Abstract
Objective:
Climate change and urbanization increasingly cause extreme conditions hazardous to health. The bedroom environment plays a key role for high-quality sleep. Studies objectively assessing multiple descriptors of the bedroom environment as well as sleep are scarce.
Methods:
Particulate matter with a particle size <2.5 μm (PM2.5), temperature, humidity, carbon dioxide (CO2), barometric pressure, and noise levels were continuously measured for 14 consecutive days in the bedroom of 62 participants (62.9% female, mean ± SD age 47.7 ± 13.2 years) who wore a wrist actigraph and completed daily morning surveys and sleep logs.
Results:
In a hierarchical mixed effect model that included all environmental variables and adjusted for elapsed sleep time and multiple demographic and behavioral variables, sleep efficiency calculated for consecutive one-hour periods decreased in a dose-dependent manner with increasing levels of PM2.5, temperature, CO2 and noise. Sleep efficiency in the highest exposure quintiles was 3.2% (PM2.5, p<0.05); 3.4% (temperature; p<0.05), 4.0% (CO2, p<0.01) and 4.7% (noise, p<0.0001) lower compared to the lowest exposure quintiles (all p-values adjusted for multiple testing). Barometric pressure and humidity were not associated with sleep efficiency. Bedroom humidity was associated with subjectively assessed sleepiness and poor sleep quality (both p<0.05), but otherwise environmental variables were not statistically significantly associated with actigraphically assessed total sleep time and wake after sleep onset or with subjectively assessed sleep onset latency, sleep quality and sleepiness. Assessments of bedroom comfort suggest subjective habituation irrespective of exposure levels.
Conclusions:
These findings add to a growing body of evidence highlighting the importance of the bedroom environment–beyond the mattress–for high-quality sleep.
Keywords: air quality, noise, actigraphy, PM2.5, CO2, temperature
Introduction
Countless experimental1–5 and epidemiologic6–8 studies demonstrated that regular high-quality sleep of sufficient duration is of paramount importance for cognition, well-being, and health, including cardiovascular health.9 Yet, one-third of US Americans report sleeping less than the recommended minimum of 7 hours per night.10 The sleep (ie, bedroom) environment plays a key role in promoting high-quality sleep, nonetheless large parts of the population sleep in environments that disturb sleep and impair sleep recuperation.11–13
The term “sleep hygiene” refers to a set of behavioral and environmental conditions that promote healthy sleep.14 Healthy restorative sleep not only refers to sufficient duration, but also to sufficient quality, a term that relates to sleep that is minimally interrupted, fragmented, or disturbed.12
Most environmental sleep hygiene recommendations focus on the bedroom and include optimizing air quality (AQ), darkness, noise, temperature, and humidity. Sleep is uniquely sensitive to noise as the auditory system has a watchman function and is constantly monitoring the environment for potential threats during sleep.15 We and others have shown that nocturnal noise exposure disturbs sleep,12,16,17 impairs sleep recuperation,13 and increases the risk for cardiovascular disease.18,19 Cooling of core body temperature through peripheral vasodilation is a critical step in the process of falling asleep, and high bedroom temperature and humidity may interfere with this process and also cause arousals from sleep.20 CO2 is an AQ indicator but also a potent respiratory stimulant that can, in high concentrations, cause headaches and other central nervous system symptoms.21 Several studies suggest that air pollution is associated with poor sleep quality and short sleep duration.11,22,23 How air pollution interferes with sleep is less well understood, but is proposed to include effects on the central nervous system through direct impacts on neurotransmitter levels as well as inflammation of the respiratory system.24
Despite the importance of the bedroom environment for sleep quality and duration, high-quality field studies with objective measurements of both the bedroom environment and sleep are largely missing. Furthermore, prior studies often concentrate on a single (eg, air pollution) or a few (eg, temperature and humidity) descriptors of the bedroom environment. The lack of objective exposure and outcome measurements coupled with the limited assessment of the bedroom environment preclude detailed recommendations for an optimal sleep environment and were a main motivation for this study.25,26
The data presented here were collected as baseline data for an ongoing prospective study in Louisville, KY. It is one of the few studies objectively assessing multiple variables describing the bedroom environment (with an AQ monitor and sound level meter) as well as sleep (with actigraphy). Parameters concurrently measured in the bedroom included particulate matter with a particle size <2.5 μm (PM2.5), temperature, humidity, carbon dioxide (CO2), barometric pressure, and noise levels. The main goal of this analysis was to explore the relationship between variables describing the bedroom environment and sleep quality and duration while adjusting for a number of relevant individual and behavioral confounders.
Participants and methods
Participants
Participants were recruited from the ongoing Green Heart Project (GHP; clinicaltrials.gov: NCT03670524). The GHP is a prospective intervention trial that assesses over 700 participants for the cardiovascular effects of installing mature trees and shrubs in a 1.5 square mile area in Louisville, KY relative to a sociodemographically matched control group with no deliberate change in greenness. The data presented here are from the pregreening baseline data acquisition of the Green Sleep Project (GSP), an ancillary study that investigates the effects of greening on sleep in a subgroup of GHP participants.
The GSP approached 343 GHP participants at their pregreening baseline measurement and recruited 130 participants (see study flow chart in Figure S1; 62.3% female, mean ± SD age 46.8 ± 13.1 years; mean ± SD body mass index [BMI] 30.4 ± 7.1 kg/m2) who wore a wrist actigraph 24 hours per day for 14 consecutive days and completed a brief sleep log and survey each morning. Every GHP subject was approached; however, limited GSP study equipment was available. A subset of 78 participants recorded bedroom AQ and SLs. One or more of the study measurements (actigraphy, AQ, noise) failed in 16 subjects for technical reasons (Figure S1). Thus, 62 subjects (62.9% female, mean ± SD age 47.7 ± 13.2 years; mean ± SD body mass index [BMI] 30.5 ± 6.4 kg/m2) provided data for actigraphy, AQ, and SL measurements for at least part of the 14-day measurement period and contributed to data analysis. Data for these participants were sampled between mid-June and the end of September 2021.
The GHP has several inclusion criteria–25–70 years and living within the targeted study location. The following were the exclusion criteria: HIV/AIDS, active treatment for cancer, active bleeding including wounds, body weight less than 100 pounds or BMI > 40. The following were additional exclusion criteria for the GSP: (1) use of prescribed or over the counter sleep aids 3 times or more per week over the preceding month; (2) one or more of the following sleep disorders diagnosed by a physician: sleep apnea, narcolepsy, restless leg syndrome, periodic limb movement syndrome; or (3) average self-reported sleep duration on weekday nights >9 hours. The protocol was approved by the Institutional Review Board (IRB) of the University of Pennsylvania, which served as the IRB of record. Participants provided written informed consent prior to participation. They were compensated with up to $175 if they completed all study-related measures.
Study procedures
Participants were provided with an actigraph (ActiGraph wGT3X-BT, Pensacola, FL; sample rate set to 30 Hz), the AQ monitor and SL meter, and a manual with detailed instructions for setting up the equipment in the bedroom and starting measurements. The manual also included daily surveys. Participants were instructed to wear the actigraph on the nondominant wrist 24/7 but to take it off during impact sports or activities that immersed it in water. They were asked to recharge the watch for 1 hour after the first study week. At the end of the 2-week measurement period, participants returned all equipment to the study team.
Measures of the bedroom environment
The AQ monitor PCE-AQD 20 (PCE Instruments, Alicante, Spain) was used to measure PM2.5, temperature, relative humidity, CO2 and barometric pressure in the bedroom with a 1/min sampling rate. As the device fan produces some noise, participants were instructed to place the device in their bedroom but not too close to their head, and not on window sills or in front of air conditioning units or fans. The Decibel Meter PCE-SLD 10 (PCE Instruments, Alicante, Spain) was used to measure A-weighted SL in the bedroom with a 12/min sampling rate, fast time constant, and range set to auto (see Supplementary Materials for AQ and SL monitor specifications). SL meters were checked for accuracy before and after each measurement period with a calibrator (CAL200, Larson Davis, Provo, UT). Participants were asked to place the SL meter close to their pillow, preferably on a bedside table and not in front of AC units, fans, or directly behind the AQ monitor. Both AQ and SL meters were connected to power outlets but could run on battery power for a few hours if needed. As both meters were found to significantly drift forward in time (mean ± SD drift AQ 4.8 ± 0.7 s/day; SL 4.6 ± 0.8 s/day), the time drift of both devices relative to atomic clock time was recorded before deployment and after participants returned them. Actigraphs did not drift relevantly in time (mean ± SD 0.0 ± 0.5 s/day).
