Abstract
Objectives
To evaluate the effect of a health maintenance reminder (HMR) on Human Papillomavirus (HPV) vaccine administration and completion across different age, insurance, and race cohorts.
Study Design
Retrospective pre-post analysis
Setting
Academic primary care
Methods
Patients aged 9-26 who had initiated the HPV vaccine series from 2016 to 2021 were analyzed, based on current age-based standards. Cohort was divided based on vaccine uptake before and after implementation of HMR program in February 2020. Multivariate analysis estimated odds of vaccine completion based on sociodemographic factors, and variable interactions were investigated to determine independent associations between sociodemographic factors and HMR implementation.
Results
There were 7,654 individual patients (mean age was 15.8 years; 46.7 were males; and 50.7% were White). HPV vaccine completion rates increased post-HMR implementation by 59.2% (37% pre-, and 58.9% post-HMR; p<0.001) in the entire cohort. Overall, Black patients (aOR = 0.68; 95% CI 0.60, 0.70) and patients ≥18 years (aOR = 0.13; 95% CI 0.11, 0.15) were significantly less likely to complete their vaccine series; however, this improved significantly following HMR in these groups (p<0.001). Post-HMR, race and insurance status were not independently associated with disparate vaccine completion rates, however, age was, and patients ≤14 or younger had higher odds of vaccine completion (aOR = 3.54; 95% CI 2.91, 4.32).
Conclusion
The implementation of an HMR was associated with increased HPV vaccine uptake across age and race groups in this single institution study. Future research should explore barriers to implementing HMRs in different healthcare settings.
Keywords: Human Papillomavirus, Oropharyngeal Cancer, HPV Vaccine, Preventative Health, Health Maintenance, Quality Improvement
Introduction:
Human papillomavirus (HPV) remains one of the most common sexually transmitted infections,1 with many high-risk variants classically associated with the development of diseases such as oropharyngeal and anogenital cancers.2 For these indications, the implementation of the HPV vaccine in adolescents has proven effective for the prevention of long-term infectious and oncologic sequalae.3,4 More recently, the growing prevalence of HPV-associated oropharyngeal cancer (OPC) has prompted the expansion of indications to include the prevention of OPC and recommendation of catch-up vaccines in adults aged up to 261 and previously unvaccinated adults aged up to 45.5 Given that over 40,000 incident cases of HPV-associated cancers occur yearly,6 all qualified individuals should be encouraged to complete the HPV vaccine series, including individuals beyond the age of primary vaccination.
Despite the expansion of clinical indications, current vaccine completion rates remain relatively low. Recent National Immunization Survey-Teen data demonstrate that only 61.4% and 56.0% of adolescent females and males, respectively, were up to date on HPV vaccination in 2020.7 Furthermore, national claims data suggest that timely receipt of subsequent doses of the vaccine is low for both females and males.8 Pertinent barriers to vaccine completion include patient concerns over vaccine safety and efficacy, cost constraints, disparities in healthcare access, and other social factors.5,9 Specifically, race has been linked as an important negative predictor of HPV vaccine completion, with Black and Hispanic patients having reduced completion rates compared to their White counterparts.10 Additionally, the ongoing COVID-19 pandemic has been associated with a reduction in the utilization of preventative health services,11 and a decline in median doses administered of the HPV vaccine has been observed during the Spring of 2020 compared to previous years.12
Provider recommendation remains an integral factor in addressing existing barriers to HPV vaccination initiation and completion.13 Given the longitudinal nature of such care, the implementation of electronic health record (EHR) health maintenance reminder (HMR) systems has demonstrated improved vaccine uptake in different adolescent cohorts.14,15 Despite this, the effect of HMR on young adult cohorts encompassed by the expanded vaccination guidelines has not been well characterized, particularly in males up to 26 years old. Furthermore, few studies have assessed the impact of a standardized intervention like an HMR on existing racial disparities in vaccine completion.
Thus, the primary goal of this study was to assess the effect of an institution-wide HMR reminder on HPV vaccination completion rates, especially as it relates to newly covered age cohorts and historically marginalized racial groups. Ultimately, although otolaryngologists primarily manage HPV-associated OPC, by remaining removed from vaccination discussions, we are missing opportunities to prevent these conditions from occurring in the first place. This study explores the impact of a collaborative effort to improve HPV vaccination among otolaryngologists and primary care physicians at one institution.
Materials and Methods:
Study Participants and HPV Health Maintenance Reminder
Following approval from the Duke University Institutional Review Board, a retrospective analysis was conducted using patient data extracted from April 2016 to August 2021. A total of 7,654 individual patients aged 9 to 26 from 40 Duke Primary Care outpatient centers were included, all utilizing a common EHR. Pre- and post-intervention cohorts were defined with relation to the implementation of the EHR HPV HMR system in February 2020. The HMR was designed after prior alerts on health maintenance topics that had been implemented previously at our institution and was added to an existing interface containing other HMRs (for example, Diabetes screenings and childhood vaccines) by EHR superusers, who are employees that provide EHR operational support. After implementation, the reminder was pushed out to all physician, mid-level, and nursing staff of clinics using the EHR. When an eligible patient’s chart was accessed, the provider would be automatically prompted to make a decision on outstanding HPV vaccination tasks, based on the patient’s age, vaccine type, and vaccine dose in series. Providers were required to mark the task as “complete” or “incomplete” and provide a reason for the latter. If not completed, the alert would again populate on the next encounter with the patient.
