Abstract
Although the definitions of sinonasal neuroendocrine and neuroectodermal neoplasms did not change substantially in the 5th edition WHO Classification of Head and Neck Tumours, the diagnosis of olfactory neuroblastoma (ONB), small cell neuroendocrine carcinoma, and large cell neuroendocrine carcinoma remains quite challenging in practice. Ambiguities surrounding the amount of keratin expression allowable in ONB and the amount of neuroendocrine differentiation seen in sinonasal undifferentiated carcinoma (SNUC) lead to significant diagnostic discrepancies at the high grade end of this tumor spectrum. Furthermore, a group of problematic neuroepithelial tumors that show overlapping features of ONB and neuroendocrine carcinoma have never been recognized in formal classification schemes. Since publication of the 5th edition WHO, two new tumor entities have been proposed that help resolve these problems. Olfactory carcinoma is defined by high grade keratin-positive neuroectodermal cells with frequent intermixed glands and shows recurrent Wnt pathway, ARID1A, and RUNX1 alterations. IDH2-mutant sinonasal carcinoma is a molecularly-defined category that encompasses tumors with undifferentiated (SNUC), large cell neuroendocrine, and neuroepithelial phenotypes. This review will provide a practical overview of these emerging entities and their application to diagnostic challenges in the post-WHO sinonasal neuroendocrine and neuroectodermal tumor classification.
Keywords: Nasal neoplasms, Olfactory neuroblastoma, Neuroendocrine carcinoma, Sinonasal undifferentiated carcinoma, Olfactory carcinoma, IDH2-mutant sinonasal carcinoma, Immunohistochemistry, Molecular diagnostics
Introduction
The 5th edition WHO Classification of Head and Neck Tumours formalizes several dramatic changes to the sinonasal tumor taxonomy that have been introduced over the last decade, with increasingly precise and nuanced histologic categories and heavy emphasis on molecular findings in tumor diagnosis [1]. However, the classification of sinonasal neuroendocrine and neuroectodermal neoplasms remains largely untouched by these updates. In the 5th edition WHO, there are no substantive alterations to the definition of olfactory neuroblastoma (ONB)—a neuroectodermal neoplasm that is still regarded as unique to the sinonasal tract [2]. Additionally, while the 5th edition WHO shifts sinonasal small cell and large cell neuroendocrine carcinoma chapters into a separate neuroendocrine tumor section as part of the effort to harmonize neuroendocrine tumor terminology across organ systems, their diagnostic criteria are unchanged [3–5]. Furthermore, molecular data does not yet play an important role in the diagnosis and classification of sinonasal neuroendocrine and neuroectodermal tumors. No recurrent genetic drivers have been identified in ONB to inform diagnosis. Additionally, unique molecular findings recently reported in sinonasal small cell and large cell neuroendocrine carcinomas do not impact their current classification with neuroendocrine tumors of other sites.
Despite this seemingly static classification, diagnosis of sinonasal neuroendocrine and neuroectodermal neoplasms remains one of the most challenging areas of head and neck pathology. Ambiguities in diagnostic criteria can make it difficult to distinguish between these tumors and non-neuroendocrine neoplasms such as sinonasal undifferentiated carcinoma (SNUC), particularly at the high grade end of the spectrum. Furthermore, a problematic group of neuroepithelial tumors suggests genuine overlap between ostensibly discrete entities, including mixed neuroectodermal elements reminiscent of ONB and epithelial features suggestive of neuroendocrine carcinoma. Consequently, high grade sinonasal neuroendocrine and neuroectodermal tumors represent one of the most frequent areas of diagnostic discrepancy in head and neck pathology [6, 7], leading to clinical confusion about tumor classification and divergent treatment recommendations. Fortunately, even in the short time since publication of the 5th edition WHO Classification, two novel entities, olfactory carcinoma and IDH2-mutant sinonasal carcinoma, have been proposed that may help resolve these issues and substantially clarify the pathogenesis of this spectrum of tumors. This review presents a practical overview of current challenges in the diagnosis of sinonasal neuroendocrine and neuroectodermal neoplasms and how these emerging entities may resolve classification conundrums.
Terminology
Use of widely variable terminology in the literature has been one factor that may have contributed to confusion surrounding the sinonasal neuroendocrine and neuroectodermal differential diagnosis. As such, it is necessary to establish a common vocabulary for the tumors discussed in this review. Neuroendocrine tumors represent the broadest group in this differential diagnosis and include any tumors that show similarities to the body’s normal neuroendocrine cells on morphologic, immunohistochemical, or ultrastructural levels. Neuroectodermal tumors do fall under the larger neuroendocrine umbrella as they express neuroendocrine markers such as synaptophysin, chromogranin, and INSM1. However, neuroectodermal refers to a more specific group of tumors that recapitulate primitive or developing neural tissue, with frequent production of neurofibrillary matrix and rosette formation. Finally, the term neuroepithelial is frequently used synonymously with neuroectodermal, which may be accurate from a developmental perspective. However, for the purpose of clarifying this complicated tumor classification, this discussion will reserve neuroepithelial to exclusively refer to tumors that show both neuroectodermal features as well as epithelial differentiation in the form of keratin positivity or gland formation.
Traditional Categories of Sinonasal Neuroendocrine Neoplasms
Understanding challenges and new developments in the diagnosis of sinonasal neuroendocrine and neuroectodermal neoplasms first requires clear definitions of existing categories. This review will focus on the three most common such tumors that occur in the sinonasal tract: ONB, small cell neuroendocrine carcinoma, and large cell neuroendocrine carcinoma.
Olfactory Neuroblastoma
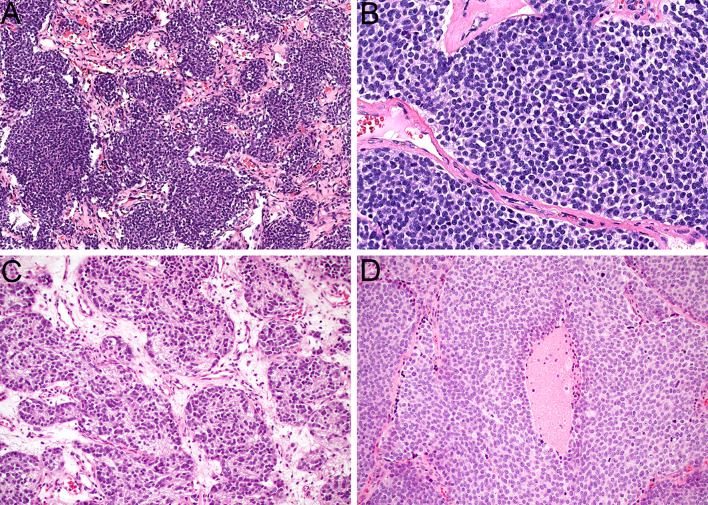
ONB is a neuroectodermal neoplasm that is unique to the sinonasal tract. It is thought to originate from the globose basal cells of the olfactory membrane [8, 9], resulting in very consistent localization in the superior aspect of the nasal cavity around the cribriform plate. Histologically, ONB is composed of anastomosing nests and lobules of neuroectodermal cells that are embedded in hypervascular stroma (Fig. 1A). These cells have round to oval nuclei with speckled chromatin and syncytial borders (Fig. 1B) and frequently show overt neural differentiation, including variable amounts of neurofibrillary matrix and rosette formation. ONB can demonstrate a broad range of histologic grades as defined by the Hyams system, with bland cytology and prominent neurofibrillary matrix in low grade tumors (Fig. 1C) and increasing nuclear pleomorphism, mitotic activity, and necrosis in high grade tumors (Fig. 1D). By immunohistochemistry, ONB consistently shows strong and diffuse positivity for all neuroendocrine markers, including synaptophysin, chromogranin, and INSM1 [10]. The vast majority of ONB are negative for pankeratin, although controversy exists about the degree of keratin expression that is allowable in this tumor, as discussed in detail below. Classically, ONB is surrounded by a delicate network of sustentacular cells that are positive for S100 protein and SOX10. ONB is also consistently positive for calretinin, which can help differentiate it from overlapping small round blue cell tumors [11]. Across several studies, no dominant oncogenic driver mutations have been identified in ONB, with alterations in TP53, PIK3CA, NF1, CDKN2A, CDKN2C, CCND1, and FGFR3 each only seen in small subsets of cases [12–15]. Recently, frequent deletion of the dystrophin (DMD) gene was reported in ONB, although the functional implications of this finding are unclear [16]. Tumor stage, as assessed by the Kadish-Morita system, is the most powerful predictor of outcomes in ONB, with Hyams grade having a smaller but definitive impact [17–22]. The 5-year survival for ONB is approximately 70 to 75% [23, 24].
