Introduction
Antimuscarinic toxicity is among the most frequently observed toxidromes in the emergency department (ED). Overdoses of medications including diphenhydramine, cyclobenzaprine, quetiapine, olanzapine, and tricyclic antidepressants antagonize central muscarinic cholinergic receptors and cause delirium [1]. Historically, physostigmine was available to reverse antimuscarinic delirium (AD). The peripheral manifestations of toxicity, including tachycardia, anhidrosis, and ileus, would also improve.
Akorn, the sole US manufacturer of physostigmine, has stopped production, and many hospitals have depleted their supplies. Although it may be possible to obtain this medication via import or from a compounding pharmacy, these are not without logistical challenges. Rivastigmine, however, is readily available and may be able to fill the antidotal void.
Rivastigmine is a centrally acting cholinesterase inhibitor used in the management of Alzheimer’s dementia. Unlike physostigmine, there is no parenteral formulation; the medication is administered orally or transdermally. There are no large studies evaluating rivastigmine’s role in acute antimuscarinic toxicity, but several small case reports have suggested it is both safe and effective [2, 3]. The purpose of this study was to provide additional evidence that rivastigmine is a viable alternative to physostigmine in the treatment of AD.
Methods
This is a retrospective review of patients treated with rivastigmine for AD between January 1, 2021, and December 31, 2022. Cases were identified through a patient log. All patients were evaluated by a solo medical toxicologist who established a consultation service at this suburban community teaching hospital the previous month. Patients were reassessed every 15–30 min until symptoms resolved. Delirium was defined as an acute, transient disturbance of consciousness characterized by confusion, agitation, disorientation, and/or hallucinations. The diagnosis of antimuscarinic toxicity required the presence of at least four of the following: tachycardia, anhidrosis, absent or hypoactive bowel sounds, urinary retention, mydriasis, and “mumbling” speech. Miosis did not exclude the diagnosis, because there are several muscarinic antagonists, e.g., olanzapine, quetiapine, and chlorpromazine, that also antagonize peripheral α1-adrenergic receptors, producing miosis. Patients without any peripheral antimuscarinic features and those whose delirium was believed to be multifactorial were excluded. Rivastigmine would not have been administered to patients with AD if bradycardia or heart block had been present. Symptoms were considered resolved when patients had normal mentation, clear speech, and were able to ambulate and tolerate oral intake. Demographic information, likely ingestant(s), laboratory data, electrocardiographic findings, treatment, adverse reactions, and length of hospitalization, were recorded. The study was determined to be exempt or excluded from Institutional Review Board oversight in accordance with current regulations and institutional policy.
Results
There were 22 patients with AD, including 15 (68%) nonpregnant females. Ages ranged from 16–63 years old, with a median age of 29 years old (IQR: 21–50 years old). All patients were treated with rivastigmine, as our practice is to aggressively treat AD.
Diphenhydramine was the most common source of antimuscarinic toxicity. The various antimuscarinic ingestants are listed in Table 1.
Table 1.
Ingestants and clinical features
| Ingestant(s) (number of cases) | Miosis | Mydriasis | Tachycardia | QTc > 500 ms | Potassium < 3.5 mmol/L |
|---|---|---|---|---|---|
| Diphenhydramine (7) | 0 cases (0%) | 7 cases (100%) | 7 cases (100%) |
4 cases (57%) |
2 cases (29%) |
| Diphenhydramine and chlorpromazine (1) | 0 cases (0%) |
0 cases (0%) |
1 case (100%) |
1 case (100%) |
0 cases (0%) |
| Diphenhydramine and quetiapine (1) | 0 cases (0%) |
0 cases (0%) |
1 case (100%) |
0 cases (0%) |
0 cases (0%) |
| Quetiapine (5) | 4 cases (80%) |
0 cases (0%) |
5 cases (100%) |
1 case (20%) |
0 cases (0%) |
| Quetiapine and benztropine (1) | 0 cases (0%) |
1 case (100%) |
1 case (100%) |
0 cases (0%) |
0 cases (0%) |
| Benztropine (1) |
0 cases (0%) |
1 case (100%) |
1 case (100%) |
0 cases (0%) |
0 cases (0%) |
| Carbamazepine (1) | 0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
1 case (100%) |
| Hydroxyzine (1) | 0 cases (0%) |
0 cases (0%) |
1 case (100%) |
0 cases (0%) |
0 cases (0%) |
| Olanzapine (1) | 1 case (100%) |
0 cases (0%) |
1 case (100%) |
1 case (100%) |
0 cases (0%) |
| Prochlorperazine (1) | 1 case (100%) |
0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
| Promethazine (1) | 0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
0 cases (0%) |
| Unknown (1) | 0 cases (0%) |
0 cases (0%) |
1 case (100%) |
0 cases (0%) |
1 case (100%) |
| Total (22) |
6 cases (27%) |
9 cases (41%) |
19 cases (86%) |
7 cases (32%) |
4 cases (18%) |
All patients exhibited mumbling speech. The median maximal heart rate was 128 beats per minute (range 86–168). Tachycardia was observed in 19 (86%) patients. Seven (32%) patients had QT interval prolongation > 500 ms. There was no worsening of QT prolongation following treatment with rivastigmine.