Morning surveys
Participants completed a brief survey every morning (see Supplementary Materials). The survey asked about bedtimes, daytime naps, times the actigraph was taken off, about the last night’s sleep (quality, sleep onset latency, intermittent awakenings, recuperation), and additional questions about the last wake period (eg, stress levels, exercise, caffeine, alcohol, and medication use). Participants were asked how they rated the humidity and temperature in the bedroom and how much outside noise disturbed their sleep during the last night (all 5-point Likert scales). The Karolinska Sleepiness Scale27 was also administered with the survey to evaluate subjective sleepiness.
Data analysis
Actigraphy data were automatically scored in 1-minute epochs with a software developed by one of the authors (M.B.) and classified as wake, sleep, or off-wrist. The software uses activity counts in the current as well as 30 preceding and 30 future 1-minute epochs to determine the likelihood of an epoch being sleep or wake. Long periods without movement outside of sleep periods are considered off-wrist by the software. Wrongly classified epochs were manually corrected by one experienced scorer using the sleep log to inform corrections. A sleep period was included in data analysis if the start and the end fell within ±2 hours windows relative to sleep log entries, respectively, and there were 30 or fewer epochs classified as off-wrist. AQ data were cleaned and corrected for differences between AQ monitors (see Supplementary Materials for detailed description). Finally, AQ and SL data were corrected for time drift and resampled to full 1-minute periods to match 1-minute actigraphy epochs. BMI was missing for one female participant was imputed with the grand mean for BMI for all female participants.
Statistical analysis
Sleep efficiency (SE), defined as the time spent sleeping relative to the time available for sleep, was chosen as the primary outcome of interest. High values of SE indicate high-quality, high-continuity and restorative sleep. In contrast, low SE indicates fragmented and disturbed sleep. As SE is positively correlated with sleep duration (r=0.41 in this study, see Figure S3), it captures aspects of both sleep duration and sleep continuity/quality. In contrast to sleep duration, SE is a relative metric and as such less prone to be influenced by factors that determine sleep opportunity (e.g., work and family demands). As both sleep pressure and the bedroom environment change systematically over the course of the night, SE was calculated for each full hour since sleep onset in addition to the whole sleep period (time between the first and last epoch classified as sleep). Excluding 1 h periods with ≥ 30 min off-wrist, 4,710 1 h sleep periods contributed to data analysis.
Hierarchical mixed models with 1-hour SE estimates nested within sleep periods nested within participants were run. For models that were based on the whole night, linear mixed effect models with random subject intercept were used. To account for nonlinear effects, quintiles were generated for each environmental variable and tested for differences to reference (lowest quintile). For PM2.5, quintiles were assigned based on accepted annual PM2.5 standards set by the Environmental Protection Agency instead (PM2.5 values <12 μg/m3 are considered good AQ). The derived exposure quintiles can be found in Table S1.
Several models were run based on the 1-hour SE data: the basic model (Model 1) was run for each environmental variable separately and only adjusted for elapsed sleep time and its quadratic term. A quadratic term was included as SE, after an initial decline across the first hours of sleep, tended to increase again at the end of long sleep periods. Model 2 additionally adjusted for all other bedroom environmental variables. Model 3 additionally adjusted for age, sex, race, BMI, household income, educational attainment, and the number of household members. Model 4 additionally adjusted for self-reported daytime exercise, naps, stress levels, alcohol and caffeine intake; whether the bed was shared; whether it was a weekday or weekend night, window position (closed/open); whether AC (central or window) or a fan was used, and whether a subject was classified as having a high risk for obstructive sleep apnea according to the BERLIN questionnaire,28 which subjects filled out prior to the first study night.
Model 4 was also run with z-standardized environmental exposures as linear predictors (PM2.5 values were log+1 transformed prior to standardization) after establishing with AIC that linear models fit better than quadratic models. Furthermore, 2-way linear interactions between environmental predictors were tested in separate models. These interaction models only adjusted for the other environmental variables, and the statistically significant covariables elapsed sleep time and alcohol consumption on the previous day. It was also investigated how environmental variables in the bedroom (except for barometric pressure) changed depending on window opening position and the use of central AC, window AC, or a fan. These analyses were based on the whole night.
Finally, subjective assessments of humidity and temperature levels as well as the degree of noise disturbance were investigated in relation to exposure quintiles. It was also investigated whether those who rated their bedroom as slightly too hot or too hot, slightly too humid or too humid, or who felt very or extremely disturbed by noise differed in SE compared to those who did not. These models were based on fully adjusted Model 4 but excluded bedroom environment measurement variables.
All models were run in SAS 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was assumed at α=0.05. P-values of post-hoc comparisons were adjusted for multiple testing with the false discovery rate (FDR) method.29
Results
The 62 participants with both AQ and SL data provided on average 13.8 days of actigraphy data (range: 7.0–16.4 days; excluding off-wrist), 13.3 days of AQ data (range: 1.1–20.0 days) and 12.8 days of SL data (range: 0.1–22.0 days). Tests for collinearity were negative (all |r| <0.65). No relevant correlations between environmental exposure variables both within nights and across nights were found (all |r| <0.2) except for humidity and PM2.5 (r=0.37 within nights and r=0.40 across nights; see Figure S2), which has been described for the relevant exposure range before.30 Of expected morning surveys, 97.2% were received.
Across all participants, total sleep time (TST) averaged (mean ± SD) 6.32 ± 1.43 hours on weekdays and 6.51 ± 1.47 hours on weekends. SE averaged 82.4 ± 8.0% on weekdays and 82.8 ± 7.0% on weekends (range: 40.9%–94.8%). On average, participants napped on 16.9% and exercised on 43.0% of weekdays; they also reported consuming caffeine and alcohol in the 6-hour period prebed in 28.2% and 19.0% of weekdays, respectively. Participants reported sharing the bed in 62.2% of nights. On a 5-point Likert scale (1 = “best”; 5 = “worst”) participants averaged 2.1 for stress, 2.4 for sleep quality, 2.8 for tiredness, and 1.4 for noise disturbance.
The following were average values of the different variables measured in the bedroom (mean ± SD): PM2.5 10.6 ± 47.0 μg/m3; relative humidity 51.2% ± 7.3%; temperature 73.3°F ± 4.2°F; CO2 1194.0 ± 523.5 ppm; barometric pressure 999.8 ± 2.7 hPa; and sound pressure level 49.3 ± 7.8 dBA. Some environmental variables changed systematically across the sleep period (Figure 1). During nighttime sleep periods, PM2.5 and sound pressure levels showed decreasing trends, humidity and CO2 showed increasing trends, and temperature and barometric pressure showed a slightly decreasing followed by a slightly increasing trend.