Data Extraction
Data were extracted from all eligible patients using EHR SlicerDicer® (Epic)16 functionality. Relevant patient demographic features, including age, sex, race, and insurance status were collected. Additional treatment data, including date of HPV vaccination, dose in series, vaccine type, and location of administration were extracted, along with data on total Duke Primary Care visits before and during the pandemic.
Cohort Division and Series Completion Definition
Patients included in the study must have had all doses of their vaccine administered within the Duke Health network to be included. Any patient starting the first dose of the HPV vaccine series within a year of the study’s conclusion period was excluded from the study as it was not possible for these patients to receive the full complement of vaccines.
For our analysis, patients were subdivided along race-based, insurance-based, and age-based categories. Completion was defined based on the total number of vaccine doses received and the recorded age at first dose administration. In accordance with the age-based recommendations set by the Center for Disease Control’s Advisory Committee on Immunization Practices, patients 14 or younger at their first dose required two doses to be considered complete. Comparatively, patients 15 or older needed three doses. Patients were considered complete upon receipt of all required doses within 12 months. Additionally, individuals were subcategorized based on when they received their last recorded HPV vaccine dose; patients were placed into “Before” and “After” categories with relation to the HMR implementation in February 2020.
Statistical Analysis
Relevant demographic characteristics were summarized with respect to HMR. Differences in proportions in the “Before” and “After” categories were interrogated using Fisher’s Exact and Pearson’s Chi Square test as appropriate based on contingency table parameters. Student’s two-tailed t-tests were performed to determine the difference between continuous variables as needed. A multivariate logistic regression model was created to investigate the effect of race, insurance, gender, vaccine type, HMR implementation, and age on vaccination completion. Additionally, pairwise interaction covariance was included in subsequent mult-variate modeling to determine the independent effect of HMR implementation on vaccination completion. A post-hoc contrast analysis was computed for the effect modifiers age, race, and insurance. Data analysis was performed in SAS (v. 9.4, SAS Analytics Software and Solutions, Cary, NC) while figure preparation was performed in R (v. 4.0.3, The R Foundation for Statistical Computing, Vienna, Austria).
Results:
Patient Population
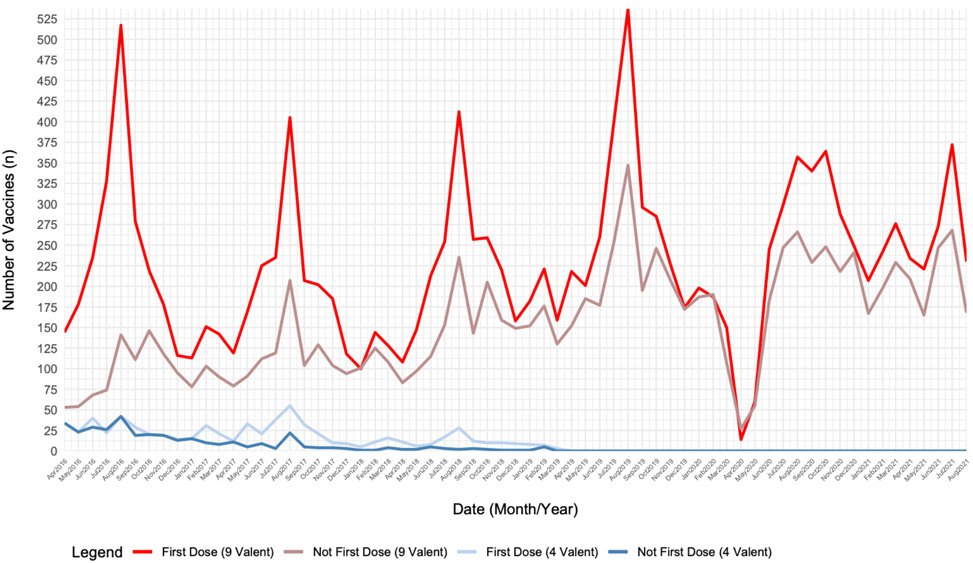
During the study, relevant demographic data were collected from 7654 patients before and after the implementation of the HPV vaccine HMR. There were significant differences according to race (p=0.006), insurance status (p<0.0001), vaccine type (p<0.0001), and age (15.85 [4.16] years vs. 15.59 [4.52] years, p=0.032) before and after HMR. Overall, there was a slightly higher racial representation of White (51.7% vs 50.4%), Asian (8.7% vs 7.1%), and Other (12.2% vs 11.0) after the HMR. Additionally, there were more patients with private (73.7% vs 66.5%) or other (1.5% vs 1.1%) forms of insurance, and less patients that were self-paid (4.7% vs 8.2%) after the change. After the HMR, only HPV 9-valent vaccines were administered (100% vs 97.8%). The gender distributions were not significantly different before and after the HMR (p=0.819). A full demographic breakdown can be appreciated in Table 1. Notably, outpatient visits decreased by 18% in 2020 during the pandemic compared to the prior 3 years. Total number of HPV vaccine doses administered concomitantly fell with outpatient visits during the pandemic (Figure 1).
