Abstract
Background
Respiratory Epithelial Adenomatoid Hamartoma (REAH) is an uncommon, benign tumor of the sinonasal tract. It can, however, be confused with a sinonasal malignancy causing undo morbidity to patients. Therefore, the clinical as well as histological diagnosis is crucial in order to correctly care for patients.
Methods
This review of a patient, to include their clinical pictures, radiologic pictures, and histologic pictures, allow for the clinician to accurately evaluate and diagnose REAH.
Results
Our patient presented with a classic bilateral olfactory cleft mass on endoscopic exam. CT was obtained showing a non-enhancing homogenous mass, widening the olfactory cleft, with no evidence of skull base defects or bony erosion. MRI was additionally obtained, given the location, showing a homogenous cribriform mass with clearly defined borders with post-contrast enhancement on T1-weighted images and hyperintense T2-weighted images. A biopsy in clinic was done, showing small to medium, round to oval shaped glands lined with ciliated respiratory epithelium and separated by stroma. The surface epithelium extends into the submucosa, communicating with the proliferating glands.
Conclusion
Our patient, presented in this case report, shows a classic presentation of REAH. Using these findings, patients can be better counseled on this benign entity, ranging from observation to surgical intervention.
Keywords: Respiratory epithelial adenomatoid hamartoma, Sinonasal mass, Nasal cavity mass
History
A 68 year old male with a history of seasonal allergic rhinitis and cardiac disease presented to the ENT clinic after an incidental finding of bilateral olfactory recess masses on computed tomography (CT) of the sinuses. The masses were originally identified as non-enhancing on positron emission tomography (PET)/CT in 2016 and appeared unchanged on recent CT. The patient also endorsed a history of nasal congestion. On endoscopic exam, a polypoid mass was seen filling the olfactory cleft medial to the middle turbinate on the right side (Fig. 1). Complete visualization of the left side was difficult due to a septal deviation.
Fig. 1.
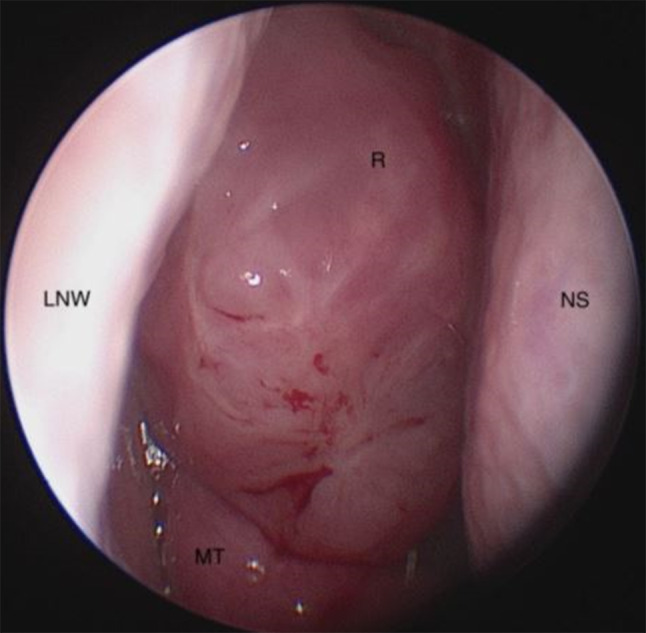
Endoscopic view of the patient's right nasal cavity showing REAH (R) anterior and medial to the middle turbinate (MT), filling the olfactory cleft with the nasal septum (NS) and lateral Nasal Wall (LNW) visible
Radiographic Features
A CT maxillofacial with contrast was obtained showing bilateral polypoid soft tissue masses within the bilateral olfactory recesses (Fig. 2a). There was no evidence of bony remodeling. The cribriform plate was intact and there was no evidence of intracranial extension. The olfactory recesses were enlarged. Magnetic Resonance Imaging was performed to further evaluate the soft tissue masses and skull base (Fig. 2b–d). The masses appeared predominantly isointense compared to brain parenchyma on T1 with some areas of hyperintensity, likely reflecting the trapped proteinaceous material. On T2, the mass was noted to be homogeneously hyperintense without evidence of intracranial or intraorbital extension. The mass was noted to be bilateral and appeared to emanate from the olfactory recess causing widening of the olfactory groove.
Fig. 2.
Coronal non-contrasted computed tomography (A), coronal T1 magnetic resonance imaging (MRI) with a mass isointense to brain (B), coronal T2 short tau inversion recovery MRI with homogenous hyperintensity (C), and coronal T1 post-contrast fat suppressed MRI with homogeneous enhancement (D) demonstrating a mass symmetrically filling and expanding the olfactory recesses and extending inferiorly into the nasal cavity (asterisk 2A) with no evidence of intracranial extension
Diagnosis
The masses were identified in clinic using a zero degree rigid Hopkins rod. It was noted to be non-vascular and not easily friable. After discussion with the patient and known imaging characteristics, the decision was made to biopsy the mass in clinic. Under local anesthesia and using direct visualization with a rigid endoscope, submucosal biopsies were taken of the right nasal mass. Histological examination of a hematoxylin and eosin stained slide demonstrated focal glandular hyperplasia, confirming the diagnosis of respiratory epithelial adenomatoid hamartoma (REAH) (Figs. 3, 4).