Figure 1:
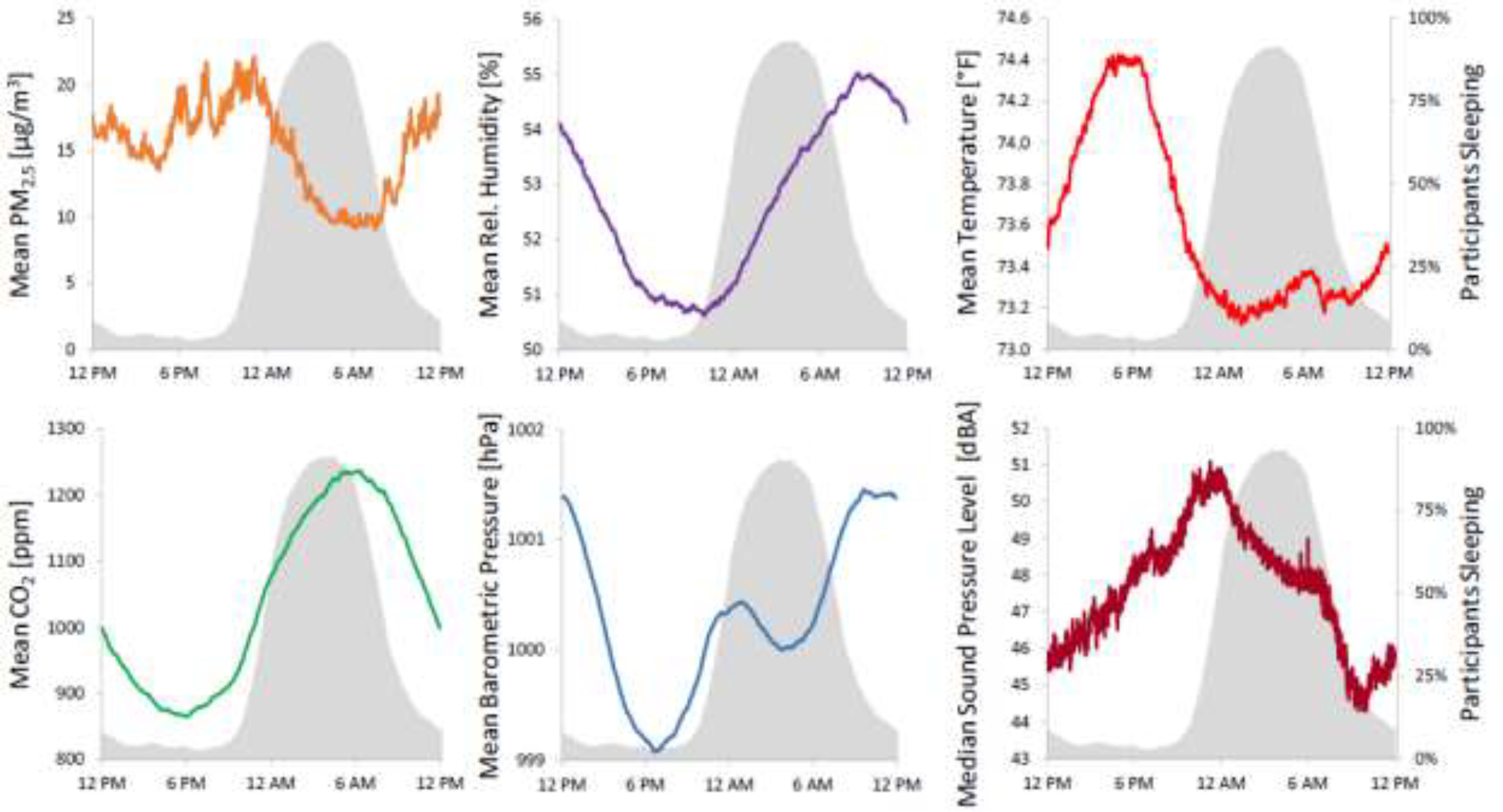
Bedroom environment by time of day. Values of mean fine particulate matter (PM2.5), mean relative humidity, mean temperature, mean CO2, mean barometric pressure, and median sound pressure levels are plotted against time of day (the median was chosen for sound pressure levels as they are strongly affected by outliers). Values were derived from all subjects with valid air quality and sound level data. Percent of participants sleeping is shown in gray. PM2.5 and sound pressure levels show decreasing trends, humidity and CO2 show increasing trends while temperature and barometric pressure show a slightly decreasing followed by a slightly increasing trend during participants’ sleep periods.
Results of hierarchical regression models of bedroom environmental variables on one-hour SE values are shown in Table 1. In the fully adjusted model (Model 4, Table S2), there was a statistically significant association of temperature, CO2 and noise with SE such that increasing exposure levels were associated with lower SE (Figure 2). In the highest exposure quintiles SE was 3.2% (PM2.5, p<0.05); 3.4% (temperature; p<0.05), 4.0% (CO2, p<0.01) and 4.7% (noise, p<0.0001) lower compared to the lowest exposure quintiles. Humidity and barometric pressure were not statistically significantly associated with SE.
Table 1:
Associations of the bedroom environment with sleep efficiency.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
|
| ||||
| Estimate in % (95% CI) | Estimate in % (95% CI) | Estimate in % (95% CI) | Estimate in % (95% CI) | |
| PM2.5 [μg/m3] | ||||
| ≤ 0.1 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 0.1 ≤ 4.0 | −1.06 (−2.44; 0.32) | −1.31 (−2.69; 0.08) | −1.38 (−2.77; 0.01) | −1.44 (−2.83; −0.05) |
| > 4.0 ≤ 8.0 | −0.88 (−2.73; 0.97) | −1.61 (−3.47; 0.26) | −1.7 (−3.58; 0.17) | −1.65 (−3.54; 0.23) |
| > 8.0 ≤ 12.0 | −1.44 (−3.61; 0.74) | −2.42 (−4.61; −0.22) | −2.51 (−4.72; −0.3) | −2.51 (−4.74; −0.29) |
| > 12.0 | −3.1 (−5.26; −0.93)* | −3.43 (−5.60; −1.25)** | −3.34 (−5.55; −1.13)* | −3.27 (−5.51; −1.03)* |
| p = 0.0510 | p = 0.0417 | p = 0.0551 | p = 0.0633 | |
| Relative Humidity [%] | ||||
| ≤ 44.7 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 44.7 ≤ 48.5 | −0.40 (−1.77; 0.98) | −0.39 (−1.78; 1.00) | −0.27 (−1.67; 1.12) | −0.31 (−1.71; 1.08) |
| > 48.5 ≤ 52.2 | −0.47 (−2.17; 1.23) | −0.41 (−2.12; 1.30) | −0.28 (−2.01; 1.44) | −0.35 (−2.08; 1.39) |
| > 52.2 ≤ 57.1 | −0.57 (−2.42; 1.27) | −0.39 (−2.26; 1.48) | −0.25 (−2.13; 1.64) | −0.26 (−2.16; 1.63) |
| > 57.1 | −2.27 (−4.46; −0.09) | −1.31 (−3.52; 0.89) | −1.14 (−3.4; 1.12) | −1.17 (−3.45; 1.1) |
| p = 0.2252 | p = 0.7773 | p = 0.8388 | p = 0.8273 | |
| Temperature [°F] | ||||
| ≤ 69.7 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 69.7 ≤ 72.2 | −1.15 (−2.61; 0.31) | −1.16 (−2.63; 0.31) | −1.06 (−2.54; 0.41) | −0.98 (−2.46; 0.5) |
| > 72.2 ≤ 74.4 | −1.02 (−2.79; 0.74) | −0.90 (−2.68; 0.88) | −0.66 (−2.46; 1.13) | −0.5 (−2.32; 1.31) |
| > 74.4 ≤ 76.6 | −2.33 (−4.36; −0.31)* | −2.64 (−4.68; −0.61)* | −2.3 (−4.36; −0.24) | −2.08 (−4.16; 0) |
| > 76.6 | −3.98 (−6.14; −1.82)** | −4.02 (−6.20; −1.84)** | −3.61 (−5.83; −1.4)** | −3.4 (−5.64; −1.16)* |
| p = 0.0046 | p = 0.0035 | p = 0.0110 | p = 0.0182 | |
| CO2 [ppm] | ||||
| ≤ 777.3 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 777.3 ≤ 984.0 | −1.63 (−3.11; −0.14)* | −1.54 (−3.02; −0.05)* | −1.71 (−3.21; −0.21)* | −1.82 (−3.33; −0.32)* |
| > 984.0 ≤ 1184.3 | −2.04 (−3.73; −0.35)* | −2.04 (−3.74; −0.34)* | −2.25 (−3.97; −0.53)* | −2.39 (−4.13; −0.65)* |
| > 1184.3 ≤ 1514.2 | −2.27 (−4.10; −0.44)* | −2.28 (−4.12; −0.44)* | −2.52 (−4.39; −0.64)* | −2.65 (−4.54; −0.75)** |
| > 1514.2 | −3.99 (−6.07; −1.91)*** | −3.72 (−5.82; −1.62)** | −3.89 (−6.04; −1.75)** | −4.04 (−6.21; −1.87)** |
| p = 0.0061 | p = 0.0159 | p = 0.0116 | p = 0.0083 | |
| Barometric Pressure [hPa] | ||||
| ≤ 997.9 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 997.9 ≤ 999.3 | 0.85 (−0.39; 2.10) | 0.60 (−0.66; 1.86) | 0.61 (−0.66; 1.87) | 0.63 (−0.63; 1.9) |
| > 999.3 ≤ 1000.4 | 1.09 (−0.20; 2.37) | 0.97 (−0.33; 2.27) | 0.98 (−0.32; 2.28) | 1.09 (−0.22; 2.4) |
| > 1000.4 ≤ 1002.1 | 0.99 (−0.32; 2.29) | 0.88 (−0.44; 2.20) | 0.92 (−0.4; 2.24) | 1.11 (−0.23; 2.44) |
| > 1002.1 | −0.23 (−1.56; 1.11) | −0.29 (−1.63; 1.06) | −0.24 (−1.59; 1.11) | −0.18 (−1.55; 1.2) |
| p = 0.1834 | p = 0.2907 | p = 0.3018 | p = 0.2165 | |
| Sound Pressure Level [dBA] | ||||
| ≤ 41.7 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| > 41.7 ≤ 46.5 | −0.26 (−1.65; 1.12) | −0.53 (−1.92; 0.86) | −0.54 (−1.93; 0.86) | −0.56 (−1.96; 0.84) |
| > 46.5 ≤ 51.8 | −2.69 (−4.35; −1.03)** | −2.97 (−4.63; −1.32)*** | −3.05 (−4.72; −1.38)*** | −3.13 (−4.81; −1.44)*** |
| > 51.8 ≤ 56.0 | −4.88 (−6.76; −3.00)**** | −4.92 (−6.80; −3.04)**** | −5.12 (−7.02; −3.21)**** | −5.22 (−7.14; −3.29)**** |
| > 56.0 | −4.41 (−6.29; −2.53)**** | −4.65 (−6.53; −2.78)**** | −4.55 (−6.45; −2.65)**** | −4.68 (−6.61; −2.74)**** |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
Models were adjusted for the following variables: Model 1 was run for each environmental variable separately and only adjusted for elapsed sleep time (including a quadratic term). Model 2 additionally adjusted for all other environmental variables. Model 3 additionally adjusted for age, sex, race, BMI, household income, educational attainment, and the number of household members. Model 4 additionally adjusted for daytime exercise, naps, stress levels, alcohol and caffeine intake; whether the bed was shared; whether it was a weekday or weekend night, window position (closed/open) and whether air conditioning (central or window) or a fan were used, and high risk for Obstructive Sleep Apnea based on the BERLIN questionnaire (all variables added to Model 4 were self-reported). Ref.: reference category; PM2.5: fine particulate matter; p-values reflect type-III tests of fixed effects; asterisks reflect statistical significance of post-hoc tests contrasting quintiles 2–5 to Ref. (after false-discovery rate29 adjustment; *adjusted p<0.05; **adjusted p<0.01; ***adjusted p<0.001; ****adjusted p<0.0001).