Table 1:
Characteristics of HPV Cohort Before and After HPV Vaccination HMR; N=7654
| Characteristic: | Number of Patients, n (%) | ||||
|---|---|---|---|---|---|
| Total | Before | After | p-value | ||
| Race | |||||
| White | 3879 (50.7) | 3038 (50.4) | 796 (51.7) | ||
| Asian | 571 (7.5) | 437 (7.1) | 134 (8.7) | ||
| Black | 2347 (30.7) | 1925 (31.5) | 422 (27.4) | ||
| Other | 857 (11.2) | 670 (11.0) | 187 (12.2) | 0.006 | |
| Insurance Status | |||||
| Medicaid | 1788 (23.4) | 1480 (24.2) | 308 (20.0) | ||
| Private | 5199 (67.9) | 4064 (66.5) | 1135 (73.7) | ||
| Self-Paid | 574 (7.5) | 501 (8.2) | 73 (4.7) | ||
| Other | 93 (1.2) | 70 (1.1) | 23 (1.5) | <0.0001 | |
| Vaccine Type | |||||
| HPV 4-Valent | 132 (1.7) | 132 (2.2) | 0 (0) | ||
| HPV 9-Valent | 7522 (98.3) | 5983 (97.8) | 1539 (100) | <0.0001 | |
| Gender | |||||
| Male | 3568 (46.7) | 2855 (46.7) | 713 (46.3) | ||
| Female | 4086 (53.3) | 3260 (53.3) | 826 (53.7) | 0.819 | |
| Age | Mean (SD) | ||||
| 15.80 (4.2) | 15.85 (4.2) | 15.59 (4.5) | 0.032 | ||
| Number of Patients, n (%) | |||||
| 14 or Younger | 3690 (31.1) | 2879 (47.1) | 811 (52.7) | ||
| 15 to 17 | 1587 (20.7) | 1337 (21.9) | 250 (16.2) | ||
| 18 or Older | 2377 (31.1) | 1899 (31.1) | 478 (31.1) | <0.0001 | |
| Vaccine Completion | |||||
| Yes | 3167 (41.4) | 2260 (37.0) | 907 (58.9) | ||
| No | 4487 (58.6) | 3855 (63.0) | 632 (41.1) | <0.0001 | |
Abbreviations: HPV= Human Papillomavirus; HMR = Health Maintenance Reminder.
Data presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values.
Figure 1. Total number of HPV vaccine doses administered at study locations by month and dose in series.
Identifying Categorical Vaccine Completion Disparities
Multivariate analysis of completion identified reduced completion rates in Black (aOR = 0.68; 95% CI 0.60, 0.77), Other [Race] (aOR = 0.76; 95% CI 0.64, 0.90), patients 18 and older (aOR = 0.13; 95% CI 0.11, 0.15), and patients receiving the quadrivalent vaccine series (aOR = 0.17; 95% CI 0.10, 0.29). Patients with private insurance compared to Medicaid (aOR = 1.70; 95% CI 1.49,1.93), those receiving vaccines after the HMR (aOR = 2.51; 95% CI 2.21,1.85), and female patients (aOR = 1.12; 95% CI 1.01, 1.25) were more likely to complete their vaccine series. (see Table 2).