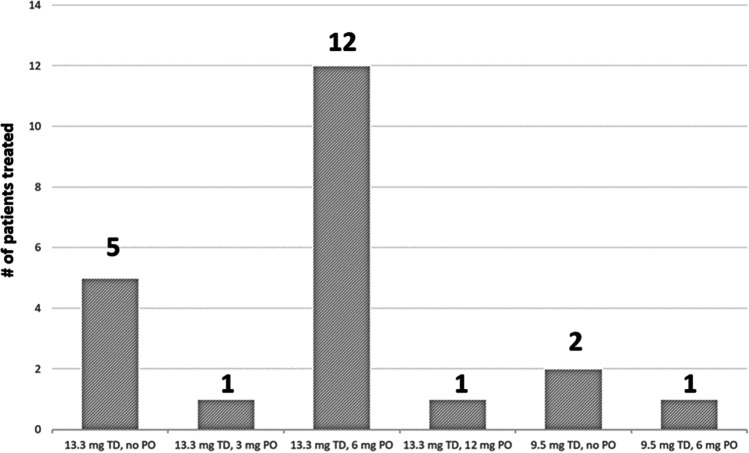
All patients were treated with a rivastigmine transdermal patch, which was placed on the back to prevent premature removal. The 13.3 mg patch was used in 19 (86%) cases, based on the assumption it would provide the most benefit. Three patients received the 9.5 mg patch when the 13.3 mg patch was unavailable. Patients and nursing staff were instructed to remove the patches after 24 h. Because rivastigmine capsules should not be crushed or altered in any way, we elected to forego oral rivastigmine in seven (32%) patients who were unable or unwilling to swallow the intact capsules. The number of patients treated with each regimen of rivastigmine dosing is illustrated in Fig. 1.
Fig. 1.
Rivastigmine dosing regimens
Fourteen (64%) patients were admitted; 13 were managed on the general medicine service. One patient who required intubation and mechanical ventilation after ingesting quetiapine and fluoxetine was admitted to the intensive care unit (ICU). All the patients who were initially unable to tolerate oral rivastigmine were admitted, but eight (53%) patients treated with the oral medication in the ED avoided hospitalization. There was no relationship between ingestant(s) and the need for hospital admission.
The median time to symptom resolution was 2 h (range 1–4.5 h) for patients treated with oral rivastigmine. Median recovery time for patients who received only the transdermal patch was 5 h (range 3 h–23.75 h). The one outlier was symptomatic from concomitant fluoxetine toxicity. All patients recovered completely. No patient required any medical treatment for more than 24 h. No complications, including seizures, vomiting, diarrhea, symptomatic bradycardia, or new electrocardiographic abnormalities, were reported. No patients returned to the hospital for recurrent symptoms.
All patients were treated with maintenance intravenous fluids. No patients required fluid resuscitation. Patients with QT prolongation were treated with magnesium sulfate 2 g. Potassium was also repleted as needed to maintain potassium > 4.0 mmol/L. The ICU team performed an elective intubation for airway protection in the patient who ingested quetiapine and fluoxetine. Propofol was initially used for sedation before the patient was transitioned to midazolam and fentanyl.
Discussion
Antimuscarinic toxicity is commonly observed in the ED, and untreated cases can result in delirium, prolonged hospitalization, and higher rates of intubation [1, 4–7]. Patients with olanzapine or quetiapine ingestions often have prolonged symptoms. The median hospital length of stay was 2 days (range 1–4 days) in a review of 14 quetiapine overdoses, and 43% of patients were admitted to the ICU [8]. The median duration of delirium was 21 h (interquartile range 14–42 h) in a study of 108 olanzapine ingestions [9].
Centrally acting cholinesterase inhibitors can reverse toxicity by raising the acetylcholine concentration to levels at which it can compete with the offending muscarinic antagonist(s). When administered intravenously, physostigmine often works within several minutes. Yet, despite multiple studies demonstrating its effectiveness, physostigmine often went unused. Unfamiliarity with physostigmine and concerns about its safety are the most likely explanations for its historic underuse. The latter is likely the reason physostigmine was not used more by medical toxicologists. Physostigmine was administered in only 171 (21%) cases of antimuscarinic toxicity reported to the Toxicology Investigators Consortium registry administered by the American College of Medical Toxicology [7].
There is evidence that these safety concerns may be exaggerated. Transient emesis, diaphoresis, diarrhea, asymptomatic bradycardia, and increased respiratory secretions were reported in one patient each in a study of 45 patients treated with physostigmine [4]. No serious complications were noted in a study of 39 adult patients treated with physostigmine, although one patient had a brief seizure without adverse sequelae [10].
Rapid administration of physostigmine can precipitate seizures. The bioavailability of oral rivastigmine is ~ 36%, and absorption takes ~ 1 h, while transdermal rivastigmine is not fully absorbed for up to 8 h [11, 12]. Because of rivastigmine’s slower absorption, seizures are unlikely. In the case reports describing rivastigmine use in AD, no adverse events were noted.