Figure 2:
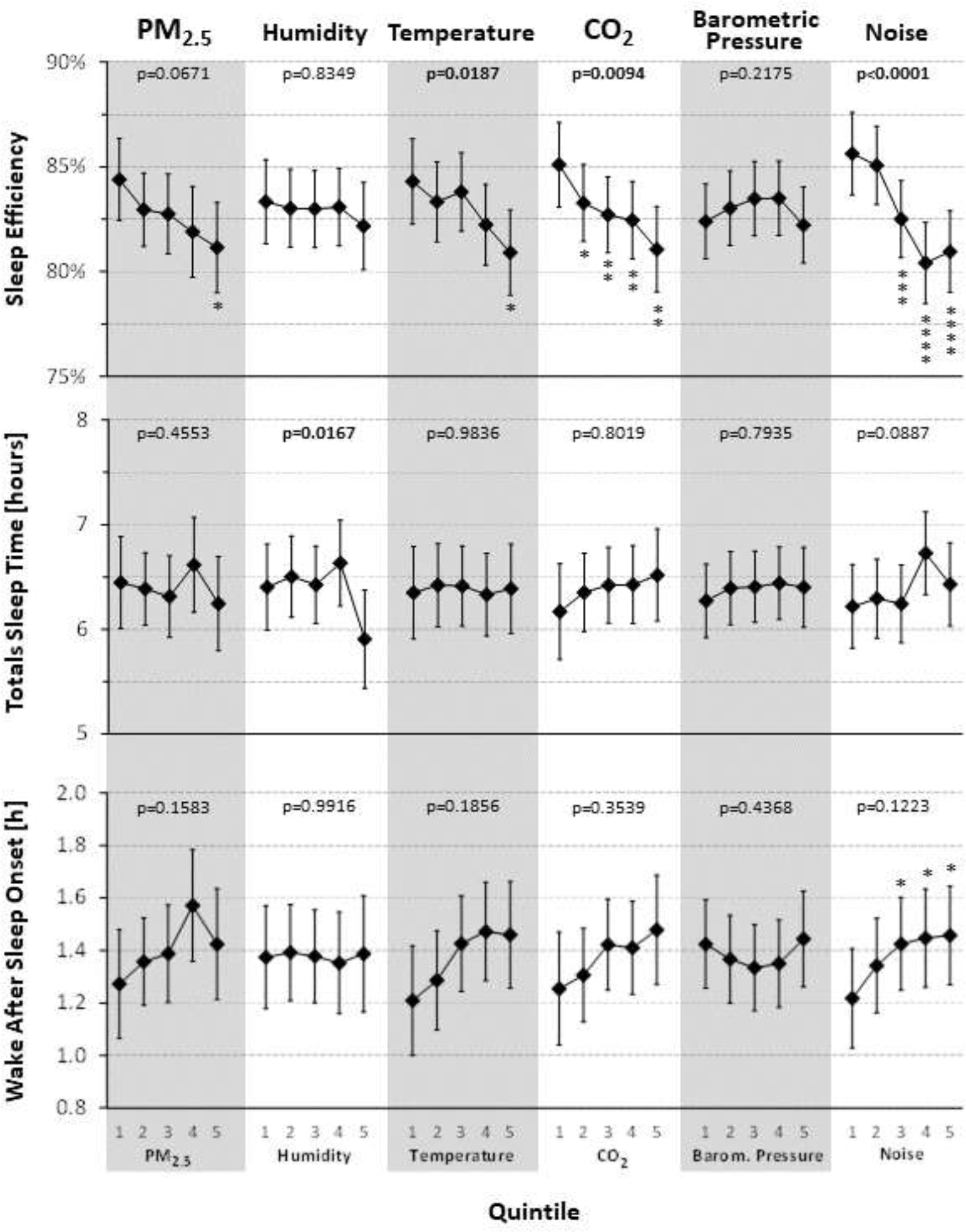
Associations of the bedroom environment with objectively assessed (via actigraphy) SE, total sleep time and wake after sleep onset. P-values reflect type-III tests for fixed effects. Estimates are based on the fully adjusted model (Model 4), using observed marginal means for all covariates. PM2.5: fine particulate matter; asterisks reflect statistical significance of post-hoc tests contrasting quintiles 2–5 to quintile 1 (after false-discovery rate29 adjustment; *adjusted p<0.05; **adjusted p<0.01; ***adjusted p<0.001; ****adjusted p<0.0001)
After z-transformation, significant linear associations with SE were observed for PM2.5 (β=−2.15%, p<0.0001, log+1 transformed), noise (β=−1.85%, p<0.0001) and temperature (β=−1.73%, p<0.0001), while humidity (β=−0.14%, p=0.7372), barometric pressure (β=−0.24%, p=0.2817) and CO2 (β=−0.32%, p=0.3801) showed no significant linear association with SE. After adjusting for multiple testing, none of the 2-way interactions between environmental exposures were statistically significant at an FDR of 0.05 (Table S4) suggesting additive effects. The only covariates with significant associations with SE were elapsed sleep time and pre-bed alcohol consumption.
No statistically significant associations between the bedroom environment and actigraphically assessed TST and WASO (Figure 2) were found. While the omnibus test did not suggest that WASO in any of the noise exposure quintiles differed from the mean across quintiles, post-hoc tests show statistically significantly higher WASO in the three highest noise exposure quintiles relative to the lowest quintile (all adjusted p<0.05). With the exception of humidity, no statistically significant associations were observed between the bedroom environment and subjectively assessed sleep onset latency, sleep quality and sleepiness (Figure 3). For humidity, sleep quality was assessed significantly worse in the three highest exposure quintiles relative to the lowest quintiles, and participants rated themselves sleepier in exposure quintiles 3 and 5 relative to the lowest quintile.