Table 2:
Multivariate Analysis of HPV Vaccine Series Completion, Main Effect Model
| Characteristic: | Vaccine Series Completion | ||
|---|---|---|---|
| Odds Ratio (95% CI) | p-value | ||
| Race | |||
| White | REF | ||
| Asian | 1.03 (0.84-1.26) | 0.77 | |
| Black | 0.68 (0.60-0.77) | <0.0001 | |
| Other | 0.76 (0.64-0.90) | 0.001 | |
| Insurance Status | |||
| Medicaid | REF | ||
| Private | 1.70 (1.49-1.93) | <0.0001 | |
| Self-Paid | 1.10 (0.87-1.38) | 0.43 | |
| Other | 1.03 (0.63-1.69) | 0.91 | |
| Gender | |||
| Male | REF | ||
| Female | 1.12 (1.01-1.25) | 0.027 | |
| Vaccine Type | |||
| Nine-Valent | REF | ||
| Quadrivalent | 0.17 (0.10-0.29) | <0.0001 | |
| HMR Notification | |||
| Before | REF | ||
| After | 2.51 (2.21-2.85) | <0.0001 | |
| Age | |||
| 14 or Younger | REF | ||
| 15 to 17 | 0.19 (0.17-0.22) | <0.0001 | |
| 18 or Older | 0.13 (0.11-0.15) | <0.0001 | |
Abbreviations: HPV= Human Papillomavirus; HMR= Health Maintenance Reminder
The Effect of HMR on Completion by Age, Race, and Insurance Status
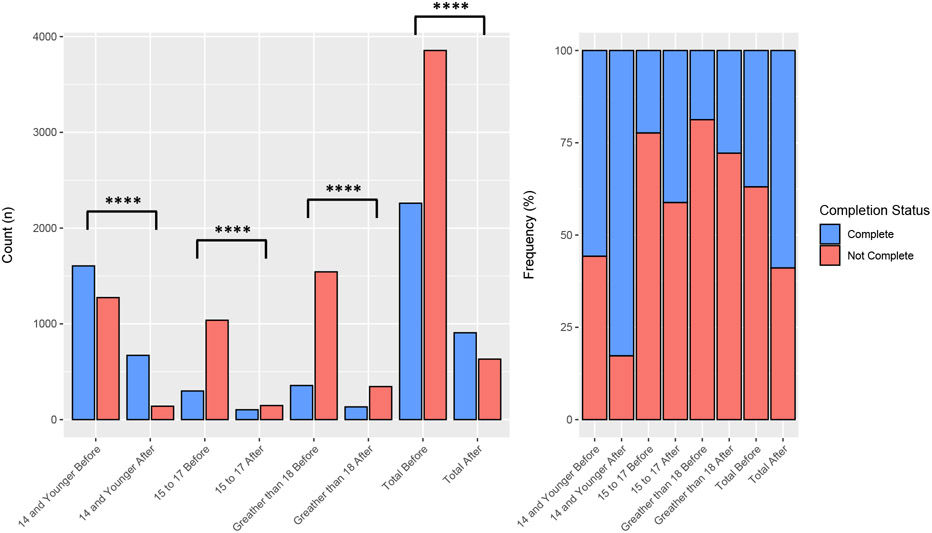
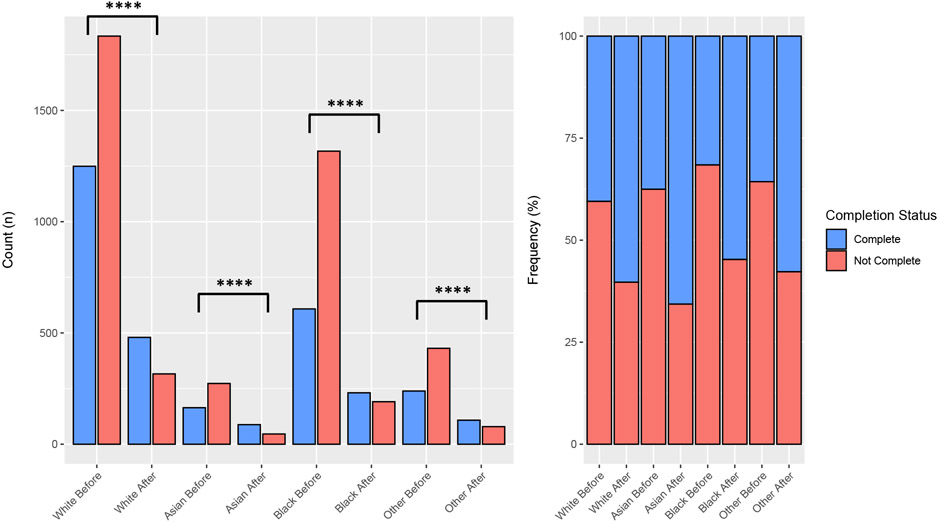
Vaccine series completion was increased in all age cohorts after the implementation of the HPV vaccine HMR (37.0% vs 58.9%, p<0.0001). Statistically significant changes in completion were appreciated for patients 14 and under (55.8% vs 82.7%, p<0.0001), 15 to 17 (22.4% vs 41.2%, p<0.0001), and those 18 and over (18.8% vs 27.8%, p<0.0001). A breakdown of the complete vs. non-complete age counts and proportions is available in Figure 2. The proportion of complete vaccine series increased after HMR for White (40.5 % vs 60.3%, p<0.0001), Asian (37.5% vs 65.7 p<0.0001), Black (31.6% vs 54.7%, p<0.0001), and Other (35.7% vs 57.8%, p<0.0001) patients. A breakdown of the complete vs. non-complete counts and proportions for race is seen in Figure 3. Patients who were self-paid (27.5% vs 49.3%, p<0.0001), had Medicaid (34.8% vs 62.0%, p<0.0001), or private insurance (39.0% vs 58.9%, p<0.0001) all had improved completion rates after HMR. Patients with other insurance had a less significant rise in completion (30.0% vs 47.8, p=0.13).