Fig. 1.
ONB is composed of lobules and nests of neuroectodermal cells that are surrounded by hypervascular stroma (A, × 10). The tumor cells have syncytial borders and monotonous round to oval nuclei with speckled chromatin (B, × 40). Low grade ONB tends to have abundant neurofibrillary stroma with pseudorosette formation (C, × 20). High grade ONB shows increased mitotic activity and necrosis (D, × 20)
Small Cell Neuroendocrine Carcinoma
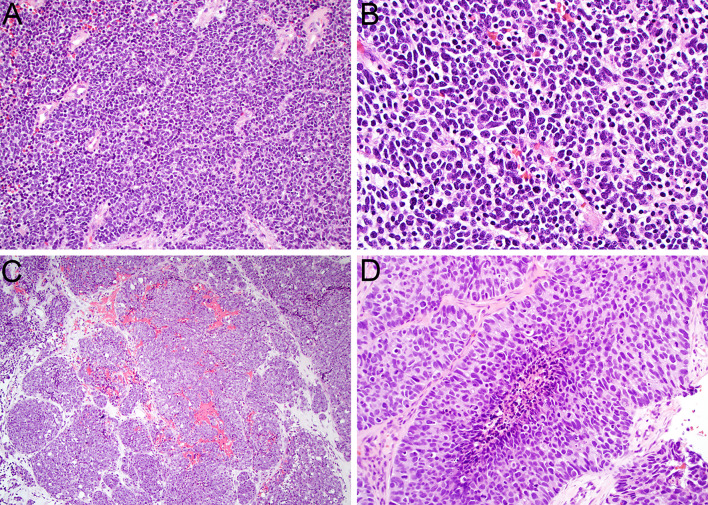
Small cell neuroendocrine carcinoma is a high grade neuroendocrine carcinoma that rarely arises in the sinonasal tract. As noted above, sinonasal small cell carcinoma is currently assumed to be analogous to small cell carcinoma of other histologic sites and was moved out of the sinonasal chapter in the 5th edition WHO classification to emphasize this unified classification [3, 4]. It most frequently affects the superior nasal cavity and ethmoid sinuses and occur in patients of a variety of ages [25, 26]. Histologically, small cell carcinoma is defined by the presence of sheets of high grade epithelial cells that have a very high nuclear-cytoplasmic ratio with scant cytoplasm (Fig. 2A). The tumor nuclei are large and hyperchromatic, with angulated borders, coarse chromatin, and extensive molding between adjacent nuclei (Fig. 2B). The presence of greater than 10 mitoses per 2 mm2 is required for diagnosis, and numerous apoptotic bodies and broad zones of necrosis are frequently seen. While it is well-established in other organs, particularly the lung, that small cell carcinomas can display minimal immunohistochemical evidence of epithelial differentiation, some degree of keratin positivity is essentially required in sinonasal small cell carcinoma in light of the extensive overlap with other sinonasal small round blue cell tumors [4]. Small cell carcinoma is also positive for neuroendocrine markers synaptophysin, chromogranin, and INSM1, although expression of all these markers can sometimes be quite focal. INSM1 seems to have highest sensitivity in high grade end neuroendocrine tumors [10]. Interestingly, recent sequencing studies have identified unique molecular features in sinonasal small cell carcinoma, with frequent alterations in ARID1A and Wnt pathway genes rather than the TP53 and RB1 mutations common at other sites [27]. A subset are also driven by high-risk human papillomavirus [28]. These tumors are aggressive but also have somewhat better outcomes than other extra-pulmonary small cell carcinomas, with a 5-year survival of approximately 40–50% [29–32].
Fig. 2.
Sinonasal small cell neuroendocrine carcinoma displays sheets of markedly atypical epithelial cells with scant cytoplasm (A, × 20). The nuclei are hyperchromatic with frequent molding, mitotic figures, and apoptotic bodies (B, × 40). Large cell neuroendocrine carcinoma tends to show nested architecture (C, × 10). The tumor cells have abundant cytoplasm and large nuclei with vesicular chromatin and prominent nucleoli that demonstrate peripheral palisading (D, × 40)
Large Cell Neuroendocrine Carcinoma
Large cell neuroendocrine carcinoma is the rarest form of high grade neuroendocrine carcinoma that arises in the sinonasal tract. Like small cell carcinoma, it has been moved out of the sinonasal tumor classification to reflect harmonization with similar tumors at other anatomic sites [3, 5]. The clinicopathologic features of these tumors are not well-established due to their rarity. The definition of large cell neuroendocrine carcinoma varies somewhat throughout the body, with some organ systems applying this terminology for any high-grade neuroendocrine carcinoma that does not meet criteria for small cell carcinoma, and others only invoking it in tumors that demonstrate characteristic neuroendocrine morphologic patterns. As in the lung, the WHO Classification of Head and Neck Tumours has always required specific morphologic features for a diagnosis of large cell neuroendocrine carcinoma. Histologically, sinonasal large cell neuroendocrine carcinoma consists of nests or trabeculae composed of large cells with abundant eosinophilic to amphophilic cytoplasm (Fig. 2C). These cells frequently demonstrate peripheral palisading, rosette formation, and central comedo-pattern necrosis and have large nuclei with vesicular chromatin and frequent prominent nucleoli (Fig. 2D). The mitotic rate should be greater than 10 mitoses per 2 mm2. By definition, large cell neuroendocrine carcinoma should be positive for both keratin and neuroendocrine markers synaptophysin, chromogranin, and INSM1, although the degree of expression of all of these markers is quite variable [10]. Recently, a majority of sinonasal large cell neuroendocrine carcinoma has been shown to have recurrent IDH2 R172X mutations [27]—a finding that will be discussed further below. Rare cases also harbor transcriptionally active high-risk human papillomavirus [33].
Problems in Classification
Despite the seeming simplicity of the sinonasal neuroendocrine tumor classification, this differential diagnosis can be quite challenging in practice. These difficulties center around three issues: how much epithelial differentiation can be seen in ONB, how much neuroendocrine differentiation is allowable in SNUC, and how to classify tumors with overlapping neuroepithelial features.
How much Epithelial Differentiation is Acceptable in Olfactory Neuroblastoma?