Fig. 3.
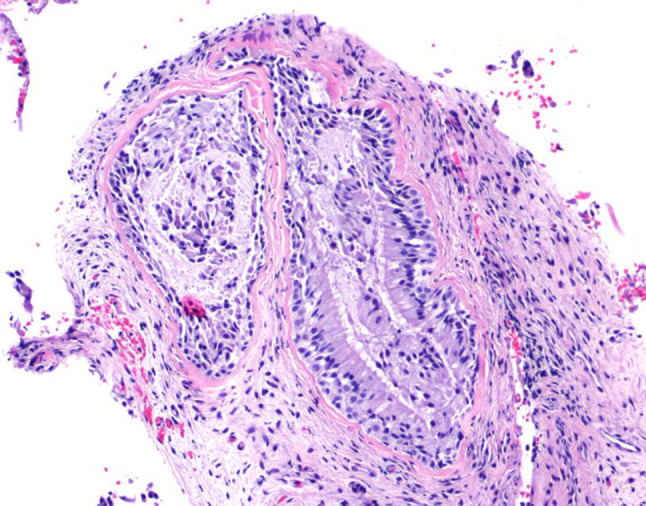
Medium power hematoxylin and eosin (H&E) view of glandular elements within fibrous connective tissue lined by enlarged respiratory epithelium with prominent cilia
Fig. 4.

High power H&E view of glandular element within fibrous connective tissue lined by enlarged respiratory epithelium with prominent cilia
Treatment
Due to the benign nature of REAH, the lack of bothersome symptoms, and significant comorbidities of the patient, no surgical treatment was undertaken. The patient will return for evaluation annually to ensure the mass is not enlarging or is becoming symptomatic.
Discussion
REAH is a benign tumor of the sinonasal tract, often misdiagnosed as nasal polyps or various sinonasal malignancies. REAH was first described in a case series by Wenig and Heffner in 1995, with only 13 cases of nasal hamartoma being described in the literature at that time [1]. A more recent retrospective evaluation published in 2016 by Davison et al. which showed an additional 23 cases, with most being reported between the years of 2011 to 2015 further demonstrating increased recognition of this entity [2]. REAH most often occurs in men in the third to ninth decades and is often found bilaterally in up to 50% of cases [1, 3]. Classically, REAH has been described as originating from the superior/posterior nasal septum and olfactory cleft. This tumor has also been reported to arise from the lateral nasal wall, nasopharynx, and paranasal sinuses [4].
Its etiology is controversial as it has been associated with both chronic sinonasal inflammation and neoplasia. Very limited data has been published regarding the genetics of this tumor. Ozolek et al. compared the molecular profiles of REAH, sinonasal adenocarcinoma and inflamed sinonasal mucosa in 2006 [5]. This study found higher than expected similarities between REAH and sinonasal adenocarcinoma in the percentage of fractional allelic loss and loss of heterozygosity, suggesting REAH may be a benign neoplasm rather than a hamartoma [5]. Other studies have attempted to delineate inflammatory etiologies of this neoplasm. One prospective study by Nguyen et al. compared nasal polyposis patients with and without olfactory cleft REAH. Their data showed the REAH group had a longer duration of nasal polyposis disease, increased number of previous sinus surgeries and increased incidence of asthma, supporting the hyperplastic theory of etiology [6]. A study published by Schertzer et al. showed REAH most commonly affects a location within the central compartment, and those affected also had a high incidence of allergy, concluding long-standing inflammatory changes may be the cause of REAH, as was seen in our patient [3]. Patients can present with a variety of symptoms, depending on the location of the tumor, including nasal obstruction, rhinorrhea, hyposmia, headache, facial pain, and epistaxis [4]. On gross examination, this tumor is often described as polypoid, with various consistencies (fleshy, firm), colors (white, yellow, and pink), and sizes [1, 4, 6]. Radiography is essential in the work up of this tumor. Differential diagnosis of a mass in this location includes a bilobed middle turbinate, nasal polyps, inverted papilloma, olfactory cleft tumor, low grade carcinoma, and encephalocele. Imaging can not only prevent morbidity of biopsies, but pathognomonic radiographic characteristics of REAH can differentiate it from other nasal masses. Radiologically, this slow growing tumor shows local bony expansion without intracranial or other local invasion [7]. Lateralization of the middle turbinate is a frequent radiographic finding [3]. REAH is often found in the olfactory cleft, and one study has suggested olfactory cleft widening (greater than 10 mm) can assist with distinguishing this benign process from a malignant one [8, 9]. This olfactory cleft widening is also seen in aspirin-exacerbated respiratory disease (AERD) and central compartment atopic disease, which are associated with allergic and chronic rhinitis [3].