Figure 2. Proportions of Vaccine Completion Before and After HMR Implementation by Age Group.
The left side of the graph represents the raw counts per group while the right represents the relative scaled proportions. **** indicates statistically significant differences where p < 0.0001.
Figure 3. Proportions of Vaccine Completion Before and After HMR Implementation by Race.
The left side of the graph represents the raw counts per group while the right represents the relative scaled proportions. **** indicates statistically significant differences where p < 0.0001.
Assessment of Inter-variable Interactions on Vaccine Completion
Post-hoc sub-analyses contrasting the effect of HMR controlling for age group revealed a disproportionate increase in vaccination completion rates among younger patients. Patients aged 14 or younger had the highest relative increase in odds of vaccine completion (aOR 3.54, 95% CI 2.91– 4.32) compared to those aged 15 to 17 (aOR 2.35, 95% CI 1.76– 3.13) or 18 or older (aOR 1.60, 95% CI 1.27– 2.20). Conversely, there was an appreciable age-dependent effect when comparing vaccine completion between different age categories when controlling for HMR. Patients aged 15 to 17 (aOR 0.66, 95% CI 0.47-0.94) and 18 or older (aOR 0.45, 95% CI 0.33-0.61) had relative reduced aORs when compared to those aged 14 or younger. Additionally, separate multivariate models were constructed to account for potential covariate relationships between HMR implementation and age, race, and insurance status, respectively, affecting vaccination rates. Only age was determined to be statistically significantly linked (p<0.0001) to differential HMR effects on vaccine completion. A summary of both sub-analyses can be seen in Table 3.
Table 3:
Contrast Analysis of the Effects of HMR Implementation by Age and Multivariate Interaction Modeling
| Groups: | Vaccine Series Completion | |||
|---|---|---|---|---|
| HMR | Age | Odds Ratio (95% CI) | p-value | |
| After | Before | 14 and Younger | 3.54 (2.91-4.32) | <0.0001 |
| After | Before | 15 to 17 | 2.35 (1.76-3.13) | <0.0001 |
| After | Before | 18 or Older | 1.60 (1.27-2.02) | <0.0001 |
| Age | HMR | |||
| 15 to 17 | 14 or Younger | HMR Effect | 0.66 (0.47-0.94) | <0.0207 |
| 18 or Older | 15 to 17 | HMR Effect | 0.67 (0.56-0.80) | <0.0001 |
| 18 or Older | 14 or Younger | HMR Effect | 0.45 (0.33-0.61) | <0.0001 |
| Interaction Terms: | F Statistic | p-value | ||
| Age | HMR | 13.10 | <0.0001 | |
| Race | HMR | 2.35 | 0.07 | |
| Insurance | HMR | 1.95 | 0.12 | |
Abbreviations: HMR= Health Maintenance Reminder
Discussion:
In this large, single healthcare system analysis of patients who initiated the HPV vaccine, sociodemographic factors, specifically Black race and age 15 years or older, were associated with reduced series completion rates. Implementation of an EHR-based HMR for the HPV vaccine helped bolster immunization completion rates in both males and females across all age-groups, insurance status, and self-identified race groups. When accounting for interaction, the before and after effect of the HMR on vaccine completion was significantly modified by patient age, with the greatest effect at younger ages. However, the effect of the HMR was not impacted by insurance status or race, with consistently improved completion rates for all groups in the main effects model. Overall, in contrast to prior studies, our study uniquely assesses the efficacy of HMRs on HPV vaccine completion in both males and females across a broad age-range of eligibility in relation to sociodemographic influences.
While improving primary vaccine series initiation remains the gold standard for the prevention of HPV-associated malignancy, vaccine series completion is necessary to ensure adequate immunologic response.17 Although some reports have shown modest improvement in completion rates of a 3-dose HPV vaccine series in women over time,14 a significant deficit in series completion among vaccine initiators remains.18 Several barriers to HPV vaccine completion have been identified previously.19-21 In our study, patients over the age of 15 years were less likely to complete their vaccine series when compared to those initiating their primary vaccine series, especially those over 18. This corroborates national reports that have shown only 16% completion rates in men and women ages 18-26 years, which is far less than that of adolescents.22 Those over age 18 represent a unique patient population that is newly autonomous in making healthcare decisions. Amplified by the propensity for insurance coverage to change during young adulthood,23 this transition point represents an opportunity for patients to place a diminished priority on preventive care services. Providers play an important role in guiding these patients as they learn to navigate the healthcare system, especially as these patients may not be aware of HPV-associated cancers and the benefits of a vaccine for which they remain eligible.