One persistent issue that has dogged the classification of high grade neuroendocrine and neuroectodermal neoplasms is the amount of epithelial differentiation that is allowable in ONB. Classically, the presence of epithelial differentiation is one of the most important inflection points for classification of neuroendocrine neoplasms across organ systems, with strict lines separating keratin-positive and keratin-negative entities. Under this conceptualization, ONB is strictly regarded as a keratin-negative neuroendocrine neoplasm. However, the existence of ONB with some degree of epithelial differentiation has long been acknowledged in the literature. Older studies reported highly variable rates of keratin positivity in ONB, with prevalence ranging from 0 to 40% [34–40]. Although most cases show only focal low molecular weight keratin staining, more diffuse pankeratin expression has also been noted. Recent reports of a subset of putative ONB that were strongly keratin positive and harbored IDH2 mutations further exacerbated this issue, as discussed below [41–43]. Additionally, otherwise conventional ONB can also demonstrate epithelial differentiation in the form of well-developed glands that are intermixed with the keratin-negative neuroectodermal cells [23, 44]. In some ways, this phenomenon is unsurprising, as the normal olfactory membrane is home to several keratin-positive cell types including glandular cells [9]. Moreover, ONB is prone to divergent differentiation in general, with other rare but well-documented elements including melanin pigmentation, rhabdomyoblastic cells, and ganglion cells [23, 44, 45]. Nevertheless, the question of how much keratin expression to allow in ONB creates fundamental challenges for its definition and diagnosis.
How much Neuroendocrine Differentiation is Allowed in Sinonasal Undifferentiated Carcinoma?
Another key problem that confounds the diagnosis of these tumors is the degree of neuroendocrine differentiation that is allowable in SNUC. Currently, SNUC is defined as a carcinoma that lacks any specific line of differentiation [46]—a description that seems to negate the possibility of a genuine neuroendocrine component. However, initial description of SNUC by Frierson et al. characterized SNUC as a high grade tumor that lacked squamous or glandular differentiation, but actually allowed these tumors to show neuroendocrine differentiation, largely due to some neuron-specific enolase expression by immunohistochemistry and the presence of rare neurosecretory granules by electron microscopy [47]. Mills later proposed that such abortive neuroendocrine differentiation in SNUC could qualify it for the large cell neuroendocrine carcinoma category [48]. While allowance for significant neuroendocrine differentiation was removed from later definitions of this entity, the tendency to classify SNUC as a neuroendocrine tumor has persisted in both clinical and pathologic literature [7, 23, 32, 44]. Although diffuse positivity for neuroendocrine markers certainly excludes tumors from the SNUC category, there is still no consensus as to whether SNUC can show focal expression of neuroendocrine markers—and if it should truly be regarded within the neuroendocrine tumor spectrum.
How Should Tumors with Mixed Neuroectodermal and Epithelial Features be Classified?
The final problem that complicates neuroendocrine tumor diagnosis is the existence of tumors that genuinely straddle the boundaries between ONB and neuroendocrine carcinoma. Over the years, occasional challenging tumors have been reported that demonstrate neuroepithelial differentiation, including neuroectodermal elements that overlap with ONB as well as epithelial features in the form of extensive keratin positivity or abundant gland formation. Of course, this issue dovetails with the question of epithelial differentiation in ONB, and some observers have fully lumped tumors with these findings into the ONB category or described them as ONB with divergent epithelial differentiation [38, 49–51]. However, the epithelial elements in these tumor are generally so well-developed that many authors have recognized them as outside the conventional ONB spectrum, with some regarding them as hybrid tumors under the names combined ONB and carcinoma [52–54] or mixed lineage ONB [55] and others using entirely separate terminology, including blastomatous variant of sinonasal adenocarcinoma [56], olfactory neuroepithelioma [57–60], and olfactory carcinoma [55, 61]. Because these divergent classifications have largely been presented in case reports and small series, it has been unclear whether these neuroepithelial tumors represent a recurrent and unified pathologic phenomenon or extremely rare and individual anomalies and whether they are more closely related to ONB or sinonasal neuroendocrine carcinoma. As such, tumors with this appearance have never been formally addressed in the WHO classification, providing pathologists no guidance about their classification.
Olfactory Carcinoma
Since the publication of the 5th edition WHO Classification of Head and Neck Tumours, there has been an increasing effort to better classify sinonasal tumors with neuroepithelial differentiation. Despite the piecemeal description of these tumors in previous literature, emerging evidence suggests that most should be regarded as a distinctive entity with the proposed name olfactory carcinoma.
Characterization of Olfactory Carcinoma
Recently, our group performed a comprehensive clinicopathologic evaluation of 53 tumors with mixed neuroectodermal and epithelial elements [62]. Despite the widely divergent terminology used to describe such neuroepithelial neoplasms in the past, we determined that the vast majority of these tumors had a recurrent and recognizable histologic mix of high grade, keratin-positive neuroectodermal cells and well-formed glands, as described in greater detail in the next paragraph. They disproportionately affected young to middle aged men, with a 3.4:1 male:female ratio and 40% of cases arising in patients younger than 40. These tumors also showed consistently aggressive clinical behavior, including extension beyond the sinonasal tract at presentation in 51%, persistent, recurrent, or metastatic disease in 48%, and death from disease in 28% in limited follow up. Progression occurred at a median interval of 8 months—a substantially quicker timeline than most ONB. We subsequently evaluated 24 neuroepithelial tumors at the molecular level [63]. We determined that the aforementioned histologic and clinical characteristics are closely tied to a discrete molecular profile, encompassing recurrent Wnt pathway mutations affecting CTNNB1 and PPP2R1A, ARID1A inactivation, and RUNX1 frameshift mutations. While several tumors had additional mutations in SWI/SNF complex genes SMARCA4, SMARCB1, and ARID1B, most of these alterations were variants of uncertain significance that were not associated with immunohistochemical loss, and their functional implications are unclear. Based on these findings, we proposed that this distinctive group of neuroepithelial tumors merit recognition as a unique entity- olfactory carcinoma.
