
On study enrollment, baseline medical history, demographics such as age, gender, and clinical characteristics such as vital signs, BMI, and time of last known well symptom prior to having AIS symptoms will be extracted from the EHR. We will also collect data about enrollment criteria, clinical characteristics, prehospital care, hospital-based procedures, hospital course, hospital discharge disposition, infections, and complications sections. Data will be collected and managed through Research Electronic Data Capture (REDCap) (Harris et al., 2019; Harris et al., 2009). Stroke-specific information will be collected at baseline and at discharge from the EHR to include stroke location, infarct volume, National Institutes of Health Stroke Scale (NIHSS), and modified Rankin Scale (mRS). In addition, data about comorbid conditions (e.g., hypertension, atrial fibrillation, diabetes, hyperlipidemia, previous stroke, coronary heart disease, and COPD) and concomitant medications (e.g., statins) will be collected upon entry into the study and at each study day visit.
Lipidomic Assay (Untargeted Agilent Qtof 6545 and UPLC 1290 Platform)
The Agilent Qtof 6545 and UPLC 1290 assay detects about 700 distinct lipid species across 13 different lipid classes (e.g., lysophosphatidylcholines, lysophosphatidylethanolamines, phosphatidylcholines, phosphatidylethanolamines, sphingomyelins, diacylglycerols, triacylglycerols, free fatty acids, cholesterol, ceramides, dihydroceramides, hexosylceramides, and lactosylceramides). Each sample is run once on the platform using positive and negative ionization modes. Data acquisition lasts for 20 min per sample, and 25μL of the plasma equivalent will be injected. Data quality will be ensured by using a set of lipid standards after each cleaning, 24 hr of idling or 3 days of consecutive use, performing a quick system suitability test before each batch to ensure acceptable limit of detection for each lipid class, sample randomization for lipid extraction and data acquisition, and triplicate injection of lipids extracted from a reference plasma sample at the beginning of the batch.
Questionnaires
All self-report symptom and outcome measures are valid and reliable in a wide range of subjects across disciplines and in AIS patients (Carlozzi et al., 2020; Carlozzi et al., 2019; Muus et al., 2007; Victorson et al., 2014). The research team administering the symptom and outcome questionnaires and will be trained in a standardized manner for consistency in the study. If participants at Study Day 5 are unable to answer the questionnaires on their own, we will attempt each day thereafter to collect the data while hospitalized.
We use the National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) for ischemic stroke to select outcome measures within the domains of fatigue and depressive symptoms, upper extremity function—fine motor, activity of daily living (ADL), lower extremity function—mobility, applied cognition—executive function, and stroke-specific QOL (Table 3; (Cella et al., 2012). In addition, we will assess suicidal ideation/self-harm via the C–SSRS on all participants at each study visit. Regardless of symptom severity, all participants will be provided a list of resources for mental and behavioral health services available in the area at every study visit.
Table 3.
Variables and Measurements
| Symptoms | Instrument | Definition | Validity/Reliability |
|---|---|---|---|
| Fatigue | National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) – Fatigue Domain, takes 10 min to complete. | Self-report 8-item form evaluating tiredness to an overwhelming, debilitating, sustained sense of exhaustion that decreases one’s ability for physical, functional, social roles or activities. Scores are 1–5, ranges from 8–40; higher scores equal greater fatigue. | Good internal consistency and a reliability coefficient, Cronbach α = 0.86. |
| Depressive | National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) – Depressive Domain, takes 10 min to complete. | Self-report 8-item evaluating experience of loss and feelings of hopelessness, negative mood (sadness, guilt), decrease in positive affect (loss of interest), information-processing deficits (problems in decision-making), negative views of self (self-criticism, worthlessness), and negative social cognition (loneliness). Scores are 1–5, ranges from 8–40; higher scores indicate greater depressive symptoms. | Good internal consistency and a reliability coefficient, Cronbach α = 0.86. |
| Outcomes | |||
| Physical | National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) – Upper Extremity Function – Fine Motor, ADL Domain, takes 15 min to complete. | Self-report 8-item evaluating one’s ability to carry out various activities involving digital, manual, and reach-related functions, ranging from fine motor to activities of daily living. Scores are 1–5, ranges from 8–40; higher scores indicate better physical health. | Good internal consistency and a reliability coefficient, Cronbach α = 0.86. |
| Physical | National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) – Lower Extremity Function – Mobility Domain, takes 15 min to complete. | Self-report 8-item evaluating one’s ability to carry out various activities involving the trunk region and increasing degrees of bodily movement, ambulation, balance or endurance. Scores are 1–5, ranges from 8–40; higher scores indicate better physical health. | Good internal consistency and a reliability coefficient, Cronbach α = 0.88. |