When compared to White patients, Black patients who initiated the HPV vaccine series in our study were also less likely to complete the series. Black patients have been shown to have reduced initiation, completion, and catch-up vaccination rates for the HPV vaccine.24,25 This is particularly worrisome given the higher prevalence of high-risk HPV subtypes and disproportionate morbidity and mortality of HPV-associated cervical cancer in Black women compared to White women.21,26 Since non-Hispanic Blacks are less likely to be aware of the HPV vaccine overall,26 provider recommendation aimed at promoting knowledge in a patient-specific manner and ensuring prompt follow-up will be crucial to reducing these inequities.
There were less pronounced differences in completion according to sex, which may reflect improved awareness of HPV-associated disease in males and more intentional efforts to vaccinate this group. Payer status, however, was significantly associated with completion of a 2 or 3-dose series. In particular, patients with private insurance were significantly more likely to complete their series within 12 months across the study period. These results are in line with Mansfield and colleagues,27 who conducted a mixed methods study assessing HPV vaccine completion in the same geographic location as the current study. However, this association has been bi-directional in the broader literature.28,29
Although multi-level strategies have attempted to reduce barriers to accessing preventative health services,30,31 EHR-based clinician prompts are minimally labor-intensive interventions that have shown promise in improving reliable vaccine delivery.32 Particularly in the setting of the HPV vaccine, EHR reminder systems have been utilized to improve both vaccine initiation and completion rates in females across the age spectrum of vaccine eligibility14, as well as bolster initiation rates in young males.33 In our study, HPV vaccine completion rates significantly improved in females and males across all ages, but when accounting for the differential association of HMR and vaccine completion by patient age groups, those less than 15 years old were most likely to complete the series with HMR prompting. This is unsurprising as adolescents experience increasing disconnects from healthcare as they transition into young adulthood,34 and patients with a routine place of healthcare have significantly greater odds of being adherent to HPV vaccine recommendations.35 The effect of regular visits would compound an intervention whose impact is dependent on provider-patient interfaces, such as an EHR HMR. This contrast may be further explained by provider perceptions, as efforts to improve vaccination rates have historically been aimed at those receiving the primary vaccine series. However, the overall positive impact of HMR on those receiving catch-up vaccinations is promising and is in line with prior research.14
In addition, HMR implementation was associated with consistently increased vaccine completion rates across all race groups. This is important because known racial disparities had no impact on the effect of the HMR. For example, despite having lower completion rates overall, Black patients had a comparable increase in vaccine completion to White patients after HMR implementation. In agreement with Ruffin and colleagues,14 who found that EHR reminder systems improved HPV vaccine initiation rates in Black females, our findings suggest that standardized alert systems may provide an unbiased method to ensure the timely delivery of follow up vaccine doses. Given that non-Hispanic Black males with HPV-associated OPC have been found to have higher cancer-specific mortality compared to other races, regardless of stage at presentation or treatment modality,36 any effort to improve preventative measures such as HPV vaccination in this population is critical.
Key limitations to this study include that this was an analysis of a single-healthcare system, which was associated with a large academic center. Thus, our findings are unique to this high-resource practice setting with a centralized EHR. Other limitations include the effect of unmeasured confounders on vaccine completion rates. In particular, the beginning of the COVID-19 pandemic was near-concurrent with the implementation of the EHR HMR. Although we demonstrate that overall clinic visits were decreased during the period, it is difficult to determine the exact impact of the pandemic on provider practices and patient preferences during this time. Additionally, variables such as socioeconomic status, healthcare utilization metrics and clinical variables were not captured in our dataset, which may have informed more complete conclusions. Finally, this was a retrospective, pre-post study design without randomization, thus limiting our conclusions on causality. Despite these limitations, this study has key strengths, including the large sample size and granularity with respect to vaccine administration data, rather than relying on self-reported data. As a result, this is the first study to examine HPV vaccine completion barriers and the effect of an EHR reminder system on improving series completion despite these barriers in males and females across expanded ages of vaccine eligibility. Therefore, EHR HMR represents a scalable, provider-level intervention that improves provider awareness of eligible populations and mitigates barriers to HPV vaccine uptake.
Overall, provider recommendation remains at the heart of HPV vaccination efforts. The incidence of tobacco-associated head and neck cancers has declined by over 50%37, owing to public health campaigns and patient education efforts that otolaryngologists have actively participated in. As experts on the diagnosis and treatment of HPV-positive OPC, otolaryngologists are well-poised to deliver strong vaccine recommendations. Although over 90% of head and neck surgeons discuss HPV as a risk factor for OPC, less than half actually discuss the HPV vaccine with patients, according to national survey data.38,39 While otolaryngologists may feel removed from preventative health interventions, we stand to make a significant impact by actively participating in vaccination discussions and recognizing eligible patients. Even via actions as simple as mentioning the vaccine during routine pediatric otolaryngology visits, we may reduce the suffering and debility of conditions like OPC. Through collaboration between the otolaryngology and primary care communities, as demonstrated in the current study, evidence-based and sustainable interventions can be developed to increase HPV vaccine coverage.