Figure 3:
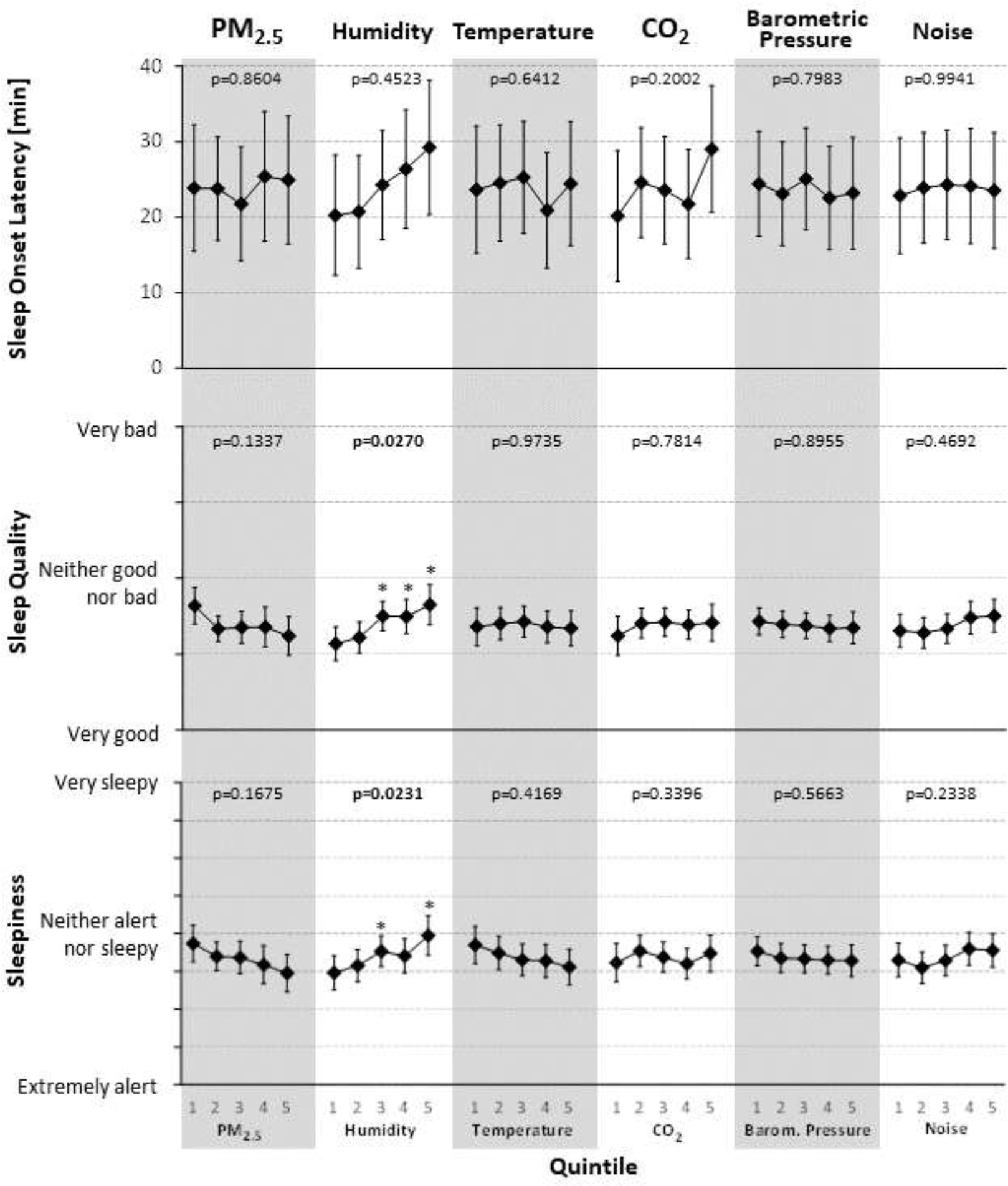
Associations of the bedroom environment with self-reported sleep onset latency, sleep quality and sleepiness (via daily morning surveys). P-values reflect type-III tests for fixed effects. Estimates are based on the fully adjusted model (Model 4) using observed marginal means for all covariates. PM2.5: fine particulate matter; Q1–5: quintiles 1–5; asterisks reflect statistical significance of post-hoc tests contrasting quintiles 2–5 to quintile 1 (after false-discovery rate29 adjustment; *adjusted p<0.05; **adjusted p<0.01; ***adjusted p<0.001; ****adjusted p<0.0001)
Participants overwhelmingly rated humidity (78.6%) and temperature (68.9%) in the bedroom as “just right” and did “not at all” feel disturbed by outside noise (73.9%) (Table 2). Humidity comfort ratings were similar irrespective of measured bedroom humidity levels. Likewise, sleep disturbance by outside noise ratings were similar across measured noise exposure quintiles. There was an increased tendency to select “slightly too hot” and “too hot” instead of “just right” in the highest two temperature quintiles. Participants who rated temperature and humidity slightly too hot/humid or too hot/humid or noise as very or extremely disturbing had lower SE compared to those who did not, albeit not statistically significantly (temperature: β=−0.7%, p=0.2629; humidity: β=−1.3%, p=0.1004; noise: β=−1.4%, p=0.4678).
Table 2:
Subjective ratings of nighttime humidity comfort, temperature comfort and sleep disturbance by outside noise depending on bedroom exposure quintiles.
| Relative | How would you rate the humidity in your bedroom last night? | |||||
|
| ||||||
| Humidity [%] | N | Too dry | Slightly too dry | Just right | Slightly too humid | Too humid |
| ≤ 45.8 | 177 | 0.0% | 3.4% | 74.0% | 21.5% | 1.1% |
| > 45.8 ≤ 49.6 | 187 | 0.0% | 4.8% | 81.8% | 11.8% | 1.6% |
| > 49.6 ≤ 53.3 | 185 | 1.6% | 8.6% | 70.8% | 17.8% | 1.1% |
| > 53.3 ≤ 58.0 | 181 | 0.0% | 5.0% | 83.4% | 10.5% | 1.1% |
| > 58.0 | 183 | 0.0% | 1.6% | 83.1% | 15.3% | 0.0% |
| All | 913 | 0.3% | 4.7% | 78.6% | 15.3% | 1.0% |
| How would you rate the temperature in your bedroom last night? | ||||||
| Temperature [°F] | N | Too cold | Slightly too cold | Just right | Slightly too hot | Too hot |
| ≤ 69.4 | 187 | 0.0% | 10.2% | 81.3% | 8.6% | 0.0% |
| > 69.4 ≤ 71.9 | 178 | 0.6% | 4.5% | 72.5% | 21.9% | 0.6% |
| > 71.9 ≤ 74.4 | 180 | 0.0% | 3.3% | 78.9% | 16.7% | 1.1% |
| > 74.4 ≤ 76.7 | 181 | 0.6% | 6.6% | 58.6% | 32.6% | 1.7% |
| > 76.7 | 184 | 0.5% | 3.8% | 53.3% | 38.6% | 3.8% |
| All | 910 | 0.3% | 5.7% | 68.9% | 23.6% | 1.4% |
| How much did outside noise disturb your sleep last night? | ||||||
| Noise [dBA] | N | Not at all | Slightly | Moderately | Very | Extremely |
| ≤ 44.2 | 145 | 77.9% | 15.9% | 4.8% | 1.4% | 0.0% |
| > 44.2 ≤ 48.6 | 140 | 80.7% | 13.6% | 5.0% | 0.7% | 0.0% |
| > 48.6 ≤ 53.2 | 144 | 71.5% | 20.1% | 4.9% | 2.8% | 0.7% |
| > 53.2 ≤ 58.0 | 143 | 66.4% | 21.7% | 9.1% | 2.8% | 0.0% |
| > 58.0 | 148 | 73.0% | 10.1% | 13.5% | 1.4% | 2.0% |
| All | 720 | 73.9% | 16.3% | 7.5% | 1.8% | 0.6% |
The 5 dominant window opening/AC/fan usage patterns were: central AC only (42.3%); central AC plus fan (29.9%); window AC plus fan (7.6%), window AC only (6.4%); and fan only (4.6%). Windows were opened (alone or in combination) in only 6.7% of nights. Opening the window and the use of central AC were not statistically significantly associated with PM2.5, humidity, temperature, CO2 or noise in the bedroom (Figure 4). The use of window AC was associated with significantly higher bedroom noise levels (+3.84 dBA, adjusted p<0.05). The use of a fan was associated with a significantly higher bedroom temperature (+1.07 °F, adjusted p<0.05) and significantly higher bedroom noise levels (+1.89 dBA, adjusted p<0.05).