Pathologic Features of Olfactory Carcinoma
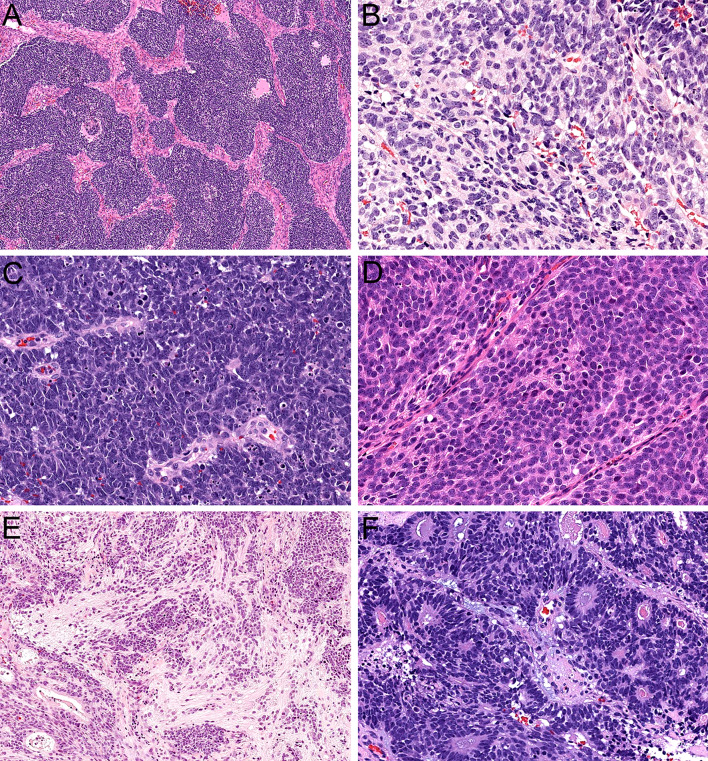
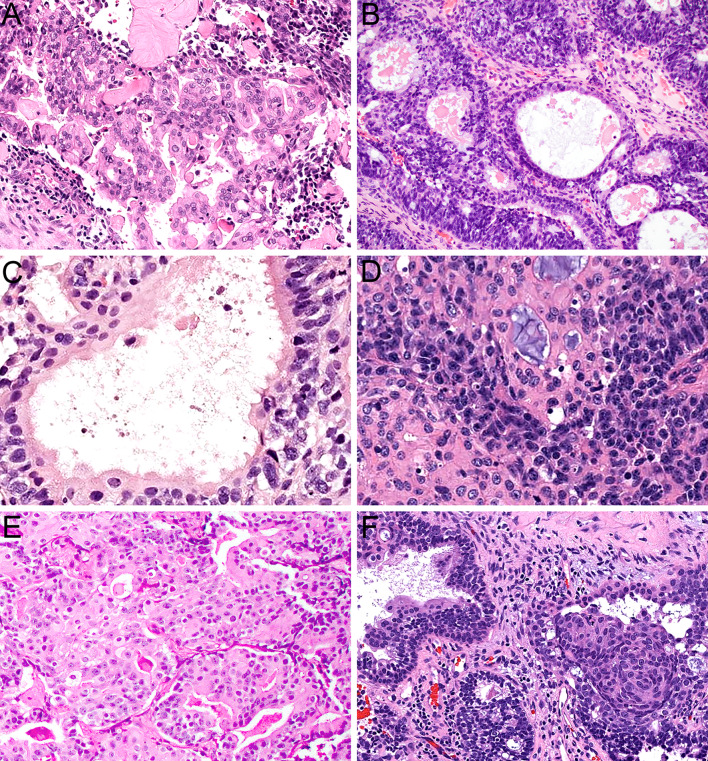
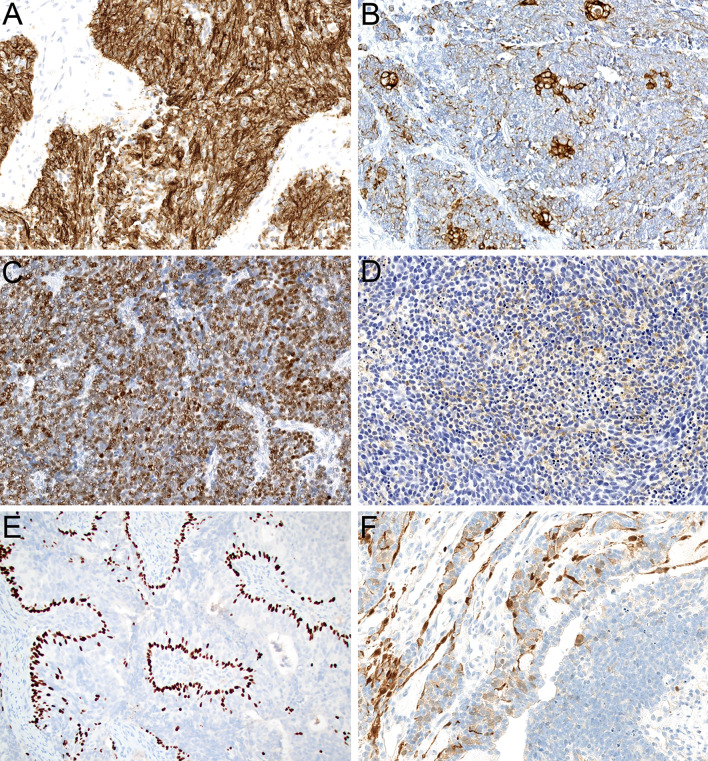
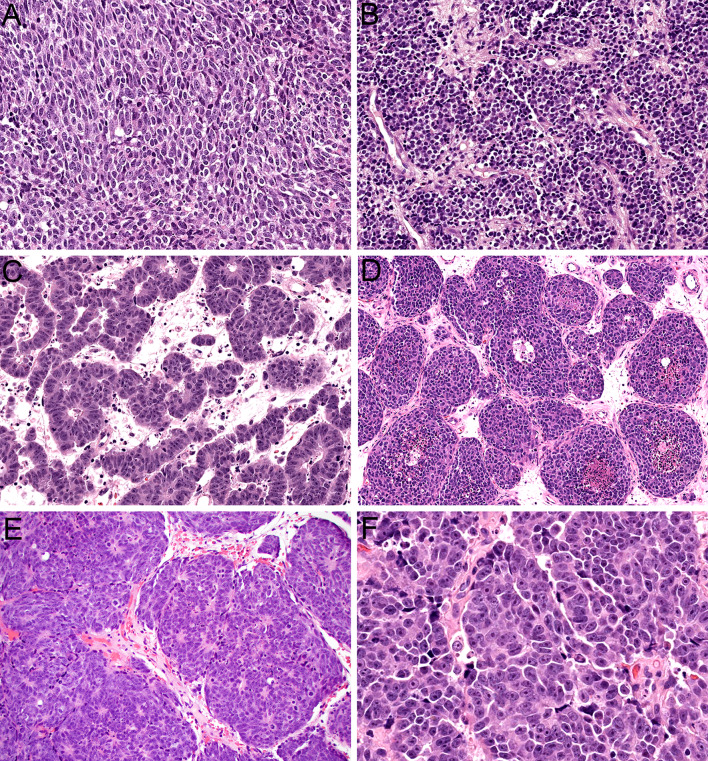
Histologically, olfactory carcinoma is defined by a predominant population of primitive neuroectodermal cells. These cells are arranged in expansile sheets and large lobules (Fig. 3A) and have syncytial cytoplasm that ranges from scant to abundant (Fig. 3B). They are uniformly high grade, with large, hyperchromatic, angulated nuclei that show cell-cell wrapping, nuclear molding, speckled chromatin, abundant apoptotic bodies, and frequent mitotic activity (Fig. 3C). Additionally, these neuroectodermal cells often show overt neural differentiation with variable amounts of neurofibrillary matrix material (Fig. 3D) including occasional expansile areas with intermixed ganglion cells (Fig. 3E) as well as prominent rosette formation (Fig. 3F). Almost all cases of olfactory carcinoma also display well-formed glandular structures intermixed with these neuroectodermal elements. These glands include a mix of complex, expansile proliferations of back-to-back acini (Fig. 4A) as well as ducts and tubules that are intimately embedded among the neuroectodermal cells (Fig. 4B). They often show well-formed cilia (Fig. 4C) and mucin production and generally have more low-grade cytology than surrounding neuroectodermal cells with round to oval nuclei and uniform chromatin (Fig. 4D). The degree of architectural complexity and confluence seen in the glands and the extent to which they are enmeshed with the neuroectodermal cells differentiates these glands from surface mucosa and supports that they are part of the neoplasm despite their relatively bland cytology. In areas, it can also be quite challenging to differentiate glands from true rosettes (Fig. 4E), but the cytologic features can help resolve this issue as well. Rare cases also have squamous morules that are closely associated with the glands (Fig. 4F). Immunohistochemically, olfactory carcinomas are consistently positive for keratin. They generally show strong expression of low molecular weight keratin Cam 5.2 (Fig. 5A) but have variable staining for pankeratin AE1/AE3 with consistently stronger expression in glandular structures compared to neuroectodermal cells (Fig. 5B). They also show positivity for neuroendocrine markers in the neuroectodermal cells that can range from diffuse to focal, including INSM1 (Fig. 5C), synaptophysin (Fig. 5D), and chromogranin. Interestingly, a majority of cases demonstrate patchy expression of p63 and p40 at the periphery of the neuroectodermal elements (Fig. 5E). Additionally, olfactory carcinomas display frequent positivity for calretinin similar to ONB and a subset have S100-protein-positive sustentacular networks (Fig. 5F). Importantly, these histologic and immunohistochemical features are indistinguishable across tumors with Wnt pathway, ARID1A, or RUNX1 alterations.