Histologically, REAH is characterized by glandular proliferation of ciliated respiratory epithelium. The glands are often round to oval in shape, separated by stromal tissue [1]. There are no significant nuclear features found in REAH [1]. Focal glandular structures may be seen surrounded by a variably thickened, eosinophilic basement membrane. Immunohistochemistry shows REAH staining positive for CK7 and negative for CK20, CDX-2, and S-100 [4]. The basal cells of this tumor are p63 positive [4].
No consensus exists regarding the treatment of REAH. It is generally agreed upon that, although complete excision appears curative, surgical resection is not indicated unless local expansion causes uncontrollable symptoms [6, 10].
REAH is an uncommon nasal mass that is becoming increasingly recognized within the literature, often mimicking other nasal pathology, making the diagnosis difficult. The etiology of REAH is most likely multifactorial, therefore it is important to recognize patients who may be at increased risk in hopes that we can develop more understanding of the pathophysiology of this benign lesion. Correctly identifying REAH can avoid unnecessary and morbid treatment options. Overall, when a patient is identified with bilateral masses resulting in widening of the olfactory cleft without identifiable bone erosion on imaging, REAH should be high on the differential diagnosis.
Acknowledgements
None
Disclaimers
The views expressed in this publication reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. I am a military Service member. This work was prepared as part of my official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military Service member or employee of the U.S. Government as part of that person’s official duties.”
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KS, KT, and GC. The first draft of the manuscript was written by KZ with revisions completed by AS and GC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Code Availability
There was no data set or code used during this case study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study informed consent was obtained for any and all treatment.
Consent to Participate
For this type of study formal consent was obtained for any and all treatment.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fitzhugh VA, Mirani N. Respiratory epithelial adenomatoid hamartoma: a review. Head Neck Pathol. 2008;2:203–208. doi: 10.1007/s12105-008-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davison WL, Pearlman AN, Donatelli LA, Conley LM. Respiratory epithelial adenomatoid hamartomas: an increasingly common diagnosis in the setting of nasal polyps. Am J Rhinol Allergy. 2016;30(4):139–146. doi: 10.2500/ajra.2016.30.4338. [DOI] [PubMed] [Google Scholar]
- 3.Schertzer JS, Levy JM, Wise SK, Magliocca KR, DelGaudio JM. Is respiratory epithelial adenomatoid hamartoma related to central compartment atopic disease? Int Forum Allergy Rhinol. 2020;34(5):610–617. doi: 10.1177/1945892420914212. [DOI] [PubMed] [Google Scholar]
- 4.Bullock MJ. Low-grade epithelial proliferations of the sinonasal tract. Head Neck Pathol. 2016;10:47–59. doi: 10.1007/s12105-016-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozolek JA, Hunt JL. Tumor suppressor gene alterations in respiratory epithelial adenomatoid hamartoma (REAH): comparison to sinonasal adenocarcinoma and inflamed sinonasal mucosa. Am J Surg Pathol. 2006;30:1576–1580. doi: 10.1097/01.pas.0000213344.55605.77. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DT, Nguyen-Thi P-L, Gauchotte G, Arous F, Vignaud JM, Jankowski R. Predictors of respiratory epithelial adenomatoid hamartomas of the olfactory clefts in patients with nasal polyposis. Laryngoscope. 2014;124(11):2461–2465. doi: 10.1002/lary.24778. [DOI] [PubMed] [Google Scholar]
- 7.Hawley KA, Ahmed M, Sindwani R. CT findings of sinonasal respiratory epithelial adenomatoid hamartoma: a closer look at the olfactory clefts. AJNR Am J Neuroradiol. 2013;34:1086–1090. doi: 10.3174/ajnr.A3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safi C, Li C, Tabaee A, Ramakrishna R, Riley C. Outcomes and imaging findings of respiratory epithelial adenomatoid hamartoma: a systematic review. Int Forum Allergy Rhinol. 2019;9:1–7. doi: 10.1002/alr.22298. [DOI] [PubMed] [Google Scholar]
- 9.Braun J, Riehm S, Averous G, Billing A, Veillon F. MRI in respiratory epithelial adenomatoid hamartoma of nasal cavities. J Neurorad. 2013;40:216–227. doi: 10.1016/j.neurad.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Rom D, Lee M, Chandraratnam E, et al. Respiratory epithelial adenomatoid hamartoma: an important differential of sinonasal masses. Cureus. 2018;10(4):e2495. doi: 10.7759/cureus.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There was no data set or code used during this case study.