| Cognitive | National Institute of Neurological Disorders and Stroke (NINDS) Quality of Life (QOL) – Applied Cognition – Executive Function, takes 15 min to complete. | Self-report 8-item evaluating perceived difficulties in applications of mental function related to planning, organizing, calculating, working with memory and learning. Scores are 1–5, ranges from 8–40; higher scores indicate better cognitive health. | Good internal consistency and a reliability coefficient, Cronbach α = 0.88. |
| HRQOL | Stroke Specific Quality of Life (SS–QOL), takes 15 min to complete. | Self-report scale with 49 items in 12 domains: mobility, energy, upper extremity, work/ productivity, mood, self-care, social roles, family roles, vision, language, thinking, personality. Scores range are 1–5 and each domain has 3 to 5 questions. Higher scores indicate a better quality of life. | Good internal consistency and a reliability coefficient, Cronbach α = 0.94. |
Note. HRQOL = health-related quality of life
Data Analysis
Descriptive statistics (means, standard deviations, frequency, and percentages) will be used to characterize AIS participants. All tests of the hypothesis will be evaluated for significance with an alpha of 0.05 (two-sided test). Effect sizes in standard deviation units (Cohen’s d) will be calculated for clinical interpretation. Demographic and clinical variables associated (p < 0.05) with symptoms and outcomes will be evaluated as potential covariates (refer to the Demographic and Clinical Characteristics of Measures section).
Linear mixed models will be used to predict the linear change and compare symptoms and outcome scores across time (Study Day 5 and Months 1, 3, and 6) between both groups. Linear mixed models allow for estimating the response to AIS and if participants receive reperfusion or did not receive reperfusion intervention. We will include a group-by-time interaction term to assess and compare different group patterns. Linear mixed models are robust and account for missing data by estimating the effects with all cases—even if some answers on questionnaires are missing or if a participant drops out of a study. Potential effect modifiers and covariates will be controlled for and included in the model if associated at 3 and 6 months with significant symptom and outcome measures.
Statistical and pathway analyses for lipidomic data will be analyzed using Metaboanalyst (Chong et al., 2019). A list of lipid compounds will be generated using retention time, m/z data, and KEGG IDs (Kanehisa, 2019; National Institute of Standards and Technology, 2020). For each group and time point, positive and negative ionization mass spectrometry outputs will be explored. Multivariate supervised and unsupervised methods will be performed to identify signal detection in each group across time and on outcome, including principal component analysis (PCA), partial least squares discriminant analysis (PLS–DA), machine learning, cluster analysis, and network learning methods (Chen & Ishwaran, 2012; Hu et al., 2021; Priyanka et al., 2013).
Linear mixed models will also be used to test the predictive association of lipidomic biomarker peak mean value across time (Months 1, 3, and 6) between both groups. We will include group-by-time interaction terms between lipidomic biomarkers, symptoms, and outcomes to assess and compare different patterns in the groups at early (Study Days 1, 3, and 5) and late (Months 1, 3, and 6) time points.
Sample/Power Analysis
Based on an estimated 30% attrition rate, we planned initial enrollment of 104 participants to achieve a final sample of 40/group. Power analysis and sample size calculations were based on assumptions and requirements to satisfy specific aims. Linear mixed model is a robust method for analyzing repeated measures of longitudinal data. Based on 80 subjects, there will be 80% power for testing the null hypothesis with the mean slope of 0, effect size of 0.50, at a two-tailed test of alpha of .05. Power also depends on how strong the association is between the lipidomic biomarkers. We estimated the two groups’ average lipid biomarker concentration across three time points. A conservative approximation of power based on 80 participants gives 96% power for detecting a correlation of .40 and 80% power for detecting a correlation of .31.
Discussion
This proposal uses innovative approaches to capture longitudinal lipidomic profile signatures in the 6 months following AIS. This includes the novel method of examining the lipid profile of arterial blood within the involved artery immediately distal and proximal to the thrombus in the stroke infarct zone. Analysis of these arterial blood samples using approaches such as lipidomics may provide knowledge of early pathophysiological changes after AIS onset, the interplay of different pathways of injury, and responsiveness to reperfusion treatment. Knowledge of the underlying molecular mechanisms of AIS at the infarct site and their association with later symptom and functional outcomes could assist in the development of timely targeted therapies (e.g., dietary interventions) to complement current AIS treatments. The study team will examine the lipidomic profiles over time and their association with symptoms, functional, and HRQOL outcomes in AIS patients. We will also compare findings in those persons who received and did not receive reperfusion intervention.