Fig. 3.
Olfactory carcinoma is predominantly composed of sheets and lobules of neuroectodermal cells (A, × 4). These cells have syncytial cytoplasm that ranges from scant to abundant (B, × 40). The nuclei are hyperchromatic and angulated with prominent nuclear molding and cell-cell wrapping (C, × 40). Most cases display overt neural differentiation with variable amounts of neurofibrillary matrix (D, × 40) including occasional expansile neurofibrillary zones with ganglion cells (E, × 10). Many cases also show well-formed true rosettes (F, × 20)
Fig. 4.
Olfactory carcinoma also displays a well-developed glandular component that can include either complex, expansile proliferations of back-to-back acini (A, × 20) or simple ducts and tubules that are closely intermixed with surrounding neuroectodermal cells (B, × 20). Many of the glands show well-developed cilia formation (C, × 40). The glandular cells have abundant eosinophilic cytoplasm and round to oval nuclei that lack the atypia of surrounding neuroectodermal cells (D, × 40). It can be difficult to differentiate glands from rosettes in some cases (E, × 20). Rare cases show squamous morules intermixed with the glands (F, × 20)
Fig. 5.
Olfactory carcinoma generally shows strong positivity for low molecular weight keratin Cam 5.2 (A, × 20) with variable expression of pankeratin AE1/AE3 that is stronger in glands than neuroectodermal cells (B, × 20). The neuroectodermal cells also display variable expression of neuroendocrine markers including INSM1 (C, × 20) and synaptophysin (D, × 20). A subset of cases show peripheral p40 expression in the neuroectodermal cells (E, × 20) and S100-protein-positive sustentacular cells (F, × 20)
Taxonomy of Olfactory Carcinoma
Not only does olfactory carcinoma have a unique pathologic profile that supports its recognition as an independent entity, but its histologic and molecular features are distinct from ONB. While some observers previously lumped sinonasal neuroepithelial tumors into the ONB spectrum, the presence of high grade cytology, a complex glandular component, and keratin positivity collectively push these tumors beyond the conventional borders of ONB. Indeed, recognition of olfactory carcinoma may help resolve the controversy of how much keratin expression in allowable in ONB by providing a separate category for neuroectodermal tumors with the most well-developed epithelial features. Moreover, recurrent Wnt pathway, ARID1A, and RUNX1 alterations in olfactory carcinoma further differentiate it from ONB, which does not have a well-defined molecular profile [12–15]. Conversely, olfactory carcinoma demonstrates histologic and molecular overlap with sinonasal teratocarcinosarcoma (TCS) and sinonasal small cell neuroendocrine carcinoma. Both olfactory carcinoma and TCS are multilineage malignancies with prominent primitive neuroectodermal components and have alterations in Wnt pathway and SWI/SNF complex genes, although olfactory carcinoma lacks the mesenchymal or fetal elements and full SMARCA4 inactivation characteristic of TCS [64, 65]. Likewise, the neuroectodermal component of olfactory carcinoma is histologically indistinguishable from the presumed neuroendocrine cells of sinonasal small cell carcinoma, which also harbors overlapping Wnt pathway and ARID1A mutations despite a lack of overt neural differentiation or gland formation [27]. Interestingly, although detailed histologic descriptions were not provided, Jurmeister et al. recently reported that sinonasal neuroendocrine tumors with ARID1A, CTNNB1, and SMARCA4 mutations had overlapping methylation profiles that were distinct from ONB and IDH2-mutant carcinomas [66]. These findings raise the possibility of a spectrum of primitive and multilineage neuroectodermal and neuroendocrine neoplasms that includes the distinctive histologic and molecular subgroups of TCS, sinonasal small cell carcinoma, and olfactory carcinoma.
IDH2-Mutant Sinonasal Carcinoma
Sinonasal tumors with IDH2 mutations have also been investigated in substantially more detail since the publication of the 5th edition WHO classification of tumors. While these tumors currently are classified in separate categories of SNUC and large cell neuroendocrine carcinoma, as well as rare tumors with neuroepithelial differentiation, there is increasing consensus that IDH2-mutant sinonasal carcinomas should be regarded as a single molecularly-defined entity.
Characterization of IDH2-Mutant Sinonasal Carcinomas
In the last few years, IDH2 mutations have been documented in an increasingly-diverse spectrum of sinonasal malignancies. Jo et al. initially identified IDH2 R172X mutations in the majority of sinonasal tumors still regarded as SNUC after re-classification of various newly-recognized entities- a finding that was validated by Dogan et al. [67, 68]. Dogan et al. also reported that the majority of sinonasal large cell neuroendocrine tumors harbor IDH2 R172X mutations [27]. Subsequent reports that tumors described as keratin-positive ONB had IDH2 mutations [41, 42] complicated the understanding of this group of tumors. However, consensus review by Glöss et al. determined that the majority of these putative ONB were probably better classified as LCNEC [43]. Nevertheless, a small subset of tumors with IDH2 mutations do appear to have true neuroepithelial differentiation that overlaps with olfactory carcinoma, including neuroectodermal elements such as neurofibrillary stroma and S100 protein-positive sustentacular cells despite keratin positivity [43, 63]. Regardless of histologic pattern, sinonasal tumors with IDH2 mutations cluster together on DNA methylation analysis, showing a hypermethylator phenotype similar to IDH2-mutant gliomas [27, 41–43, 66]. They also display consistent clinical behavior across histologic patterns, with a 5-year survival of 59%- significantly better outcomes than SMARCB1-deficient sinonasal carcinomas or SNUC that lack IDH2 mutations [43]. Based on these findings, Glöss et al. proposed that IDH2-mutant sinonasal tumors should be regarded as a single entity [43].
Pathologic Features of IDH2-Mutant Sinonasal Carcinoma
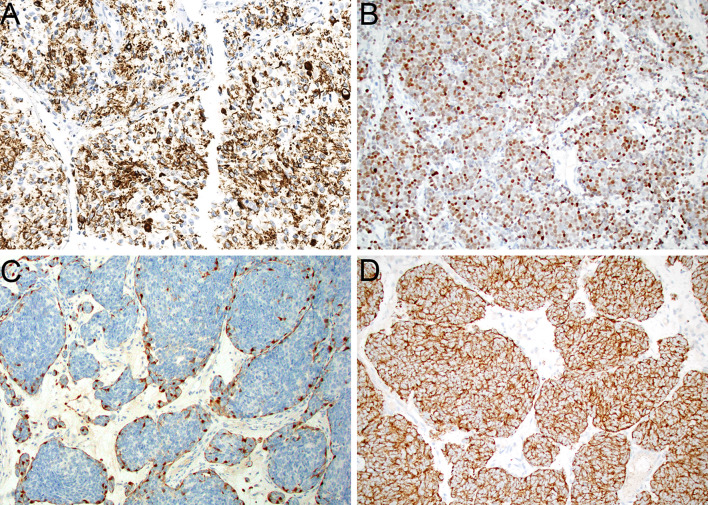
Histologically, IDH2-mutant sinonasal carcinomas fall within a diverse but unified spectrum, although they do not have extremely distinctive morphologic features. These tumors show a wide range of architectural patterns, with frequent sheet-like growth in undifferentiated carcinomas (Fig. 6A), more lobulated (Fig. 6B), trabecular, and corded architecture in neuroendocrine carcinomas (Fig. 6C), and formation of extremely compact nests (Fig. 6D) and well-developed rosettes and neurofibrillary matrix (Fig. 6E) in those cases with overt neuroepithelial differentiation. In limited experience, the neuroepithelial tumors seem to lack the prominent glandular component that is consistently present in olfactory carcinoma [63]. Regardless of line of differentiation, these tumors are high grade, with an elevated mitotic rate, abundant apoptotic bodies, and frequent zones of tumor necrosis. The tumor cells have indistinct borders with large oval nuclei with irregular nuclear membranes and vesicular chromatin. Notably, prominent macronucleoli (Fig. 6F) seem to be one of the most unique and consistent features of these tumors, being seen in 90% of cases across phenotypes [43]. Immunohistochemically, IDH2-mutant sinonasal carcinomas consistently show positivity for keratins AE1/AE3 (Fig. 7A) and Cam 5.2, although the intensity of staining can vary widely. They also demonstrate a range of expression of neuroendocrine markers that ranges from absent in tumors with an undifferentiated phenotype to variable positivity for synaptophysin, chromogranin, and INSM1 (Fig. 7B) in tumors with neuroendocrine or neuroepithelial features. Those neuroepithelial tumors also can show S100 protein positivity in a sustentacular distribution (Fig. 7C). Antibodies that recognize IDH2 R172X mutant proteins, usually in combination with its paralog IDH1 R132X, display granular cytoplasmic expression in these tumors (Fig. 7D) that provides a convenient immunohistochemical surrogate for confirming this diagnosis, although molecular testing remains more sensitive [67, 69, 70].