Figure 4:
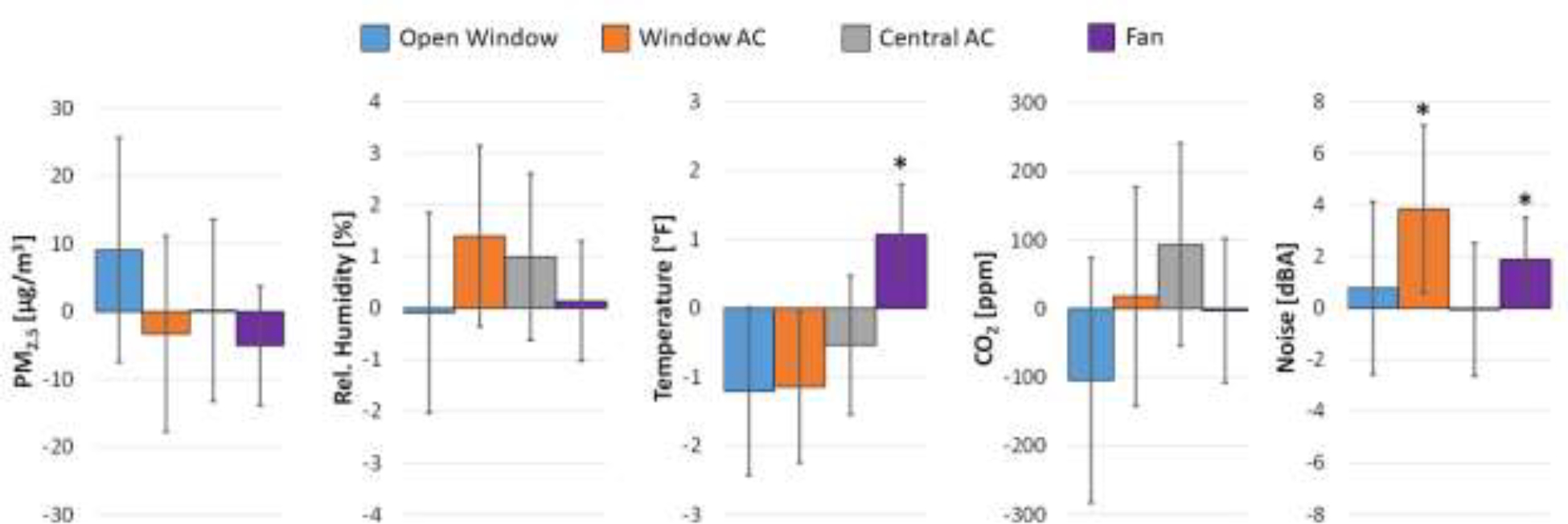
Associations of window opening behavior and the use of window air conditioning, central air conditioning or a fan with indoor measurements of fine particulate matter (PM2.5), relative humidity, temperature, CO2 and noise. * adjusted p<0.05; AC: air conditioning
Discussion
In this study, we evaluated associations between bedroom environmental variables (PM2.5, temperature, humidity, CO2, barometric pressure, and noise) and actigraphically assessed sleep in 62 participants who were each monitored over a period of 14 days. This study is one of only a few that (a) assessed both the bedroom environment and sleep objectively and (b) concurrently assessed multiple relevant descriptors of the bedroom environment.
Significant associations among bedroom PM2.5, temperature, CO2 and noise levels were found with SE, such that higher exposure levels were associated with lower SE in a dose-dependent fashion. These associations were robust and did not change relevantly across models 1–4 (ie, with the level of adjusting for confounders). Effect sizes derived from standardized linear regression suggest larger associations between noise and temperature and SE relative to PM2.5 and CO2, although effect size estimates for PM2.5 and CO2 may have been affected by larger measurement variability both within and between AQ meters (see Supplementary Materials). No significant associations with SE were found for relative humidity and barometric pressure. Also, no significant 2-way interactions were found for all possible environmental exposure pairs after adjusting for multiple testing. This suggests that associations of the investigated environmental variables were additive, although analyses likely lacked statistical power to find significant interactions.
Numerous laboratory and field studies have demonstrated that nighttime noise exposure adversely affects sleep.12,15,31 Noise exposure during sleep, which is often intermittent, causes autonomic and cortical arousals, including awakenings, with reductions in slow-wave and rapid eye movement (REM) sleep, and increases in superficial sleep stages.15,16 Disruptions of sleep due to nighttime noise exposure can lead to decrements in daytime functioning, including daytime sleepiness13 and modest slowing of psychomotor speed.16 Furthermore, translational and epidemiological research suggests that noise exposure in general, but especially intermittent noise exposure during the rest period,32 contributes to cardiovascular, metabolic, and neurodegenerative disease risk.33 In comparison to the other investigated environmental variables, the negative associations of noise with SE were the most robust. Thus, the importance of reducing noise levels in the bedroom cannot be overstated and represents a cornerstone of good sleep hygiene.
Previous studies have shown associations of the bedroom climate with sleep. Higher nighttime temperatures have been associated with more nights of self-reported insufficient sleep duration, where a +1°C increase in ambient temperature was associated with approximately 3 nights of insufficient sleep duration per 100 individuals.34 Another study in 48 households found a 1% decrease in actigraphically assessed SE for each +1°C increase in ambient temperature.35 A recent carefully controlled laboratory study did not find significant differences between 2 temperature conditions (24°C vs. 28°C) and sleep, assessed with a Fitbit, but this may be due to the small sample size (N = 10).36 Another recent study did not find a relationship between ambient temperature or humidity on sleep duration using continuous objective measures of sleep-wake behaviors across 1 year, although sleep durations during spring were significantly shorter than other seasons.37 One small study in 20 participants found reductions in subjectively assessed sleep quality and objectively assessed sleep duration with increasing bedroom temperature.38 Higher daily temperatures were also associated with increased Apnea-Hypopnea Index (AHI) scores, a marker of disrupted sleep, which was also more pronounced during summer months.39 Thus, while there are several studies hinting at negative associations of high bedroom temperatures with sleep, findings have been inconsistent, which may be explained by low sample sizes and inadequate statistical power. Our study demonstrates a dose-response-like decrease in SE with increasing ambient temperature and thus adds to the growing body of literature highlighting the importance of a cool bedroom environment.
The effects of ambient humidity on sleep are often examined along with temperature and appear to be synergistic: humid heat exposure has been associated with more wakefulness during the sleep period, as well as less REM and slow-wave sleep.40–42 In field studies, humidity, independent of temperature, had little effect on sleep quality,43,44 but when examined together more complaints of temperature and humidity were associated with worse sleep quality.45 Adjusting for the other environmental variables, no significant relationships between relative humidity and objectively assessed SE, TST, and WASO were found across the measured humidity range (33.0%–74.5%). However, statistically significant associations between humidity and self-reported poor sleep quality and sleepiness were found. Subjectively assessed SOL also increased in a dose-response-like fashion with increasing humidity, albeit not statistically significantly. Humidity was the only investigated environmental variable that was associated with any of the investigated subjective sleep outcomes. This suggests that humidity plays an important role in sleep perception.
Research on the effects of barometric pressure on sleep is scant. In one study, barometric pressure levels exhibited curvilinear patterns with sleep onset.46 Another study found a positive correlation between barometric pressure and self-reported TST.47 This study did not show associations of barometric pressure with any of the subjective or objective sleep outcomes. However, fluctuations in barometric pressure were relatively minor during the study period (mean ± SD 999.8 ± 2.7 hPa).
Several studies investigated associations between ambient AQ and sleep. One large cross-sectional study found associations between estimated PM2.5 exposure and increased self-reported sleep latency.48 An analysis of UK Biobank data found an association of atmospheric PM2.5 concentration with sleep disturbance (OR 2.39 per 10 μg/m3 increase; based on ICD10 codes) and sleep duration reduction (0.14 minutes per 10 μg/m3 increase; based on self-report).49 Two studies found an association between PM2.5 levels and increased obstructive sleep apnea severity.50,51 In contrast, a recent study in 20 participants with indoor AQ measurements and subjective as well as objective (Fitbits) assessments of sleep found improved sleep quality and increased SE with higher PM2.5 exposure levels, which the authors suggest could be partially explained by the relatively low PM2.5 levels measured during the study.38 Shorter self-reported sleep duration was associated with worse AQ index, as well as higher levels of PM2.5, PM10, and NO2; a one standard deviation increase in these air pollutants was associated with approximately 30-minute shorter sleep.52,53 Furthermore, exposure to higher levels of PM2.5, PM10, and NO2 was associated with poorer ratings of sleep quality,22 and perceived exposure to air pollution was also associated with poor sleep quality.54 Overall, the literature supports negative associations of PM2.5 and other AQ metrics with sleep duration and quality, which is corroborated by the findings of our study for SE.
Several studies investigated the effects of bedroom CO2 on sleep. Reductions in bedroom CO2 levels through window opening or running a fan were associated with higher SE in 30 students.11 Another small (N = 17) intervention study did not find differences in actigraphically assessed sleep outcomes during nights with open (average CO2 717 ppm) or closed doors or windows (average CO2 1150 ppm).55 A small but carefully controlled laboratory experiment repeatedly investigated 12 participants polysomnographically while manipulating laboratory CO2 concentrations (800, 1900, and 3000 ppm).56 In addition to decreased sleep quality, the authors found statistically significant reductions in slow-wave sleep and increases in SOL with increasing levels of CO2. Another study in 48 households found negative associations between bedroom CO2 levels and deep sleep. The latter was, however, assessed actigraphically.35 A small study in 20 participants found reductions in subjectively assessed sleep quality and objectively assessed sleep duration with increasing bedroom CO2 levels.38 A small polysomnographic study found that lowering CO2 levels from 1400 ppm to less than 1000 ppm by increasing ventilation reduced WASO and increased SE.57 Finally, a recent carefully controlled laboratory study did not find significant differences between 2 CO2 conditions (800 vs. 1700 ppm) and sleep assessed with a Fitbit, but this may be due to the small sample size (N = 10).36 Collectively, these studies suggest negative associations of high CO2 levels with sleep architecture and fragmentation, which is corroborated by the dose-response like decrease in SE with increasing levels of CO2 in our study.