Funding Source
Dr. Osazuwa-Peters reported receiving grants from the National Institute of Health/National Institute of Dental and Craniofacial Research (K01 DE030916; R01 DE032216) outside of the submitted work.
Footnotes
Disclosures: Dr. Osazuwa-Peters is a scientific advisor to Navigating Cancer
Competing Interests: none
This article was presented at the AAO-HNSF 2022 Annual Meeting & OTO Experience, Philadelphia, PA, September 13, 2022.
References:
- 1.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32): 698–702. Published 2019 Aug 16. doi: 10.15585/mmwr.mm6832a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee LY, Garland SM. Human papillomavirus vaccination: The population impact. F1000Res. 2017;6:866. Published 2017 Jun 12. doi: 10.12688/f1000research.10691.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–852. doi: 10.1016/S1473-3099(10)70219-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Batur P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Cleve Clin J Med. 2019;86(3):173–178. doi: 10.3949/ccjm.86a.18018 [DOI] [PubMed] [Google Scholar]
- 5.Osazuwa-Peters N, Graboyes EM, Khariwala SS. Expanding Indications for the Human Papillomavirus Vaccine: One Small Step for the Prevention of Head and Neck Cancer, but One Giant Leap Remains. JAMA Otolaryngol Head Neck Surg. 2020;146(12):1099–1101. doi: 10.1001/jamaoto.2020.4068 [DOI] [PubMed] [Google Scholar]
- 6.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human Papillomavirus-Attributable Cancers - United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724–728. Published 2019 Aug 23. doi: 10.15585/mmwr.mm6833a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pingali C, Yankey D, Elam-Evans LD, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183–1190. Published 2021 Sep 3. doi: 10.15585/mmwr.mm7035a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer JC, Brewer NT, Trogdon JG, Wheeler SB, Dusetzina SB. Predictors of Human Papillomavirus Vaccine Follow-Through Among Privately Insured US Patients. Am J Public Health. 2018;108(7):946–950. doi: 10.2105/AJPH.2018.304408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeudin P, Liveright E, del Carmen MG, Perkins RB. Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum Vaccin Immunother. 2013;9(7):1413–1420. doi: 10.4161/hv.24422 [DOI] [PubMed] [Google Scholar]
- 10.Lott BE, Okusanya BO, Anderson EJ, et al. Interventions to increase uptake of Human Papillomavirus (HPV) vaccination in minority populations: A systematic review. Prev Med Rep. 2020;19:101163. Published 2020 Jul 11. doi: 10.1016/j.pmedr.2020.101163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bramer CA, Kimmins LM, Swanson R, et al. Decline in child vaccination coverage during the COVID-19 pandemic - Michigan Care Improvement Registry, May 2016-May 2020. Am J Transplant. 2020;20(7):1930–1931. doi: 10.1111/ajt.16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel Murthy B, Zell E, Kirtland K, et al. Impact of the COVID-19 Pandemic on Administration of Selected Routine Childhood and Adolescent Vaccinations - 10 U.S. Jurisdictions, March-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(23):840–845. Published 2021 Jun 11. doi: 10.15585/mmwr.mm7023a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatr (Phila). 2011;50(12):1116–1124. doi: 10.1177/0009922811415104 [DOI] [PubMed] [Google Scholar]
- 14.Ruffin MT 4th, Plegue MA, Rockwell PG, Young AP, Patel DA, Yeazel MW. Impact of an Electronic Health Record (EHR) Reminder on Human Papillomavirus (HPV) Vaccine Initiation and Timely Completion. J Am Board Fam Med. 2015;28(3):324–333. doi: 10.3122/jabfm.2015.03.140082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae J, Ford EW, Wu S, Huerta T. Electronic reminder's role in promoting human papillomavirus vaccine use. Am J Manag Care. 2017;23(11):e353–e359. Published 2017 Nov 1. [PubMed] [Google Scholar]
- 16.Galaxy. Epic User Web (2021). https://galaxy.epic.com/#Search/searchWord=slicerdicer April 10, 2021. [Google Scholar]
- 17.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi: 10.1001/jama.2013.1625 [DOI] [PubMed] [Google Scholar]
- 18.Widdice LE, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127(1):77–84. doi: 10.1542/peds.2010-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempsey A, Cohn L, Dalton V, Ruffin M. Worsening disparities in HPV vaccine utilization among 19-26 year old women. Vaccine. 2011;29(3):528–534. doi: 10.1016/j.vaccine.2010.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan W, Viera AJ, Rowe-West B, Grimshaw A, Quinn B, Walter EB. The HPV vaccine: are dosing recommendations being followed?. Vaccine. 2011;29(14):2548–2554. doi: 10.1016/j.vaccine.2011.01.066 [DOI] [PubMed] [Google Scholar]