Conclusion
Should pathophysiological mechanisms related to lipid metabolism underlie development of symptoms and outcomes in AIS, relevant treatments to address changes could be tested for efficacy in AIS survivors in a future clinical trial. Interventions (e.g., dietary) that reduce or enhance lipid metabolism could be used to improve symptoms and outcomes in AIS survivors. Our study will help identify AIS survivors for targeted intervention.
Acknowledgement:
We would like to thank the University of Washington, School of Nursing Omics Pre/Postdoctoral Fellows and Faculty for critical input on the study design as it evolved through Dr. Martha’s NIH/NINR T32NR016913 fellowship.
Funding:
Dr. Sarah Martha is supported by National Institute of Nursing Research of the National Institutes of Health under the award number K23NR019864; Sigma Theta Tau International/Western Institute of Nursing Research Grant (#17775); and Agnes Marshall Walker Foundation Research Grant.
Footnotes
Conflict of Interests Statement: The authors have no conflict of interest to report.
Ethical Conduct of Research: The Institutional Review Board of the University of Illinois at Chicago approved the study (Protocol #2021-0615).
Contributor Information
Sarah R. Martha, University of Illinois at Chicago College of Nursing, Biobehavioral Nursing Science Department, Chicago, IL.
Alice Y. Pen, University of Illinois at Chicago College of Nursing, Biobehavioral Nursing Science Department, Chicago, IL.
Laura Stone McGuire, University of Illinois at Chicago College of Medicine, Department of Neurosurgery, Chicago, IL.
Ali Alaraj, University of Illinois at Chicago College of Medicine, Department of Neurosurgery, Chicago, IL.
Mark Maienschein-Cline, University of Illinois at Chicago Research Resources Center, Chicago, IL.
Sanjib Basu, University of Illinois at Chicago School of Public Health, Division of Epidemiology and Biostatistics, Chicago, IL.
Jeffrey A. Loeb, University of Illinois at Chicago College of Medicine, Department of Neurology and, Rehabilitation, Chicago, IL.
Hilaire J. Thompson, Biobehavioral Nursing and Health Informatics, Office of Academic Personnel, Harborview Injury Prevention and Research Center, University of Washington School of Nursing, Seattle, WA.
References
- Adibhatla RM, & Hatcher JF (2008). Altered lipid metabolism in brain injury and disorders. Subcell Biochemistry, 49, 241–268. 10.1007/978-1-4020-8831-5_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayerbe L, Ayis S, Crichton S, Wolfe CDA, & Rudd AG (2014). The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. Journal of Neurology, Neurosurgery & Psychiatry, 85, 514–521. 10.1136/jnnp-2013-306448 [DOI] [PubMed] [Google Scholar]
- Banerjee P, Ghosh S, Dutta M, Subramani E, Khalpada J, RoyChoudhury S, Chakravarty B & Chaudhury K (2013). Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS ONE, 8, e80940. 10.1371/journal.pone.0080940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJH, van Walderveen MAA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, … Dippel DWJ (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. New England Journal of Medicine, 372, 11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- Bhole R, Goyal N, Nearing K, Belayev A, Doss VT, Elijovich L, Hoit DA, Tsivgoulis G, Alexandrov AV, Arthur AS, & Alexandrov AW (2017). Implications of limiting mechanical thrombectomy to patients with emergent large vessel occlusion meeting top tier evidence criteria. Journal of NeuroInterventional Surgery, 9, 225–228. 10.1136/neurintsurg-2015-012206 [DOI] [PubMed] [Google Scholar]
- Borgens RB, & Liu-Snyder P (2012). Understanding secondary injury. Quarterly Review of Biology, 87, 89–127. 10.1086/665457 [DOI] [PubMed] [Google Scholar]
- Campbell BC, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, Goyal M, Mitchell PJ, Saver JL, Diener HC, & Davis SM (2015). Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet Neurology, 14, 846–854. 10.1016/s1474-4422(15)00140-4 [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, Boileau NR, Chou KL, Ready RE, Cella D, McCormack MK, Miner JA, & Dayalu P (2020). HDQLIFE and neuro-QoL physical function measures: Responsiveness in persons with Huntington’s disease. Movement Disorders, 35, 326–336. 10.1002/mds.27908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Boileau NR, Paulsen JS, Downing NR, Ready R, Perlmutter JS, Cella D, Chou KL, McCormack MK, Barton S & Lai J-S (2019). Psychometric properties and responsiveness of Neuro-QoL Cognitive Function in persons with Huntington disease (HD). Quality of Life Research, 29, 1393–1403. 