With the exception of humidity, no statistically significant association was found for any of the environmental variables and objectively assessed TST or WASO or subjectively assessed SOL, sleep quality or sleepiness. SE is only strongly correlated with sleep duration in settings where sleep opportunity is kept constant as the latter depends on several factors unrelated to the bedroom environment, especially work and family demands.58 In fact, sleep opportunity (i.e., time in bed) and SE were not correlated (r<0.01) in this study (Figure S3). Thus, sleep duration is likely an inferior outcome for studies on the effects of AQ and noise in real world settings. The fact that only humidity was statistically significantly associated with two out of three subjectively assessed sleep outcomes despite significant associations of PM2.5, temperature, CO2 and noise with objectively assessed SE question the usefulness of self-report data in studies investigating the effects of the bedroom environment on sleep. Humans are unconscious and unaware of themselves and their surroundings during the sleep period for most of the night, which is why subjective assessments have to rely on typically short periods of intermittent wakefulness. Also, self-report ratings like sleepiness have been shown to habituate quickly, especially in chronic exposure situations.59 Participants may thus assess themselves and their surrounding as “normal” even if objective assessments suggest otherwise.35 This is corroborated by our finding that most of the participants chose the “just right” category for temperature and humidity or the “not at all disturbed” category for noise regardless of actual exposure levels. In line with previous research,60 ratings of a bedroom too hot or humid or sleep very or extremely disturbed by noise were associated with lower SE, albeit not statistically significantly.
Some environmental variables changed systematically across the night. While decreasing PM2.5 concentrations and noise levels can benefit sleep, increasing CO2 levels together with lower sleep pressure could be responsible for a higher degree of sleep fragmentation in the second half of the night. This study also investigated how window opening behavior and the use of central AC, window AC, or a fan changed the bedroom environment. Room temperature was lower in nights with window AC use, but at the expense of significantly higher noise levels. Other studies found that by opening the window, opening the door to the hallway or increasing ventilation, CO2, temperature, and humidity in the bedroom decrease while PM2.5 and noise levels increase.11,55,57,61–63 While it is difficult to establish causality from these data, they do suggest that there is a trade-off with positive effects on some and negative effects on other bedroom environmental variables.
Strengths of this study include ecologically valid measurements in the home environment; objective assessments both of the bedroom environment and sleep; concurrent measurement of several environmental variables, which allowed us to separate the association of each variable by adjusting for the other variables; and the fact that subjects participated in the study for 14 consecutive days..
Although the study has strengths, it is not without limitations. Weaknesses of the study include that ambient light levels and potentially other relevant variables (eg, volatile organic compounds, whether doors were open or closed) were not measured. Prebed light exposure can suppress melatonin excretion, increase sleep onset latency, and affect sleep architecture. A recent study also demonstrated that light exposure during the sleep period was associated with lighter sleep, higher sympathovagal balance, and increased insulin resistance.64 Also, this study only conducted measurements during the summer months in a single geographic region, which limits the generalizability of our findings; the relatively high interdevice variability of PM2.5 measurements; and that sleep was not assessed polysomnographically, which prevented investigation of sleep architecture. Also, more data were lost than originally anticipated. This was most likely caused by procedures put in place to minimize the risk of Covid-19 infections that required participants to set up the equipment themselves, and by the low battery runtime of the AQ and SL meters in case of power outages or a participant forgetting to connect a device to the outlet. It is, however, unlikely that this would have systematically biased results. Furthermore, due to the lack of sufficient resources, it was not possible to approach all GHP participants and, while unlikely, it is possible that those subjects approached differed in some systematic way from those not approached. Also, while the sample size is larger than that of most previous comparable studies, the study was powered for repeated measurements in the same subjects and the sample size is still relatively small. Therefore, the results need to be interpreted with caution. Finally, while causality cannot be inferred from this cross-sectional study, at least reverse causality is unlikely, as the degree of SE is unlikely to influence the bedroom environment relevantly (aside perhaps from obstructive snoring and noise levels).
Conclusions
This study investigated associations of several environmental variables with objectively assessed sleep duration and efficiency. Temperature, noise, PM2.5 and CO2 levels in the bedroom were all found to be significantly associated with lower levels of SE in a dose-dependent manner. These findings add to a growing body of evidence highlighting the importance of the bedroom environment–beyond the mattress–for high-quality sleep. There is a need to identify interventions that can improve the bedroom environment, especially since climate change has already started to produce more extreme weather events. These interventions span a wide spectrum and include, but are not limited to, simple behavioral changes (eg, leave the door to the hallway open to lower CO2 levels), to changes in building structure (eg, triple-pane windows for noise reduction), to political interventions (eg, incentives to buy electric cars to lower air pollution and noise at low speeds), and land use planning (eg, increase in greenspace65).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank study participants.
GRANT SUPPORT
This study was supported in part by grants from the National Institutes of Health, ES029846 and ES023716 (PI: A. Bhatnagar), the Christina Lee Brown Envirome Institute, and the National Aviation and Space Administration (NASA) through grant NNX14AM81G (PI: M. Basner). C.W.J. is supported by NIH/NHLBI 5T32HL007713. M.C. is supported by NIH/NINR K99 NR019862. R.Y. is supported by NIH P30ES030283.
ABBREVIATIONS
- AC
Air conditioning
- AHI
Apnea-Hypopnea-Index
- AQ
Air quality
- BMI
Body mass index
- CO2
Carbon dioxide
- dBA
A-weighted decibels
- GHP
Green Heart Project
- IRB
Institutional Review Board
- PM2.5
Particulate matter with a particle size <2.5 μm
- SD
Standard Deviation
- SL
Sound level
Footnotes
DECLARATION OF CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare related to the work presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE
- 1.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012; 4 (129): 129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dettoni JL, Consolim-Colombo FM, Drager LF, et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol (1985). 2012; 113 (2): 232–236. [DOI] [PubMed] [Google Scholar]
- 3.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2011; 463 (1): 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St-Onge MP, O’Keeffe M, Roberts AL, Roychoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012; 35 (11): 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013; 342 (6156): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. AnnNYAcadSci. 2008; 1129: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011; 34 (5): 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010; 33 (5): 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson NF, Badr MS, Belenky G, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015; 38 (8): 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016; 65 (6): 137–141. [DOI] [PubMed] [Google Scholar]
- 11.Strom-Tejsen P, Zukowska D, Wargocki P, Wyon DP. The effects of bedroom air quality on sleep and next-day performance. Indoor Air. 2016; 26 (5): 679–686. [DOI] [PubMed] [Google Scholar]
- 12.Basner M, McGuire S. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Effects on Sleep. Int J Environ Res Public Health. 2018; 15 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basner M Nocturnal aircraft noise increases objectively assessed daytime sleepiness. Somnologie. 2008; 12 (2): 110–117. [Google Scholar]
- 14.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev. 2015; 22: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basner M Effects of noise on sleep. In: Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier; 2021. [Google Scholar]