Many patients treated with physostigmine require more than one dose [13]. Rivastigmine has a much longer duration of action—10 h for the oral formulation and 24 h for the transdermal product—so the need for repeat dosing is likely lower. The median time to clinical improvement was shorter in patients treated with oral rivastigmine compared to those who received only the transdermal patch. It is unclear if the addition of the transdermal patch provided benefit to patients who also received oral rivastigmine, although the patch may have provided prolonged protection against recurrence.
There are several limitations to this study. There is no control group from this hospital population because all identified AD patients receive antidotal therapy. Historical controls from the same hospital are unavailable because, prior to the establishment of the toxicology consultation service, many of these patients were undiagnosed or transferred. Cases were identified using the toxicology service patient log. It is standard practice at this hospital to consult the toxicology service on all overdoses. However, some patients with AD may have been managed independently in the ED or on the inpatient service. Thus, there is the potential for bias. Although no patients returned to the hospital with recurrent symptoms, there is no way to know if they sought treatment elsewhere. A similar dosing strategy may not be possible at hospitals that do not stock one or both formulations of rivastigmine. Finally, the results of this study do not prove causality, though the comparatively rapid, temporal association between rivastigmine administration and symptom resolution is highly suggestive. Rivastigmine appears to be safe in this population. Larger studies are warranted to determine the optimal dosing, including route(s) of administration, and to assess for uncommon adverse reactions.
Sources of Funding
None.
Declarations
Conflicts of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arens AM, Shah K, Al-Abri S, Olson KR, Kearney T. Safety and effectiveness of physostigmine: a 10-year retrospective review. Clin Toxicol. 2018;56(2):101–107. doi: 10.1080/15563650.2017.1342828. [DOI] [PubMed] [Google Scholar]
- 2.Van Kernebeek MW, Ghesquiere M, Vanderbruggen N, Verhoeven E, Hubloue I, Crunelle CL. Rivastigmine for the treatment of anticholinergic delirium following severe procyclidine intoxication. Clin Toxicol. 2021;59(5):447–448. doi: 10.1080/15563650.2020.1818768. [DOI] [PubMed] [Google Scholar]
- 3.Hughes AR, Moore KK, Mah ND, Birmingham AR, Clark RK, et al. Letter in response to Rivastigmine for the treatment of anticholinergic delirium following severe procyclidine intoxication. Clin Toxicol. 2021;59(9):855–856. doi: 10.1080/15563650.2020.1869757. [DOI] [PubMed] [Google Scholar]
- 4.Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374–381. doi: 10.1016/S0196-0644(00)70057-6. [DOI] [PubMed] [Google Scholar]
- 5.Boley SP, Olives TD, Bangh SA, Fahrner S, Cole JB. Physostigmine is superior to non-antidote therapy in the management of antimuscarinic delirium: a prospective study from a regional poison center. Clin Toxicol. 2019;57(1):50–55. doi: 10.1080/15563650.2018.1485154. [DOI] [PubMed] [Google Scholar]
- 6.Wang GS, Baker K, Ng P, Janis GC, Leonard J, et al. A randomized trial comparing physostigmine vs lorazepam for treatment of antimuscarinic (anticholinergic) toxidrome. Clin Toxicol. 2021;59(8):698–704. doi: 10.1080/15563650.2020.1854281. [DOI] [PubMed] [Google Scholar]
- 7.Watkins JW, Schwarz ES, Arroyo-Plascencia AM, Mullins ME, Toxicology Investigators Consortium investigators The use of physostigmine by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11:179–84. doi: 10.1007/s13181-014-0452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunfeld NG, Westerman EM, Boswijk DJ, De Haas JA, van Putten MJ, Touw DJ. Quetiapine in overdosage: a clinical and pharmacokinetic analysis of 14 cases. Ther Drug Monit. 2006;28(2):185–189. doi: 10.1097/01.ftd.0000185770.44502.51. [DOI] [PubMed] [Google Scholar]
- 9.Morgan M, Hackett LP, Isbister GK. Olanzapine overdose: a series of analytically confirmed cases. Int Clin Psychopharmacol. 2007;22(3):183–186. doi: 10.1097/YIC.0b013e32805aedf5. [DOI] [PubMed] [Google Scholar]
- 10.Schneir AB, Offerman SR, Ly BT, Davis JM, Baldwin RT, et al. Complications of diagnostic physostigmine administration to emergency department patients. Ann Emerg Med. 2003;42(1):14–19. doi: 10.1067/mem.2003.232. [DOI] [PubMed] [Google Scholar]
- 11.Medscape: Rivastigmine. Available at: https://reference.medscape.com/drug/exelon-oral-solution-rivastigmine-343069#10. Accessed on April 14, 2023.
- 12.Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer’s disease. Expert Opin. 2008;5(12):1377–1386. doi: 10.1517/17425240802542690. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum C, Bird SB. Timing and frequency of physostigmine redosing for antimuscarinic toxicity. J Med Toxicol. 2010;6:386–392. doi: 10.1007/s13181-010-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]