Fig. 6.
IDH2-mutant sinonasal carcinoma can show variable architecture, including sheet-like growth in undifferentiated cases (A, × 20). Tumors with neuroendocrine differentiation frequently display cells arranged in lobules and nests (B, × 20) or cords and trabeculae (C, × 20). Rare tumors with neuroepithelial differentiation are composed of compact nests (D, ×20) and demonstrate well-developed rosette formation and neurofibrillary matrix (E, × 20). Across histologic phenotypes, prominent macronucleoli are consistently seen (F, × 40)
Fig. 7.
IDH2-mutant sinonasal carcinomas are consistently positive for keratin (A, × 20). Tumors with neuroendocrine and neuroepithelial differentiation show expression of neuroendocrine markers such as INSM1 (B, × 20). Neuroepithelial tumors also frequently have an S100-protein-positive sustentacular network (C, × 20). Mutant IDH1/IDH2 immunohistochemistry demonstrates strong cytoplasmic positivity in tumor cells (D, × 20)
Taxonomy of IDH2-Mutant Sinonasal Carcinoma
The question of whether all sinonasal tumors with IDH2 mutations should be classified as a single category raises competing diagnostic priorities. When IDH2 mutations were initially reported only in SNUC, it did not immediately make sense to redefine a familiar entity based on a frequent but not universal molecular finding. However, with recognition of IDH2 mutations in LCNEC, the question of whether genetic alterations trump histologic phenotype became increasingly relevant. Indeed, identification of parallel mutations in SNUC and LCNEC provides some resolution to the question of how much neuroendocrine differentiation should be allowed in SNUC, as these IDH2-mutant tumors truly appear to be on a continuous spectrum of neuroendocrine differentiation. Moreover, the presence of an overt neuroepithelial phenotype in a small subset of tumors with IDH2 mutations make classification of these tumors using conventional histologic categories even more complicated, feeding into the controversy of how much epithelial differentiation can be seen in ONB. Of course, IDH2-mutant neuroepithelial tumors could be subsumed into the olfactory carcinoma category given their overlapping neuroectodermal and epithelial features—a classification that would mean IDH2 mutations would be recognized in three separate sinonasal entities. However, considering the common prognosis and methylation profile of all these IDH2-mutant tumors, it seems questionable whether they should really be divided. Pathologists are increasingly accepting that heterogeneous morphologies and immunoprofiles can be acceptable in molecularly defined tumor types, as exemplified by SWI/SNF-deficient sinonasal carcinomas. Recognition of IDH2-mutant sinonasal carcinomas as a heterogeneous category with variable undifferentiated, neuroendocrine, and neuroepithelial phenotypes seems to provide valuable clinical information while helping to resolve problematic issues in pathologic classification.
Conclusions
While the 5th edition WHO Classification of Head and Neck Tumours introduces dramatic changes in classification of sinonasal tumors in general, sinonasal neuroendocrine and neuroectodermal neoplasms have not been affected by the emerging molecular taxonomy. However, several persistent challenges that plague the diagnosis of these tumors lead to frequent disagreements in classification, suggesting that this area is ripe for improvement. Fortunately, several developments that have crystallized in the brief time since this volume was published suggest that introduction of two new entities supported by molecular analysis may help overcome these diagnostic difficulties. Recognition of olfactory carcinoma, an emerging entity defined by high-grade keratin-positive neuroectodermal cells with frequent gland formation that shows recurrent Wnt pathway, CTNNB1, and RUNX1 alterations, provides the first formal recognition of tumors with neuroepithelial features and helps resolve the question of how much epithelial differentiation should be allowed in ONB. Additionally, grouping sinonasal tumors with IDH2 mutations into a single category, regardless of undifferentiated, neuroendocrine, or neuroepithelial phenotype, eliminates the quandary of how much neuroendocrine differentiation is acceptable in SNUC. These developments would not only clarify the classification of sinonasal neuroendocrine and neuroectodermal neoplasms but also make molecular features central to their taxonomy for the first time.
Notably, these developments highlight important nuances to the sinonasal tumor classification that have implications beyond these specific entities. With recognition of neuroepithelial differentiation in both olfactory carcinoma and IDH2-mutant sinonasal carcinoma, it is evident that a neuroepithelial phenotype is not limited to a single sinonasal entity. This terminology can also be applied to classic ONB that truly show gland formation or focal keratin expression, the primitive components of sinonasal TCS, and even rare examples of solitary fibrous tumor with transdifferentiation [71]. Indeed, neuroepithelial differentiation may best be regarded as analogous to squamous or glandular differentiation—a histologic pattern that can be seen in multiple sinonasal diagnoses. Additionally, these entities highlight different ways that molecular testing can be applied to sinonasal tumor classification. In IDH2-mutant sinonasal carcinomas, the choice to let molecular findings trump divergent histologic and immunohistochemical phenotypes actually resolves quandaries in conventional classification. In contrast, while olfactory carcinoma has a recurrent molecular profile that differentiates it from ONB, its characteristic histologic findings are associated with several recurrent genetic alterations, negating the possibility of a mutation-specific diagnosis. As pathologists continue to make insights into the molecular underpinnings of sinonasal tumors in the future, it will be necessary to continue to balance molecular and histologic findings to achieve a meaningful and accessible classification.
Author Contributions
LMR performed literature review and prepared the manuscript. All authors read and approved the final paper.
Funding
This review had no specific funding.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The author certifies that she has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics Approval
Not applicable.