- 21.Rahman M, Laz TH, McGrath C, Berenson AB. Correlates of human papillomavirus vaccine series completion among young adult female initiators. Hum Vaccin Immunother. 2014;10(8):2163–2167. doi: 10.4161/hv.2963321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adjei Boakye E, Lew D, Muthukrishnan M, et al. Correlates of human papillomavirus (HPV) vaccination initiation and completion among 18-26 year olds in the United States. Hum Vaccin Immunother. 2018;14(8):2016–2024. doi: 10.1080/21645515.2018.1467203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams SH, Newacheck PW, Park MJ, Brindis CD, Irwin CE Jr. Health insurance across vulnerable ages: patterns and disparities from adolescence to the early 30s. Pediatrics. 2007;119(5):e1033–e1039. doi: 10.1542/peds.2006-1730 [DOI] [PubMed] [Google Scholar]
- 24.Cook RL, Zhang J, Mullins J, et al. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in Medicaid. J Adolesc Health. 2010;47(6):596–599. doi: 10.1016/j.jadohealth.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 25.Hirth J Disparities in HPV vaccination rates and HPV prevalence in the United States: a review of the literature. Hum Vaccin Immunother. 2019;15(1):146–155. doi: 10.1080/21645515.2018.1512453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adjei Boakye E, Tobo BB, Rojek RP, Mohammed KA, Geneus CJ, Osazuwa-Peters N. Approaching a decade since HPV vaccine licensure: Racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Hum Vaccin Immunother. 2017;13(11):2713–2722. doi: 10.1080/21645515.2017.1363133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansfield LN, Chung RJ, Silva SG, Merwin EI, Gonzalez-Guarda RM. Social determinants of human papillomavirus vaccine series completion among U.S. adolescents: A mixed-methods study. SSM Popul Health. 2022;18:101082. Published 2022 Mar 26. doi: 10.1016/j.ssmph.2022.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agawu A, Hanlon AL, Buttenheim AM, Song L, Fiks AG, Feemster KA. Disparities in Human Papillomavirus Vaccine Series Completion by Adolescent Males: A Retrospective Cohort Study. Acad Pediatr. 2020;20(3):364–373. doi: 10.1016/j.acap.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Schluterman NH, Terplan M, Lydecker AD, Tracy JK. Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine. 2011;29(21):3767–3772. doi: 10.1016/j.vaccine.2011.03.032 [DOI] [PubMed] [Google Scholar]
- 30.Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–1124. doi: 10.1542/peds.2012-3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berenson AB, Hirth JM, Kuo YF, Starkey JM, Rupp RE. Use of patient navigators to increase HPV vaccination rates in a pediatric clinical population. Prev Med Rep. 2020;20:101194. Published 2020 Aug 28. doi: 10.1016/j.pmedr.2020.101194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiks AG, Grundmeier RW, Biggs LM, Localio AR, Alessandrini EA. Impact of clinical alerts within an electronic health record on routine childhood immunization in an urban pediatric population. Pediatrics. 2007;120(4):707–714. doi: 10.1542/peds.2007-0257 [DOI] [PubMed] [Google Scholar]
- 33.Martin S, Warner EL, Kirchhoff AC, Mooney R, Martel L, Kepka D. An Electronic Medical Record Alert Intervention to Improve HPV Vaccination Among Eligible Male College Students at a University Student Health Center. J Community Health. 2018;43(4):756–760. doi: 10.1007/s10900-018-0480-6 [DOI] [PubMed] [Google Scholar]
- 34.Thakkar MY, Hao L, Marcell AV. Adolescents' and Young Adults' Routine Care Use: The Role of Their Mothers’ Care Use Behaviors. J Adolesc Health. 2019;64(1):107–115. doi: 10.1016/j.jadohealth.2018.07.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel K, Vasudevan L. Disparities in healthcare access and utilization and human papillomavirus (HPV) vaccine initiation in the United States. Hum Vaccin Immunother. 2021;17(12):5390–5396. doi: 10.1080/21645515.2021.1989919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villalona S, Stroup A, Villalona S, Ferrante J. Racial/ethnic disparities in HPV-related oropharyngeal cancer outcomes among males in the United States (2005-2016): a population-based retrospective cohort study. J Clin Oncol. 2022; 39(15). doi: 10.1200/JCO.2021.39.15_suppl.e18576 [DOI] [Google Scholar]
- 37.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malloy KM, Ellender SM, Goldenberg D, Dolan RW. A survey of current practices, attitudes, and knowledge regarding human papillomavirus-related cancers and vaccines among head and neck surgeons. JAMA Otolaryngol Head Neck Surg. 2013;139(10):1037–1042. doi: 10.1001/jamaoto.2013.4452 [DOI] [PubMed] [Google Scholar]
- 39.Shew M, Shew ML, Bur AM. Otolaryngologists and their role in vaccination for prevention of HPV associated head & neck cancer. Hum Vaccin Immunother. 2019;15(7-8):1929–1934. doi: 10.1080/21645515.2018.1526559 [DOI] [PMC free article] [PubMed] [Google Scholar]