10.1007/s11136-019-02391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Lai J-S, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, Reder AT, Sufit R, Simuni T, Holmes GL, Siderowf A, Wojna V, Bode R, McKinney N, Podrabsky T, Wortman K, Choi S, Gershon R, Rothrock N, & Moy C (2012). Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology, 78, 1860–1867. 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, & Ishwaran H (2012). Random forests for genomic data analysis. Genomics, 99, 323–329. 10.1016/j.ygeno.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Wishart DS, & Xia J (2019). Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Current Protocols in Bioinformatics, 68, e86. 10.1002/cpbi.86 [DOI] [PubMed] [Google Scholar]
- De Ryck A, Brouns R, Fransen E, Geurden M, Van Gestel G, Wilssens I, De Ceulaer L, Mariën P, De Deyn PP , & Engelborghs S (2013). A prospective study on the prevalence and risk factors of poststroke depression. Cerebrovascular Diseases Extra, 3, 1–13. 10.1159/000345557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, … Turner MB (2014). Executive summary: Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation, 129, 399–410. 10.1161/01.cir.0000442015.53336.12 [DOI] [PubMed] [Google Scholar]
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miguel MA, Donnan GA, Roos YBWEM, Bonafe A, Jahan R, Diener H-C, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, … Jovin TG (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet, 387, 1723–1731. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, & Duda SN on Behalf of the REDCap Consortium. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Sun Y, Jia W, Li D, Zou M, & Zhang M (2021). Study on the estimation of forest volume based on multi-source data. Sensors, 21, 7796. https://www.mdpi.com/1424-8220/21/23/7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain G, Wang J, Rasul A, Anwar H, Imran A, Qasim M, Zafar S, Kashif S, Kamran S, Razzaq A, Aziz N, Ahmad W, Shabbir A, Iqbal J, Baig SM, & Sun T (2019). Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids in Health and Disease, 18, 26. 10.1186/s12944-019-0965-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (1991). Disability in America: Toward a national agenda for prevention. National Academies Press. https://doi.org/doi: 10.17226/1579 [DOI] [Google Scholar]
- Kanehisa M (2019). Toward understanding the origin and evolution of cellular organisms. Protein Science, 28, 1947–1951. 10.1002/pro.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang J, Wu B, Xu H, Wu X, Zhou L, & Deng B (2020). Association between early cognitive impairment and midterm functional outcomes among Chinese acute ischemic stroke patients: A longitudinal study. Frontiers in Neurology, 11, 20. 10.3389/fneur.2020.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K, Gornbein J, & Saver JL (2017). Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: A review. Frontiers in Neurology, 8, 651. 10.3389/fneur.2017.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Diaz A, Sheinberg DL, Snelling B, Luther EM, Chen SH, Yavagal DR, Peterson EC, & Starke RM (2019). Long-term outcomes of mechanical thrombectomy for stroke: A meta-analysis. Scientific World Journal, 2019, 7403104. 10.1155/2019/7403104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus I, Williams LS, & Ringsberg KC (2007). Validation of the Stroke Specific Quality of Life Scale (SS–QOL): Test of reliability and validity of the Danish version (SS–QOL–DK). Clinical Rehabilitation, 21, 620–627. 10.1177/0269215507075504 [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. (1995). Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine, 333, 1581–1587. 10.1056/nejm199512143332401 [DOI] [PubMed] [Google Scholar]
- National Institute of Standards and Technology. (2020). NIST standard reference database 1A. https://www.nist.gov/srd/nist-standard-reference-database-1a-v17
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, & Lazarov O (2014). Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. Journal of Cerebral Blood Flow & Metabolism, 34, 1573–1584. 10.1038/jcbfm.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorson D, Cavazos JE, Holmes GL, Reder AT, Wojna V, Nowinski C, Miller D, Buono S, Mueller A, Moy C, & Cella D (2014). Validity of the Neurology Quality-of-Life (Neuro–QoL) measurement system in adult epilepsy. Epilepsy & Behavior, 31, 77–84. 10.1016/j.yebeh.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. (2021). Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation, 143, e254–e743. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]