Consent to Participate for Publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thompson LDR, Bishop JA. Update from the 5th edition of the world health organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull Base. Head Neck Pathol. 2022;16:1–18. doi: 10.1007/s12105-021-01406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D, Classe M, Perez-Ordonez B et al (2022) Olfactory neuroblastoma. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Head and Neck Tumours. Lyon, France: International Agency for Research on Cancer
- 3.Mete O, Wenig BM. Update from the 5th edition of the world health organization classification of head and neck tumors: overview of the 2022 WHO classification of head and neck neuroendocrine neoplasms. Head Neck Pathol. 2022;16:123–142. doi: 10.1007/s12105-022-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooper LM, Classe M, Nose V et al (2022) Small cell neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Head and Neck Tumours. Lyon, France: International Agency for Research on Cancer
- 5.Rooper LM, Classe M, Nose V et al (2022) Large cell neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Head and Neck Tumours. Lyon, France: International Agency for Research on Cancer
- 6.Choi KY, Amit M, Tam S, et al. Clinical implication of diagnostic and histopathologic discrepancies in sinonasal malignancies. Laryngoscope. 2021;131:E1468–E1475. doi: 10.1002/lary.29102. [DOI] [PubMed] [Google Scholar]
- 7.Turri-Zanoni M, Maragliano R, Battaglia P, et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Zunitch MJ, Fisch AS, Lin B, et al. Molecular evidence for olfactory neuroblastoma as a tumor of malignant globose basal cells. Mod Pathol. 2023;35(6):100122. doi: 10.1016/j.modpat.2023.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holbrook EH, Wu E, Curry WT, et al. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol. 2018;42:665–671. doi: 10.1097/PAS.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 11.Wooff JC, Weinreb I, Perez-Ordonez B, et al. Calretinin staining facilitates differentiation of olfactory neuroblastoma from other small round blue cell tumors in the sinonasal tract. Am J Surg Pathol. 2011;35:1786–1793. doi: 10.1097/PAS.0b013e3182363b78. [DOI] [PubMed] [Google Scholar]
- 12.Gay LM, Kim S, Fedorchak K, et al. Comprehensive genomic profiling of Esthesioneuroblastoma reveals additional treatment options. Oncologist. 2017;22:834–842. doi: 10.1634/theoncologist.2016-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur RP, Izumchenko E, Blakaj DM, et al. The genomics and epigenetics of olfactory neuroblastoma: a systematic review. Laryngoscope Investig Otolaryngol. 2021;6:721–728. doi: 10.1002/lio2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Lazo L, McHugh JB, Cani AK, et al. Comprehensive molecular profiling of olfactory neuroblastoma identifies potentially targetable FGFR3 amplifications. Mol Cancer Res. 2017;15:1551–1557. doi: 10.1158/1541-7786.MCR-17-0135. [DOI] [PubMed] [Google Scholar]
- 15.Topcagic J, Feldman R, Ghazalpour A, et al. Comprehensive molecular profiling of advanced/metastatic olfactory neuroblastomas. PLoS ONE. 2018;13:e0191244. doi: 10.1371/journal.pone.0191244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallia GL, Zhang M, Ning Y, et al. Genomic analysis identifies frequent deletions of dystrophin in olfactory neuroblastoma. Nat Commun. 2018;9:5410. doi: 10.1038/s41467-018-07578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell D, Saade R, Roberts D, et al. Prognostic utility of hyams histological grading and kadish-morita staging systems for esthesioneuroblastoma outcomes. Head Neck Pathol. 2015;9:51–59. doi: 10.1007/s12105-014-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32:706–714. doi: 10.1227/00006123-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kaur G, Kane AJ, Sughrue ME, et al. The prognostic implications of hyam’s subtype for patients with kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013;20:281–286. doi: 10.1016/j.jocn.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malouf GG, Casiraghi O, Deutsch E, et al. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013;49:1324–1334. doi: 10.1016/j.ejca.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Van Gompel JJ, Giannini C, Olsen KD, et al. Long-term outcome of esthesioneuroblastoma: hyams grade predicts patient survival. J Neurol Surg B Skull Base. 2012;73:331–336. doi: 10.1055/s-0032-1321512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma: a clinical analysis of 17 cases. Cancer. 1976;37:1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::AID-CNCR2820370347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Bell D. Sinonasal neuroendocrine neoplasms: current challenges and advances in diagnosis and treatment, with a focus on olfactory neuroblastoma. Head Neck Pathol. 2018;12:22–30. doi: 10.1007/s12105-018-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell D, Hanna EY, Weber RS, et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck. 2016;38(Suppl 1):E2259–2266. doi: 10.1002/hed.24152. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell EH, Diaz A, Yilmaz T, et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012;34:1372–1376. doi: 10.1002/hed.21940. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Ordonez B, Caruana SM, Huvos AG, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998;29:826–832. doi: 10.1016/S0046-8177(98)90452-X. [DOI] [PubMed] [Google Scholar]
- 27.Dogan S, Vasudevaraja V, Xu B, et al. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol. 2019;32:1447–1459. doi: 10.1038/s41379-019-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuan EC, Alonso JE, Tajudeen BA, et al. Small cell carcinoma of the head and neck: a comparative study by primary site based on population data. Laryngoscope. 2017;127:1785–1790. doi: 10.1002/lary.26406. [DOI] [PubMed] [Google Scholar]
- 30.Rivero A, Liang J. Sinonasal small cell neuroendocrine carcinoma: a systematic review of 80 patients. Int Forum Allergy Rhinol. 2016;6:744–751. doi: 10.1002/alr.21734. [DOI] [PubMed] [Google Scholar]
- 31.van der Laan TP, Bij HP, van Hemel BM, et al. The importance of multimodality therapy in the treatment of sinonasal neuroendocrine carcinoma. Eur Arch Otorhinolaryngol. 2013;270:2565–2568. doi: 10.1007/s00405-013-2554-5. [DOI] [PubMed] [Google Scholar]
- 32.van der Laan TP, Iepsma R, Witjes MJ, et al. Meta-analysis of 701 published cases of sinonasal neuroendocrine carcinoma: the importance of differentiation grade in determining treatment strategy. Oral Oncol. 2016;63:1–9. doi: 10.1016/j.oraloncology.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Thompson ED, Stelow EB, Mills SE, et al. Large cell neuroendocrine carcinoma of the Head and Neck: a clinicopathologic series of 10 cases with an emphasis on HPV Status. Am J Surg Pathol. 2016;40:471–478. doi: 10.1097/PAS.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argani P, Perez-Ordonez B, Xiao H, et al. Olfactory neuroblastoma is not related to the ewing family of tumors: absence of EWS/FLI1 gene fusion and MIC2 expression. Am J Surg Pathol. 1998;22:391–398. doi: 10.1097/00000478-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Axe S, Kuhajda FP. Esthesioneuroblastoma: intermediate filaments, neuroendocrine, and tissue-specific antigens. Am J Clin Pathol. 1987;88:139–145. doi: 10.1093/ajcp/88.2.139. [DOI] [PubMed] [Google Scholar]
- 36.Frierson HF, Jr, Ross GW, Mills SE, et al. Olfactory neuroblastoma: additional immunohistochemical characterization. Am J Clin Pathol. 1990;94:547–553. doi: 10.1093/ajcp/94.5.547. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima S, Sugita Y, Niino D, et al. Clincopathological analysis of olfactory neuroblastoma. Brain Tumor Pathol. 2012;29:207–215. doi: 10.1007/s10014-012-0083-3. [DOI] [PubMed] [Google Scholar]