- 16.Basner M, Muller U, Elmenhorst EM. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011; 34 (1): 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MG, Cordoza M, Basner M. Environmental Noise and Effects on Sleep: An Update to the WHO Systematic Review and Meta-Analysis. Environ Health Perspect. 2022; 130 (7): 76001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S, Bai L, Oiamo TH, et al. Association Between Road Traffic Noise and Incidence of Diabetes Mellitus and Hypertension in Toronto, Canada: A Population-Based Cohort Study. J Am Heart Assoc. 2020; 9 (6): e013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempen EV, Casas M, Pershagen G, Foraster M. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Cardiovascular and Metabolic Effects: A Summary. Int J Environ Res Public Health. 2018; 15 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008; 12 (4): 307–317. [DOI] [PubMed] [Google Scholar]
- 21.Scully RR, Basner M, Nasrini J, et al. Effects of acute exposures to carbon dioxide on decision making and cognition in astronaut-like subjects. npj Microgravity. 2019; 5 (1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Xiang H, Mao Z, et al. Is long-term exposure to air pollution associated with poor sleep quality in rural China? Environ Int. 2019; 133 (Pt B): 105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canha N, Lage J, Candeias S, Alves C, Almeida SM. Indoor air quality during sleep under different ventilation patterns. Atmospheric Pollution Research. 2017; 8 (6): 1132–1142. [Google Scholar]
- 24.Liu J, Wu T, Liu Q, Wu S, Chen JC. Air pollution exposure and adverse sleep health across the life course: A systematic review. Environ Pollut. 2020; 262: 114263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caddick ZA, Gregory K, Arsintescu L, Flynn-Evans EE. A review of the environmental parameters necessary for an optimal sleep environment. Build Environ. 2018; 132: 11–20. [Google Scholar]
- 26.Dautovich ND, Dzierzewski JM, MacPherson A. Chapter 11 - Bedroom environment and sleep health. In: Nieto FJ, Petersen DJ, eds. Foundations of Sleep Health. Academic Press; 2022: 239–264. [Google Scholar]
- 27.Gillberg M, Anderzen I, Akerstedt T. Recovery within day-time sleep after slow wave sleep suppression. Electroencephalogr Clin Neurophysiol. 1991; 78 (4): 267–273. [DOI] [PubMed] [Google Scholar]
- 28.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999; 131 (7): 485–491. [DOI] [PubMed] [Google Scholar]
- 29.Curran-Everett D Multiple comparisons: philosophies and illustrations. AmJ Physiol RegulIntegrComp Physiol. 2000; 279 (1): R1–R8. [DOI] [PubMed] [Google Scholar]
- 30.Lou C, Liu H, Li Y, Peng Y, Wang J, Dai L. Relationships of relative humidity with PM2.5 and PM10 in the Yangtze River Delta, China. Environ Monit Assess. 2017; 189 (11): 582. [DOI] [PubMed] [Google Scholar]
- 31.Hume K Sleep disturbance due to noise: current issues and future research. Noise Health. 2010; 12 (47): 70–76. [DOI] [PubMed] [Google Scholar]
- 32.Kroller-Schon S, Daiber A, Steven S, et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J. 2018; 39 (38): 3528–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Münzel T, Kröller-Schön S, Oelze M, et al. Adverse Cardiovascular Effects of Traffic Noise with a Focus on Nighttime Noise and the New WHO Noise Guidelines. Annu Rev Public Health. 2020; 41 (1): 309–328. [DOI] [PubMed] [Google Scholar]
- 34.Obradovich N, Migliorini R, Mednick SC, Fowler JH. Nighttime temperature and human sleep loss in a changing climate. Sci Adv. 2017; 3 (5): e1601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong J, Lan L, Lian Z, De dear R. Associations of bedroom temperature and ventilation with sleep quality. Science and Technology for the Built Environment. 2020; 26 (9): 1274–1284. [Google Scholar]
- 36.Fan X, Shao H, Sakamoto M, et al. The effects of ventilation and temperature on sleep quality and next-day work performance: pilot measurements in a climate chamber. Build Environ. 2022; 209. [Google Scholar]
- 37.Mattingly SM, Grover T, Martinez GJ, et al. The effects of seasons and weather on sleep patterns measured through longitudinal multimodal sensing. NPJ Digit Med. 2021; 4 (1): 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz H, Kinney KA, Wu CY, Schnyer DM, Nagy Z. Data fusion of mobile and environmental sensing devices to understand the effect of the indoor environment on measured and self-reported sleep quality. Build Environ. 2022; 214. [Google Scholar]
- 39.Weinreich G, Wessendorf TE, Pundt N, et al. Association of short-term ozone and temperature with sleep disordered breathing. Eur Respir J. 2015; 46 (5): 1361–1369. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012; 31 (1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto-Mizuno K, Mizuno K, Michie S, Maeda A, Iizuka S. Effects of humid heat exposure on human sleep stages and body temperature. Sleep. 1999; 22 (6): 767–773. [PubMed] [Google Scholar]
- 42.Tsuzuki K, Okamoto-Mizuno K, Mizuno K. Effects of humid heat exposure on sleep, thermoregulation, melatonin, and microclimate. J Therm Biol. 2004; 29 (1): 31–36. [Google Scholar]
- 43.Cao T, Lian ZW, Ma S, Bao JK. Thermal comfort and sleep quality under temperature, relative humidity and illuminance in sleep environment. J Build Eng. 2021; 43. [Google Scholar]
- 44.Kim M, Chun C, Han J. A Study on Bedroom Environment and Sleep Quality in Korea. Indoor and Built Environment. 2010; 19 (1): 123–128. [Google Scholar]
- 45.Matsumoto Y, Uchimura N, Ishida T, et al. The relationship of sleep complaints risk factors with sleep phase, quality, and quantity in Japanese workers. Sleep Biol Rhythms. 2017; 15 (4): 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb WB, Ades H. Sleep Tendencies: Effects of Barometric Pressure. Science. 1964; 143 (3603): 263–264. [DOI] [PubMed] [Google Scholar]
- 47.Pandey J, Grandner M, Crittenden C, Smith MT, Perlis ML. Meteorologic factors and subjective sleep continuity: a preliminary evaluation. Int J Biometeorol. 2005; 49 (3): 152–155. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Liu XT, Chen GB, et al. Association of long-term exposure to ambient air pollutants with prolonged sleep latency: The Henan Rural Cohort Study. Environ Res. 2020; 191. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Zhang W, Xie L, et al. Effects of atmospheric particulate matter pollution on sleep disorders and sleep duration: a cross-sectional study in the UK biobank. Sleep Med. 2020; 74: 152–164. [DOI] [PubMed] [Google Scholar]
- 50.Billings ME, Gold D, Szpiro A, et al. The Association of Ambient Air Pollution with Sleep Apnea: The Multi-Ethnic Study of Atherosclerosis. Ann Am Thorac Soc. 2019; 16 (3): 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen YL, Liu WT, Lee KY, Chuang HC, Chen HW, Chuang KJ. Association of PM2.5 with sleep-disordered breathing from a population-based study in Northern Taiwan urban areas. Environ Pollut. 2018; 233: 109–113. [DOI] [PubMed] [Google Scholar]
- 52.Yu H, Chen P, Paige Gordon S, Yu M, Wang Y. The Association between Air Pollution and Sleep Duration: A Cohort Study of Freshmen at a University in Beijing, China. Int J Environ Res Public Health. 2019; 16 (18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong C, Longcore T, Benbow J, et al. Environmental influences on sleep in the California Teachers Study Cohort. Am J Epidemiol. 2021; 191 (9): 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju YJ, Lee JE, Choi DW, Han KT, Lee SY. Association between perceived environmental pollution and poor sleep quality: results from nationwide general population sample of 162,797 people. Sleep Med. 2021; 80: 236–243. [DOI] [PubMed] [Google Scholar]
- 55.Mishra AK, van Ruitenbeek AM, Loomans M, Kort HSM. Window/door opening-mediated bedroom ventilation and its impact on sleep quality of healthy, young adults. Indoor Air. 2018; 28 (2): 339–351. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Lian Z, Shen J, et al. Experimental study on sleep quality affected by carbon dioxide concentration. Indoor Air. 2020: 31 (2): 440–453. [DOI] [PubMed] [Google Scholar]
- 57.Lan L, Sun Y, Wyon DP, Wargocki P. Pilot study of the effects of ventilation and ventilation noise on sleep quality in the young and elderly. Indoor Air. 2021; 31 (6): 2226–2238. [DOI] [PubMed] [Google Scholar]
- 58.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007; 30 (9): 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26 (2): 117–126. [DOI] [PubMed] [Google Scholar]
- 60.Alhasan DM, Gaston SA, Jackson CL. Investigate the complexities of environmental determinants of sleep health disparities. Sleep. 2022; 45 (8): zsac145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao C, Delghust M, Wargocki P, Laverge J. Effects of window opening on the bedroom environment and resulting sleep quality. Science and Technology for the Built Environment. 2021; 27 (7): 995–1015. [Google Scholar]
- 62.Sekhar C, Akimoto M, Fan X, et al. Bedroom ventilation: Review of existing evidence and current standards. Build Environ. 2020; 184: 107229. [Google Scholar]
- 63.Mainka A, Zajusz-Zubek E. Keeping doors closed as one reason for fatigue in teenagers - a case study. Appl Sci. 2019; 9 (17): 3533. [Google Scholar]
- 64.Mason IC, Grimaldi D, Reid KJ, et al. Light exposure during sleep impairs cardiometabolic function. Proc Natl Acad Sci U S A. 2022; 119 (12): e2113290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin JC, Parab KV, An R, Grigsby-Toussaint DS. Greenspace exposure and sleep: A systematic review. Environ Res. 2020; 182: 109081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