- 38.Hirose T, Scheithauer BW, Lopes MB, et al. Olfactory neuroblastoma. An immunohistochemical, ultrastructural, and flow cytometric study. Cancer. 1995;76:4–19. doi: 10.1002/1097-0142(19950701)76:1<4::AID-CNCR2820760103>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 39.Taxy JB, Bharani NK, Mills SE, et al. The spectrum of olfactory neural tumors: a light-microscopic immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1986;10:687–695. doi: 10.1097/00000478-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Wick MR, Stanley SJ, Swanson PE. Immunohistochemical diagnosis of sinonasal melanoma, carcinoma, and neuroblastoma with monoclonal antibodies HMB-45 and anti-synaptophysin. Arch Pathol Lab Med. 1988;112:616–620. [PubMed] [Google Scholar]
- 41.Capper D, Engel NW, Stichel D, et al. DNA methylation-based reclassification of olfactory neuroblastoma. Acta Neuropathol. 2018;136:255–271. doi: 10.1007/s00401-018-1854-7. [DOI] [PubMed] [Google Scholar]
- 42.Classe M, Yao H, Mouawad R, et al. Integrated multi-omic analysis of esthesioneuroblastomas identifies two subgroups linked to cell ontogeny. Cell Rep. 2018;25:811–821. doi: 10.1016/j.celrep.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 43.Gloss S, Jurmeister P, Thieme A, et al. IDH2 R172 mutations across poorly differentiated Sinonasal tract malignancies: forty molecularly homogenous and histologically variable cases with favorable outcome. Am J Surg Pathol. 2021;45:1190–1204. doi: 10.1097/PAS.0000000000001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faragalla H, Weinreb I. Olfactory neuroblastoma: a review and update. Adv Anat Pathol. 2009;16:322–331. doi: 10.1097/PAP.0b013e3181b544cf. [DOI] [PubMed] [Google Scholar]
- 45.Shah K, Perez-Ordonez B. Neuroendocrine neoplasms of the sinonasal Tract: neuroendocrine carcinomas and olfactory neuroblastoma. Head Neck Pathol. 2016;10:85–94. doi: 10.1007/s12105-016-0696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jo VY, Agaimy A, Franchi A et al (2022) Sinonasal undifferentiated carcinoma. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Head and Neck Tumours. Lyon, France: International Agency for Research on Cancer
- 47.Frierson HF, Jr, Mills SE, Fechner RE, et al. Sinonasal undifferentiated carcinoma. an aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–779. doi: 10.1097/00000478-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol. 2002;15:264–278. doi: 10.1038/modpathol.3880522. [DOI] [PubMed] [Google Scholar]
- 49.Charles NC, Petris CK, Kim ET. Aggressive esthesioneuroblastoma with divergent differentiation: a taxonomic dilemma. Orbit. 2016;35:357–359. doi: 10.1080/01676830.2016.1193537. [DOI] [PubMed] [Google Scholar]
- 50.Meyer C, Hamersley ERS, Manosalva RE, et al. Olfactory neuroblastoma with divergent differentiation: an unusual histologic finding in a rare tumor. Head Neck Pathol. 2017;11:531–536. doi: 10.1007/s12105-017-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura K, Mineta H, Yokota N, et al. Olfactory neuroblastoma with epithelial and endocrine differentiation transformed into ganglioneuroma after chemoradiotherapy. Pathol Int. 2001;51:942–947. doi: 10.1046/j.1440-1827.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 52.Attwood JE, Jeyaretna DS, Sheerin F, et al. Mixed olfactory neuroblastoma and adenocarcinoma with in situ neuroendocrine hyperplasia. Head Neck Pathol. 2020;14:792–798. doi: 10.1007/s12105-019-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhoke CS, Dewan A, Gupta D, et al. A rare case report of mixed olfactory neuroblastoma: carcinoma with review of literature. Surg Neurol Int. 2017;8:83. doi: 10.4103/sni.sni_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller DC, Goodman ML, Pilch BZ, et al. Mixed olfactory neuroblastoma and carcinoma. A report of two cases. Cancer. 1984;54:2019–2028. doi: 10.1002/1097-0142(19841101)54:9<2019::AID-CNCR2820540940>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Seethala RR, Wenig BM, Barnes EL, et al. Olfactory neuroblastoma with divergent differentiation: from ganglioneuroblastoma to carcinoma. Mod Pathol. 2007;20:229A. [Google Scholar]
- 56.Stelow EB, Mills SE, Jo VY, et al. Adenocarcinoma of the upper aerodigestive tract. Adv Anat Pathol. 2010;17:262–269. doi: 10.1097/PAP.0b013e3181e3bf80. [DOI] [PubMed] [Google Scholar]
- 57.Hassoun J, Gambarelli D, Grisoli F, et al. Esthesioneuroepithelioma, a true neurosensorial tumor. Light- and electron-microscopic study of a case with endocranial extension. Acta Neuropathol. 1981;55:77–80. doi: 10.1007/BF00691536. [DOI] [PubMed] [Google Scholar]
- 58.Sugita Y, Kusano K, Tokunaga O, et al. Olfactory neuroepithelioma: an immunohistochemical and ultrastructural study. Neuropathology. 2006;26:400–408. doi: 10.1111/j.1440-1789.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi H, Ohara S, Yamada M, et al. Esthesioneuroepithelioma: a tumor of true olfactory epithelium origin. An ultrastructural and immunohistochemical study. Acta Neuropathol. 1987;75:147–155. doi: 10.1007/BF00687075. [DOI] [PubMed] [Google Scholar]
- 60.Utsuki S, Kawano N, Oka H, et al. Olfactory neuroepithelioma arising from the olfactory placode. Clin Neuropathol. 2000;19:7–12. [PubMed] [Google Scholar]
- 61.Wenig BM. Atlas of head and neck pathology. Cambridge: Elsevier; 2015. Olfactory carcinoma; pp. 155–156. [Google Scholar]
- 62.Rooper LM, Bishop JA, Faquin WC, et al. Sinonasal tumors with neuroepithelial differentiation (olfactory carcinoma): delineation of their pathologic and clinical features with insights into their relationship to olfactory neuroblastoma and sinonasal carcinoma. Am J Surg Pathol. 2022;46:1025–1035. doi: 10.1097/PAS.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 63.Rooper LM, Gagan J, Nishino M, et al. USCAP 2022 abstracts: head and neck pathology (800–850) Mod Pathol. 2022;35:960–1015. doi: 10.1038/s41379-022-01040-8. [DOI] [PubMed] [Google Scholar]
- 64.Rooper LM, Agaimy A, Gagan J, et al. Comprehensive molecular profiling of sinonasal teratocarcinosarcoma highlights recurrent SMARCA4 inactivation and CTNNB1 mutations. Am J Surg Pathol. 2022;47:224–233. doi: 10.1097/PAS.0000000000001976. [DOI] [PubMed] [Google Scholar]
- 65.Rooper LM, Uddin N, Gagan J, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44:1331–1339. doi: 10.1097/PAS.0000000000001508. [DOI] [PubMed] [Google Scholar]
- 66.Jurmeister P, Gloss S, Roller R, et al. DNA methylation-based classification of sinonasal tumors. Nat Commun. 2022;13:7148. doi: 10.1038/s41467-022-34815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dogan S, Chute DJ, Xu B, et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol. 2017;242:400–408. doi: 10.1002/path.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo VY, Chau NG, Hornick JL, et al. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017;30:650–659. doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 69.Dogan S, Frosina D, Geronimo JA, et al. Molecular epidemiology of IDH2 hotspot mutations in cancer and immunohistochemical detection of R172K, R172G, and R172M variants. Hum Pathol. 2020;106:45–53. doi: 10.1016/j.humpath.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mito JK, Bishop JA, Sadow PM, et al. Immunohistochemical detection and molecular characterization of IDH-mutant sinonasal undifferentiated carcinomas. Am J Surg Pathol. 2018;42:1067–1075. doi: 10.1097/PAS.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 71.Stevens TM, Rooper LM, Bacchi CE, et al. Teratocarcinosarcoma-like and adamantinoma-like head and neck neoplasms harboring NAB2::STAT6: unusual variants of solitary fibrous tumor or novel tumor entities? Head Neck Pathol. 2022;16(3):746–754. doi: 10.1007/s12105-022-01444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.